Abstract
OBJECTIVE
Previous prospective studies on the association of white rice intake with incident diabetes have shown contradictory results but were conducted in single countries and predominantly in Asia. We report on the association of white rice with risk of diabetes in the multinational Prospective Urban Rural Epidemiology (PURE) study.
RESEARCH DESIGN AND METHODS
Data on 132,373 individuals aged 35–70 years from 21 countries were analyzed. White rice consumption (cooked) was categorized as <150, ≥150 to <300, ≥300 to <450, and ≥450 g/day, based on one cup of cooked rice = 150 g. The primary outcome was incident diabetes. Hazard ratios (HRs) were calculated using a multivariable Cox frailty model.
RESULTS
During a mean follow-up period of 9.5 years, 6,129 individuals without baseline diabetes developed incident diabetes. In the overall cohort, higher intake of white rice (≥450 g/day compared with <150 g/day) was associated with increased risk of diabetes (HR 1.20; 95% CI 1.02–1.40; P for trend = 0.003). However, the highest risk was seen in South Asia (HR 1.61; 95% CI 1.13–2.30; P for trend = 0.02), followed by other regions of the world (which included South East Asia, Middle East, South America, North America, Europe, and Africa) (HR 1.41; 95% CI 1.08–1.86; P for trend = 0.01), while in China there was no significant association (HR 1.04; 95% CI 0.77–1.40; P for trend = 0.38).
CONCLUSIONS
Higher consumption of white rice is associated with an increased risk of incident diabetes with the strongest association being observed in South Asia, while in other regions, a modest, nonsignificant association was seen.
Introduction
Globally, 425 million people currently have diabetes and this number is expected to increase to 629 million by 2045 (1). China and India, two countries in Asia where rice is the staple food, are also the top two countries in terms of the number of people with diabetes in the world (2). Rapid urbanization and economic development, especially in developing countries of the world, have led to a dramatic change in nutrition and dietary intake as well as in physical inactivity, both of which are related to the obesity and diabetes epidemics (3).
Carbohydrate forms 70–80% of the calories consumed in many South Asian countries (4). Till the early 1970s, most of the traditional diets, especially in India and some other Asian countries, were less milled or polished as it was manually hand pounded (5,6). Undermilled rice (2% degree of polishing) is nutritionally superior (higher in fiber, γ-oryzanol, other polyphenols, and vitamin E) than the fully milled white rice (7). The polishing process strips the grains of dietary fiber by removing the bran and alters the structure of the grain kernel (8). Interestingly, during the last four to five decades of replacing hand-pounded or undermilled rice with highly milled white rice, the prevalence of diabetes in urban areas in India increased from 2% in the 1970s to 25% in 2015 and in rural areas from 1% to 14–16% (9,10). Undoubtedly, this secular trend in the increase in the diabetes rates cannot be solely attributed to increased intake of polished white rice as several other diabetogenic factors (e.g., a marked decrease in physical activity [PA] and increase in obesity rates) also occurred during this period, due to the improved socioeconomic status and lifestyle modification of the people. Thus, rice (carbohydrate) consumption was possibly only one of the many factors contributing to the diabetes epidemic.
It is known that consumption of foods high in glycemic index (GI) and glycemic load (GL) leads to elevated postprandial blood glucose levels (11). A meta-analysis of cohort studies from Western countries showed that diets high in GI and GL, mostly from carbohydrate sources, were associated with higher risk of type 2 diabetes (12). In contrast, reports from a study conducted in eight European countries show that carbohydrate intake was not associated with diabetes risk (13).
Specifically, consumption of high amounts of white rice has been shown to increase the risk of diabetes in some studies (14–18) but not all (19–22). In their meta-analysis that pooled results from four studies in China, Japan, U.S., and Australia, Hu et al. (14) showed that each extra serving of white rice increased the risk for diabetes by 11%. By contrast, a large prospective cohort study of >45,000 participants from Singapore reported that higher consumption of white rice (above 500 g/day) did not substantially increase the risk of incident diabetes (19). Two different cohort studies from Iran also showed opposing results with one showing an increased risk while the other did not (21). Many of these studies were conducted in single countries and predominantly in Asia where consumption of white rice is higher than most other regions of the world. Our aim was to assess the association of white rice consumption with risk of diabetes in the large multiethnic, multinational Prospective Urban Rural Epidemiology (PURE) study with data on 132,373 individuals, enrolled from 21 counties, representing different geographies and continents.
Research Design and Methods
Study Design and Participants
The design and methods of the PURE study have been described previously (23,24). In this report, we include data on 132,373 individuals who had complete information on diet from 21 countries (Argentina, Bangladesh, Brazil, Canada, Chile, China, Colombia, India, Iran, Malaysia, occupied Palestine territory, Pakistan, Philippines, Poland, South Africa, Saudi Arabia, Sweden, Tanzania, Turkey, United Arab Emirates, and Zimbabwe) and who had completed at least one follow-up visit. Data were collected at the community, household, and individual levels using standardized questionnaires. Standard case-report forms were used to record data on health outcomes during follow-up. For the current analysis, we included all outcome events (i.e., incident diabetes) until 3 July 2019.
Procedures
In the PURE study, the participants’ habitual food intake was recorded using country-specific validated food frequency questionnaires (FFQs) at baseline. For countries where a validated FFQ was not available, we developed and validated FFQs using a standard method (Supplementary Table 1). For validation of FFQ, we followed the Hu et al. (25) classification and classified starchy foods as refined grains (which included white rice when we started the PURE study in 2005) and whole grains. Deattenuated correlation coefficients of nutrient and food intake are presented in Supplementary Table 2. Participants were asked “during the past year, on average, how often have you consumed the following foods or drinks” and were asked to select their response from a list of food items. The format of the FFQ was the same for all countries, and the frequency of consumption of each food item varied from “never” to “more than six times per day.” Standard serving sizes were assigned to each food item. The reported frequency of consumption for each food item was converted to daily intake and was then multiplied by the portion size (U.S. Department of Agriculture) to calculate the daily intake of that particular food. For the present analysis, rice was not included in the refined grains group, and it was computed separately. Mixed dishes prepared with rice (such as rice with beans, rice with vegetables, and so on) were disaggregated into their constituents, and a proportional weight was assigned to the white rice component, which was included in the white rice definition. The list of FFQ validation studies is provided in the supplementary material (Supplementary Table 1). Regarding types of rice, during the FFQ development, we collected a 24-h dietary recall from 100 participants residing in urban and rural areas of each country. The most commonly reported food items were compiled as a food list and predefined portion sizes were assigned for each food item. To ensure face and content validity of the short FFQ, two expert nutritionists (M.D. and a local nutritionist) checked the food list, and if nutrient-rich or discriminating foods were missing, those foods were added to the list. Then, they structured the food list as a short FFQ. Brown rice was reported as a commonly consumed food only in very few countries, e.g., Brazil, and hence it was not included in the list.
Outcome
The main outcome of this study was incident diabetes. Incident diabetes was deemed to have occurred in those who had no diabetes at baseline but subsequently, on follow-up, reported having a diagnosis of diabetes made by a physician, used oral antidiabetic agents or insulin, or had a documented fasting plasma glucose level of ≥7.0 mmol/L (126 mg/dL) (26). Of the 6,129 cases of incident diabetes, 5,563 (90.7%) were diagnosed based on documented evidence of the use of hypoglycemic agents or insulin and/or a documented elevated plasma glucose level, while in 566 (9.3%), it was based on self-reported diabetes.
Statistical Analysis
One cup of cooked white rice is roughly equivalent to 150 g, and hence white rice consumption was categorized into the following groups: <150 g/day, ≥150 to <300 g/day, ≥300 to <450 g/day, and ≥450 g/day (equivalent to less than one cup, one to two cups, two to three cups, and greater than three cups of cooked rice), with the lowest intake group, i.e., <150 g/day, used as the reference group. We estimated the median intakes of white rice consumption across these four different categories of white rice intake. We examined the association between white rice intake and incident diabetes in the entire PURE cohort and examined it separately in South Asia (India, Bangladesh, Pakistan), the rest of the world (South East Asia, Middle East, South America, North America/Europe, and Africa), and China.
We calculated the hazard ratios (HRs) for incident diabetes using multivariable Cox frailty model with random intercepts to account for center clustering (which also adjusts for region and country) and evaluated the association of white rice consumption with incident diabetes. Multivariable models were adjusted for age, sex, BMI, waist-to-hip ratio, family history of diabetes, smoking, location, wealth index, education, PA, energy intake, whole grains and refined grains, vegetable and fruit intake, and study center as random effect.
Location refers to urban/rural area. PA was assessed using the long form International Physical Activity Questionnaire and was calculated as a total of occupation, transportation, housework, and recreational activity reported in metabolic equivalents (MET) × minutes per week. Total PA was then categorized into physically inactive (<600 MET × minutes per week) or physically active (>600 MET × minutes per week), corresponding to <150 min per week or >150 min per week of moderate intensity PA.
Results
Dietary information was recorded in 148,858 individuals in the PURE study. After excluding participants who had baseline diabetes (n = 16,485), 132,373 individuals were included in the analysis. The overall mean age of participants was 50 ± 9 years. Baseline characteristics of participants across regions and in South Asia, the rest of the world, and China are presented in Supplementary Tables 3 and 4.
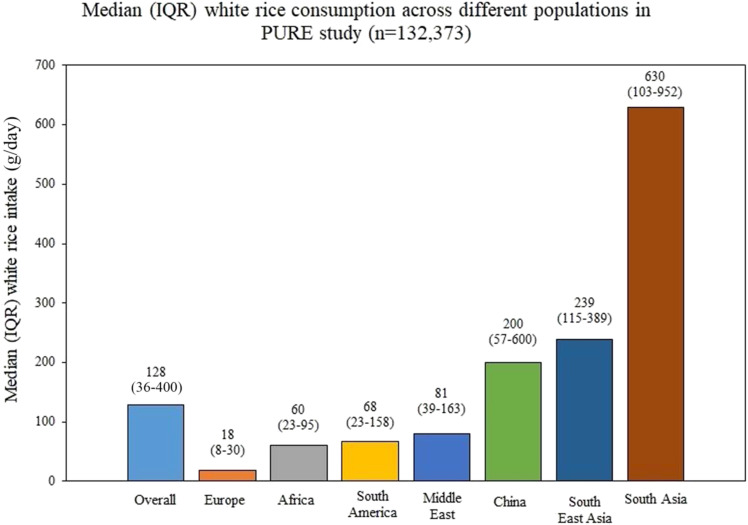
Overall, the median (interquartile range [IQR]) consumption of white rice was 128 (36–400) g/day among all PURE participants. The highest median (IQR) consumption of white rice was seen in South Asia at 630 (103–952) g/day, followed by South East Asia at 239 (115–389) g/day, and China at 200 (57–600) g/day (Fig. 1).
Figure 1.
Consumption of white rice (g/day) in different geographic regions. South Asia includes India, Pakistan, and Bangladesh. South East Asia includes Malaysia, Philippines.
Table 1 shows the characteristics of participants with different levels of white rice consumption. Those who consumed ≥450 vs. <150 g/day were younger, had lower BMI (23.1 ± 4.3 vs. 26.5 ± 5.4 kg/m2), and lower smoking rates (4.9% vs. 15.0%). These clinical characteristics probably reflect the profile of Asians, particularly South Asians who consume the maximum amount of rice. The higher category of rice consumers also consumed lower amounts of most other foods, such as whole and refined wheat products, fiber, red meat, and dairy products. Additionally, those who consumed ≥450 g/day of white rice consumed the highest percentage of their energy from carbohydrate and a lower percentage from fat and protein.
Table 1.
Characteristics of study participants by levels of white rice consumption in 132,373 participants
| White rice intake (g/day) | ||||
|---|---|---|---|---|
| <150 g/day (n = 71,914) | ≥150 to <300 g/day (n = 16,976) | ≥300 to <450 g/day (n = 14,010) | ≥450 g/day (n = 29,473) | |
| Median intake (g/day) | 42.8 (18.7–82.6) | 200 (171.9–233.5) | 395 (341.0–400) | 900 (609.8–991.4) |
| Age (years) | 50.3 (10.0) | 50.3 (9.7) | 50.8 (9.8) | 48.8 (9.8) |
| BMI (kg/m2) | 26.5 ± 5.4 | 25.9 ± 4.6 | 25.3 ± 4.5 | 23.1 ± 4.3 |
| Men | 29,192 (40.3) | 6,693 (40.5) | 5,547 (39.6) | 13,470 (45.7) |
| Urban | 40,509 (56.0) | 9,993 (60.5) | 7,737 (55.2) | 10,621 (36.0) |
| Physical inactivity | 11,474 (17.4) | 2,780 (17.7) | 2,035 (15.3) | 5,182 (18.4) |
| Current smoker | 10,811 (15.0) | 1,374 (8.4) | 1,555 (11.2) | 1,431 (4.9) |
| Fasting plasma glucose (mmol/L) | 4.9 ± 0.8 | 4.9 ± 0.8 | 4.9 ± 0.7 | 5.0 ± 0.7 |
| Diet components | ||||
| Energy intake (kcal) | 1,963 (1,497–2,546) | 2,048 (1,579–2,619) | 2,065 (1,586–2,658) | 2,120 (1,693–2,741) |
| %E from carbohydrate | 57.6 (50.0–66.1) | 58.2 (52.6–64.6) | 61.8 (56.0–68.2) | 71.4 (63.3–78.5) |
| %E from fat | 26.7 (19.4–32.4) | 25.9 (20.0–30.6) | 22.4 (16.9–27.7) | 15.2 (9.6–23.5) |
| %E from protein | 15.7 (13.6–17.9) | 16.2 (14.0–18.2) | 15.5 (13.2–17.5) | 12.0 (10.5–14.2) |
| Fiber intake (g/day) | 24.2 (15.6–34.5) | 21.1 (14.3–29.4) | 16.9 (10.4–24.9) | 10.8 (7.8–14.7) |
| Refined wheat products (g/day) | 146 (66–300) | 171 (88–279) | 102 (56–182) | 43 (12–107) |
| Whole wheat products (g/day) | 27 (0–125) | 15 (0–71) | 11 (0–33) | 7 (0–33) |
| Red meat (g/day) | 42.8 (14.4–87.8) | 51.4 (16.4–108.7) | 48.0 (16.4–107.7) | 15 (2.0–52.4) |
| White meat (g/day) | 39.0 (12.1–74.8) | 39.9 (13.9–82.7) | 44.4 (18.8–79.8) | 26.2 (6.9–67.2) |
| Processed meat (g/day) | 2.8 (0–12.1) | 0 (0–6) | 1.9 (0–9.6) | 0 (0–3.3) |
| Fish (g/day) | 11.4 (0–26) | 12.8 (2.8–36.7) | 11.3 (0–28.7) | 8.6 (0–39.7) |
| Dairy products (g/day) | 145.3 (29.5–290.0) | 137.1 (13.1–289.9) | 97.8 (4–252.9) | 15.7 (0–118.6) |
Data are median (IQR), mean ± SD, or n (%). E, energy.
During mean follow-up of 9.5 years, 6,129 cases of incident diabetes were recorded. Table 2 shows the association of white rice consumption with incident diabetes. In the overall PURE cohort, after adjusting for lifestyle and dietary factors, higher consumption of white rice (≥450 vs. <150 g/day) was significantly associated with an increased risk of incident diabetes (HR 1.20; 95% CI 1.02–1.40; P for trend = 0.003). The subgroup analysis by regions showed that the association was most pronounced in South Asia (HR 1.61; 95% CI 1.13–2.30; P for trend = 0.02) followed by the rest of the world, which includes South East Asia, Middle East, South America, North America, Europe, and Africa (HR 1.41; 95% CI 1.08–1.86; P for trend = 0.01). However, in China, the effect was minimal and did not reach statistical significance (HR 1.04; 95% CI 0.77–1.40; P for trend = 0.38).
Table 2.
Association of white rice consumption with incident diabetes in the overall PURE cohort, China, South Asia, and the rest of the world
| White rice intake (g/day) | P for trend | ||||
|---|---|---|---|---|---|
| <150 g/day | ≥150 to <300 g/day | ≥300 to <450 g/day | ≥450 g/day | ||
| Overall PURE cohort (N = 132,373) | n = 71,914 | n = 16,976 | n = 14,010 | n = 29,473 | |
| Median intake (g/day) | 42.8 (18.7–82.6) | 200 (171.9–233.5) | 395 (341.0–400.0) | 900 (609.8–991.4) | |
| Diabetes events | 2,960 (4.1) | 922 (5.4) | 628 (4.5) | 1,619 (5.5) | |
| Minimally adjusted model | 1.00 | 1.13 (1.03–1.24) | 1.22 (1.09–1.37) | 1.19 (1.05–1.34) | 0.001 |
| Fully adjusted model* | 1.00 | 1.12 (1.01–1.24) | 1.25 (1.10–1.43) | 1.20 (1.02–1.40) | 0.003 |
| South Asia (N = 26,419)† | n = 7,227 | n = 1,672 | n = 2,046 | n = 15,474 | |
| Median intake (g/day) | 34 (15–64) | 200 (173–246) | 356 (328–395) | 379 (694–1,099) | |
| Diabetes events | 343 (4.8) | 114 (6.8) | 139 (6.8) | 1,243 (8.0) | |
| Minimally adjusted model | 1.00 | 1.19 (0.93–1.52) | 1.17 (0.90–1.53) | 1.23 (0.98–1.55) | 0.12 |
| Fully adjusted model* | 1.00 | 1.26 (0.86–1.86) | 1.70 (1.14–2.52) | 1.61 (1.13–2.30) | 0.02 |
| Rest of the world (N = 64,227)‡ | n = 46,798 | n = 8,004 | n = 7,137 | n = 2,288 | |
| Median intake (g/day) | 42 (19–79) | 187 (158–234) | 395 (327–395) | 675 (550–786) | |
| Diabetes events | 2,097 (4.5) | 577 (7.2) | 317 (4.4) | 108 (4.7) | |
| Minimally adjusted model | 1.00 | 1.21 (1.07–1.36) | 1.18 (1.00–1.38) | 1.46 (1.16–1.83) | 0.0006 |
| Fully adjusted model* | 1.00 | 1.19 (1.04–1.36) | 1.13 (0.95–1.35) | 1.41 (1.08–1.86) | 0.01 |
| China (N = 41,727) | n = 17,889 | n = 7,300 | n = 4,827 | n = 11,711 | |
| Median intake (g/day) | 57 (20–86) | 200 (200–228) | 400 (400–402) | 800 (600–905) | |
| Diabetes events | 520 (2.91) | 231 (3.2) | 172 (3.6) | 268 (2.3) | |
| Minimally adjusted model | 1.00 | 1.02 (0.86–1.21) | 1.42 (1.15–1.74) | 0.99 (0.79–1.23) | 0.53 |
| Fully adjusted model* | 1.00 | 0.97 (0.80–1.17) | 1.34 (1.05–1.70) | 1.04 (0.77–1.40) | 0.38 |
Data are median (IQR) or n (%).
The fully adjusted model includes the following: adjusted for age, sex, BMI, waist-to-hip ratio, family history of diabetes, smoking, location, education, wealth index, PA, energy intake, whole grains, refined grains, fruits and vegetables, and study center as random effect.
South Asia includes India, Pakistan, and Bangladesh.
The rest of the world includes South East Asia, Middle East, South America, North America, Europe, and Africa.
The association between rice intake and incident diabetes was seen even when stratified based on family history of diabetes, PA, BMI, or waist-to-hip ratio, particularly in South Asia (Supplementary Tables 5A, 5B, 5C, and 5D). Further subgroup analysis by different regions showed the direction of association to be similar in South East Asia, Middle East, and South America, but the results did not reach statistical significance. In North America, Europe, and Africa, the amount of white rice consumed was much less, and therefore the model did not provide meaningful results (Supplementary Table 6). A pooled analysis showed no significant heterogeneity between the regions (I2 = 7.7%; P = 0.369) (Supplementary Fig. 1).
Conclusions
Data from this large multinational prospective cohort study of 21 countries show that in the overall PURE cohort, higher consumption of white rice is associated with an increased risk of diabetes, which was most marked and driven by the strong association seen in South Asia. In other regions, like South East Asia, Middle East, South America, North America, Europe, and Africa, the association was in a similar direction, but it did not reach statistical significance except when pooled. In China, there was no significant association between white rice consumption and incident diabetes.
Overall, our findings are consistent with results from some of the previous studies conducted in Asia and Europe and North America (14–18), but not all (19–22). A meta-analysis by Hu et al. (14), which included data on 352,384 participants with 13,284 incident diabetes from four studies in China, Japan, U.S., and Australia, showed that each extra serving of white rice (equivalent to about 150 g of cooked rice) increased the risk for diabetes by 11%. The Shanghai Women’s Health Study, one of the earliest studies conducted on 64,227 Chinese women, showed a relative risk of 1.78 among women who consumed 750 g of cooked white rice compared with 500 g/day (15). A similar association was seen in a Japanese study among women, where women consuming >437 g of white rice had a 1.65 times higher risk of diabetes than those consuming <200 g/day (16). It is important to note that in the meta-analysis by Hu et al. (14), however, a direct association of risk was observed only in one study, the Nurses’ Health Study II, which showed a higher risk (odds ratio 1.40; 95% CI 1.09–1.80; P = 0.01). The Japanese study reported an effect only in women and not in men (16). Prospective data from a south Indian cohort with a follow-up of 10 years showed a doubling in the rate of incident diabetes with increasing quartiles (416 vs. 222 g/day) of white rice consumption (18).
There are also some studies that do not corroborate our results (19–22). The Singapore Chinese Health Study of 45,411 Chinese participants followed up for 11 years, with 5,207 cases of incident diabetes, reported no increase in the risk of diabetes (HR 0.98; 95% CI 0.90–1.08), although the median intake in the lowest and highest quartile was substantial (236 vs. 649 g/day) (19). Another study from China showed that a diet high in white rice was associated with a lower prevalence of diabetes in certain parts of China (20). In the current study also, in China, there was no significant association between rice intake and incident diabetes. It is possible that the type of rice is different in China (sticky rice), that the vegetables, pulses, or meat consumed with the rice blunts the GL of the rice, or that the consumption of rice itself has decreased in China in recent times.
Data from two prospective studies from Iran reported opposing results (21). While data from Tehran showed significantly higher risk for >250 g/day of white rice, the Golestan Cohort Study showed no significant increase in risk at 210 g/day intake of white rice (21). A lack of association between white rice intake and incident diabetes was also reported in a study conducted in southern Spain (22). However, this again is not a predominantly rice-eating region, and the comparison was between rice consumed two to three times per week and rice consumed once a week. Hence, this would not compare with the predominantly rice-eating populations, like South Asia, that we have reported in our study. Unmeasured confounding caused by other dietary factors, characteristics of the population and ethnicity could also explain the discrepancy in these findings. Finally, the inconsistent reports from these different studies could also be attributed to different amounts of white rice consumed among the different study population.
What could be the possible mechanism by which excess rice intake leads to diabetes?
It is known that excess rice consumption leads to postprandial glucose spikes that, in turn, lead to compensatory hyperinsulinemia to maintain euglycemia (27,28). Over time, β-cells become exhausted, leading to β-cell failure and diabetes. There are some reports that suggest that rice consumption leads to high arsenic exposure due to the arsenic-contaminated groundwater that is used for rice cultivation (29–31). Some authors believe that this is an alternative explanation for the link between rice intake and diabetes, as arsenic is known to damage β-cells (32) or to act as an endocrine disruptor (33). However, further studies are needed to look at this hypothesis by measuring the arsenic content of soil and water and the risk of diabetes.
Traditional diets earlier consisted of mainly hand-pounded rice and other coarse grains like barley, rye, and maize. These have now been replaced by highly polished white rice in several Asian countries (34). It has been shown that replacing white rice with unpolished brown rice decreases the glycemic response by 23% and the fasting insulin response by 57% in overweight Asian Indians (35). However, the consumer acceptance of brown rice is poor (36). Longer cooking duration, decreased visual appeal, and greater difficulty in chewing the grain are some of the barriers for the wider acceptance of brown rice (36,37).
One of the earliest studies on GI showed that the GI of rice was higher or similar to white bread (38). Consumption of white bread has also been associated with an increased risk of diabetes (39). A recent study showed that a unique high-fiber white rice variety had a significantly higher dietary fiber and lower GI than regular polished white rice (40). Further, a continuous glucose monitoring study assessing 24-h glycemic responses showed that this high-fiber white rice had a 34% lower 24-h glucose response and a 30% reduction in adjusted mean plasma insulin levels (41). While replacing white rice with other cereals, such as wheat or millets, may not be an acceptable option due to taste preferences in some cultures, modifying the diet quality by replacing the staple white rice with less polished brown rice (36) or healthier varieties of rice may be viable options in countries where highly polished white rice constitutes the bulk (>70%) of the calories in the diet. All legumes, as a class, have a low GI (42) and, thus, adding legumes to rice not only increases the fiber and protein content but also lowers the GI of the rice-containing meal (28,35).
Our study has several strengths. This is the largest prospective study on rice and incident diabetes, and it covers 21 countries from five continents, with a broad range of white rice consumption. Second, several potential confounders have been included in the multivariable analysis. Third, the sample size is large, and there is a fairly long period of follow-up. However, there are also limitations of our study, which include the following: measurement of diet was done only at baseline and changes in diet and other lifestyle factors could have subsequently occurred. Despite extensive adjustment for confounding factors, residual confounding due to unmeasured dietary factors, such as alcohol use, or the newly emerging risk factors like air pollution (43) or use of pesticides (44) cannot be completely ruled out. Third, the costs and logistics involved in carrying out glucose tolerance tests or A1C tests in all participants is prohibitive in a large, multinational study such as this, and hence these tests could not be done. Nevertheless, the majority of the participants in the study (97.3%) were tested for diabetes using fasting blood glucose. Fourth, information on different types of white rice would have further enhanced the results of this study, for example, whether parboiled rice or raw rice was used, as there are nutritional differences between the two. However, unfortunately, this information was not collected at the time of baseline data collection as country-specific FFQs were used, which did not have this level of granularity. Obviously, these unanswered questions provide opportunities for further research in this field.
In conclusion, we report that consumption of higher amounts of white rice was associated with increased risk of incident diabetes with the risk being most pronounced in South Asia, while in other regions the risk was modest and failed to reach statistical significance, the most notable example of this being China. Replacing highly polished white rice with other cereals or healthier varieties of rice or by adding adequate legumes and pulses may not only help to reduce the GI of the meal but also, possibly, to reduce the actual quantity of white rice consumed. These may be important public health strategies to be adopted in South Asian and other populations with rice as the staple food, which, if combined with measures to increase PA, could help to slow down the rapidly rising epidemic of type 2 diabetes in these regions.
Article Information
Acknowledgments. A full list of investigators and institutions of the PURE study is available in the supplementary material online, in addition to the list below. PURE Project Office Staff, National Coordinators, Investigators, and Key Staff: Project office (Population Health Research Institute, Hamilton Health Sciences and McMaster University, Hamilton, Canada): S. Yusuf* (Principal Investigator), S. Rangarajan (Program Manager), K.K. Teo, S.S. Anand, C.K. Chow, M. O’Donnell, A. Mente, D. Leong, A. Smyth, P. Joseph, M. Duong, R. D’Souza, M. Walli-Attaei, S. Islam (Statistician), W. Hu (Statistician), C. Ramasundarahettige (Statistician), P. Sheridan (Statistician), S. Bangdiwala, L. Dyal, B. Liu (Biometric Programmer), C. Tang (Biometric Programmer), X. Yang (Biometric Programmer), R. Zhao (Biometric Programmer), L. Farago (ICT), M. Zarate (ICT), J. Godreault (ICT), M. Haskins (ICT), M. Jethva (ICT), G. Rigitano (ICT), A. Vaghela (ICT), M. Dehghan (Nutrition Epidemiologist), A. Aliberti, A. Reyes, A. Zaki, B. Connolly, B. Zhang, D. Agapay, D. Krol, E. McNeice, E. Ramezani, F. Shifaly, G. McAlpine, I. Kay, J. Rimac, J. Swallow, M. Di Marino, M. Jakymyshyn, M(a). Mushtaha, M(o). Mushtaha, M. Trottier, N. Aoucheva, N. Kandy, P. Mackie, R. Buthool, R. Patel, R. Solano, S. Gopal, S. Ramacham, S. Trottier. Core Laboratories: G. Pare, M. McQueen, S. Lamers, J. Keys (Hamilton), X. Wang (Beijing, China), A. Devanath (Bangalore, India). Argentina: R. Diaz*, A. Orlandini, P. Lamelas, M.L. Diaz, A. Pascual, M. Salvador, C. Chacon; Bangladesh: O. Rahman*, R. Yusuf*, S.A.K.S. Ahmed, T. Choudhury, M. Sintaha, A. Khan, O. Alam, N. Nayeem, S.N. Mitra, S. Islam, F. Pasha; Brazil: A. Avezum*, C.S. Marcilio, A.C. Mattos, G.B. Oliveira; Canada: K. Teo*, S. Yusuf*, Sumathy Rangarajan, A. Arshad, B. Bideri, I. Kay, J. Rimac, R. Buthool, S. Trottier, G. Dagenais, P. Poirier, G. Turbide, A.S. Bourlaud, A. LeBlanc De Bluts, M. Cayer, I. Tardif, M. Pettigrew, S. Lear, V. de Jong, A.N. Saidy, V. Kandola, E. Corber, I. Vukmirovich, D. Gasevic, A. Wielgosz, A. Pipe, A. Lefebvre, A. Pepe, A. Auclair, A. Prémont, A.S. Bourlaud; Chile: F. Lanas*, P. Serón, M.J. Oliveros, F. Cazor, Y. Palacios; China: Liu Lisheng*, Li Wei*, Chen Chunming#, Zhao Wenhua, Hu Bo, Yin Lu, Zhu Jun, Liang Yan, Sun Yi, Wang Yang, Deng Qing, Jia Xuan, He Xinye, Zhang Hongye, Bo Jian, Wang Xingyu, Liu Xu, Gao Nan, Bai Xiulin, Yao Chenrui, Cheng Xiaoru, Wang Chuangshi, Li Sidong, Liu Weida, Lang Xinyue, Liu Xiaoyun, Zhu Yibing, Xie Liya, Liu Zhiguang, Ren Yingjuan, Dai Xi, Gao Liuning, Wang Liping, Su Yuxuan, Han Guoliang, Song Rui, Cao Zhuangni, Sun Yaya, Li Xiangrong, Wang Jing, Wang Li, Peng Ya, Li Xiaoqing, Li Ling, Wang Jia, Zou Jianmei, Gao Fan, Tian Shaofang, Liu Lifu, Li Yongmei, Bi Yanhui, Li Xin, Zhang Anran, Wu Dandan, Cheng Ying, Xiao Yize, Lu Fanghong, Li Yindong, Hou Yan, Zhang Liangqing, Guo Baoxia, Liao Xiaoyang, Chen Di, Zhang Peng, Li Ning, Ma Xiaolan, Lei Rensheng, Fu Minfan, Liu Yu, Xing Xiaojie, Yang Youzhu, Zhao Shenghu, Xiang Quanyong, Tang Jinhua, Liu Zhengrong, Qiang Deren, Li Xiaoxia, Xu Zhengting, Aideeraili Ayoupu, Zhao Qian; Colombia: P. Lopez-Jaramillo*, P.A. Camacho-Lopez, M. Perez, J. Otero-Wandurraga, D.I. Molina, C. Cure-Cure, J.L. Accini, E. Hernandez, E. Arcos, C. Narvaez, A. Sotomayor, F. Manzur, H. Garcia, G. Sanchez, F. Cotes, A. Rico, M. Duran, C. Torres; India: Bangalore - P. Mony*, M. Vaz*, S. Swaminathan, A.V. Bharathi, K. Shankar, A.V. Kurpad, K.G. Jayachitra, H.A.L. Hospital, A.R. Raju, S. Niramala, V. Hemalatha, K. Murali, C. Balaji, A. Janaki, K. Amaranadh, P. Vijayalakshmi; Chennai - V. Mohan*, R.M. Anjana, M. Deepa, K. Parthiban, L. Dhanasekaran, S.K. Sundaram, M. Rajalakshmi, P. Rajaneesh, K. Munusamy, M. Anitha, S. Hemavathy, T. Rahulashankiruthiyayan, D. Anitha, R. Dhanasekar, S. Sureshkumar, D. Anitha, K. Sridevi; Jaipur - R. Gupta, R.B. Panwar, I. Mohan, P. Rastogi, S. Rastogi, R. Bhargava, M. Sharma, D. Sharma; Trivandrum - V. Raman Kutty, K. Vijayakumar, Kamala R., Manu M.S., Arunlal A.R., Veena A., Sandeep P. Kumar, Leena Kumari, Tessi R., Jith S., K. Ajayan, G. Rajasree, A.R. Renjini, A. Deepu, B. Sandhya, S. Asha, H.S. Soumya; Chandigarh - R. Kumar, M. Kaur, P.V.M. Lakshmi, V. Sagar, J.S. Thakur, B. Patro, R. Mahajan, A. Josh, G. Singh, K. Sharma, P. Chaudary. Iran: R. Kelishadi*, A. Bahonar, N. Mohammadifard, H. Heidari; Kazakhstan: K. Davletov*, B. Assembekov, B. Amirov; Kyrgyzstan: E. Mirrakhimov*, S. Abilova, U. Zakirov, U. Toktomamatov; Malaysia: UiTM - K. Yusoff*, T.S. Ismail, K. Ng, A. Devi, N. Mat-Nasir, A.S. Ramli, M.N.K. Nor-Ashikin, R. Dasiman, M.Y. Mazaouspavina, F. Ariffin, M. Miskan, H. Abul-Hamid, S. Abdul-Razak, N. Baharudin, N.M.N. Mohd-Nasir, S.F. Badlishah-Sham, M. Kaur, M. Koshy, F.A. Majid, N.A. Bakar, N. Zainon, R. Salleh, S.R. Norlizan, N.M. Ghazali, M. Baharom, H. Zulkifli, R. Razali, S. Ali, C.W.J.C.W. Hafar, F. Basir; UKM - Noorhassim Ismail, M.J. Hasni, M.T. Azmi, M.I. Zaleha, R. Ismail, K.Y. Hazdi, N. Saian, A. Jusoh, N. Nasir, A. Ayub, N. Mohamed, A. Jamaludin, Z. Rahim; Occupied Palestinian Territory: R. Khatib*, U. Khammash, R. Giacaman; Pakistan: R. Iqbal*, R. Khawaja, I. Azam, K. Kazmi; Peru: J. Miranda*, A. Bernabe Ortiz, W. Checkley, R.H. Gilman, L. Smeeth, R.M. Carrillo, M. de los Angeles, C. Tarazona Meza; Philippines: A. Dans*, H.U. Co, J.T. Sanchez, L. Pudol, C. Zamora-Pudol, L.A.M. Palileo-Villanueva, M.R. Aquino, C. Abaquin, S.L. Pudol, K. Manguiat, S. Malayang; Poland: W. Zatonski*, A. Szuba, K. Zatonska, R. Ilow#, M. Ferus, B. Regulska-Ilow, D. Różańska, M. Wolyniec; Saudi Arabia: K.F. AlHabib*, M. Alshamiri, H.B. Altaradi, O. Alnobani, N. Alkamel, M. Ali, M. Abdulrahman, R. Nouri; South Africa: L. Kruger*, A. Kruger#, P. Bestra, H. Voster, A.E. Schutte, E. Wentzel-Viljoen, F.C. Eloff, H. de Ridder, H. Moss, J. Potgieter, A. Roux, M. Watson, G. de Wet, A. Olckers, J.C. Jerling, M. Pieters, T. Hoekstra, T. Puoane, R. Swart*, E. Igumbor, L. Tsolekile, K. Ndayi, D. Sanders, P. Naidoo, N. Steyn, N. Peer, B. Mayosi#, B. Rayner, V. Lambert, N. Levitt, T. Kolbe-Alexander, L. Ntyintyane, G. Hughes, J. Fourie, M. Muzigaba, S. Xapa, N. Gobile, K. Ndayi, B. Jwili, K. Ndibaza, B. Egbujie; Sweden: A. Rosengren*, K. Bengtsson Boström, A. Rawshani, A. Gustavsson, M. Andreasson, L. Wirdemann; Tanzania: K. Yeates*, M. Oresto, N. West; Turkey: A. Oguz*, N. Imeryuz, Y. Altuntas, S. Gulec, A. Temizhan, K. Karsidag, K.B.T. Calik, A.K. Akalin, O.T. Caklili, M.V. Keskinler, K. Yildiz; United Arab Emirates: A.H. Yusufali, F. Hussain, M.H.S. Abdelmotagali, D.F. Youssef, O.Z.S. Ahmad, F.H.M. Hashem, T.M. Mamdouh, F.M. AbdRabbou, S.H. Ahmed, M.A. AlOmairi, H.M. Swidan, M. Omran, N.A. Monsef; Zimbabwe: J. Chifamba*, T. Ncube, B. Ncube, C. Chimhete, G.K. Neya, T. Manenji, L. Gwaunza, V. Mapara, G. Terera, C. Mahachi, P. Murambiwa, R. Mapanga, A. Chinhara.
*National Coordinator.
#Deceased.
Funding and Duality of Interest. S.Y. is supported by the Mary W. Burke endowed chair of the Heart and Stroke Foundation of Ontario. The PURE study is an investigator-initiated study that is funded by the Population Health Research Institute, Hamilton Health Sciences Research Institute, the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, support from Canadian Institutes of Health Research’s Strategy for Patient-Oriented Research, through the Ontario Strategy for Patient-Oriented Research Support Unit, as well as the Ontario Ministry of Health and Long-Term Care and through unrestricted grants from several pharmaceutical companies, with major contributions from AstraZeneca (Canada), Sanofi (France and Canada), Boehringer Ingelheim (Germany and Canada), Servier, and GlaxoSmithKline, and additional contributions from Novartis and King Pharma and from various national or local organizations in participating countries. These include the following: Argentina: Fundación ECLA (Estudios Clínicos Latino America); Bangladesh: Independent University, Bangladesh, and Mitra and Associates; Brazil: Unilever Health Institute, Brazil; Canada: this study was supported by an unrestricted grant from Dairy Farmers of Canada and the National Dairy Council (U.S.), Public Health Agency of Canada, and Champlain Cardiovascular Disease Prevention Network; Chile: Universidad de La Frontera (DI13-PE11); China: National Center for Cardiovascular Diseases and ThinkTank Research Center for Health Development; Colombia: Colciencias (6566-04-18062 and 6517-777-58228); India: Indian Council of Medical Research; V.M. is involved in the promotion of healthier varieties of rice; Malaysia: Ministry of Science, Technology and Innovation of Malaysia (100-IRDC/BIOTEK 16/6/21 [13/2007], and 07-05-IFN-BPH 010), Ministry of Higher Education of Malaysia (600-RMI/LRGS/5/3 [2/2011]), Universiti Teknologi MARA, Universiti Kebangsaan Malaysia (UKM-Hejim-Komuniti-15-2010); Occupied Palestinian Territory: the United Nations Relief and Works Agency for Palestine Refugees in the Near East, Occupied Palestinian Territory, and International Development Research Centre, Canada; Philippines: Philippine Council for Health Research and Development; Poland: Polish Ministry of Science and Higher Education (290/W-PURE/2008/0), Wroclaw Medical University; Saudi Arabia: Saudi Heart Association, Dr. Mohammad Alfagih Hospital, The Deanship of Scientific Research at King Saud University, Riyadh (research group number RG -1436-013), Saleh Hamza Sarafi Chair for Research of Coronary Heart Disease, Umm AlQura University, Makkah; South Africa: The North-West University, South African- Netherlands Programme on Alternatives in Development, National Research Foundation, Medical Research Council of South Africa, The South Africa Sugar Association, Faculty of Community and Health Sciences; Sweden: grants from the Swedish state under the agreement concerning research and education of doctors, the Swedish Heart and Lung Foundation, the Swedish Research Council, the Swedish Council for Health, Working Life and Welfare, King Gustaf V and Queen Victoria Freemasons’ Foundation, AFA Insurance; Turkey: Metabolic Syndrome Society, AstraZeneca, Sanofi Aventis; United Arab Emirates: Sheikh Hamdan Bin Rashid Al Maktoum Award For Medical Sciences and Dubai Health Authority, Dubai. No other potential conflicts of interest relevant to this article were reported.
The external funders and sponsors of the study had no role in study design and conduct of the study; in the collection, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Author Contributions. B.B. carried out data analyses. B.B. and V.M. had primary responsibility for writing of the article with the support of M.D. V.M. conceived the idea and initiated the analysis plan for the current study. M.D. coordinated the entire nutrition component of the PURE study. S.R. coordinated the worldwide study and reviewed and commented on drafts. S.Y. conceived and initiated the PURE study, supervised its conduct, and reviewed and commented on draft. All other authors coordinated the study in their respective countries, provided comments on drafts of the manuscript, and have read and approved the manuscript. B.B. and V.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying article, p. 2625.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12654227.
References
- 1.International Diabetes Federation IDF Diabetes Atlas, 8th edition, 2017. Accessed 25 June 2020. Available from https://www.diabetesatlas.org
- 2.Hills AP, Arena R, Khunti K, et al. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol 2018;6:966–978 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34:1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan V, Unnikrishnan R, Shobana S, Malavika M, Anjana RM, Sudha V. Are excess carbohydrates the main link to diabetes & its complications in Asians? Indian J Med Res 2018;148:531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya K. Parboiling of rice. In Rice Chemistry and Technology. Juliano BO, Ed. St. Paul, MN, AACC, Inc, 1985, pp. 289–348 [Google Scholar]
- 6.Achaya K. The Illustrated Food of India A-Z, New Delhi, India, Oxford University Press, 2009 [Google Scholar]
- 7.Shobana S, Malleshi NG, Sudha V, et al. Nutritional and sensory profile of two Indian rice varieties with different degrees of polishing. Int J Food Sci Nutr 2011;62:800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidhuber J, Shetty P. The nutrition transition to 2030. Why developing countries are likely to bear the major burden. Food Econ - Acta Agric Scand Sect C 2005;2:150–166 [Google Scholar]
- 9.Mohan V, Deepa M, Deepa R, et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India--The Chennai Urban Rural Epidemiology Study (CURES-17). Diabetologia 2006;49:1175–1178 [DOI] [PubMed] [Google Scholar]
- 10.Deepa M, Grace M, Binukumar B, et al.; CARRS Surveillance Research Group . High burden of prediabetes and diabetes in three large cities in South Asia: the Center for cArdio-metabolic Risk Reduction in South Asia (CARRS) Study. Diabetes Res Clin Pract 2015;110:172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolever TM, Mehling C. Long-term effect of varying the source or amount of dietary carbohydrate on postprandial plasma glucose, insulin, triacylglycerol, and free fatty acid concentrations in subjects with impaired glucose tolerance. Am J Clin Nutr 2003;77:612–621 [DOI] [PubMed] [Google Scholar]
- 12.Barclay AW, Brand-Miller JC, Wolever TMS. Glycemic index, glycemic load, and glycemic response are not the same. Diabetes Care 2005;28:1839–1840 [DOI] [PubMed] [Google Scholar]
- 13.Sluijs I, van der Schouw YT, van der A DL, et al. Carbohydrate quantity and quality and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL) study [published correction appears in Am J Clin Nutr 2011;93:676]. Am J Clin Nutr 2010;92:905–911. [DOI] [PubMed] [Google Scholar]
- 14.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 2012;344:e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villegas R, Liu S, Gao Y-T, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med 2007;167:2310–2316 [DOI] [PubMed] [Google Scholar]
- 16.Nanri A, Mizoue T, Noda M, et al.; Japan Public Health Center-based Prospective Study Group . Rice intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 2010;92:1468–1477 [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anjana RM, Sudha V, Nair DH, et al. Diabetes in Asian Indians-how much is preventable? Ten-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES-142). Diabetes Res Clin Pract 2015;109:253–261 [DOI] [PubMed] [Google Scholar]
- 19.Seah JYH, Koh W-P, Yuan J-M, van Dam RM. Rice intake and risk of type 2 diabetes: the Singapore Chinese Health Study. Eur J Nutr 2019;58:3349–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong F, Howard AG, Herring AH, Popkin BM, Gordon-Larsen P. White rice intake varies in its association with metabolic markers of diabetes and dyslipidemia across region among Chinese adults. Ann Nutr Metab 2015;66:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golozar A, Khalili D, Etemadi A, et al. White rice intake and incidence of type-2 diabetes: analysis of two prospective cohort studies from Iran. BMC Public Health 2017;17:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soriguer F, Colomo N, Olveira G, et al. White rice consumption and risk of type 2 diabetes. Clin Nutr 2013;32:481–484 [DOI] [PubMed] [Google Scholar]
- 23.Dehghan M, Mente A, Zhang X, et al.; Prospective Urban Rural Epidemiology (PURE) study investigators . Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–2062 [DOI] [PubMed] [Google Scholar]
- 24.Miller V, Mente A, Dehghan M, et al.; Prospective Urban Rural Epidemiology (PURE) study investigators . Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet 2017;390:2037–2049 [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–249 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association 13. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S137–S143 [DOI] [PubMed] [Google Scholar]
- 27.Thompson SV, Winham DM, Hutchins AM. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: a cross-over study. Nutr J 2012;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moravek D, Duncan AM, VanderSluis LB, et al. Carbohydrate replacement of rice or potato with lentils reduces the postprandial glycemic response in healthy adults in an acute, randomized, crossover trial. J Nutr 2018;148:535–541 [DOI] [PubMed] [Google Scholar]
- 29.Rahman MA, Hasegawa H. High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci Total Environ 2011;409:4645–4655 [DOI] [PubMed] [Google Scholar]
- 30.Nachman KE, Ginsberg GL, Miller MD, Murray CJ, Nigra AE, Pendergrast CB. Mitigating dietary arsenic exposure: current status in the United States and recommendations for an improved path forward. Sci Total Environ 2017;581–582:221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MA, Signes-Pastor AJ, Argos M, et al. Assessment of human dietary exposure to arsenic through rice. Sci Total Environ 2017;586:1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol 2004;198:424–433 [DOI] [PubMed] [Google Scholar]
- 33.Davey JC, Bodwell JE, Gosse JA, Hamilton JW. Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci 2007;98:75–86 [DOI] [PubMed] [Google Scholar]
- 34.Popkin BM, Horton S, Kim S, Mahal A, Shuigao J. Trends in diet, nutritional status, and diet-related noncommunicable diseases in China and India: the economic costs of the nutrition transition. Nutr Rev 2001;59:379–390 [DOI] [PubMed] [Google Scholar]
- 35.Mohan V, Spiegelman D, Sudha V, et al. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: a randomized controlled trial. Diabetes Technol Ther 2014;16:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sudha V, Spiegelman D, Hong B, et al. Consumer Acceptance and Preference Study (CAPS) on brown and undermilled Indian rice varieties in Chennai, India. J Am Coll Nutr 2013;32:50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang G, Malik VS, Pan A, et al. Substituting brown rice for white rice to lower diabetes risk: a focus-group study in Chinese adults. J Am Diet Assoc 2010;110:1216–1221 [DOI] [PubMed] [Google Scholar]
- 38.Jenkins DJA, Wolever TMS, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–366 [DOI] [PubMed] [Google Scholar]
- 39.Hodge AM, English DR, O’Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004;27:2701–2706 [DOI] [PubMed] [Google Scholar]
- 40.Mohan V, Anjana RM, Gayathri R, et al. Glycemic index of a novel high-fiber white rice variety developed in India--a randomized control trial study. Diabetes Technol Ther 2016;18:164–170 [DOI] [PubMed] [Google Scholar]
- 41.Anjana RM, Gayathri R, Lakshmipriya N, et al. Effect of a novel high fiber rice diet on 24-hour glycemic responses in Asian Indians using continuous glucose monitoring: a randomized clinical trial. Diabetes Technol Ther 2019;21:177–182 [DOI] [PubMed] [Google Scholar]
- 42.Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H. Exceptionally low blood glucose response to dried beans: comparison with other carbohydrate foods. Br Med J 1980;281:578–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang BY, Fan S, Thiering E, et al. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res 2020;180:108817. [DOI] [PubMed] [Google Scholar]
- 44.Evangelou E, Ntritsos G, Chondrogiorgi M, et al. Exposure to pesticides and diabetes: a systematic review and meta-analysis. Environ Int 2016;91:60–68 [DOI] [PubMed] [Google Scholar]