Abstract
Aims
Rodent studies propose potential mechanisms linking excessive drinking and pain hypersensitivity (hyperalgesia), such that stress hormones (i.e. epinephrine and cortisol) mediate induction and maintenance of alcohol withdrawal-induced hyperalgesia. The first aim of this study was to examine whether hyperalgesia would occur within 48 h after a drinking episode in healthy young adult binge drinkers. The second was to examine whether stress hormones and negative effect would be associated with binge drinking or alcohol withdrawal-associated hyperalgesia.
Methods
A cross-sectional experiment was conducted in five groups with naturally occurring drinking (mean age = 19.6, range 18–29 years): abstainers (n = 43, 54% female), moderate drinkers with (n = 50, 50% female) or without recent drinking (i.e. within 48 h, n = 23, 26% female) and binge drinkers with (n = 36, 58% female) or without recent drinking (n = 25, 44% female). All types of drinkers endorsed drinking about 2–3 times a month and 2–3 years of drinking history.
Results
Muscle pressure pain thresholds were significantly lower in the binge group with recent drinking compared to other groups, but cutaneous mechanical and heat pain thresholds were not significantly different across the five groups. Basal epinephrine levels were significantly higher in binge groups regardless of recent drinking, but cortisol and negative effect were not significantly different across the five groups.
Conclusions
This is the first study to show that alcohol withdrawal-associated muscle hyperalgesia may occur in healthy episodic binge drinkers with only 2–3 years of drinking history, and epinephrine may play a role in binge drinking-associated hyperalgesia.
Short Summary This study suggests that transient alcohol withdrawal-associated muscle hyperalgesia exists in young adult binge drinkers with as little as a 2- or 3-year history of drinking, and the stress hormone epinephrine may play a role in binge drinking-associated hyperalgesia.
INTRODUCTION
Up to 35% of young adults report binge drinking, a significant health risk behavior (Substance Abuse and Mental Health Services Administration, 2019). Binge drinking is defined as consuming at least 4(women)/5(men) standard drinks within 2 h (National Institute on Alcohol Abuse and Alcoholism, 2004), which increases blood alcohol concentrations (BAC) to 0.08 g% and often causes blackouts (Hingson et al., 2016) and neurotoxicity (Alfonso-Loeches et al., 2013). Following binge drinking, unpleasant physical, neurocognitive and psychological symptoms develop and persist several hours after BAC returns to 0 g% (Swift and Davidson, 1998). These alcohol withdrawal symptoms, commonly called hangover symptoms in the context of young adults’ binge drinking, disappear within 2 days (Sellers, 1988).
Rodent studies show that pain hypersensitivity (hyperalgesia) occurs during alcohol withdrawal. This hyperalgesia during withdrawal may explain the relationship between excessive drinking and pain. Reviews, largely based on rodent research, highlight the disrupted stress system as a possible mechanism linking alcohol to pain (Egli et al., 2012; Edwards et al., 2020; Robins et al., 2019). Repeated exposure to alcohol intoxication and withdrawal episodes enhances stress signaling and leads to hyperalgesia. Rats show analgesic responses after an ethanol diet, followed by hyperalgesic responses during alcohol withdrawal (Gatch and Lal, 1999). A study also shows that rats develop hyperalgesia to muscle pressure pain during withdrawal after 1 cycle of an ethanol binge diet, and this hyperalgesia worsens with repeated binges up to 4 cycles (Dina et al., 2006). Surprisingly, rats on a continuous ethanol diet do not develop hyperalgesia, highlighting the role of ‘withdrawal’, not excessive drinking, in the development of hyperalgesia. Rodent studies have also identified several mechanisms driving the development of alcohol withdrawal-induced hyperalgesia, including increased circulating levels of stress hormones (epinephrine and glucocorticoids, Dina et al., 2008). Blocking the secretion and action of epinephrine or cortisol reverses already occurring hyperalgesia and prevents the occurrence of withdrawal-induced hyperalgesia despite continued cycles of binge and withdrawal (Dina et al., 2008).
Alcohol withdrawal-induced hyperalgesia has received limited attention in the human literature. One human study demonstrated evidence of alcohol withdrawal-associated hyperalgesia in which middle-aged men with alcohol dependence without neuropathy showed reversible pain hypersensitivity during alcohol detoxification (Jochum et al., 2010). This study demonstrated that withdrawal-associated hyperalgesia occurs before the development of alcoholic neuropathic pain. However, it is unknown whether withdrawal-associated hyperalgesia would occur even before the development of alcohol dependence such as in episodic binge drinkers and whether stress hormones are similarly implicated in alcohol withdrawal-associated hyperalgesia of humans. If so, lowering stress hormones by psychological and pharmacological intervention might provide a preventative approach for binge drinkers who are at risk for transitioning from episodic binge drinking to alcohol dependence (e.g. family history of alcoholism, childhood adversity, Fenton et al., 2013) and alcoholic neuropathic pain (e.g. female, Ammendola et al., 2000). Without these preventive approaches, recurrent alcohol intoxication and withdrawal episodes may lead to dysregulation of brain reward and stress systems during the development of alcohol dependence (Egli et al., 2012). Over time, negative effect and persistent pain may play key roles in continued drinking because alcohol provides immediate relief from them, which persist and worsen during alcohol withdrawal (Egli et al., 2012). Eventually, in advanced stages of drinking, irreversible and painful alcoholic neuropathy may develop.
The primary aim of this study was to investigate whether alcohol withdrawal-associated hyperalgesia would occur in healthy young adult binge drinkers. Second, we examined whether measures of psychological and physiological stress would be elevated in binge drinkers who drank alcohol within 2 days. To achieve these aims, we conducted a cross-sectional study to evaluate pain sensitivity after naturally occurring alcohol use. We hypothesized that binge drinkers who drank alcohol within 2 days would show (a) pain hypersensitivity and (b) elevated psychological (negative effect) and physiological (stress hormones) stress levels compared to binge drinkers who did not drink within 2 days, moderate drinkers and abstainers.
METHODS
Recruitment
The Texas A&M University Institutional Review Board approved the study procedures, which were conducted in accordance with the Helsinki Declaration. All participants provided informed consent. Inclusion criteria were healthy adults between 18 and 30 years of age. Exclusion criteria were (a) any chronic physical and mental health issues, (b) prescription medication use (except contraceptives and vitamins), (c) needle phobia and (d) an injury or skin condition on the feet (pain testing site).
Potential participants completed an online prescreening survey evaluating health status and drinking history. They were asked to select one that describes their drinking pattern, with options never, former and current drinker. Those who endorsed ‘Never’ (57%) were classified as abstainers. ‘Current’ drinkers (37%) were asked to complete the Daily Drinking Questionnaires (DDQ, Collins et al., 1985). On the DDQ, participants reported the number of standard drinks and hours spent drinking for a typical week in the last 30 days. Moderate drinking was defined as consuming <4 (women)/5 (men) standard drinks on any occasion.
Of the total 2226 people who completed the prescreening (12% current binge drinkers), 177 people participated in this study. Before the laboratory visit, participants were instructed to avoid any pain and allergy medicine 3 days before the experiment and avoid caffeine and vigorous exercise 4 h before the experiment. After quantitative sensory testing (QST), participants reported their last drinking on the exit survey. Most participants (85%) received class credit for their participation, while the rest received the opportunity to win one of three $100 gift cards in a raffle.
Questionnaires
The following self-report measures assessed binge drinkers’ baseline psychological characteristics and emotional responses to pain testing. The Depression Anxiety Stress Scales (DASS) assessed the severity of depression, anxiety and life stress symptoms over the past week (Antony et al., 1998). Total scores range from 0 to 42 for each symptom domain, with normal ranges <10 for depressive, <8 for anxiety and <15 for stress symptoms (Lovibond and Lovibond, 1995). The Hangover Symptoms Scale (HSS) was administered to assess the frequency of 13 hangover symptoms over the past 12 months (Slutske et al., 2003), with scores ranging from 0 (0% of the time) to 4 (100% of the time). Items were dichotomized to identify symptom presence (1 = 1–4) or absence (0 = 0) and then summed. Therefore, total scores range from 0 to 13, with higher scores indicating more hangover symptoms. The Acute Hangover Scale (AHS) was used to assess nine acute hangover symptoms (i.e. hangover, thirsty, tired, headache, dizziness, loss of appetite, stomachache, nausea and heart racing) rated on a 0: None to 7: Incapacitating scale (Rohsenow et al., 2007). The Spielberger State-Trait Anxiety Inventory-6 (STAI) evaluated emotional responses to pain testing, with total scores ranging from 6 to 24 and higher scores indicating higher anxiety (Marteau and Bekker, 1992).
Quantitative Sensory Testing
Because alcoholic polyneuropathy often affects lower extremities (Chopra and Tiwari, 2012), pain testing was conducted on the dorsum of the nondominant foot (L5 dermatome). The QST protocol of the German Research Network was used to measure cutaneous mechanical, heat and muscle pressure pain thresholds (PPTs) (Rolke et al., 2006). Briefly, the cutaneous mechanical pain threshold (MPT) was measured by applying von Frey filaments (0.6, 1.4, 4, 6, 15, 26 and 60 gF, Stoelting Co. USA) in an ascending, and then a descending order. To prevent sensory fatigue and local sensitization, each threshold test occurred at a different location with an intertrial interval ≥ 10s. Individual MPT was calculated as the geometric mean of the three series of ascending and descending stimulus intensity ratings. If participants reported no painful sensation to the highest force von Frey (60 gF), the next highest force (100 gF) in the series was used as their pain threshold (Cameron et al., 2008). Heat pain threshold (HPT) was measured using a 9-cm2 Peltier thermode (ATS, Medoc Ltd., Israel). Temperature was ramped at 1 °C/s (baseline temperature 32°C) until participants indicated their first pain sensation. Tests were repeated three times at 30 s intervals and the values were averaged to calculate individual HPT. Muscle PPT was measured three times using a handheld algometer (FPX 50 model, Wagner Instrument, USA) at 30 s intervals and the values averaged to calculate individual PPT.
Blood collection
Blood was collected in a chilled EDTA tube before and after QST. The blood samples were centrifuged for 15 min at 1000 × g in a cold room (4°C), aliquoted into 0.5-ml tubes, and stored in a −80°C freezer until assayed.
Enzyme-linked immunosorbent assay
Duplicate samples were analyzed according to the manufacturer’s manual. Abnova (No KA1877) and IBL international (RE52061) kits were used for Epinephrine and Cortisol, respectively.
Physiological measures
Continuous heart rate (HR), skin conductance level (SCL) and respiration rate (RR) were sampled at 1000 Hz using a MP150 (BIOPAC Systems, USA), interfaced with AcqKnowledge 4.2 for data acquisition. LabVIEW 8.0 was also used to generate a digital signal from the National Instrument 6008USB device connected to a BIOPAC Systems STP100C and UIM100C. A finite impulse response (FIR) bandpass filter was used to remove movement artifacts from HR (0.5 and 35 Hz) (Ruha et al., 1997) and RR (0.05 and 1.0 Hz). To remove artifacts from SCL, a 1 Hz FIR lowpass filter was used. After filtering, all data were visually inspected. Filtering corrected most noise in RR and SCL. However, HR data from three subjects containing uncorrectable noises (e.g. extreme movement artifacts) were not analyzed.
Procedures
To recruit participants who voluntarily consumed alcohol, the experiment was conducted between Thursday and Sunday. For ethical reasons, participants did not receive any instructions about alcohol consumption. Participants who chose to consume alcohol within 2 days were classified in ‘recent’ alcohol drinking groups. Additionally, the experiment time was between 12 pm and 6 pm to minimize the effect of diurnal variation on pain and stress hormones. Participants were blinded to the study hypotheses and the experimenter conducting QSTs was blinded to participants’ drinking patterns.
Figure 1 depicts the experiment timeline. Before and after the blood draw, BP, HR and body temperature were measured to make sure the levels were within normal limits. In the testing room, baseline physiological data were collected 5 min after the survey. The interval between different QSTs was 5 min.
Fig. 1.
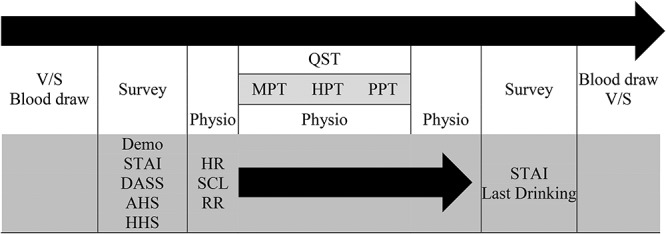
Experiment timeline [1 h]. V/S: Vital Sign including Blood Pressure, HR, and BT. MPT: Cutaneous Mechanical Pain Threshold, HPT: Heat Pain Threshold, PPT: Muscle Pressure Pain Threshold. Demo: Demographics, STAI: State-Trait Anxiety Inventory-6; DASS (Depression Anxiety Stress Scales); AHS: Acute Hangover Scale; HSS: Hangover Symptom Scale; HR: Heart Rate; BT: Body Temperature; SCL: Skin Conductance Level; RR: Respiration Rate.
Statistical analysis
To examine the first hypothesis, a multivariate analysis of variance (MANOVA) with three pain thresholds was followed by a least significant difference (LSD) test. For the second hypothesis, a repeated-measures analysis of variance (ANOVA) compared the group difference in stress hormones and effective responses to QST. Then, an analysis of covariance (ANCOVA) examined how much of the group difference in pain sensitivity would be accounted for by stress hormones and negative effect. All statistical tests were two-tailed and P values < 0.05 were considered significant. SPSS 22 was used for all analyses.
Sample size calculation
This study was designed to recruit five naturally occurring groups: (a) abstainers, (b) moderate drinkers with a recent drinking episode, (c) moderate drinkers without a recent drinking episode, (d) binge drinkers with a recent drinking episode (main group of interest) and (e) binge drinkers without a recent drinking episode. Large effects of alcohol withdrawal on pain threshold were reported (Cohen’s d > 1.65, Dina et al., 2006, Dina et al., 2008; Jochum et al., 2010). Sample size calculation indicated that at least 25 participants were needed in each group to achieve 95% power and an α of 0.05 for a between-subjects design. Our main interest was to evaluate group differences by drinking patterns and we sought to recruit an equal number of men and women per group. Thus, all the data were analyzed and presented by drinking group, but gender and gender by group interactions were examined and significant results were noted, if any.
Missing data
There were few missing values (~1.7%), which were replaced with overall means. For pain data, three participants did not undergo HPT tests because of equipment malfunctioning. Therefore, these three participants’ physiological data were replaced with the overall means. For psychological state variables, one binge drinker did not complete the STAI at baseline.
Participants
Table 1 depicts the demographics. There were no group differences in gender, race/ethnicity or cigarette use (Ps > 0.097). The abstainer group was about 1 year younger than the other groups, but all were young adults.
Table 1.
Demographics and drinking patterns
| Abstainers | Moderate drinkers | Binge drinkers | |||||
|---|---|---|---|---|---|---|---|
| No recent drinking | Recent drinking | No recent drinking | Recent drinking | ||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | χ 2 or F | P | |
| N | 43 | 50 | 23 | 36 | 25 | ||
| (Male/Female) | (20/23) | (25/25) | (17/6) | (15/21) | (14/11) | 6.69 | 0.153 |
| % Caucasian | 60.5 | 64.0 | 73.9 | 63.9 | 80.0 | 3.57 | 0.467 |
| % Cigarette use | 2.3 | 6.0 | 13.0 | 13.9 | 8.0 | 9.87 | 0.043 |
| Age | 18.7(1.2)a | 20.1(2.5)b | 20.3(2.0)b | 19.8(1.8)b | 19.6(2.3)ab | 4.35 | 0.002 |
| M(SD) | M(SD) | M(SD) | M(SD) | M(SD) | F | P | |
| Ages at first drink | – | 18.0(1.9)a | 16.8(1.9)b | 17.1(1.3)b | 16.5(1.0)b | 4.87 | 0.003 |
| Years of drink | – | 2.2(2.1) | 2.8(1.9) | 2.6(1.9) | 3.0(2.3) | 0.89 | 0.448 |
| Frequency | – | 2.0(1.4) | 2.0(1.6) | 2.6(1.5) | 2.2(2.4) | 0.82 | 0.488 |
| HSS | – | 3.0(2.8)a | 4.0(3.0)a | 5.1(3.0)b | 6.6(2.9)c | 10.22 | <0.001 |
| AHS | – | 5.6(3.7)a | 5.6(3.0)a | 6.3(4.0)a | 10.2(9.3)b | 4.94 | 0.003 |
Recent drinking: Alcohol consumption within 48 h before the experiment; χ2 statistics were used for gender, ethnicity and cigarette use; superscripts of a, b and c indicate significant difference in post-hoc analysis. HSS: Hangover Symptom Scale; AHS: Acute Hangover Scale.
The age of first alcohol use was somewhat older in the moderate drinkers than the others, but no difference was found in years of regular alcohol use (P = 0.448). An ANOVA indicated no group difference in drinking frequency endorsed on the DDQ (P = 0.488); all types of drinkers endorsed drinking about 2–3 times a month. The respective means of typical drinks were 2.5 (SD = 1.6) and 3.1 drinks (SD = 2.4) for female and male moderate drinkers, and 6.6 (SD = 3.2) and 10.3 (SD = 10.2) for female and male binge drinkers. On the HSS, binge drinkers reported more hangover symptoms for the past 12 months. Notably, the HSS scores were even higher in the binge drinkers with recent drinking than those without it. Additionally, more hangover symptoms were reported on the AHS by binge drinkers with recent drinking, being consistent with the self-reported recent drinking episode. The respective means of recent drinks were 1.8 (SD = 1.0) and 2.0 (SD = 1.5) for female and male moderate drinkers, and 5.0 (SD = 1.6) and 6.0 (SD = 4.9) for female and male binge drinkers.
Baseline psychological and physiological characteristics
Baseline psychological characteristics were similar between the groups (Kruskal–Wallis tests’ Ps > 0.136) except for depressive symptoms (P = 0.022). Post-hoc comparisons with the Mann–Whitney test indicated that moderate and binge drinker groups reported more depressive symptoms than the abstainer group (Table 2). Depressive symptoms did not differ between abstainers and moderate drinkers with recent drinking (P = 0.075).
Table 2.
Experiment 1 baseline psychological and physiological characteristics
| Abstainers | Moderate drinkers | Binge drinkers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No recent drinking | Recent drinking | No recent drinking | Recent drinking | |||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Psychological variables | ||||||||||
| DASS-DEP | 3.8a | 5.2 | 6.2b | 7.2 | 5.9ab | 6.5 | 5.3b | 5.1 | 7.9b | 7.3 |
| DASS-ANX | 4.1 | 4.5 | 4.8 | 5.0 | 3.7 | 3.7 | 4.6 | 5.1 | 6.3 | 4.5 |
| DASS-STR | 8.9 | 6.0 | 8.9 | 6.3 | 8.6 | 6.6 | 9.3 | 6.6 | 11.0 | 6.7 |
| Psychological states | ||||||||||
| STAI | 9.2 | 2.3 | 9.2 | 2.3 | 9.4 | 2.5 | 9.4 | 3.1 | 9.4 | 2.6 |
| Physiological states | ||||||||||
| HR (beat/min) | 72.7 | 8.9 | 75.6 | 10.7 | 72.0 | 9.5 | 74.3 | 10.1 | 72.8 | 9.5 |
| SCL (sqrtμS) | 2.0a | 0.7 | 2.5b | 0.8 | 2.5b | 0.6 | 2.1ab | 0.6 | 2.4b | 0.8 |
| RR (breath/min) | 13.6 | 3.9 | 14.2 | 2.8 | 14.1 | 3.3 | 13.2 | 3.2 | 13.9 | 3.0 |
| SBP (mmHg)* | 112.0 | 10.0 | 114.5 | 13.1 | 119.4 | 13.7 | 113.9 | 12.7 | 116.8 | 12.9 |
| DBP (mmHg)* | 70.6 | 5.7 | 71.8 | 7.4 | 72.7 | 10.2 | 72.1 | 9.0 | 71.0 | 6.5 |
The means with the different superscript letter are significantly different at P value of 0.05. DASS (Depression Anxiety Stress Scales)-DEP: Depression, ANX: Anxiety, STR: Stress; STAI: State-Trait Anxiety Inventory-6; HR: Heart Rate, SCL: Skin Conductance Level, RR: Respiration Rate; SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure.
*Kruskal–Wallis followed by Mann–Whitney U test.
In comparing the baseline physiological states, a one-way ANOVA indicated difference only in SCLs, F(4, 283) = 3.12, P = 0.017. LSD post-hoc analysis indicated that higher SCLs were observed in the two moderate drinker groups and the binge drinker group with recent drinking, Ps < 0.05 (Table 2).
RESULTS
Pain sensitivity
To examine group differences in cutaneous mechanical, heat and muscle PPTs, a MANOVA was conducted. The results revealed a significant main effect of group, Wilk’s Λ = 0.80, P < 0.001. Follow-up univariate analysis indicated that only muscle PPT was different between the groups, F(4, 165) = 7.10, P < 0.001, partial η2 = 0.14. LSD post-hoc analysis indicated that the binge group without recent drinking, lnM = 1.02, lnSD = 0.50, showed lower muscle PPTs when compared to the moderate drinking group without recent drinking, lnM = 1.27, lnSD = 0.47, P = 0.013 (Fig. 2). These results indicate the divergent effects of alcohol consumption on muscle PPT. Specifically, moderate drinking was associated with an increase in PPT, whereas binge drinking was associated with a decrease in PPT. Notably, the binge group with recent drinking, lnM = 0.67, lnSD = 0.51, showed reduced PPT compared to all the other groups, Ps < 0.005. Lastly, neither cutaneous MPT (P = 0.148) nor heat pain threshold (P = 0.393) differed significantly between groups, suggesting that binge drinking and alcohol withdrawal were unrelated to cutaneous mechanical and heat pain thresholds in young adult binge drinkers.
Fig. 2.
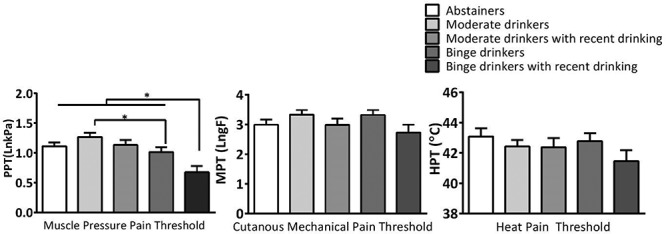
Comparison of muscle pressure pain, cutaneous mechanical and heat pain thresholds between the groups with different drinking patterns (Error bars = SEM).
Stress hormones in relation to drinking patterns and pain sensitivity
A time × gender × group repeated measures ANOVA was conducted for epinephrine (Fig. 3, Upper Left). The results showed no significant time (P = 0.296) nor interaction effects (Ps > 0.245, Fig. 3, upper right). There were significant main effects of group, F(4, 167) = 2.66, P = 0.035, and gender, F(1, 167) = 10.62, P = 0.001, but no significant gender x group interaction (P = 0.834). The main effect of gender on basal epinephrine was driven by higher levels in men, 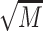 = 14.91,
= 14.91, 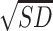 = 6.15, compared to women,
= 6.15, compared to women, 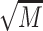 = 11.78,
= 11.78, 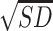 = 6.58.
= 6.58.
Fig. 3.
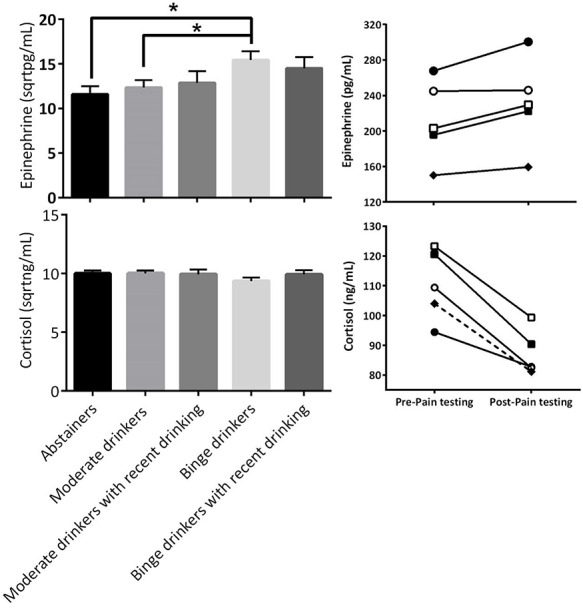
Comparison of epinephrine (upper left) and cortisol concentrations (lower left) between the groups with different drinking patterns (Error bar = SEM).
An LSD post-hoc analysis showed that binge drinkers without recent drinking, 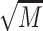 = 15.43,
= 15.43, 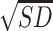 = 5.99, showed elevated epinephrine levels compared to abstainers,
= 5.99, showed elevated epinephrine levels compared to abstainers, 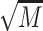 = 11.56,
= 11.56, 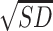 = 5.67, and moderate drinkers without recent drinking,
= 5.67, and moderate drinkers without recent drinking, 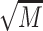 = 12.34,
= 12.34, 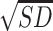 = 5.95. Different from expectation, no significant difference was found between the binge group with and without recent drinking (P = 0.562). These results suggest that binge drinking, but not alcohol withdrawal, was associated with an increase in basal epinephrine levels. A subsequent ANCOVA controlling for basal epinephrine levels indicated that the group difference in muscle PPT remained significant, F(4, 171) = 6.21, P < 0.001, but the effect size was reduced by 7%, partial η2 = 0.13, suggesting basal epinephrine levels explained a small portion of the variance in binge drinking-associated hyperalgesia.
= 5.95. Different from expectation, no significant difference was found between the binge group with and without recent drinking (P = 0.562). These results suggest that binge drinking, but not alcohol withdrawal, was associated with an increase in basal epinephrine levels. A subsequent ANCOVA controlling for basal epinephrine levels indicated that the group difference in muscle PPT remained significant, F(4, 171) = 6.21, P < 0.001, but the effect size was reduced by 7%, partial η2 = 0.13, suggesting basal epinephrine levels explained a small portion of the variance in binge drinking-associated hyperalgesia.
Next, a time x gender x group repeated measures ANOVA was conducted on cortisol. The results showed a significant effect of time, F(4, 167) = 127.85, P < 0.001, but no other significant main or interaction effects (ps > 0.138). Cortisol levels decreased after the QSTs (M = −26.8, SD = 30.5, Fig. 3, Lower right). The lack of a group difference in cortisol suggests cortisol was unrelated to the group difference in PPT.
Negative effect in relation to drinking patterns and pain sensitivity
Because baseline depressive symptoms were significantly higher in drinkers regardless of drinking patterns, no additional tests were conducted. No significant changes in STAI scores by the three QSTs were observed (M = 0.07, SD = 2.42), P = 0.732.
Physiological responses to pain testing
Changes in HR, SCL and RR were compared between the groups with a 3 (time: baseline, pain testing and final) by 5 (group) repeated measures ANOVA. Results revealed significant effects only on time, Ps < 0.001. LSD post-hoc analyses indicated that HR was elevated during pain testing (M = 74.7, SD = 9.5) and after pain testing (M = 74.5, SD = 9.7) compared to baseline (M = 73.6, SD = 9.8). Secondly, compared to baseline (M = 2.3, SD = 0.7), SCL was elevated during (M = 2.8, SD = 0.6) and after pain testing (M = 2.7, SD = 0.6). Thirdly, RRs were elevated during (M = 14.9, SD = 2.3) and after pain testing (M = 14.6, SD = 2.8) compared to baseline (M = 13.8, SD = 3.3). A post-hoc test was conducted for SCL because a significant time by group interaction effect was observed, F(5, 214) = 3.17, P = 0.009. The results indicated that initially different SCLs were no longer different during, P = 0.382, and after pain testing, P = 0.414.
Taken together, psychophysiological responses to pain testing were similar across the groups and paralleled the lack of group differences observed in epinephrine and cortisol responses to pain testing.
DISCUSSION
This study was the first to examine whether hyperalgesia would occur within 2 days of drinking in young adult episodic binge drinkers. We also examined whether this hyperalgesia would be associated with epinephrine and cortisol hormones or negative effect. Our results indicated that hypersensitivity to muscle pressure pain occurred in episodic binge drinkers who reported drinking within 2 days and experiencing acute alcohol withdrawal symptoms at the time of testing. Specifically, PPTs were lower in binge drinkers with recent drinking compared to binge drinkers without it. Therefore, hyperalgesia may occur in episodic binge drinkers during withdrawal. Hypersensitivity to muscle pressure pain was also observed in episodic binge drinkers without recent drinking compared to the moderate drinkers without recent drinking. Yet, binge drinking was not associated with persistent hyperalgesia, as muscle pressure thresholds were not significantly different between abstainers and binge drinkers without recent drinking. Cutaneous mechanical and heat pain thresholds were not associated with binge drinking and alcohol withdrawal. Next, binge drinking was associated with an elevation of plasma epinephrine concentration, but alcohol withdrawal was not associated with its further elevation. In contrast, plasma cortisol concentrations were unrelated to binge drinking and alcohol withdrawal. Finally, we did not find significant group differences in psychological distress levels with one exception. Binge and moderate drinkers reported more depressive symptoms compared to abstainers, suggesting depressive symptoms were associated with alcohol use.
We found that alcohol withdrawal was associated with transitory hypersensitivity to muscle pressure pain, consistent with findings in rodents (Gatch and Lal, 1999; Dina et al., 2000, Dina et al., 2006, Dina et al., 2008;). Our finding is also consistent with previous human research showing reversible withdrawal-associated hyperalgesia in alcohol-dependent men (Jochum et al., 2010). This study extends that work by showing that hypersensitivity to muscle pressure pain may occur during alcohol withdrawal periods even in healthy young adults with only 2–3 years of episodic binge drinking history. With persistent binge drinking, persistent hyperalgesia may develop (Chopra and Tiwari, 2012) as subjective symptoms of alcoholic neuropathy are reported by 20% of alcoholics during the first 5 years of misuse and 40% after 10 years (Vittadini et al., 2001). Thus, future studies could explore the development of alcohol withdrawal-associated hyperalgesia from transitory to persistent.
Rodent studies show that the stress axes can mediate alcohol withdrawal-induced hyperalgesia (Dina et al., 2006; Dina et al., 2008). Our findings suggest that some of these preclinical findings translate to humans, as epinephrine was elevated in binge drinkers regardless of recent drinking. Therefore, elevated epinephrine in binge drinking may be related to binge drinking-associated with hyperalgesia and explained some of the variance in withdrawal-induced hyperalgesia, albeit only to a small degree (7%). Although we found no further elevation of epinephrine levels with alcohol withdrawal symptoms, others observed elevated epinephrine and norepinephrine levels during alcohol withdrawal (Hawley et al., 1981; Heikkonen et al., 1989; Patkar et al., 2003). Evidence linking HPA axis activation to drinking is mixed. Some observed elevated cortisol levels during withdrawal (Adinoff et al., 1991; Adinoff et al., 2003), whereas others observed blunted cortisol levels in young adult binge drinkers (Orio et al., 2018). We found no group differences in cortisol levels. The inconsistency between ours and other studies is potentially due to differences in the time after the last drinking, the number of years of alcohol use and age of the drinkers. This study defined the window of alcohol withdrawal to include the period up to 48 h after the last drink. Despite withdrawal symptoms, epinephrine and cortisol levels might be recovered in our healthy young adults who engaged in episodic binge drinking. Additionally, the role of stress hormones may be more pronounced in the forced ethanol diet used in rodent studies (Dina et al., 2006, Dina et al., 2008). Indeed, a mouse study showed that after a voluntary ethanol diet, withdrawal-induced hyperalgesia occurred without elevation of corticosterone levels (Smith et al., 2016). However, even in the case of self-administration, blocking the signaling pathway of corticotropin release reduced hyperalgesia in ethanol-dependent rats (Edwards et al., 2012), suggesting cortisol may play a role in later stages of drinking.
Unexpectedly, we did not observe greater negative effect in binge relative to moderate drinkers. Drinkers reported slightly higher depressive symptoms compared to abstainers. Previous findings are mixed, with some finding an association between college binge drinking and depression (Cranford et al., 2009) and others finding a U-shaped pattern, with abstainers and heavy drinkers reporting more depressive symptoms than moderate drinkers (O'Donnell et al., 2006) or no association (Dawson et al., 2005; Geisner et al., 2012). Our null finding in negative effect may be due to our exclusion of individuals with psychiatric diagnoses or treatments. Therefore, binge drinkers in this study may be different from typical college binge drinkers.
Limitations of the current study should be acknowledged. First, we were unable to recruit an equal number of men and women to control for potential sex differences. Therefore, the absence of sex effects in this study may be due to insufficient power. Additionally, participants’ self-reported last drinking episode might be inaccurate because it was based on recall. However, the binge drinkers who endorsed drinking within 2 days reported greater acute hangover symptoms at the time of experiment compared to binge drinkers who endorsed no recent drinking and moderate drinkers. Therefore, endorsement of recent drinking was consistent with acute hangover symptoms. Future studies could assess a biomarker (e.g. phosphatidylethanol) to verify self-reported alcohol consumptions (Schröck et al., 2017). More importantly, natural drinking allows to study the effect of ‘typical’ alcohol withdrawal on pain sensitivity ethically in young adult binge drinkers, and their typical binge drinking was far greater than the minimum binge drinking definition (White et al., 2006). Therefore, our study design has greater external validity. Lastly, this between subject-design was limited in examining the effect of alcohol withdrawal on pain sensitivity because of unknown individual differences in pain sensitivity at baseline.
In summary, this was the first study to demonstrate that alcohol withdrawal-associated hyperalgesia to muscle pressure pain existed in young adult binge drinkers. Additionally, elevated basal concentration of epinephrine was associated with binge drinking. However, cortisol concentration and negative effect were unrelated to either binge drinking or alcohol withdrawal. Additional studies are warranted to disentangle the role and the timeline of stress hormones and negative effect in alcohol withdrawal-induced hyperalgesia.
FUNDING
Preparation of this article was funded by the National Institutes of Health National Research Service Award (NIH NRSA), National Institute on Alcohol Abuse and Alcoholism (1F31AA023709) and Dissertation Enhancement Award from College of Liberal Arts, Texas A&M University to the first author and Texas A&M University research support to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- Adinoff B, Risher-Flowers D, De Jong J, et al. (1991) Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry 148:1023–5. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, et al. (2003) Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res 27:1420–7. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C (2013) Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 311:27–34. [DOI] [PubMed] [Google Scholar]
- Ammendola A, Gemini D, Iannaccone S, et al. (2000) Gender and peripheral neuropathy in chronic alcoholism: A clinical–electroneurographic study. Alcohol Alcohol 35:368–71. [DOI] [PubMed] [Google Scholar]
- Antony MM, Bieling PJ, Cox BJ, et al. (1998) Psychometric properties of the 42-item and 21-item versions of the depression anxiety stress scales in clinical groups and a community sample. Psychol Assess 10:176–81. doi: 10.1037/1040-3590.10.2.176. [DOI] [Google Scholar]
- Cameron DM, Brennan TJ, Gebhart GF (2008) Hind paw incision in the rat produces long-lasting colon hypersensitivity. J Pain 9:246–53. doi: 10.1016/j.jpain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K, Tiwari V (2012) Alcoholic neuropathy: Possible mechanisms and future treatment possibilities. Br J Clin Pharmacol 73:348–62. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RL, Parks GA, Marlatt GA (1985) Social determinants of alcohol consumption: The effects of social interaction and model status on the self-administration of alcohol. J Consult Clin Psychol 53:189–200. doi: 10.1037//0022-006X.53.2.189. [DOI] [PubMed] [Google Scholar]
- Cranford JA, Eisenberg D, Serras AM (2009) Substance use behaviors, mental health problems, and use of mental health services in a probability sample of college students. Addict Behav 34:134–45. doi: 10.1016/j.addbeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, et al. (2005) Psychopathology associated with drinking and alcohol use disorders in the college and general adult populations. Drug Alcohol Depend 77:139–50. doi: 10.1016/j.drugalcdep.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Dina OA, Barletta J, Chen X, et al. (2000) Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci 20:8614–9. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Alessandri-Haber N, et al. (2008) Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci 27:83–92. doi: 10.1111/j.1460-9568.2007.05987.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Messing RO, Levine JD (2006) Ethanol withdrawal induces hyperalgesia mediated by PKCε. Eur J Neurosci 24:197–204. doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Gilpin NW, et al. (2020) Alcohol and pain: A translational review of preclinical and clinical findings to inform future treatment strategies. Alcohol Clin Exp Res 44:368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, et al. (2012) Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: Alleviation by CRF1 receptor antagonism. Neuropharmacology 62:1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S (2012) Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 36:2179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton M, Geier T, Keyes K, et al. (2013) Combined role of childhood maltreatment, family history, and gender in the risk for alcohol dependence. Psychol Med 43:1045–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Lal H (1999) Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res 23:328–33. doi: 10.1111/j.1530-0277.1999.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Geisner IM, Mallett K, Kilmer JR (2012) An examination of depressive symptoms and drinking patterns in first year college students. Issues Ment Health Nurs 33:280–7. doi: 10.3109/01612840.2011.653036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley RJ, Major LF, Schulman EA, et al. (1981) CSF levels of norepinephrine during alcohol withdrawal. Arch Neurol 38:289–92. doi: 10.1001/archneur.1981.00510050055008. [DOI] [PubMed] [Google Scholar]
- Heikkonen E, Mäki T, Kontula K, et al. (1989) Effect of acute ethanol intake and hangover on the levels of plasma and urinary catecholamines and lymphocytic β-adrenergic receptors. Alcohol Clin Exp Res 13:20–4. doi: 10.1111/j.1530-0277.1989.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Hingson R, Zha W, Simons-Morton B, et al. (2016) Alcohol-induced blackouts as predictors of other drinking related harms among emerging young adults. Alcohol Clin Exp Res 40:776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, et al. (2010) Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain 14:713–8. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH (1995) The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 33:335–43. doi: 10.1016/0005-7967(94)00075-U. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Bekker H (1992) The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 31:301–6. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2004) NIAAA Councile Approves Definition of Binge Drinking. NIAAA Newsletter, No. 3. Bethesda MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- O'Donnell K, Wardle J, Dantzer C, et al. (2006) Alcohol consumption and symptoms of depression in young adults from 20 countries. J Stud Alcohol 67:837–40. [DOI] [PubMed] [Google Scholar]
- Orio L, Antón M, Rodríguez-Rojo IC, et al. (2018) Young alcohol binge drinkers have elevated blood endotoxin, peripheral inflammation and low cortisol levels: Neuropsychological correlations in women. Addict Biol 23:1130–44. doi: 10.1111/adb.12543. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Gopalakrishnan R, Naik PC, et al. (2003) Changes in plasma noradrenaline and serotonin levels and craving during alcohol withdrawal. Alcohol Alcohol 38:224–31. doi: 10.1093/alcalc/agg055. [DOI] [PubMed] [Google Scholar]
- Robins MT, Heinricher MM, Ryabinin AE (2019) From pleasure to pain, and back again: The intridcate relationship between alcohol and nociception. Alcohol Alcohol 54:625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Minsky SJ, et al. (2007) The Acute Hangover Scale: A new measure of immediate hangover symptoms. Addict Behav 32:1314–20. doi: 10.1016/j.addbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, et al. (2006) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 123:231–43. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Ruha A, Sallinen S, Nissilä S (1997) A real-time microprocessor QRS detector system with a 1-ms timing accuracy for the measurement of ambulatory HRV. IEEE Trans Biomed Eng 44:159–67. doi: 10.1109/10.554762. [DOI] [PubMed] [Google Scholar]
- Schröck A, Thierauf-Emberger A, Schürch S et al. (2017) Phosphatidylethanol (PEth) detected in blood for 3 to 12 days after single consumption of alcohol—a drinking study with 16 volunteers. Int J Legal Med 131:153–60. [DOI] [PubMed] [Google Scholar]
- Sellers EM. (1988) Alcohol, barbiturate and benzodiazepine withdrawal syndromes: Clinical management. CMAJ 139:113–8. [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Hunt-Carter EE (2003) Development and initial validation of the hangover symptoms scale: Prevalence and correlates of hangover symptoms in college students. Alcohol Clin Exp Res 27:1442–50. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Smith ML, Hostetler CM, Heinricher MM, et al. (2016) Social transfer of pain in mice. Sci Adv 2:e1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2019) Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; https://www.samhsa.gov/data/ (13 May 2020, date last accessed). [Google Scholar]
- Swift R, Davidson D (1998) Alcohol hangover. Alcohol Health Res World 22:54–60. [PMC free article] [PubMed] [Google Scholar]
- Vittadini G, Buonocore M, Colli G, et al. (2001) Alcoholic polyneuropathy: A clinical and epidemiological study. Alcohol Alcohol 36:393–400. doi: 10.1093/alcalc/36.5.393. [DOI] [PubMed] [Google Scholar]
- White AM, Kraus CL, Swartzwelder HS (2006) Many college freshmen drink at levels far beyond the binge threshold. Alcohol Clin Exp Res 30:1006–10. [DOI] [PubMed] [Google Scholar]


