Highlights
-
•
Multi-domain profiles advance retrospective prediction of emergent literacy.
-
•
DCDC2 and KIAA0319 risk variants influence emergent spelling skills.
-
•
Combined DYX2 and auditory brainstem measures enhance predictive model fits.
-
•
Additional benefit of preliterate phonological awareness on predictive power.
Keywords: Reading, Spelling, Auditory brainstem responses, KIAA0319, DCDC2, Longitudinal
Abstract
Literacy acquisition is impaired in children with developmental dyslexia resulting in lifelong struggle to read and spell. Proper diagnosis is usually late and commonly achieved after structured schooling started, which causes delayed interventions. Legascreen set out to develop a preclinical screening to identify children at risk of developmental dyslexia. To this end we examined 93 preliterate German children, half of them with a family history of dyslexia and half of them without a family history. We assessed standard demographic and behavioral precursors of literacy, acquired saliva samples for genotyping, and recorded speech-evoked brainstem responses to add an objective physiological measure. Reading and spelling was assessed after two years of structured literacy instruction. Multifactorial regression analyses considering demographic information, genotypes, and auditory brainstem encoding, predicted children’s literacy skills to varying degrees. These predictions were improved by adding the standard psychometrics with a slightly higher impact on spelling compared to reading comprehension. Our findings suggest that gene-brain-behavior profiling has the potential to determine the risk of developmental dyslexia. At the same time our results imply the need for a more sophisticated assessment to fully account for the disparate cognitive profiles and the multifactorial basis of developmental dyslexia.
1. Introduction
Developmental dyslexia is a polygenetic disorder that manifests in disparate neural and cognitive profiles with a missing clear-cut pattern of inheritance. The underlying neural mechanisms and risk factors of dyslexia are well described due to extensive research in the last decades (see Centanni, 2020; Richlan, 2020 for recent reviews). The exact composition, complex interplay and individual impact of these factors leading to the diverse phenotypes of dyslexia, however, are not completely understood. Likewise, standardized and commonly applied preventive measures are still missing. It is highly desirable to identify and treat children at risk sufficiently early, i.e., before literacy acquisition, because literacy is a key qualification for an integrated life and career. Potential biomarkers of dyslexia lie in the genetic and in the neurophysiological domain, as measures in both domains are comparably easy to acquire, inexpensive, and likely tolerable for children at kindergarten age. The current study is a longitudinal follow-up on the project Legascreen, which aims at developing an early multifactorial diagnostic instrument in the triangle of behavior, brain, and genetics. In a previous report (Neef et al., 2017a), we investigated the relation of genotypes and auditory brainstem responses with family risk for dyslexia in preliterate children. Here we report the outcome of those children after two years of schooling. We thus involved children from families with and without family history of dyslexia, with the objective to compile demographic and cognitive profiles, genotype them, and to acquire established electrophysiological markers of reading difficulties.
Commonly, demographic and cognitive measures compose the primary source to determine a potential risk of developing a reading or writing disorder. Home literacy environment, parental education, and socioeconomic status explain variance in literacy as well as in precursor skills of literacy (Dilnot et al., 2017; Hamilton et al., 2016; Ozernov-Palchik et al., 2019; van Bergen et al., 2012, 2011). Likewise, several cognitive-linguistic predictors of literacy such as rapid auditory processing (Pugh et al., 2013; van der Leij et al., 2013), visual and auditory attention (Franceschini et al., 2012; Lallier et al., 2013) or visual motion perception (Boets et al., 2011; Gori et al., 2016) have been described. In the present study, we focus on phonological awareness and rapid automatized naming as cardinal behavioral predictors of literacy. Their strong relationship materializes in multiple large-scale cross-linguistic studies at a cross-sectional (Landerl et al., 2013; Moll et al., 2014; Saksida et al., 2016) as well as a longitudinal level (Caravolas et al., 2012; Landerl et al., 2018; van Bergen et al., 2011). However, in particular psychometric data is less reliable when acquired from children at preliterate age. Thus, an objective measure that is less susceptible to variations in the children’s compliance is highly desirable.
Today, the genetic basis of dyslexia is undisputed based on several studies suggesting different dyslexia susceptibility loci (see Carrion-Castillo et al., 2017, 2013; Kere, 2014; Mascheretti et al., 2017 for an overview). But only a few of the great number of potential genetic risk variants have been replicated in at least one unrelated dataset and might thus be more reliable although the direction of their effect on literacy (protective vs. detrimental) remains inconclusive. Among these are KIAA0319 (Cope et al., 2005; Dennis et al., 2009; Harold et al., 2006; Lim et al., 2014; Paracchini et al., 2008) and DCDC2 (Cope et al., 2012; Meng et al., 2005; Mueller et al., 2016; Riva et al., 2019; Scerri et al., 2011), DYX1C1 (Gialluisi et al., 2019; Taipale et al., 2003; Zhang et al., 2012), ROBO1 (Bates et al., 2011; Hannula-Jouppi et al., 2005; Tran et al., 2014), and FOXP2 (Peter et al., 2011; Sánchez-Morán et al., 2018; Wilcke et al., 2012). In the present study, genotyping focussed on risk variants of KIAA0319 and DCDC2 because these two genes constitute the best replicated susceptibility loci of dyslexia (Cope et al., 2005; Francks et al., 2004; Harold et al., 2006; Mueller et al., 2016; Newbury et al., 2011; Scerri et al., 2011). A deeper rational of focussing on these two genes lies in its specific link to rapid sensory processing, which relates them to the sensory theory of developmental dyslexia (Goswami, 2015; Tallal, 2012). Specifically, the knockdown of these genes in rodents results in degraded neural spike timing and impaired auditory processing (Centanni et al., 2016, 2014; Truong et al., 2014). Literature suggests low-level sensory-processing deficits to precede and underlie phonological problems and resulting reading deficits, a view that was recently integrated in the neural noise hypothesis of developmental dyslexia (Hancock et al., 2017). The third reason for choosing risk variants of KIAA0319 and DCDC2 is the robust relationship between genetic burden of risk alleles and the precision of temporal coding of speech at the level of the auditory brainstem (Neef et al., 2017a) as well as the primary auditory cortex (Centanni et al., 2018). Increased variability of stimulus-driven responses on these levels of the auditory pathway are associated with the genetic risk variants of KIAA0319 and DCDC2. Hence, genetic variability on those dyslexia susceptibility loci constitutes a promising objective method to predict literacy outcome already early on.
On the neurophysiological level association analyses showed a strong linkage between basic auditory processing and later reading development not only in kindergarten (Hämäläinen et al., 2013) but also in newborn event-related potentials (Guttorm et al., 2010; Leppänen et al., 2012). An additional early and objective marker of dyslexia possibly is the method of recording speech-evoked brainstem responses. A number of cross-sectional studies demonstrated the strong relationship between the precision of auditory sound encoding at the level of the auditory brainstem and reading skills or behavioral prerequisites of reading such as phonological awareness and rapid automatized naming (see Table 1). Of these studies only one used longitudinal design reporting a possible prediction of literacy outcome by these electrophysiological metrics (White-Schwoch et al., 2015). In the present study we therefore used such auditory brainstem metrics recorded during preliterate age and tested their potential to predict literacy skills as acquired through structured instruction in school.
Table 1.
Various auditory brainstem measures relate to literacy skills.
| Psychological Measure | Brainstem measure | N | Participants | Age (years) | |
|---|---|---|---|---|---|
| Cross-sectional studies | |||||
| (Chandrasekaran et al., 2009) | Reading | Consonant in quiet in repetitive or variable context | 51 | Good readers | 8 to 13 |
| Speech-in-noise | |||||
| *verbal IQ | Poor readers | ||||
| H200 ratio | |||||
| (Hornickel et al., 2009) | Phonological awareness | Consonant differentiation | 43 | Dyslexic children | 8 to 13 |
| Speech-in-noise | Neural timing | Control children | |||
| (Strait et al., 2011) | Reading | Consonant in quiet in repetitive or variable context | 42 | Good readers | 8 to 13 |
| Auditory | |||||
| Poor readers | |||||
| working memory | |||||
| H200 ratio | |||||
| Attention | |||||
| Music aptitude | |||||
| (Hornickel et al., 2011) | Reading | Consonant in quiet in repetitive or variable context | 81 | No classification | 8 to 13 |
| Speech-in-noise | |||||
| H200 ratio | |||||
| Consonant differentiation | |||||
| Neural timing | |||||
| Consonant in quiet vs. consonant in noise | |||||
| Neural timing | |||||
| Consonant in quiet | |||||
| H200 magnitude | |||||
| Onset precision | |||||
| (Hornickel et al., 2012) | Phonological awareness | Consonant in quiet | 38 | Dyslexic children | 8 to 14 |
| Neural stability | |||||
| Vowel in quiet | |||||
| Neural stability | |||||
| (Hornickel and Kraus, 2013) | Reading | Consonant in quiet | 100 | Good readers Average readers | 6 to 13 |
| *IQ | Neural stability Vowel in silence | ||||
| Neural stability | |||||
| Poor readers | |||||
| (Lam et al., 2017) | Reading fluency | Consonant in quiet Neural stability | 87 | Good RANPoor RAN | 8 to 14 |
| Phonological awareness | |||||
| Rapid automatize naming | |||||
| Processing speed aIQ, processing speed | |||||
| (Neef et al., 2017b) | Phonological awareness | Consonant in quiet | 62 | Good readers | 11 to 13 |
| delta cross-phase | |||||
| Poor readers | |||||
| anon-verbal IQ, age, sex, parental education, family risk | |||||
| Longitudinal studies | |||||
| (White-Schwoch et al., 2015) | Reading | Consonant in noise | 112 | Preschoolers | 3 to 14 |
| Spelling | Neural stability | 2nd grade | |||
| Phonological awareness | |||||
| Control | |||||
| H400 to H700 | |||||
| Neural timing | |||||
| Rapid automatized naming | |||||
explanatory variable.
2. Material and methods
2.1. Participants
In total, 98 native German-speaking children were enrolled in the study, which is part of the longitudinal Legascreen project (www.legascreen.de). At the first time point of assessment (T1), children were at preschool age and recruited from kindergarten on a voluntary basis (4.3–7.5 years; M = 5.8; 49 female). Forty-six of the children were at family risk to develop dyslexia meaning that at least one first-degree relative was affected by developmental dyslexia as reported in a parental questionnaire. The remaining 52 children had neither first- nor second-degree relatives with developmental dyslexia. All participants had normal hearing, passing a hearing screening at a 25 dB hearing level (air conduction) for octaves from 250 to 4000 Hz. Click-evoked brainstem responses were normal. No neurological diseases were known or evident. Three children with nonverbal intelligence scores < 80 were excluded from the sample. Two additional children were excluded as data quality of auditory brainstem measurement of one child did not met recording standards and DNA could not be extracted from the saliva sample of one further child. This resulted in a final sample of 93 children analyzed before the onset of formal reading instruction in elementary school (4.3–7.5 years old; M = 5.8; 46 female). Of these children 44 had a family history of dyslexia (FHD+). Eighty-one of these children could be re-invited for the second time point (T2) at the end of the first year of primary school (6.1–8.9 years old; M = 7.6; 33 female; 37 FHD+), 60 children (7.9–9.2 years old; M = 8.5; 34 female; 28 FHD+) returned for further sessions at the end of the second (T3) and 44 children (8.9–11.0 years old; M = 10.3; 28 female, 14 FHD+) at the end of the third year of school (T4). In average school children (T2-T4; N = 81) were 8.5 years old (6.3–9.9 years). For seventy-five of these children we had additional information about behavioral precursors of literacy measured at T1. A summary of demographic, psychometric and electrophysiological data is given in Table 2. All parents gave written informed consent while children gave additional documented verbal assent to participate in the study. The University of Leipzig Ethical Review Board approved experimental procedures.
Table 2.
Demographic and cognitive characteristics before and after literacy acquisition.
| Sample Description | N | M (SD) |
|---|---|---|
| Age (Years; Month) | 93 | 5.8 (0.9) |
| Age (min – max) | 4.3–7.5 | |
| Sex (male/female) | 47 / 46 | |
| Family history of dyslexia (yes/no) | 46 / 47 | |
| Parental education (Score) | 92 | 4.6 (1.7) |
| Non-verbal intelligence | 81 | |
| Intelligence score (median) | 103 (13) | |
| Range (min – max) | 81−133 | |
| Psychometrics before literacy | ||
| Speech-in-noise perception | 91 | |
| OLKISA (dB) | −4.2 (1.6) | |
| Language development | 93 | |
| SETK – pseudoword repetition (RW) | 11 (4.0) | |
| HSET – sentence repetition (PR) | 40 (30) | |
| WISC - Digit span (Score) | 76 | 9 (2.5) |
| Precursors of literacy | 84 | |
| BISC – pseudoword repetition (RW) | 5.5 (2.2) | |
| BISC – visual attention (RW) | 11 (1.4) | |
| BISC – visual attention time (RW) | 6.2 (3.2) | |
| BISC – RAN objects (RW) | 6.8 (1.1) | |
| BISC – RAN colors (RW) | 9.3 (2.6) | |
| BISC - rhyming (RW) | 9.1 (1.3) | |
| BISC – sound synthesis (RW) | 7.8 (3.3) | |
| BISC – syllable segmentation (RW) | 7.8 (2.1) | |
| BISC – word sound association (RW) | 9.2 (1.4) | |
| BISC – risk score for dyslexia | 1.4 (1.4) | |
| Psychometrics after literacy acquisition | ||
| WISC - Digit span (Score) | 78 | 8.4 (2.5) |
| Literacy acquisition | 81 | |
| ELFE - reading comprehension (PR) | 44 (28) | |
| DERET - spelling (PR) | 35 (25) | |
Environmental-demographics and psychometrics of children before (T1) and after (T2 – T4) literacy acquisition. Average results (M) and standard deviation (SD) are shown if not indicated otherwise. Median of nonverbal intelligence assessed with the Wechsler intelligence scale for children before and after literacy acquisition is reported (T1 and T2) and for reading and spelling tests (T2 – T4) is reported. Raw data is reported when no age-normed values were available. RW raw data, PR age-normed percentile rank.
2.2. Psychometrics
Psychometric assessment was conducted at three longitudinal developmental time points: before literacy acquisition (T1) and up to three times within the first three years of primary school (T2-T4).
Before literacy acquisition we tested non-verbal intelligence, language development, rapid automatized naming, phonological short-term memory, pseudoword repetition, and phonological awareness along with brainstem measures and genotyping.
Children were tested with the German adaptation of the Wechsler Preschool and Primary Scale of Intelligence (WISC-IV; Petermann and Petermann, 2011; Wechsler, 2011, 2009). In the group of preliterate children ten individuals (seven children with FHD+) yielded a nonverbal intelligence below 85. Because the brainstem measure varies with intelligence as reported in previous studies (Hornickel and Kraus, 2013; White-Schwoch and Kraus, 2013), we controlled for it by adding non-verbal intelligence to the regression models (cf. 2.7 statistical analysis). Short-term memory was assessed using forward and backward digit span of the WISC-IV (Petermann and Petermann, 2011; Wechsler, 2011, 2009). Handedness was assessed using a modified version of the Edinburgh Handedness Inventory (Oldfield, 1971).
All children completed a battery of standardized tests evaluating general language development. To assess phonological processing and verbal short-term memory children repeated pseudowords with increasing length and complexity (SETK: Sprachentwicklungstest für drei- bis fünfjährige; subscale Phonologisches Arbeitsgedächtnis für Nichtwörter; Grimm, 2001). Sentence repetition was conducted to evaluate morpho-syntactic development using a subtest of the HSET (Heidelberger Sprachentwicklungstest; Grimm and Schöler, 1977). Since we only conducted specific subtests of these more comprehensive speech and language diagnostics raw values are reported in Table 2. In addition, speech-in-noise perception was assessed using the OLKISA (Oldenburger Kinder-Satztest; HörTech). Speech-in-noise perception was not available for two children due to technical problems. Lower values of the OLKISA score represent higher performance. For statistical analyses missing data was substituted by the sample mean. Furthermore, behavioral precursors of literacy were tested using the BISC (Bielefelder Screening zur Früherkennung von Lese-Rechtschreibschwierigkeiten; Jansen, 2002) in a subsample of 84 children (5.0–7.0 years old; M = 5.10; 37 female; 35 FHD+). This standardized screening evaluates phonological awareness skills, rapid automatized naming, phonetic recoding in short-term memory, and control of visual attention at preliterate age and enables to calculate an age-normed risk score for the development of developmental dyslexia. Children showed a wide range of general language development (see Table 2). No group differences occurred with regard to nonverbal intelligence. Parents filled out a questionnaire to document professional education of families, which was operationalized with an ordinal scale (1, without professional education; 2, Professional School, Vocational School; 3, Master Craftsman, Technical College, Bachelor, University of Cooperative Education; 4, Higher classes of civil service; 5, University of Applied Sciences; 6, University Degree; State Examination).
At the second, third and fourth time point, after the start of literacy instruction in elementary school, children were tested for their actual reading and spelling performance using two well-established diagnostic tools. Reading comprehension was assessed on three levels of increasing complexity from word meaning to text comprehension (Ein Leseverständnistest für Erst- und Sechstklässler: ELFE 1–6; Lenhard and Schneider, 2006). To test spelling performance children had to write a short dictation (Deutscher Rechtschreibtest für das erste und zweite Schuljahr: DERET 1−2+; Stock and Schneider, 2008). Furthermore, assessment of nonverbal intelligence was repeated using the German adaptation of the WISC-IV (Petermann and Petermann, 2014) at T2. To ensure valid measurement of reading and spelling performance the median of all time points was computed whenever possible. In sum, reading and spelling ability of 81 children could be used to retrospectively explore factors influencing literacy development.
2.3. Acoustic stimulus
We used the Klatt-synthesized syllables [da] and [ba], which have been engineered by the laboratory of Nina Kraus (Hornickel et al., 2009; Hornickel and Kraus, 2013). The length of the syllables was 170 ms with a pitch onset of 100 Hz at 10 ms. The formant transition duration was 50 ms composed of a linear rising F1 (400–720 Hz), a linear falling F3 (2580 – 2500 Hz), and flat F4 (3300 Hz), F5 (3750 Hz), and F6 (4900 Hz). The syllables differed in the starting point of F2, 900 Hz for [ba] and 1700 Hz for [da] shifting to 1240 Hz. The steady-state vowel lasted 110 ms.
2.4. Neurophysiological data recording
Children were seated comfortably in a reclining chair in an electrically shielded, soundproof booth. A train of 2000 clicks was presented before and after stimulation with the target syllable to test the integrity of the auditory pathway and to ensure stable recording conditions throughout the experiment. Click and target syllable were presented to the right ear through Etymotic ER-3 insert earphones (Etymotic Research, Elk Grove Village, IL) at an intensity level of 80 dB SPL. The frequency of the syllable presentation was 4.35 Hz. The syllable was presented with both polarities (condensation and rarefaction). Auditory stimulation with speech started with the syllable train [da] and was followed by the syllable train [ba]. Seventeen children finished only the first syllable train. Throughout the recording session, children watched a movie of their choice. The tone of the movie was set to an intensity of 45 dB SPL and presented via loudspeakers.
Brainstem responses were collected using BrainVision V-Amp in combination with an EP-PreAmp, an extremely low-level noise bipolar amplifier (BrainVision) at 20 kHz sampling rate. Three single multitrode Ag/AgCl electrodes were attached to the scalp from Cz to the ipsilateral earlobe, with the forehead as the ground. Impedances were down-regulated (< 5 kΩ) and the inter-electrode impedance difference was not higher than 1.5 kΩ. The continuous signal was filtered off-line with the firfilt EEGLAB plugin (Windowed Sinc FIR-filter, bandpass 70–2000 Hz, Kaiser window, beta = 7.8572, filter order = 100300, fs =20 kHz), epoched from -40 to 190 ms, and baseline corrected to a 40 ms interval preceding sound onset. Epochs with any activity exceeding the range of 35 μV were rejected and a total of 6000 epochs per syllable was considered for further analyses. A comparison of click-evoked wave V latencies, measured before and after the presentation of the target syllable, showed that recording parameters were comparable throughout the session (t = -1.5, p = 0.14).
2.5. Neurophysiological data analysis and reduction
Brainstem responses to the syllable [da] were processed to extract the main electrophysiological features involving spectral features, i.e. the harmonics, and measures of neural stability. All these measures considered the brainstem response to the formant transition in the syllable, which was 10–60 ms post stimulus onset (Hornickel and Kraus, 2013; Johnson et al., 2008). Metrics of all brainstem measures are visualized in Fig. 1a-c and are summarized in Table 3.
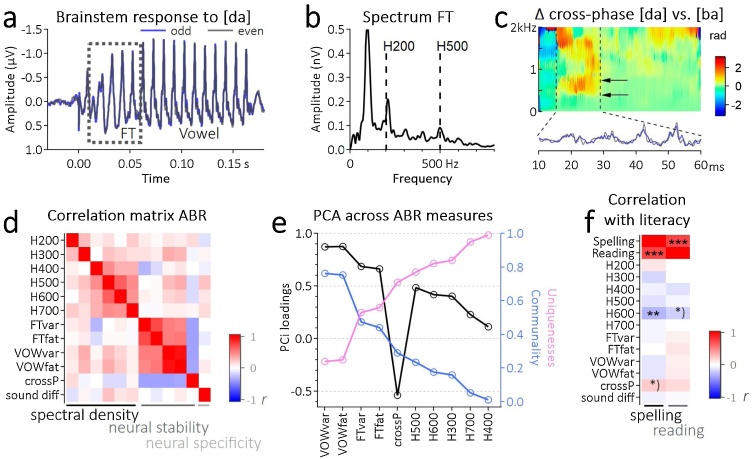
Fig. 1.
Neurophysiological data acquisition and analysis. a) Grand average auditory brainstem response to the syllable [da]. The dotted square indicates the critical time range of the syllable’s formant transition. Blue and gray curve display the sub average across odd and even responses. b) Spectrum of the fast Fourier transformation applied to the grand average of the brainstem response from 10 to 60 ms. c) Crossphaseogram indicates the physiological differentiation between [da] and [ba] in the phase of the formant transition. d) Correlation matrix for all auditory brainstem metrics. e) Loadings, communality and uniqueness for the principal component analyses with one factor and without rotation. f) Correlation matrix shows relationships between auditory brainstem metrics and literacy. Abbreviations: ABR = auditory brainstem responses; FT = formant transition; H200 – H700 = harmonics from 200 to 700 Hz; FTvar = odd versus even trials during FT; FTfat = first-half versus last-half trials during FT; VOWvar = odd versus even trials during vowel; VOWfat = first-half versus last-half trials during vowel; crossP = delta cross-phase of [da] for odd versus even trials during FT; sound diff = delta cross-phase of [da] versus [ba] during FT. Significant correlation is coded as *)p < 0.1, **p < 0.01, ***p < 0.001 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Table 3.
Brainstem measures before literacy acquisition.
| N | FHD-(N = 47) | N | FHD+(N = 46) | P | |
|---|---|---|---|---|---|
| Spectral features | |||||
| H100 (μV) | 47 | 0.48 (0.10) | 46 | 0.48 (0.14) | 0.954 |
| H200 (μV) | 0.15 (0.07) | 0.17 (0.07) | 0.224 | ||
| H300 (μV) | 0.09 (0.04) | 0.07 (0.04) | 0.012 | ||
| H400 (μV) | 0.07 (0.04) | 0.07 (0.04) | 0.503 | ||
| H500 (μV) | 0.09 (0.05) | 0.09 (0.05) | 0.912 | ||
| H600 (μV) | 0.05 (0.03) | 0.05 (0.03) | 0.558 | ||
| H700 (μV) | 0.02 (0.01) | 0.03 (0.02) | 0.360 | ||
| Neural stability to [da] | |||||
| Trial-by-trial variability (Z) for consonant | 0.99 (0.26) | 0.94 (0.30) | 0.308 | ||
| Neural fatigue (Z) for consonant | 1.00 (0.30) | 0.99 (0.29) | 0.797 | ||
| Trial-by-trial variability (Z) for vowel | 0.99 (0.32) | 0.89 (0.34) | 0.121 | ||
| Neural fatigue (Z) for vowel | 0.99 (0.32) | 0.90 (0.31) | 0.120 | ||
| Phase consistency (rad) for consonant | 0.36 (0.19) | 0.47 (0.28) | 0.030 | ||
| Physiological discrimination [da] vs. [ba] | |||||
| Δ cross-phase (rad) | 39 | 0.67 (0.32) | 37 | 0.64 (0.26) | 0.535 |
| Peak-V-latency | |||||
| Pre-speech-evoked BR (ms) | 47 | 5.42 (0.21) | 46 | 5.37 (0.26) | 0.310 |
| Post-speech-evoked BR (ms) | 5.43 (0.20) | 5.40 (0.28) | 0.508 |
Different aspects of the auditory brainstem response to [da] are shown; values are ordered according to family risk for dyslexia (FHD-: no history, FHD+: family history of dyslexia) and represent group averages (±SD); Z z-standardized correlation coefficient, rad radian; BR: brain response.
Harmonics at 200, 300, 400, 500, 600 and 700 Hz were extracted by applying a fast Fourier transformation (FFT) on the individual average across 6000 trials. The FFT was calculated without windowing and after data were padded to 230 ms. To extract the spectral magnitude of the harmonics from H200 to H700, three data points centred around the respective peak were averaged, which corresponds to a 10 Hz window.
Neural stability was computed as Pearson’s correlation coefficient across subaverages of the auditory brainstem responses. According to Hornickel and colleagues (2013), subaverages across 3000 responses were built from the first and last halves of the recordings (neural fatigue) and from the odd and even pairs of the responses to both polarities (trial-by-trial variability). Coefficients can range from 0 to 1 with higher values indicating a higher neural stability. For further statistical analyses, all correlation coefficients were transformed to Fisher’s Z.
The delta cross-phase between subaverages of the brainstem responses is a further indicator of neural stability. The delta cross-phase quantifies a frequency dependent difference of phase angles, and, thus, captures frequency-specific time delays. Delta cross-phase was quantified with MATLAB 8.2 (Mathworks, Natick, MA). The cross-power spectral density (CPSD) function was used in a sliding-window fashion (Skoe et al., 2011) across the subaverages of even and odd responses to [da] (Neef et al., 2017a). Baseline-corrected, detrended data were separated into 211 windows with the first window beginning at 40 ms pre-stimulus onset and the last window beginning at 170 ms, and a 1 ms step size. Each data window of 20 ms was divided into 8 sub-windows overlapping by 50 percent and tapered by a hamming window, resulting in a frequency resolution of 225 Hz. A Fourier transformation was employed with a virtual frequency resolution of 4 Hz. The angle function was applied to extract the cross-phase from the complex cross-spectral densities. To extract phase shifts we considered mean radians at 10−60 ms between 70 and 720 Hz and calculated the circular mean (circ_mean function of the Circular Statistics Toolbox; Berens, 2009) thereby considering the real distance between angels on a circle for odd versus even trials of [da]. A phase shift would indicate an inconsistent representation of the stimulus. In contrast, no phase shift would indicate a consistent representation. Accordingly, the smaller the value the higher the neural stability.
Eventually, we quantified the physiological discrimination between sounds by extracting the delta cross-phase between responses to [da] versus [ba]. We applied a similar routine as described above to determine phase shifts. This time we considered mean radians at 20−40 ms between 400 and 720 Hz because this is the most sensitive domain for a physiological distinction between the two consonants (Neef et al., 2017a; Skoe et al., 2011; White-Schwoch and Kraus, 2013). In contrast to the delta cross-phase within [da] where small values indicate neural stability, big values of phase shift between [da] and [ba] indicate a finer physiological discrimination between speech sounds.
To identify the latent variable structure of the features capturing the auditory brainstem response to speech signals (H200-H500, neural fatigue and variability, cross-phase) we conducted a principal component analysis (PCA). Bartlett’s test of sphericity confirmed a systematic relationship of variables (χ2(66) = 461, p < 0.0001). Inspection of the correlation matrix and Kaiser-Meyer-Olkin criterion (KMO) resulted in the exclusion of two parameters (H200 and delta cross-phase between [da] and [ba]) as they did not met the requirements for a valid PCA (MSA(H200)= 0.48, MSA(crossPhase)= 0.28). This resulted in ten features of auditory brainstem encoding entered into the PCA. The scores of the first factor were extracted for further statistical analyses representing the aggregated unique information of the auditory brainstem indices (Eigenvalue = 3.36, explained variance of 34 %).
2.6. DNA extraction and genotyping
We reported the DNA extraction and genotyping in an earlier publication (Neef et al., 2017a). The following SNPs were selected: KIAA0319 (rs761100, rs2179515, rs2143340, rs3212236, rs9461045, and 6,935,076) and DCDC2 (rs807724, rs1087266, rs807701, rs793842, rs1091047, and rs6922023). We selected these SNPs because they are associated with the neuro-genetic trait for neural variability in humans (Centanni et al., 2018). DNA was extracted from saliva samples using standard procedures (Quinque et al., 2006) or using Oragene DNA Genotek Kits (Kanata, Ontario, Canada). Genotyping was performed first with the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry system iPLEX (Agena, Hamburg, Germany). Genotyping data fulfilled the quality measures: SNP-wise Hardy-Weinberg-Equilibrium (HWE; p < 0.05 Bonferroni corrected), SNP-wise call rate > 97 %, individual-wise: call-rate > 90 % and minor allele frequency (MAF) > 0.05. We excluded KIAA0319 rs9461045 because of HWE violation and DCDC2 rs807701 because of multicollinearity (cf. 2.7 statistical analyses).
2.7. Statistical analyses
Data was analyzed using R Studio (Version 1.1.453; R Core Team, 2018; R version 3.5.0) extended with the packages afex (Singmann and Klauer, 2011) car (Fox et al., 2018), corpora (Evert, 2018), Hmisc (Harrell, with contributions from Charles Dupont and many, 2019), lmtest (Hothorn et al., 2018), and psych (Revelle, 2019). For all statistical analyses behavioral test data and demographic information was standardized or centered. To enhance data quality and validity, the results of reading comprehension, spelling and writing tests were aggregated by taking the median of all timepoints (T2-T4) available for the respective child.
To explore the predictive power of demographic factors, genetic susceptibility genes, DCDC2 and KIAA0319, and brainstem encoding of speech, a series of multiple linear regressions was conducted. In a first basic model environmental-demographic information (age, sex, nonverbal intelligence, parental education, and family history of dyslexia) was entered to predict literacy scores for reading and spelling, respectively. In a next step either DCDC2 or KIA0319 SNPs complemented the genetic model to test the effect of genetic burden on literacy acquisition. Finally, the third model included the principal component scores representing the brainstem response to syllables to test predictive power of auditory encoding of speech at preliterate age. To compare the model goodness of fit was inspected using likelihood ratio test for linear models. To ensure validity of the results independence of errors (Durbin Watson Test) and multicollinearity (VIF and tolerance statistic) was tested for each linear model and number of predictors was adapted when appropriate. This led to exclusion of one SNP of DCDC2 (rs807701) due to multicollinearity resulting in five SNPs entered into the linear regressions for each of the two selected genes (cf. Neef et al., 2017a).
3. Results
3.1. Prediction of reading comprehension
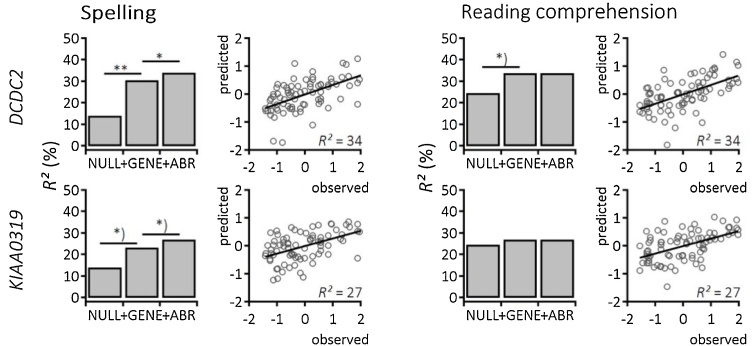
In a first step environmental-demographic information was entered into the model to predict reading comprehension of the emergent readers. This basic model explained 25 % of variance in reading performance (R2 = 0.246, adj. R2 = 0.20, F(5,75) = 4.89, p < 0.001). This effect was mainly driven by a significant influence of parental education (B = 0.37, SE = 0.10, t = 3.63, p < 0.001). Furthermore, scores of nonverbal intelligence tested at preliterate age (T1) had a significant influence (B = 0.02, SE = 0.01, t = 2.245, p = 0.03) and sex was marginally related to reading comprehension (B = -0.39, SE = 0.20, t = -1.93, p = 0.06). None of the further demographic variables showed a significant influence on reading comprehension. In a next step, we explored the influence of the dyslexia susceptibility gene DCDC2 on reading performance. This model explained 34 % of variance (R2 = 0.340, adj. R2 = 0.246, F(10,70) = 3.60, p < 0.001) and model comparison using likelihood ratio test yielded that adding genetic information of DCDC2 did marginally improve prediction (χ2(5) = 10.8, p = 0.056). More specifically SNPs rs807724 (t = -2.9, p = 0.03) and rs793842 (t = 2.1, p = 0.04) had significant impacts on reading comprehension. The last model was complemented by the factor loadings of the auditory brainstem response to speech measured at preliterate age (R2 = 0.340, adj. R2 = 0.235, F(11,69) = 3.23, p = 0.001). Neither comparison with the basic demographic model (χ2(6) = 10.8, p = 0.095) nor with the DCDC2 genetic model (χ2(1) = 0.004, p = 0.96) indicated a significant improvement.
Evaluation of the genetic influence of KIAA0319 and neural encoding of speech in combination with demographic information yielded similar results. Again, neither the addition of variance in the KIAA0319 genotype to the model (R2 = 0.272, adj. R2 = 0.168, F(10,70) = 2.61, p = 0.009) nor the additional extension with variance in the auditory brainstem response (R2 = 0.272, adj. R2 = 0.156, F(11,69) = 2.34, p = 0.02) improved prediction of reading comprehension compared to the basic demographic model (KIAA0319: χ2(5) = 2.82, p = 0.73; ABR: χ2(1) = 0.003, p = 0.96).
To sum up, preliterate nonverbal intelligence and parental education had an important impact on the development of reading comprehension in the cohort of children followed from preschool up to the end of third grade of elementary school. Dyslexia susceptibility genes (DCDC2, KIAA0319) and neural encoding of speech at the level of the auditory brainstem enhanced the explained variance in reading comprehension, but compared to the basic demographic model these effects did not reach significance.
3.2. Prediction of spelling
In a second series of analyses, we explored the influence of the same variables on spelling performance. Similar to reading, the demographic model showed a significant effect of parental education on spelling performance (B = 0.27, SE = 0.11, t = 2.52, p = 0.01). In sum, demographic information explained about 14 % of variance (R2 = 0.141, adj. R2 = 0.08, F(5,75) = 2.47, p = 0.04). In a next step genetic information of DCDC2 was added to the model. This improved prediction of spelling performance and the genetic model explained 31 % of variance (R2 = 0.307, adj. R2 = 0.208, F(10,70) = 3.10, p = 0.003). Here, rs807724 (t = -2.7, p = 0.008), rs793842 (t = 3.76, p = 0.0003), and rs1091047 (t = -2.88, p = 0.007) significantly contributed to the prediction of spelling. The likelihood ratio test confirmed a significant improvement in goodness of fit comparing the demographic and the genetic DCDC2 model (χ2(5) = 17.4, p = 0.004). In a last step the auditory brainstem response to speech was included. Adding factor scores of the first principal component of the auditory brainstem response representing neural stability was marginally significant (B = -0.20, SE = 0.10, t = -1.91, p = 0.06). Nevertheless, adding the information of neural encoding of speech at preliterate age did significantly improve goodness of model fit (χ2(1) = 4.19, p = 0.04; R2 = 0.342, adj. R2 = 0.237, F(11,69) = 3.26, p = 0.001).
The second genetic model exploring the effect of KIAA0319 on spelling improved prediction of performance in comparison to the basic model comprising demographic information (R2 = 0.234, adj. R2 = 0.124, F(10,70) = 2.14, p = 0.03) with a significant effect of rs6935076 (t = -2.77, p = 0.007). SNP rs761100 was only marginally significant (t = 1.9, p = 0.06). This effect was not significant (χ2(5) = 9.26, p = 0.099). However, preliterate auditory brainstem encoding of speech did significantly improve prediction of spelling (χ2(1) = 4.02, p = 0.04; R2 = 0.271, adj. R2 = 0.155, F(11,69) = 2.33, p = 0.02).
In sum, DCDC2 influenced spelling performance of emergent school children. In addition, the combination of genetic information and the auditory brainstem response enhanced predictive power Fig. 2.
Fig. 2.
Multifactorial prediction of literacy. Multiple linear regression analyses resulted in increasing R² for spelling (left column) and reading comprehension (right column). Bar plots show the NULL model including environmental-demographic factors, the + GENE model adding genetic information of DCDC2 (upper row) or KIAA0319 (lower row), and the + ABR model with the aggregated information of auditory brainstem response to [da]. Significant model improvement is coded as *) p < 0.1, *p < 0.05, **p < 0.01. Scatter plots show relationship between predicted values based on the overall model against observed data.
3.3. Multifactorial prediction of literacy including behavioral precursors
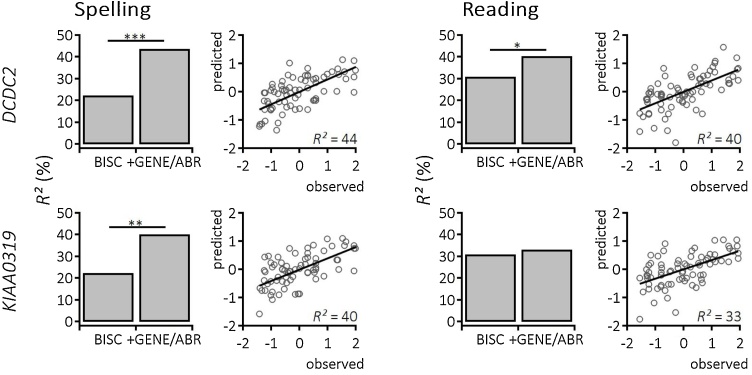
The results of the described series of analyses showed an impact of environmental-demographic, genetic and neurophysiologic factors on the prediction of future literacy to differing degrees. Reading, spelling, and writing are highly complex skills and prediction accuracy did not reach a ceiling effect, thus, we decided to run additional regressions including behavioral precursors of literacy and incorporated the age-normed and z-standardized combined score of preliterate measures (BISC risk score for dyslexia; Jansen, 2002) for a subsample of 75 children (see Table 2). In fact, adding preliterate performance boosted all four predictive models (see Fig. 3). Prediction of reading comprehension raise to 41 % (DEMO/DCDC2/ABR/preliterate skills: R2 = 0.405, adj. R2 = 0.290, F(12,62) = 3.51, p < 0.001) and 33 % (DEMO/KIAA0319/ABR/preliterate skills: R2 = 0.332, adj. R2= 0.203, F(12,62) = 2.57, p < 0.001), respectively. Adding the information of behavioral precursors of literacy to the prediction model of writing and spelling enhanced estimation to 44 % (DEMO/DCDC2/ABR/preliterate skills: R2 = 0.439, adj. R2 = 0.33, F(62,12) = 4.04, p < 0.001) and 40 % (DEMO/KIAA0319/ABR/preliterate skills: R2 = 0.402, adj. R2 = 0.286, F(12,62) = 3.48, p < 0.001). When comparing model fit excluding and including preliterate behavior the latter was more suitable in all four cases (all p’s< 0.05).
Fig. 3.
Multifactorial prediction of literacy improved when considering behavioral precursors. Extended linear regression analyses showed a significant increment of explained variance in spelling (left column) and reading (right column) when additionally considering preliterate psychometric skills. The extended regression model included environmental-demographic factors together with a combined score for phonological awareness, phonological recoding, and rapid automatized naming (BISC model; Bielefelder Screening zur Früherkennung von Lese-Rechtschreibschwierigkeiten; (Jansen, 2002). The + GENE/ABR model adds genotypes of DCDC2 (upper row) or KIAA0319 (lower row), and aggregated information of auditory brainstem response. Significant model improvement is coded as *p < 0.05, **p < 0.01, ***p < 0.001. Scatter plots show the relationship between predicted values based on the overall model against observed data.
4. Discussion
The aim of the present study was to construct an early diagnostic instrument of developmental dyslexia in the triangle of genetics, brain and behavior.
With our current longitudinal approach, we tested the predictive value of multiple dyslexia risk factors on literacy acquisition in a large cohort of German speaking children. Specifically, we tested the impact of (a) environmental-demographic factors, (b) genetic risk variants of DCDC2 and KIAA0319, and (c) neurophysiological measures of preliterate neuronal speech encoding on the development of dyslexia. Our longitudinal design allowed us to track literacy development, starting at preliterate kindergarten age to the end of the third grade of primary school. As commonly acknowledged, our results validate that demographic-environmental factors consistently support prediction of literacy acquisition (Dilnot et al., 2017; Hamilton et al., 2016; Ozernov-Palchik et al., 2019; van Bergen et al., 2012, 2011). On the genetic level, we find that DCDC2 and KIAA0319 expression affects literacy development, especially the acquisition of spelling and writing. Gene expression and neuronal encoding of speech at kindergarten age particularly boosted prediction of writing and spelling, emphasizing their important role in the acquisition of stable grapheme-to-phoneme connections and establishment of sublexical reading strategies. We will discuss the impact of these different factors in turn, before discussing multifactor models.
4.1. Demographic-environmental factors support risk assessment
When considering the demographic-environmental factors parental education showed the strongest impact on early literacy acquisition as measured by several tests. Demographic information predicted 25 % of the reading performance. This is in line with former studies investigating the influence of various environmental factors on literacy (Olson et al., 2014; Pan et al., 2017; Taylor et al., 2010). Family history of dyslexia, though, did not significantly contribute to the prediction of reading, writing and spelling. This seems surprising at first sight because family history of dyslexia is often reported as one of those predictive factors that most reliably dissociate at risk children that will or will not face reading difficulties (e.g., Lyytinen et al., 2015, 2006; Torppa et al., 2010; Psyridou et al., 2018; Thompson et al., 2015). However, several studies report the same pattern as we observe and one has to keep in mind that family history of dyslexia was classified dichotomously into high risk and no risk. Likewise, we only classified children with at least one first-degree relative as high risk to develop dyslexia. Since phenotypes of developmental dyslexia are very heterogeneous (Heim et al., 2008), binary classification might not be sufficient to reliably and specifically predict later literacy acquisition of children and future work should look at family history in more detail, evaluating the characteristics and severity of the literacy impairment in risk families.
The present investigation of multifactorial predictors of literacy showed that all sources of information – environment, genotype, and neurophysiology – influence literacy acquisition to differing degrees. An important advantage of this set of predictors is that they can be assessed quite easily and resemble objective data independent of possibly unreliable compliance of very young children. We will discuss the multifactorial models below.
4.2. Impact of risk gene variants of DCDC2 and KIAA0319
Our results support the assumption that genetic risk variants of DCDC2 and KIAA0319 restrain literacy acquisition. Comparing both genes, expression of DCDC2 had a stronger influence on spelling and writing than KIAA0319. Reading comprehension was generally less associated with gene expression. Likewise, prediction of reading did not significantly improve when adding the aggregated information representing the auditory brainstem response to complex speech sounds. In contrast, prediction of writing and spelling, fostering auditory-based sublexical encoding, improved considerably when adding the information of the auditory brainstem response - especially in combination with information of DCDC2. Similar to Schumacher and colleagues (2006) reporting an association between dyslexia and DCDC2 but not KIAA0319, our results indicate stronger impact of DCDC2 than KIAA0319 on early literacy development in German. The authors claim that the transparency of the respective orthography might lead to distinct processes and reading strategies (Landerl et al., 2018, 2013; Ziegler and Goswami, 2006, 2005), which in turn might be differentially affected by genetic variants (Ludwig et al., 2008; Schumacher et al., 2006). To successfully acquire literacy skills in English, with its opaque orthography, children have to focus on slow sublexical grapheme-to-phoneme conversion (Ziegler et al., 2013, 2001; Ziegler and Goswami, 2006). Therefore, KIAA0319 and DCDC2, which are related to neural precision of auditory processing (Centanni et al., 2016, 2014), might specifically capture this phonological and auditory-based requirements for English reading (Hancock et al., 2017). German, however, has a more transparent orthographic system. Thus, German children quickly shift to rapid and automatized whole-word reading (Grainger et al., 2012; Grainger and Ziegler, 2011) requiring different strategies that may be associated with different genetic traits. This also explains the observed stronger effect of both gene variants on writing and spelling as compared to reading comprehension: Auditory-based sublexical decoding is still required to successfully accomplish oral dictation. A similar pattern of the relationship between genetic risk variants and literacy was found by Neef and colleagues (2017a) who investigated the development in an older cohort of German speaking children. While KIAA0319 showed no correlation with reading it did show a correlation with writing and spelling. In contrast DCDC2 was not associated with spelling and writing but showed a positive relationship with single word reading. Comparing the impact of KIAA0319 and DCDC2 in different languages and phases of literacy acquisition shows that the influence of the same genetic variants might vary with the language specific orthographic system, the particular component skill of literacy investigated, and might also change over the course of literacy development.
4.3. Objective neurophysiological measures contribute to risk assessment in early preliterate state
Similar to a former study (White-Schwoch et al., 2015), we also see associations of neurophysiological assessment of the auditory brainstem response in an early preliterate state with later literacy acquisition, specifically writing and spelling. Since, however, literacy is a highly complex and multifactorial cognitive ability (Pennington, 2006), our approach was multifactorial considering the triangle of gene, brain and behavior rather than looking at the unique prediction by the auditory brainstem.
In our multifactorial approach we see the same tendency although the observed relationship between auditory brainstem responses and literacy is less pronounced than previously reported (e.g., Chandrasekaran et al., 2009; Strait et al., 2011). These studies, however, look at the relationship between the auditory brainstem response and literacy in a cross-sectional design (see Table 1). The present study, in contrast, used the auditory brainstem response to retrospectively predict literacy acquisition two years later. During these two years, formal literacy instruction at school most certainly leads to major changes in children’s development. They acquire knowledge of phoneme-to-grapheme correspondences leading to substantial changes in auditory-phonological processing. Moreover, phonological processing gets more fine-grained and sharpens continuously possibly in complex non-linear dynamic ways (Dehaene et al., 2015; Liebig et al., 2017). Thus, it is plausible to assume that the longitudinal correlation between the preliterate auditory brainstem response and literacy two years later may not be as strong as in a cross-sectional design. In their study, White-Schwoch and colleagues (2015) studying English children evaluated both cross-sectional and longitudinal relationships between various psychological measures and neural encoding of speech. In general, their results show the same tendency as described above: the cross-sectional predictive power is stronger than the longitudinal prediction. However, the strength of association between neural encoding of speech and literacy might also depend on the underlying orthographic system and the resulting reading strategy. It appears that the auditory brainstem response is particularly suitable to predict sublexical grapheme-to-phoneme conversion being predominant during literacy acquisition in the opaque English orthography. In contrast, it might be less directly connected to lexico-semantic whole-word processing, rapidly acquired in transparent orthographies (Grainger et al., 2012; Grainger and Ziegler, 2011). This reasoning is also supported when looking at the stronger predictive power of the auditory brainstem response with respect to spelling and writing compared to reading comprehension in the present study with German children. Finally, it is important to acknowledge that the precision of the auditory brainstem response only captures one specific subcomponent of literacy – auditory-based phonological processing. Since literacy is a highly complex ability involving various cognitive abilities information about visual attention or memory might further improve prediction (Heim et al., 2008). Likewise, multi-methodological approaches combining different neurocognitive methods (e.g. Centanni et al., 2019, 2018) will help to complement the complex picture.
4.4. Multifactorial models of literacy
In the present study we measured several factors in preliterate children in order to test their predictive power on literacy acquisition during later development. In addition to those factors already discussed a number of preliterate behavioral measures were considered such as the well-established precursors of literacy, phonological awareness, phonological recoding, and rapid automatized naming (e.g., (Landerl et al., 2018, 2013). We wondered whether these might help to further improve prediction of literacy. In fact, preliterate skills improved all predictive models of literacy illustrating that behavioral predictors bear an additive potential to estimate future competence in literacy, which complements the prediction by the standard environmental-demographic, genetic and neurophysiological markers. Still, our multifactorial approach did not exhaustively capture cognitive profiles of developmental reading and writing disorders (Doehla et al., 2018; Heim et al., 2008). Here we focused on phonological and auditory prerequisites of literacy und thus our retrospective regression models captured a serious proportion of children that were at risk to develop a reading or writing disorder at kindergarten age. Future longitudinal studies might improve predictive models and achieve an extensive prediction of literacy outcome by expanding the preliterate behavioral assessment and thereby considering further cognitive domains such as vision, attention and memory.
5. Conclusions and future directions
To our knowledge, this is the first study investigating the influence of the complex composition of environment, genotype, neurophysiology and behavior to predict the development of reading comprehension, spelling and writing – thereby capturing the developmental trajectory from the preliterate state up to emergent literacy after two years of schooling. We think that our results are a good starting point to inspire future studies to look at the complex interplay of different predictors of literacy instead of focusing on isolated factors. Likewise, assessing gene-by-environment interactions (Mascheretti et al., 2018) beyond parental education, e.g. home literacy environment or prenatal biological factors, could help to increase our understanding of dyslexia. Certainly, it will be highly important to disentangle the specific impact of these predictors on the different component skills of reading in order to get a more sustained understanding of the triangle of gene, brain and behavior during literacy acquisition and its core deficits.
Funding
This work was supported by the Max Planck Society and theFraunhofer Society (Legascreen (M.FE.A.NEPF0001) as a project within the framework of the “Pakt für Forschung und Innovation”).
Authors contributions
J.L., A.D.F., and N.E.N. conceived and designed the experiments; N.E.N. and J.L., performed the experiments; J.L. and N.E.N. analyzed the data; J.L. and N.E.N. wrote the paper; All authors commented on the paper.
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Acknowledgments
We thank all members of the child laboratory for help with experiments.
Contributor Information
Johanna Liebig, Email: johanna.liebig@fu-berlin.de.
Angela D. Friederici, Email: friederici@cbs.mpg.de.
Nicole E. Neef, Email: nicole.neef@med.uni-goettingen.de.
References
- Bates T.C., Luciano M., Medland S.E., Montgomery G.W., Wright M.J., Martin N.G. Genetic variance in a component of the language acquisition device: ROBO1 polymorphisms associated with phonological buffer deficits. Behav. Genet. 2011;41:50–57. doi: 10.1007/s10519-010-9402-9. [DOI] [PubMed] [Google Scholar]
- Berens P. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 2009 doi: 10.18637/jss.v031.i10.https://doi.org/10.18637/jss.v031.i10. [DOI] [Google Scholar]
- Boets B., Vandermosten M., Cornelissen P., Wouters J., Ghesquière P. Coherent motion sensitivity and reading development in the transition from prereading to reading stage. Child Dev. 2011;82:854–869. doi: 10.1111/j.1467-8624.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- Caravolas M., Lervåg A., Mousikou P., Efrim C., Litavsky M., Onochie-Quintanilla E., Salas N., Schöffelová M., Defior S., Mikulajová M., Seidlová-Málková G., Hulme C. Common patterns of prediction of literacy development in different alphabetic orthographies. Psychol. Sci. 2012;23:678–686. doi: 10.1177/0956797611434536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion-Castillo A., Franke B., Fisher S.E. Molecular genetics of dyslexia: an overview. Dyslexia. 2013;19:214–240. doi: 10.1002/dys.1464. [DOI] [PubMed] [Google Scholar]
- Carrion-Castillo A., Maassen B., Franke B., Heister A., Naber M., Van der Leij A., Francks C., Fisher S.E. Association analysis of dyslexia candidate genes in a Dutch longitudinal sample. Eur. J. Hum. Genet. 2017;25:452–460. doi: 10.1038/ejhg.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T.M. Neural and genetic mechanisms of dyslexia. In: Argyropoulos G.P.D., editor. Translational Neuroscience of Speech and Language Disorders. Springer International Publishing, Cham; 2020. pp. 47–68. [DOI] [Google Scholar]
- Centanni T.M., Booker A.B., Sloan A.M., Chen F., Maher B.J., Carraway R.S., Khodaparast N., Rennaker R., LoTurco J.J., Kilgard M.P. Knockdown of the dyslexia-associated gene Kiaa0319 impairs temporal responses to speech stimuli in rat primary auditory cortex. Cereb. Cortex. 2014;24:1753–1766. doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T.C., Booker A.B., Chen F., Sloan A.M., Carraway R.S., Rennaker R.L., LoTurco J.J., Kilgard M.P. Knockdown of dyslexia-gene Dcdc2 interferes with speech sound discrimination in continuous streams. J. Neurosci. 2016;36:4895–4906. doi: 10.1523/JNEUROSCI.4202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T.M., Pantazis D., Truong D.T., Gruen J.R., Gabrieli J.D.E., Hogan T.P. Increased variability of stimulus-driven cortical responses is associated with genetic variability in children with and without dyslexia. Dev. Cogn. Neurosci. 2018;34:7–17. doi: 10.1016/j.dcn.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T.M., Norton E.S., Ozernov-Palchik O., Park A., Beach S.D., Halverson K., Gaab N., Gabrieli J.D. Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. Neuroimage Clin. 2019;22 doi: 10.1016/j.nicl.2019.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B., Hornickel J., Skoe E., Nicol T., Kraus N. Context-dependent encoding in the human auditory brainstem relates to hearing speech in noise: implications for developmental dyslexia. Neuron. 2009;64:311–319. doi: 10.1016/j.neuron.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope N., Harold D., Hill G., Moskvina V., Stevenson J., Holmans P., Owen M.J., O’Donovan M.C., Williams J. Strong evidence that KIAA0319 on chromosome 6p is a susceptibility gene for developmental dyslexia. Am. J. Hum. Genet. 2005;76:581–591. doi: 10.1086/42913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope N., Eicher J.D., Meng H., Gibson C.J., Hager K., Lacadie C., Fulbright R.K., Constable R.T., Page G.P., Gruen J.R. Variants in the DYX2 locus are associated with altered brain activation in reading-related brain regions in subjects with reading disability. Neuroimage. 2012;63:148–156. doi: 10.1016/j.neuroimage.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Cohen L., Morais J., Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 2015;16:234. doi: 10.1038/nrn3924. [DOI] [PubMed] [Google Scholar]
- Dennis M.Y., Paracchini S., Scerri T.S., Prokunina-Olsson L., Knight J.C., Wade-Martins R., Coggill P., Beck S., Green E.D., Monaco A.P. A common variant associated with dyslexia reduces expression of the KIAA0319 gene. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilnot J., Hamilton L., Maughan B., Snowling M.J. Child and environmental risk factors predicting readiness for learning in children at high risk of dyslexia. Dev. Psychopathol. 2017;29:235–244. doi: 10.1017/S0954579416000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehla D., Willmes K., Heim S. Cognitive profiles of developmental dysgraphia. Front. Psychol. 2018;9:2006. doi: 10.3389/fpsyg.2018.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert S. 2018. Corpora: Statistics and Data Sets for Corpus Frequency Data. [Google Scholar]
- Fox J., Weisberg S., Price B., Adler D., Bates D., Baud-Bovy G., Bolker B., Ellison S., Firth D., Friendly M., Gorjanc G., Graves S., Heiberger R., Laboissiere R., Maechler M., Monette G., Murdoch D., Nilsson H., Ogle D., Ripley B., Venables W., Walker S., Winsemius D., Zeileis A., R-Core . 2018. Car: Companion to Applied Regression. [Google Scholar]
- Franceschini S., Gori S., Ruffino M., Pedrolli K., Facoetti A. A causal link between visual spatial attention and reading acquisition. Curr. Biol. 2012;22:814–819. doi: 10.1016/j.cub.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Francks C., Paracchini S., Smith S.D., Richardson A.J., Scerri T.S., Cardon L.R., Marlow A.J., MacPhie I.L., Walter J., Pennington B.F. A 77-kilobase region of chromosome 6p22. 2 is associated with dyslexia in families from the United Kingdom and from the United States. Am. J. Hum. Genet. 2004;75:1046–1058. doi: 10.1086/426404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialluisi A., Andlauer T.F., Mirza-Schreiber N., Moll K., Becker J., Hoffmann P., Ludwig K.U., Czamara D., St Pourcain B., Brandler W. Genome-wide association scan identifies new variants associated with a cognitive predictor of dyslexia. Transl. Psychiatry. 2019;9:1–15. doi: 10.1038/s41398-019-0402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S., Seitz A.R., Ronconi L., Franceschini S., Facoetti A. Multiple causal links between magnocellular–dorsal pathway deficit and developmental dyslexia. Cereb. Cortex. 2016;26:4356–4369. doi: 10.1093/cercor/bhv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. Sensory theories of developmental dyslexia: three challenges for research. Nat. Rev. Neurosci. 2015;16:43–54. doi: 10.1038/nrn3836. [DOI] [PubMed] [Google Scholar]
- Grainger J., Lété B., Bertand D., Dufau S., Ziegler J.C. Evidence for multiple routes in learning to read. Cognition. 2012;123:280–292. doi: 10.1016/j.cognition.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Grainger J., Ziegler J.C. A dual-route approach to orthographic processing. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm H. Gött. Hogrefe; 2001. Sprachentwicklungstest Für Drei-bis Fünfjährige Kinder (SETK 3–5) [Language Development Test for 3–5-year-olds] [Google Scholar]
- Grimm H., Schöler H. Hogrefe; 1977. Heidelberger Sprachentwicklungstest (HSET) [Google Scholar]
- Guttorm T.K., Leppänen P.H., Hämäläinen J.A., Eklund K.M., Lyytinen H.J. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. J. Learn. Disabil. 2010;43:391–401. doi: 10.1177/0022219409345005. [DOI] [PubMed] [Google Scholar]
- Hämäläinen J.A., Salminen H.K., Leppänen P.H. Basic auditory processing deficits in dyslexia: systematic review of the behavioral and event-related potential/field evidence. J. Learn. Disabil. 2013;46:413–427. doi: 10.1177/0022219411436213. [DOI] [PubMed] [Google Scholar]
- Hamilton L.G., Hayiou-Thomas M.E., Hulme C., Snowling M.J. The home literacy environment as a predictor of the early literacy development of children at family-risk of dyslexia. Sci. Stud. Read. Off. J. Soc. Sci. Study Read. 2016;20:401–419. doi: 10.1080/10888438.2016.1213266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R., Pugh K.R., Hoeft F. Neural noise hypothesis of developmental dyslexia. Trends Cogn. Sci. (Regul. Ed.) 2017;21:434–448. doi: 10.1016/j.tics.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula-Jouppi K., Kaminen-Ahola N., Taipale M., Eklund R., Nopola-Hemmi J., Kääriäinen H., Kere J. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D., Paracchini S., Scerri T., Dennis M., Cope N., Hill G., Moskvina V., Walter J., Richardson A.J., Owen M.J. Further evidence that the KIAA0319 gene confers susceptibility to developmental dyslexia. Mol. Psychiatry. 2006;11:1085. doi: 10.1038/sj.mp.4001904. [DOI] [PubMed] [Google Scholar]
- Harrell, with contributions from Charles Dupont and many . 2019. Hmisc: Harrell Miscellaneous. [Google Scholar]
- Heim S., Tschierse J., Amunts K., Wilms M., Vossel S., Willmes K., Grabowska A., Huber W. Cognitive subtypes of dyslexia. Acta Neurobiol. Exp. (Warsz.) 2008;68:73–82. doi: 10.55782/ane-2008-1674. [DOI] [PubMed] [Google Scholar]
- Hornickel J., Kraus N. Unstable representation of sound: a biological marker of dyslexia. J. Neurosci. 2013;33:3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., Skoe E., Nicol T., Zecker S., Kraus N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proc. Natl. Acad. Sci. 2009;106:13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., Chandrasekaran B., Zecker S., Kraus N. Auditory brainstem measures predict reading and speech-in-noise perception in school-aged children. Behav. Brain Res. 2011;216:597–605. doi: 10.1016/j.bbr.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., Zecker S.G., Bradlow A.R., Kraus N. Assistive listening devices drive neuroplasticity in children with dyslexia. Proc. Natl. Acad. Sci. 2012;109:16731–16736. doi: 10.1073/pnas.1206628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T., Zeileis A., Farebrother (pan.f) R.W., Cummins (pan.f) C., Millo G., Mitchell D. 2018. lmtest: Testing Linear Regression Models. [Google Scholar]
- Jansen H. Hogrefe, Verlag für Psychologie; 2002. BISC: Bielefelder Screening Zur Früherkennung Von Lese-rechtschreibschwierigkeiten. [Google Scholar]
- Johnson K.L., Nicol T., Zecker S.G., Bradlow A.R., Skoe E., Kraus N. Brainstem encoding of voiced consonant–vowel stop syllables. Clin. Neurophysiol. 2008;119:2623–2635. doi: 10.1016/j.clinph.2008.07.277. [DOI] [PubMed] [Google Scholar]
- Kere J. The molecular genetics and neurobiology of developmental dyslexia as model of a complex phenotype. Biochem. Biophys. Res. Commun. 2014;452:236–243. doi: 10.1016/j.bbrc.2014.07.102. [DOI] [PubMed] [Google Scholar]
- Lallier M., Thierry G., Tainturier M.-J. On the importance of considering individual profiles when investigating the role of auditory sequential deficits in developmental dyslexia. Cognition. 2013;126:121–127. doi: 10.1016/j.cognition.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Lam S.S.-Y., White-Schwoch T., Zecker S.G., Hornickel J., Kraus N. Neural stability: a reflection of automaticity in reading. Neuropsychologia. 2017;103:162–167. doi: 10.1016/j.neuropsychologia.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K., Ramus F., Moll K., Lyytinen H., Leppänen P.H.T., Lohvansuu K. Predictors of developmental dyslexia in European orthographies with varying complexity: cross-linguistic predictors of dyslexia. J. Child Psychol. Psychiatry. 2013;54:686–694. doi: 10.1111/jcpp.12029. [DOI] [PubMed] [Google Scholar]
- Landerl K., Freudenthaler H.H., Heene M., De Jong P.F., Desrochers A., Manolitsis G., Parrila R., Georgiou G.K. Phonological awareness and rapid automatized naming as longitudinal predictors of reading in five alphabetic orthographies with varying degrees of consistency. Sci. Stud. Read. 2018:1–15. doi: 10.1080/10888438.2018.1510936. [DOI] [Google Scholar]
- Lenhard W., Schneider W. Hogrefe Göttingen; 2006. Ein Leseverständnistest Für Erst-und Sechstklässler: ELFE 1-6. [Google Scholar]
- Leppänen P.H.T., Hämäläinen J.A., Guttorm T.K., Eklund K.M., Salminen H., Tanskanen A., Torppa M., Puolakanaho A., Richardson U., Pennala R. Infant brain responses associated with reading-related skills before school and at school age. Neurophysiol. Clin. Neurophysiol. 2012;42:35–41. doi: 10.1016/j.neucli.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Liebig J., Froehlich E., Morawetz C., Braun M., Jacobs A.M., Heekeren H.R., Ziegler J.C. Neurofunctionally dissecting the reading system in children. Dev. Cogn. Neurosci. 2017;27:45–57. doi: 10.1016/j.dcn.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.K.-P., Wong A.M.-B., Ho C.S.-H., Waye M.M.-Y. A common haplotype of KIAA0319 contributes to the phonological awareness skill in Chinese children. Behav. Brain Funct. 2014;10:23. doi: 10.1186/1744-9081-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig K.U., Roeske D., Schumacher J., Schulte-Körne G., König I.R., Warnke A., Plume E., Ziegler A., Remschmidt H., Müller-Myhsok B., Nöthen M.M., Hoffmann P. Investigation of interaction between DCDC2 and KIAA0319 in a large German dyslexia sample. J. Neural Transm. 2008;115:1587–1589. doi: 10.1007/s00702-008-0124-6. [DOI] [PubMed] [Google Scholar]
- Lyytinen H., Erskine J., Tolvanen A., Torppa M., Poikkeus A.M., Lyytinen P. Trajectories of reading development: a follow-up from birth to school age of children with and without risk for dyslexia. Merrill. Q. 2006;1982-:514–546. [Google Scholar]
- Lyytinen H., Erskine J., Hämäläinen J., Torppa M., Ronimus M. Dyslexia—early identification and prevention: highlights from the Jyväskylä longitudinal study of dyslexia. Curr. Dev. Disord. Rep. 2015;2(4):330–338. doi: 10.1007/s40474-015-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascheretti S., De Luca A., Trezzi V., Peruzzo D., Nordio A., Marino C., Arrigoni F. Neurogenetics of developmental dyslexia: from genes to behavior through brain neuroimaging and cognitive and sensorial mechanisms. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2016.240. e987–e987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascheretti S., Andreola C., Scaini S., Sulpizio S. Beyond genes: A systematic review of environmental risk factors in specific reading disorder. Res. Dev. Disabil. 2018;82:147–152. doi: 10.1016/j.ridd.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Meng H., Smith S.D., Hager K., Held M., Liu J., Olson R.K., Pennington B.F., DeFries J.C., Gelernter J., O’Reilly-Pol T. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc. Natl. Acad. Sci. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll K., Ramus F., Bartling J., Bruder J., Kunze S., Neuhoff N. Cognitive mechanisms underlying reading and spelling development in five European orthographies. Learn. Instr. 2014;29:65–77. doi: 10.1016/j.learninstruc.2013.09.003. [DOI] [Google Scholar]
- Mueller B., Wilcke A., Czepezauer I., Ahnert P., Boltze J., Kirsten H. Association, characterisation and meta-analysis of SNPs linked to general reading ability in a German dyslexia case-control cohort. Sci. Rep. 2016;6:27901. doi: 10.1038/srep27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef N.E., Müller B., Liebig J., Schaadt G., Grigutsch M., Gunter T.C., Wilcke A., Kirsten H., Skeide M.A., Kraft I., Kraus N., Emmrich F., Brauer J., Boltze J., Friederici A.D. Dyslexia risk gene relates to representation of sound in the auditory brainstem. Dev. Cogn. Neurosci. 2017;24:63–71. doi: 10.1016/j.dcn.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef N.E., Schaadt G., Friederici A.D. Auditory brainstem responses to stop consonants predict literacy. Clin. Neurophysiol. 2017;128:484–494. doi: 10.1016/j.clinph.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Newbury D.F., Paracchini S., Scerri T.S., Winchester L., Addis L., Richardson A.J., Walter J., Stein J.F., Talcott J.B., Monaco A.P. Investigation of dyslexia and SLI risk variants in reading-and language-impaired subjects. Behav. Genet. 2011;41:90–104. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson R.K., Keenan J.M., Byrne B., Samuelsson S. Why do children differ in their development of reading and related skills? Sci. Stud. Read. 2014;18:38–54. doi: 10.1080/10888438.2013.800521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov-Palchik O., Norton E.S., Wang Y., Beach S.D., Zuk J., Wolf M., Gabrieli J.D.E., Gaab N. The relationship between socioeconomic status and white matter microstructure in pre-reading children: a longitudinal investigation. Hum. Brain Mapp. 2019;40:741–754. doi: 10.1002/hbm.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Kong Y., Song S., McBride C., Liu H., Shu H. Socioeconomic status, parent report of children’s early language skills, and late literacy skills: a long term follow-up study among Chinese children. Read. Writ. 2017;30:401–416. doi: 10.1007/s11145-016-9682-4. [DOI] [Google Scholar]
- Paracchini D., Phil S., Steer C.D., Buckingham L.-L., Morris A.P., Ring S., Scerri D Phil T., Stein J., Pembrey M.E., Ragoussis J., Golding J. Association of the KIAA0319 dyslexia susceptibility gene with reading skills in the general population. Am. J. Psychiatry. 2008;165:1576–1584. doi: 10.1176/appi.ajp.2008.07121872. [DOI] [PubMed] [Google Scholar]
- Pennington B.F. From single to multiple deficit models of developmental disorders. Cognition. 2006;101:385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Peter B., Raskind W.H., Matsushita M., Lisowski M., Vu T., Berninger V.W., Wijsman E.M., Brkanac Z. Replication of CNTNAP2 association with nonword repetition and support for FOXP2 association with timed reading and motor activities in a dyslexia family sample. J. Neurodev. Disord. 2011;3:39–49. doi: 10.1007/s11689-010-9065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F., Petermann U. 2011. Wechsler Intelligence Scale for Children®–Fourth Edition. Frankf. M Pearson Assess. [Google Scholar]
- Petermann F., Petermann U. Pearson; 2014. Wechsler Intelligence Scale for Children-: Manual 1: Grundlagen, Testauswertung Und Interpretation: Übersetzung Und Adaptation Der WISC-IV Von David Wechsler. [Google Scholar]
- Psyridou M., Eklund K., Poikkeus A.-M., Torppa M. Reading outcomes of children with delayed early vocabulary: a follow-up from age 2-16. Res. Dev. Disabil. 2018;78:114–124. doi: 10.1016/j.ridd.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Landi N., Preston J.L., Mencl W.E., Austin A.C., Sibley D., Fulbright R.K., Seidenberg M.S., Grigorenko E.L., Constable R.T. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang. 2013;125:173–183. doi: 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinque D., Kittler R., Kayser M., Stoneking M., Nasidze I. Evaluation of saliva as a source of human DNA for population and association studies. Anal. Biochem. 2006;353:272–277. doi: 10.1016/j.ab.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Revelle W. 2019. psych: Procedures for Psychological, Psychometric, and Personality Research. [Google Scholar]
- Richlan F. The functional neuroanatomy of developmental dyslexia across languages and writing systems. Front. Psychol. 2020;11:155. doi: 10.3389/fpsyg.2020.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva V., Mozzi A., Forni D., Trezzi V., Giorda R., Riva S., Mascheretti S. The influence of DCDC2 risk genetic variants on reading: Testing main and haplotypic effects. Neuropsychologia. 2019;130:52–58. doi: 10.1016/j.neuropsychologia.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Saksida A., Iannuzzi S., Bogliotti C., Chaix Y., Démonet J.-F., Bricout L. Phonological skills, visual attention span, and visual stress in developmental dyslexia. Dev. Psychol. 2016;52:1503–1516. doi: 10.1037/dev0000184. [DOI] [PubMed] [Google Scholar]
- Sánchez-Morán M., Hernández J.A., Duñabeitia J.A., Estévez A., Bárcena L., González-Lahera A., Bajo M.T., Fuentes L.J., Aransay A.M., Carreiras M. Genetic association study of dyslexia and ADHD candidate genes in a Spanish cohort: implications of comorbid samples. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri T.S., Morris A.P., Buckingham L.-L., Newbury D.F., Miller L.L., Monaco A.P., Bishop D.V.M., Paracchini S. DCDC2, KIAA0319 and CMIP are associated with reading-related traits. Biol. Psychiatry. 2011;70:237–245. doi: 10.1016/j.biopsych.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Anthoni H., Dahdouh F., König I.R., Hillmer A.M., Kluck N., Manthey M., Plume E., Warnke A., Remschmidt H. Strong genetic evidence of DCDC2 as a susceptibility gene for dyslexia. Am. J. Hum. Genet. 2006;78:52–62. doi: 10.1086/498992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singmann H., Klauer K.C. Deductive and inductive conditional inferences: two modes of reasoning. Think. Reason. 2011;17:247–281. doi: 10.1080/13546783.2011.572718. [DOI] [Google Scholar]
- Skoe E., Nicol T., Kraus N. Cross-phaseogram: objective neural index of speech sound differentiation. J. Neurosci. Methods. 2011;196:308–317. doi: 10.1016/j.jneumeth.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C., Schneider W. 2008. DERET 1-2+: Deutscher Rechtschreibtest Für Das Erste Und Zweite Schuljahr. Beltz. [Google Scholar]
- Strait D.L., Hornickel J., Kraus N. Subcortical processing of speech regularities underlies reading and music aptitude in children. Behav. Brain Funct. 2011;7:44. doi: 10.1186/1744-9081-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M., Kaminen N., Nopola-Hemmi J., Haltia T., Myllyluoma B., Lyytinen H., Muller K., Kaaranen M., Lindsberg P.J., Hannula-Jouppi K. A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proc. Natl. Acad. Sci. 2003;100:11553–11558. doi: 10.1073/pnas.1833911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallal P. Improving neural response to sound improves reading. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16406–16407. doi: 10.1073/pnas.1214122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Roehrig A.D., Hensler B.S., Connor C.M., Schatschneider C. Teacher quality moderates the genetic effects on early reading. Science. 2010;328:512–514. doi: 10.1126/science.1186149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.A., Hulme C., Nash H.M., Gooch D., Hayiou-Thomas E., Snowling M.J. Developmental dyslexia: predicting individual risk. J. Child Psychol. Psychiatry. 2015;56:976–987. doi: 10.1111/jcpp.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torppa M., Lyytinen P., Erskine J., Eklund K., Lyytinen H. Language development, literacy skills, and predictive connections to reading in Finnish children with and without familial risk for dyslexia. J. Learn. Disabil. 2010;43(4):308–321. doi: 10.1177/0022219410369096. [DOI] [PubMed] [Google Scholar]
- Tran C., Wigg K.G., Zhang K., Cate-Carter T.D., Kerr E., Field L.L., Kaplan B.J., Lovett M.W., Barr C.L. Association of the ROBO1 gene with reading disabilities in a family-based analysis. Genes Brain Behav. 2014;13:430–438. doi: 10.1111/gbb.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong D.T., Che A., Rendall A.R., Szalkowski C.E., LoTurco J.J., Galaburda A.M., Fitch R.H. Mutation of Dcdc2 in mice leads to impairments in auditory processing and memory ability. Genes Brain Behav. 2014;13:802–811. doi: 10.1111/gbb.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen E., de Jong P.F., Regtvoort A., Oort F., van Otterloo S., van der Leij A. Dutch children at family risk of dyslexia: precursors, reading development, and parental effects. Dyslexia Chichester Engl. 2011;17:2–18. doi: 10.1002/dys.423. [DOI] [PubMed] [Google Scholar]
- van Bergen E., de Jong P.F., Plakas A., Maassen B., van der Leij A. Child and parental literacy levels within families with a history of dyslexia. J. Child Psychol. Psychiatry. 2012;53:28–36. doi: 10.1111/j.1469-7610.2011.02418.x. [DOI] [PubMed] [Google Scholar]
- van der Leij A., Van Bergen E., van Zuijen T., De Jong P., Maurits N., Maassen B. Precursors of developmental dyslexia: an overview of the longitudinal Dutch dyslexia programme study. Dyslexia. 2013;19:191–213. doi: 10.1002/dys.1463. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Pearson; 2009. Wechsler Preschool and Primary Scale of Intelligence - III Deutsche Version. [Google Scholar]
- Wechsler D. 4th ed. Hogrefe; 2011. Wechsler Intelligence Scale for Children (Deutsche Ausgabe) [Google Scholar]
- White-Schwoch T., Kraus N. Physiologic discrimination of stop consonants relates to phonological skills in pre-readers: a biomarker for subsequent reading ability? Front. Hum. Neurosci. 2013;7:899. doi: 10.3389/fnhum.2013.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Schwoch T., Woodruff Carr K., Thompson E.C., Anderson S., Nicol T., Bradlow A.R., Zecker S.G., Kraus N. Auditory processing in noise: a preschool biomarker for literacy. PLoS Biol. 2015;13:1–17. doi: 10.1371/journal.pbio.1002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke A., Ligges C., Burkhardt J., Alexander M., Wolf C., Quente E., Ahnert P., Hoffmann P., Becker A., Müller-Myhsok B. Imaging genetics of FOXP2 in dyslexia. Eur. J. Hum. Genet. 2012;20:224–229. doi: 10.1038/ejhg.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li J., Tardif T., Burmeister M., Villafuerte S.M., McBride-Chang C., Li H., Shi B., Liang W., Zhang Z. Association of the DYX1C1 dyslexia susceptibility gene with orthography in the Chinese population. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J.C., Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol. Bull. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]
- Ziegler J.C., Goswami U. Becoming literate in different languages: similar problems, different solutions. Dev. Sci. 2006;9:429–436. doi: 10.1111/j.1467-7687.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- Ziegler J.C., Perry C., Jacobs A.M., Braun M. Identical words are read differently in different languages. Psychol. Sci. 2001;12:379–384. doi: 10.1111/1467-9280.00370. [DOI] [PubMed] [Google Scholar]
- Ziegler J.C., Perry C., Zorzi M. Modelling reading development through phonological decoding and self-teaching: implications for dyslexia. Philos. Trans. R. Soc. B Biol. Sci. 2013;369 doi: 10.1098/rstb.2012.0397. 20120397–20120397. [DOI] [PMC free article] [PubMed] [Google Scholar]