Abstract
The new coronavirus pandemic is affecting the entire world with more than 25 million confirmed cases in August 2020 according to the World Health Organization. It is known that the virus can affect several tissues and can progress to a respiratory failure in severe cases. To prevent the progression to this stage of the disease and minimize all the damage caused by coronavirus (SARS-CoV-2) the immune system must be in its integrity. A healthy nutritional status are fundamental to efficient immunological protection and consequently a good response to SARS-CoV-2. Micronutrients and bioactive compounds perform functions in immune cells that are extremely essential to stop SARS-CoV-2. Their adequate consumption is part of a non-pharmacological intervention to keep the immune system functioning. This review has as main objective to inform how micronutrients and bioactive compounds could act in the essential immunological pathways could stop SARS-CoV-2, focusing on the functions that have already established in the literature and transposing to this scenario.
Keywords: COVID-19, SARS-CoV-2, Immunity, Micronutrients, Bioactive compounds
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the new coronavirus pandemic, named by the World Health Organization (WHO) as coronavirus disease 2019 (COVID-19) has reached almost all countries or territories worldwide, with more than 25 million confirmed cases and more than 800 thousand deaths according to data published on the WHO website at August 31, 2020 [1]. The SARS-CoV-2 is an enveloped virus, nonsegmented, with a simple RNA strand, a phospholipid bilayer covered by “spike” glycoproteins, and its pathogenesis being studied by several researchers [2]. Figure 1 represents a schematic of the main structural components of the virus.
Fig. 1.
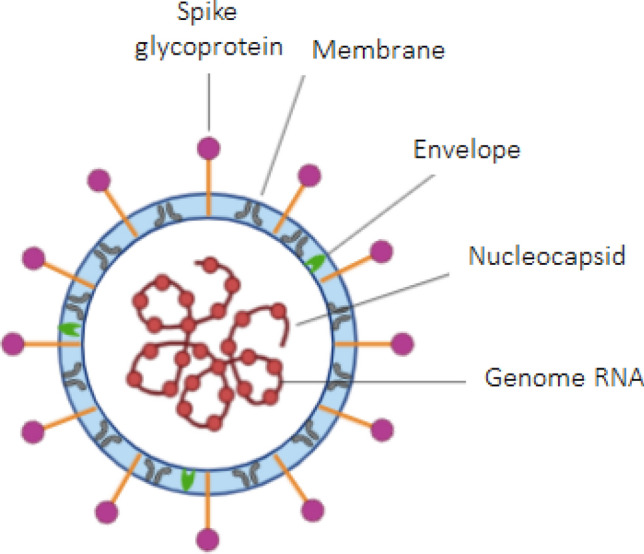
Structural scheme of the SARS-CoV-2.
Adapted from Li et al. [2]
Although the pathophysiological mechanisms associated with COVID 19 are not fully elucidated, Guan WJ and colleagues showed that not all people exposed to the SARS-Cov-2 will be infected and not all those infected will develop the most severe symptoms [3]. Based on that, the infected subject can be classified into three distinct stages, according to the symptoms and presence of the virus [4]. Stage 1 is related to the virus's incubation period, which can be detected or not, and with the absence of COVID-19′s symptoms as well. Stage 2 is the period when the virus can be detected and there are mild symptoms. Lastly, stage 3 represents the period that the subject has a high viral load and severe respiratory symptoms [4]. Besides the respiratory system, SARS-CoV-2 induced functional impairment was observed in the, nervous, cardiovascular and gastrointestinal systems [5, 6]. Once the integrity of the human immune system (IS) has a pivotal role to protect the body against the severity of the symptoms in the different systems the appropriate immune response (IR) is extremely necessary to control or to minimize the effects of the viral infection [2].
A healthy nutritional status are fundamental to efficient immunological protection. To ensure the IS functionality, the ideal contribution of macro and micronutrients should be taken into account [7, 8]. It has already been well established that during infection-induced energy demand increases, the adequate intake of carbohydrates, proteins, and lipids is essential for the activation of IS [7]. On the other hand, the malnutrition status is associated with IR commitment [7]. In addition to the macronutrients, micronutrients also have an important role in the IS. Even so, based on the dietary pattern, almost all subjects probably have a deficient intake of micronutrients, which may impair the performance of IS [7]. It is important to highlight that micronutrients act synergistically, in other words, the human body needs an adequate concentration of all micronutrients involved with the IR to guarantee the immune barrier of cells [8]. Other substances present in healthy nutrition, as some bioactive compounds, especially polyphenols, also play an important role in the IS, because of their antioxidant and anti-inflammatory properties [8]. They act by regulating immune cells, gene expression, and inhibiting certain pro-inflammatory cytokines [9]. Thus, both micronutrients and bioactive compounds might contribute to the adjuvant treatment of patients that have already infected with the SARS-CoV-2, since it targets specific IS cells, such as macrophage, NK cell and T cells [2] that could have responses potentiated by certain nutrients and bioactive compounds. This non-pharmacological intervention could help to decrease the severity of symptoms by modulating the host's IS.
It is important to note that, to the present moment, there is no solid information in the scientific literature showing how adequate nutrition status or specific food consumption could help to decrease SARS-CoV-2 spreads and control the COVID-19 current pandemic scenario progression. However, there are relevant evidences recent published showing the role of some micronutrients reducing risk of COVID-19 and other viral infections (e.g., Influenza), such as vitamin D [10–13], vitamin A, selenium, zinc [14, 15], copper [16] and vitamin C [17, 18].
A systematic review about potential interventions for COVID-19 suggests the importance of individually evaluation of nutritional status before administration of any treatment proposed [19], highlighting the synergic role of nutrients in IR. Besides, avoid nutritional deficiencies using effective and safe nutrition strategies may help to decrease the number of infected people [20].
Therefore, this review has as main objective to inform how micronutrients and bioactive compounds could act in the essential immunological pathways could stop SARS-CoV-2, focusing on the functions that have already established in the literature and transposing to this scenario.
Search strategy
Search strategy included PubMed using main keywords related to immunological responses, COVID-19, micronutrients and bioactive compounds (e.g., immune system, SARS-CoV-2, vitamins, minerals, polyphenols). Books and a food composition table complemented information about food sources, quantity and bioavailability of micronutrients and bioactive compounds. This review included COVID-19’s evidences published in the years 2019 and 2020. Also included micronutrients, bioactive compounds and immune responses articles published from the 1990s to 2020. In vivo (rodents and human experimental models) and in vitro studies were eligible when published in English or Portuguese. No systematic assessment and statistical analysis were performed.
Immunological responses and Covid-19
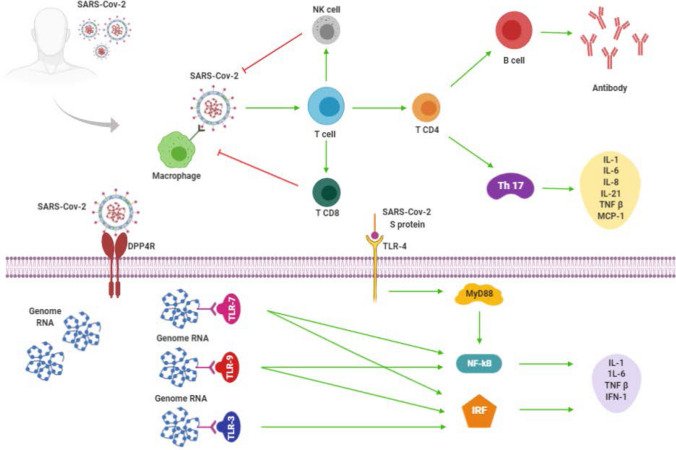
After the virus host invasion, the first recognition is made through the innate IS through pattern recognition receptors (PRRs), such as the retinoic acid-inducible gene I-like receptors [rig-I-like receptor (RLR)], which is responsible for recognizing viral genetic material [2, 21]. The inflammation cascade begins through the binding of SARS-CoV-2 to macrophages that consequently presents its antigens for TCD4 cells, generating their activation and also differentiation into T helper type 17 (Th17) cells, for example. Then, cytokines are produced to mobilize the entire adaptive IR. They are interleukins (IL) 1, 6, 8 and 21, tumor necrosis factor β (TNF-β), and monocyte chemoattractant protein-1 (MCP-1). These mediators stimulate T cells to activate natural killer cells (NK) and TCD8 cells [2]. TCD4 cells are responsible for producing specific antibodies to SARS-CoV-2 through the activation of B cells. TCD8 cells are important to viral control, since they are cytotoxic, playing a crucial role in the elimination of SARS-CoV-2 of the infected cells, and are associated with an immunological induction [2, 22].
To reach the cell's cytoplasm, the SARS-CoV-2 binds to a specific receptor, called dipeptidyl peptidase-4 (DPP4), through its spike glycoprotein (S), promoting adhesion between protein and receptor. To the viral genome presentation occurs, protein S needs to bind to the tool-like receptor (TLR)-4. The recognition of protein S triggers pro-inflammatory cytokines production through a primary response protein-dependent signaling pathway to myeloid differentiation factor-88 (MyD88). Then, it is observed the nuclear factor kappa B (NF-kB) activation, IL-1 and IL-6 production, in addition to type 1 interferon (IFN-1) and TNF-β [2].
Another pathway might be stimulated by binding viral RNA to the TLR-3 receptor, which induces the activation of inflammatory pathways through the interferon regulatory factors (IRF). Consequently, it culminates in IFN-1, IL-1, IL- 6, and TNF-β production as well. If the viral RNA binds to TLR-7 and/or TLR-9, it is also observed NF-kB and IRF activation. The presence of these cytokines attracts lymphocytes and leukocytes to the infected cell until the infection can be controlled [2]. It is important to highlight the role of IFN-1 in viral spread control. IFN-1 activate dendritic cells and NK, besides accelerate phagocytosis by macrophages of viral antigens [2, 21]. Figure 2 illustrates the process of immune activation.
Fig. 2.
Immune response to SARS-CoV-2. The scheme shows the main cells and cytokines involved in the immune defense mechanism against COVID-19 described in this review. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2; NK cell natural killer cell; Th 17 T helper type 17; IL-1 Interleukin 1; IL-6 Interleukin 6; IL-8 Interleukin 8, IL-21 Interleukin 21, TNF-β tumor necrosis factor β, MCP-1 monocyte chemoattractant protein-1, DPP4 dipeptidyl peptidase-4; S protein spike glycoprotein; TLR-3 tool-like receptor—3; TLR-4 tool-like receptor—4; TLR-7 tool-like receptor—7; TLR-9 tool-like receptor—9; MyD88 myeloid differentiation factor-88; NF-kB nuclear factor kappa B; IRF interferon regulatory factors. Red lines refer to inhibitory effects. Green lines refer to activating effects.
If there is an impairment of the immune barrier and the virus spread cannot be contained, SARS-CoV-2 proliferates and affects, principally, tissues that have a high expression of the angiotensin-converting enzyme 2 (ACE 2), like kidneys, intestine, lungs, brain, and cardiovascular system [23, 24]. The virus affinity to ACE 2 may be an explanation for patients who died of multiple organ failure [23]. Recently, Cheng H and colleagues [25] showed that the virus is dependent on the ACE 2 expression in the respiratory epithelium to be able to penetrate and start its replication. Then, the damaged cells generate the inflammatory cascade described above, starting with the presentation of the virus to lymphocytes and ending with pro-inflammatory cytokines liberation. The pro-inflammatory macrophages and granulocytes-mediated IR culminates in respiratory disorders, the main symptom in the most severe stage of the COVID-19 [24, 26]. Pro-inflammatory cytokines such as IL-1, IL-6, and TNF are found in high concentrations in the infected patient's lungs and could be a possible trigger for the formation of pulmonary mucus and fibroblasts induction [23]. Thus, strategies that allow to block or to reduce those pro-inflammatory cytokines' actions can benefit patients in more advanced stages of COVID-19 [24].
Micronutrients
Once the scientific knowledge about the mechanisms and processes involved in cells and cytokines activation and production related to the SARS-CoV-2 immune responses was previously presented, it is important to understand how some micronutrients could be fundamental for the IS homeostasis during SARS-CoV-2 infection. Some micronutrients are related to inhibiting, stimulating, or acting as a cofactor to cells and cytokine altered by that virus. Therefore, this section brings together the main vitamins and minerals that contribute to IS function.
Vitamin A
Some previous scientific reviews highlighted the importance of vitamin A to the proper function of innate IS cells, as NK cells and macrophages [8, 27]. Besides that, vitamin A is fundamental for both T and B cell performance and the mechanism of antibody production in response to the specific antigen [28, 29].
In vivo evidences
Its deficiency is associated with the reduced number and functionality of NK cells and increases of INF γ, and intervenes negatively in phagocytic and macrophages activities as well in experimental study with rodents [30].Also, deficient levels of vitamin A could affect the differentiation and quantity of T cells, reducing the generation of antibodies and consequently decreasing the response to the virus in rodents [31, 32].
In vitro evidences
Deficient status of vitamin A negatively affects the differentiation in growth of B cells [33].
Vitamin B6
Similar to vitamin A, vitamin B6 participates in the production of antibodies and NK cell activity [27, 28, 34]. It is necessary for cytokines and endogenous amino acids the synthesis and metabolism [29].
In vivo evidences
Vitamin B6 also assists in inflammation regulation, maintaining Th 1 responses and the process of lymphocyte proliferation, differentiation, and maturation in experimental studies in rodents [35, 36]. Besides, higher levels of B6 active form decreases inflammation in humans [37]. Its deficiency can lead to lymphocytopenia and consequent immune deficiency, decreasing antibody-mediated responses as well [38].
Vitamin B7
Biotin (vitamin B7 or vitamin H) is an essential co-factor for five carboxylases, known as biotin-dependent carboxylases, involved in metabolism of several pathways such as gluconeogenesis, branched-chain amino acids metabolism and fatty acid synthesis [39, 40]. Metabolic disorders can lead to changes in IS responses and inflammatory process caused by biotin deficiency [39].
In vitro evidences
Previous research shows that biotin deficiency can affect immune responses, enhancing T CD4 lymphocytes in humans’ blood samples [41]. Consequently, leading to an increased level of IFN-γ, TNF and IL-17 and enhance of proinflammatory responses [41]. Besides, dendritic cells leads to Th1 and Th17 responses through increased production of proinflammatory cytokines when biotin levels are insufficient [42].
Vitamin B9
Vitamin B9 or folate is involved in the NK cells cytotoxicity maintenance and the production and metabolism of antibodies, enabling a sufficient antigen response to the antigen [26, 27, 29].
In vivo evidences
Animal and humans studies show folate deficiency association with depressive immune response, impairing, for example, the proliferation of T cells [38]. In rodent model antibody responses can be improper in this situation [29].
Vitamin B12
As vitamin B12 is a cofactor in folate metabolism, it indirectly contributes to the production and metabolism of antibodies [28, 29, 34].
In vivo evidences
An important immunomodulator, vitamin B12 is associated with cytotoxic cells, as NK and TCD8, facilitating its production and helping to regulate the proportion between helper and cytotoxic T cells in humans [43, 44].
Vitamin C
Vitamin C is widely known for its antioxidant and immunomodulatory properties, capable to protect lymphocytes from oxidative stress [29]. It is fundamental to other antioxidant compounds regeneration, as glutathione and vitamin E, enabling conversion to their active form [45].
In vivo evidences
Vitamin C deficiency is related to increases in cell oxidative damage and in the incidence and severity of pneumonia cases in humans [46, 47].
Vitamin C is also involved in maintaining and improving the activity of NK cells and their chemotaxis [27, 28, 48, 49], removal, and macrophages-mediated neutrophils apoptosis in rodents and humans [50]. Besides, it can stimulate the production, function, and movement of leukocytes to the infection site [29, 47].
In vitro evidences
Lastly, vitamin C plays a role in T cells production, differentiation, and proliferation of T cells, resulting in cytotoxic T cell production and in the increase in the antibodies generation [29, 48, 50].
Vitamin D
Vitamin D is one of the most widely studied micronutrient involved with the IS function. Vitamin D receptors can be found in innate IS cells such as macrophages, monocytes, and dendritic cells [29].
In vitro evidences
This vitamin is able to increase the differentiation of monocytes to macrophages [48] and in its active form (i.e. calcitriol), it is associated to promote the macrophages movement and phagocytic capacity, improving their oxidative potential [28, 51–53]. Furthermore, vitamin D stimulates the proliferation of the immune cells, increases the synthesis of superoxide, and helps to protect against infection caused by pathogens [29].
About the adaptive IS, vitamin D can induce suppression in the B cells antibodies production and T cell proliferation [29].
In vivo evidences
In addition, vitamin D is related to inhibit T cytotoxic and helper cell functions and to promote T regulatory T cell production [49, 54, 55]. It can reduce the expression of pro-inflammatory cytokines and increase anti-inflammatory cytokines in vitro and in vivo experimental models [29, 51, 56–60].
Its deficiency increases the susceptibility and severity to infections, especially acute respiratory tract infections, decreases the number of lymphocytes, and increases morbidity and mortality in children [46, 61].
Vitamin E
Besides the anti-inflammatory profile, vitamin E is an important antioxidant compound, protecting cells against free radicals [29, 48].
In vivo evidences
Among its anti-inflammatory functions, vitamin E improves NK cell activity, lymphocyte proliferation, and T cell-mediated functions, helping to build immune synapses between T helper cells. Indirectly, vitamin E protects T cell functions by decreasing the production of prostaglandin E2 (PGE2) by macrophages, which has immunosuppressive activity in several studies in animal models and humans [27, 28, 48, 49, 62, 63]. Its deficiency can impair adaptive immunity, affecting the functions of T and B cells [29].
Copper
Copper is a mineral that accumulates at the site of inflammation [29, 34]. As the zinc, copper is directly related to the enzyme superoxide dismutase (SOD), important in the defense against reactive oxygen species (ROS) [28]. Thus, it is considered a free radical scavenger, capable of maintaining an intracellular antioxidant balance [28, 64].
In vivo evidences
To react against the infectious agents, copper acts on macrophages, accumulating in their phagolysosomes and improves the NK activity in rats [65, 66].
In addition, it participates in the differentiation and proliferation of T cells, the production of antibodies, and cellular immunity in animal studies [34, 35]. Its deficiency can cause an abnormal decrease in neutrophils and increase the susceptibility to infections in humans [29, 67, 68].
Iron
Iron is involved in the production and action of inflammatory cytokines such as IFN-γ, TNF-α, IL-2, and IL-10 [48], and it is also important to generate ROS that kills the pathogen that infects IS [29].
In vitro evidences
In relation to the adaptive IS, iron is involved in the differentiation and proliferation of T cells and assists to regulate the proportion between T helper and cytotoxic T cells [29, 49].
In vivo evidences
An adequate plasmatic iron level is able to modulate the IS, reducing the M1 macrophage's pro-inflammatory response in mice [69, 70]. Its deficiency can reduce the immune response in humans [38, 70]
Magnesium
In vivo evidences
In humans, magnesium is associated with DNA protection against oxidative damage [71].
In vitro evidences
In high concentrations, magnesium reduces superoxide anion production [72]. It fundamental to bind the antigens to macrophage RNA, to regulate leukocyte activation, it is a cofactor for antibodies synthesis and is involved in the regulation of apoptosis [65, 66].
Selenium
This mineral is essential for the selenoproteins activity, which is important for the host's antioxidant defense [19].
In vivo evidences
Selenoproteins may affect the NK cells and leukocytes functions and potentially reduce the exaggerated ROS production during the oxidative stress [27, 29, 34, 48, 73].
Selenium also participates in T cell differentiation and proliferation, improves T helper cell counts and antibody levels [34, 64, 74]. Its deficiency can impair cellular and humoral immunity, besides increasing the virulence during viral infections in humans and rats studies [29, 46, 74, 75].
Zinc
Zinc is an important antioxidant agent against both ROS and reactive nitrogen species (RNS) [45, 47].
In vitro evidences
Zinc is essential for the intracellular tyrosine kinase binds to T cells receptors, promoting the T lymphocytes development and activation [45, 47]. Besides that, zinc induces the T cytotoxic cells proliferation and is involved in the development of regulatory T cells (Treg) [49, 76–78].
In vivo evidences
This mineral is also an anti-inflammatory agent, able to modulate cytokines release through the regulation of development of pro-inflammatory cells, such as Th17 and Th19, and of the production of cytokines, such as IL-2, IL-6, and TNF-α in several studies with humans and animals, besides in vitro evidences [49, 76–81]. It contributes to the maintenance and improvement of the cytotoxic activity of NK cells, in the monocytes phagocytic capacity, it helps in the TCD8 cells proliferation, and it influences the activity of antioxidant proteins [27–29, 47–49, 78, 82, 83].
Zinc is also involved in the antibodies production, mainly immunoglobulin G (IgG), and in its response in animal models [28, 78, 84, 85].
Finally, adequate levels of zinc are important to maintain the host's immunological defense [49]. Its deficiency is associated with impairment of total IS, affecting the number and function of lymphocytes, particularly T cells, and leads to altered production of cytokines that contribute to oxidative stress and inflammation [29]. It may be associated with an increase in viral (particularly pneumonia) and respiratory infections in humans [38, 46].
In order for all micronutrients to perform their functions, attention must be paid to its intake according to the recommended daily dietary intake (RDA) for healthy individuals, which considers the age range of each individual. Table 1 provides this information for adults, besides to compile the main functions in the IS and food sources of each micronutrient. It is worth mentioning that, the daily values of these micronutrients might be changed, with the necessity for greater amounts of these compounds to provide ideal immune support [8].
Table 1.
Micronutrients: food sources, immune system functions and recommendations
| Micronutrient | Food sources [131] | Functions | Recommendation (RDA)—adults (18–70 years) | Quantity of micronutrients per 100 g of food [143, 144] |
|---|---|---|---|---|
| Copper | Liver, oysters, whole grains, oilseeds and chocolate |
Intracellular antioxidant [64] Contributes to the inflammatory response [34] Acts on cell differentiation and proliferation [34] Assists in the NK cells activity [145] Involved in the antibodies Productions of antibodies and cellular immunity [28] |
900 µg/day for both men and women |
Brazilian nut—1.7 mg Chocolate 2.2 mg Cooked bovine liver—9.9 mg Cooked oysters—2 mg Hazelnut—1.5 mg Oat bran—0.4 mg |
| Iron | Meat, liver, legumes, seafood, dark green vegetables |
Contributes to free radicals formation to react against viruses and bacteria [146] Participates in the T lymphocyte proliferation and differentiation [146] Participates in the enzymatic action against pathogens [147] |
8 mg/day for men and 18 mg/day for women |
Cooked beef—2.6 mg Cooked black beans—1.67 mg Cooked bovine liver—6.3 mg Cooked broccoli—0.8 mg Cooked chicken leg—2.1 mg Cooked green peas—1.63 mg Cooked pork—1.2 mg Cooked spinach—1.5 mg Steamed seafood—22 mg |
| Magnesium | Cereals and seeds, green leafy vegetables, seafood and oilseeds | Magnesium supplementation is correlated to improve lung function [148] | 420 mg/day for men and 320 mg/day for women |
Almonds—305.1 mg Cooked green beans—151 mg Cooked oysters—39 mg Cooked shrimp—34 mg Cooked spinach—68.4 mg Hazelnut—282.3 mg Pumpkin seed—532 mg Sesame—340.4 mg Sunflower seed—121 mg Wheat germ—321.4 mg |
| Selênium | Oilseeds, seafood and cereals |
Essential for several enzymes involved in the redox balance [149] Increases T cells proliferation [149] Increases the Natural Killer cells activity [149] Improves the antiviral vaccine responses (including influenza vaccine) [149] Improves antiviral immunity [149] Decreases virulence of some influenza strains and other viruses [150] |
55 µg/day for both men and women |
Brazilian nut—126 µg Brown rice—2.7 µg Canned sardine in oil—46 µg Tuna—52.5 µg Whole wheat flour—13.6 µg |
| Vitamin A | Liver, fish liver oil, egg yolk, butter, green leafy vegetables, yellow-orange vegetables and fruits |
Action in resistance to infections [151] Maintenance of the lymphocyte pool and T lymphocyte synthesis [151] Maintenance of epithelial cell differentiation and immune competence [151] Improves neutrophils, macrophages and Natural Killer cells functions [151] Role in the T lymphocytes differentiation [151] Role of prevention and treatment of respiratory tract infections [152] |
900 µg/day for men and e 700 µg/day for women |
Broccoli—279 µg Butter—1013 µg Carrot—1326 µg Cod liver oil—30,000 µg Cooked bovine liver—14,574 µg Egg yolk—148 µg kale—496 µg Manga—787 µg Spinach—484 µg |
| Vitamin B12 | Meat, liver, fish, eggs, milk and dairy products |
Immunomodulator [28] Acts on the NK cells functions [34] Assists in the T lymphocytes productions [34] Contributes indirectly to the antibodies' production and its metabolism [34] |
2.4 µg/day for both men and women |
Boiled egg—1 µg Cooked beef—2.5 µg Cooked bovine liver—112 µg Cooked chicken—0.36 µg Cooked salmon—2.8 µg Cooked trout—5 µg Cooked tuna—1.8 µg Cottage cheese—2.81 µg Steamed seafood—99 µg Whole milk—0.33 µg |
| Vitamin B6 | Meat, bananas, broccoli, carrots, spinach and oilseeds |
Helps in the inflammatory process regulation [34] Participates in the amino acids, cytokines and antibodies synthesis [34, 145] acts on the lymphocytes proliferation, differentiation, and maturation [145] Assists in NK cells activity [34] |
Men 18–50 years: 1.3 mg/day 51–70 years : 1.7 mg/day Women 18–50 years : 1.3 mg/day 50–70 years : 1.5 mg/day |
Banana—0.6 mg Bovine liver—1.43 mg Brazilian nuts—0.26 mg Carrot—0.23 mg Cooked beef—0.40 mg Cooked chicken—0.63 mg Hazelnut—0.6 mg Nuts—0.56 mg Spinach—0.15 mg |
| Vitamin B7 | Seeds, oilseeds, eggs, soy protein, salmon, carrots and sweet potatoes |
Important function in NK cells activity [42] Contributes to the generation of T lymphocytes [42] Important in the maturation and responsiveness of immune cells [42] |
30 µg/day for both men and women |
Boiled egg – 16.5 µg Carrot – 5 µg Cooked salmon – 5 µg Hazelnut – 75 µg Peanut – 101.4 µg Sweet potato—4.3 µg Wheat bran – 44.4 µg |
| Vitamin B9 | Meat, bananas, broccoli, carrots, spinach and oilseeds |
Contributes to the innate immunity maintenance [48] Contributes to the antibody response to antigen [34] Contributes to the T cells proliferation [34] |
400 µg/day for both men and women |
Boiled egg—40 µg Chicken liver—770 µg Cooked beans—149 µg Cooked bovine liver—220 µg Cooked white rice—60.7 µg Lentil—180 µg Pea—65 µg Spinach—108 µg Wheat germ—357 µg |
| Vitamin C | Citrus fruits, acerola, pineapple, strawberry, melon, tomato, pepper, broccoli and cabbage |
Positive modulation of the aging process and immunosenescence [153] Positive modulation of the low-grade inflammatory process [153] Improves phagocyte motility and chemotaxis [50] Improves antibody levels, differentiation, and proliferation [50] Modulates positively production of inflammatory cytokines [50] Role in the prevention and treatment of respiratory infections [50] Decreases the pathogenicity of viruses, bacteria, parasites and fungi [154] |
90 mg/day for men and 75 mg/day for women |
Acerola—941.4 mg Broccoli—34.3 mg Cashew—219.3 mg Green Bell pepper—100.2 mg Kale—96.7 mg lemon—38.2 mg orange—53.7 mg Papaya—82.2 mg Passion fruit—19.8 mg Pineapple—34.6 mg Strawberry—63.6 mg Tomato—21.2 mg Yellow Bell pepper—201.4 mg |
| Vitamin D | Fish liver oil, milk and dairy Products, butter, eggs, fat cheeses |
Stimulates the innate immune response in bronchial epithelial cells [155] Stimulates the monocytes to macrophages differentiation [155] Stimulates antibacterial protein synthesis [155] Stimulates the mechanisms associated with the pathogens elimination [155] Supplementation seems to have a protective effect in respiratory tract diseases (mainly in individuals with hypovitaminosis D) [156] Individuals with hypovitaminosis are more susceptible to respiratory tract infections [157] |
15 µg/day for both men and women |
Boiled egg—13 µg Butter—1.54 µg Cheddar cheese—0.32 µg Cod liver oil—252 µg Fortified milk—1 µg |
| Vitamin E | Vegetable oils, wheat germ, egg, dark green vegetables, nuts and almonds |
Membrane antioxidant—cell protection [158] Stimulates lymphocyte proliferation [158] Supplementation associated with antibacterial effects [158] Increases the activity of NK cells [158] It seems to have a protective effect against pneumonia and colds [137] |
15 mg/ day for both men and women |
Almond –5.5 mg Broccoli—1.05 mg Cabbage—0.61 mg Canola oil—21.3 mg Corn oil—21.3 mg Egg—1 mg Nuts –2.6 mg Peanut—7 mg Spinach—0.95 mg Sunflower oil—51.5 mg Wheat germ—19.1 mg |
| Zinc | Meat, seafood, oilseeds, whole grains and fortified grains |
Function in the immune cells maturation and differentiation [159] Zinc deficiency is related to impaired innate immunity [159] Increases the Natural Killer cells activity and number [159] Regulation of inflammatory cytokines [160]) Participation in anti-inflammatory pathways [160] |
11 mg/day for men and 8 mg/day for women |
Boiled flank—8 mg Cashew nuts—4.7 mg Cooked brown rice—0.7 mg Cooked oyster—39 mg Grilled fillet—5.1 mg Popcorn—2 mg Roasted Tiger fish 2.1 mg Rolled oats—2.6 mg Wholemeal bread—1.6 mg |
In addition, it is common an inadequate daily intake of vitamins and minerals, even when the accessibility to food is easy. For that case, supplementation may be necessary to improve specifics immune responses [8]. However, when this supplementation is in very high doses, it can cause unfavorable consequences for metabolism (e.g., hypervitaminosis) and IS, highlighting a bigger risk for vitamin A, iron and copper [29, 34]. To exemplify, in a small study with humans, high intake of copper for a long period was capable to reduce antibody production to an influenza vaccine [86].
If supplementation is necessary, it is important to respect safety limits previous established by Institute of Medicine (IOM) [8, 87]. Besides, it is essential to consult high quality studies previous published with consistent experimental design [8].
Bioactive compounds
Bioactive compounds are essential and nonessential compounds, widely found in fruits and vegetables [88]. They are responsible for colors, flavors, and they are related to potential pharmacological activities on human health [88, 89]. This compounds have many classifications, such as polyphenols, phytosterols, terpenoids, organosulfur compounds and alkaloids [90]. Many polyphenols have an important impact on IS through the immune cells modulation, the cytokines production, and pro-inflammatory genes expression [91, 92]. This section will present the main immune cells and pathways that are positively related to bioactive compounds.
Inflammation and oxidative stress
Some polyphenols are known for their anti-inflammatory potentials. According to in vivo and in human cells studies, resveratrol can inhibit pro-inflammatory cytokines, such as TNF-α and IL-6, while curcumin contributes to reducing TNF and IL-1. Curcumin also induces a reduction in NF-kB activation and in the TLR 2 and 4 expression [93–99]. In experimental designs using human cells, Epigallocatechin gallate (EGCG) and gingerol, present in green tea and ginger respectively, are other polyphenols that contribute to the NF-kb function [95, 100–102].
Polyphenols intake is directly associated with IS cell count and differentiation. Some studies in vitro, rodents and in humans experimental models observed alterations in the NK cells, macrophages, dendritic cells, Th1, and TCD4 cells count [91, 103–105]. Other types of T helper can be modulated, such as Th9 and Th17, by EGCG as well [106].
Several in vivo and in vitro studies show polyphenols’ anti-inflammatories properties that induce free radicals elimination, metal ions chelation, NADPH oxidase inhibition. The polyphenols contribute to the mitochondrial respiratory chain, they also induce a reduction in exaggerated ROS production, by inhibiting some enzymes involved and positively regulating antioxidant enzymes [9].
Regarding metals, curcumin can play a role in chelating transition metals, as Cu2+ and Fe2+, while quercetin and ECGC chelate Fe2+ in cell culture using THP1-monocytes [107]. Resveratrol and curcumin are the polyphenols that can inhibit NADPH oxidase in culture cells studies [108–110]. Curcumin, EGCG, phenolic acids, capsaicin, quercetin, anthocyanins, and resveratrol inhibit xanthine oxidase, an enzyme related to the ROS formation in mice and in vitro experiments [111–116]. Besides that, in vivo evidences, curcumin can stimulate the production of SOD, catalase, and glutathione peroxidase, antioxidant enzymes that are associated with decreases in ROS formation [117]. EGCG improves the activity of SOD and glutathione peroxidase, in vivo [118].
Cytokines modulation
Inflammatory cytokines modulation is one of the most studied mechanisms of polyphenols immunomodulation [9]. Their properties on macrophages were observed in an animal-model study with Chinese propolis administration in rodent “RAW 264.7” macrophages. The polyphenols present in Chinese propolis induced cyclooxygenase 2 (COX 2) and inducible nitric oxide synthase (iNOS) inhibition and a consequent reduction in the TNF-α, IL-1 β and IL-6 expression [119]. Similarly, this phenomenon was also observed when chamomile extract and quercetin alone were administrated [120]. Besides that, a clinical study highlighted that extra virgin olive oil has been related to reduce IL-6 and C-reactive protein (CRP) expression [121]. Quercetin and catechins have an effect on the balance of pro and anti-inflammatory cytokine production in vitro studies, increasing the IL-10 release and inhibiting TNF-α and IL-1 β [122, 123].
NF-kB signaling pathways
Polyphenols can modulate NF-kB at various points during the activation cascade, which induces an important anti-inflammatory effect through an alteration in the binding of the NF-kB complex to DNA, as an example [9]. In addition, in a previous study was observed a similar phenomenon when quercetin was administered in rodent BV-2 microglia cells [124]. Galangin, a flavonoid present in propolis, can control the NF-kB translocation and consequently decrease TNF-α, IL-6, IL-1β, and IL-8 expression [125]. At least in studies that were developed in cell culture, other polyphenols involved with NF-kB signaling pathways are resveratrol, catechins, and epicatechins [126, 127].
Besides that, it is important to highlight the role of EGCG on Wistar rat's respiratory epithelial cells NF-kb inhibition [128, 129], once it could improve the scientific knowledge between bioactive compounds properties and COVID-19 pandemic control.
Especially in virus infection, it is important to highlight a finding in human culture cell study and curcumin intervention [130]. This polyphenol provided an antiviral effect against enveloped viruses, inhibiting Zika and Chikungunya virus replication. This can be explained because curcumin may interferes with virus-cell binding, reducing its infectivity [130].
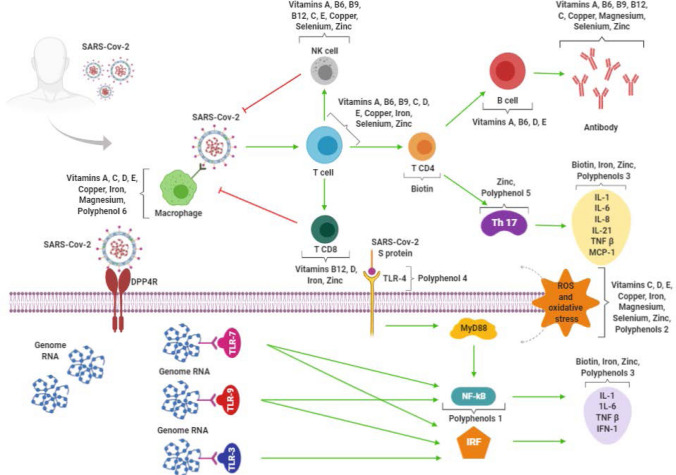
It is worth mentioning that only a healthy, varied, and fruits and vegetables-based diet is able to ensure exposure to all of these bioactive compounds [89]. In this way, an adequate supply of both bioactive compounds and micronutrients is guaranteed, as well as the synergy between vitamins and minerals [7, 8]. Table 2 provides a summary of the main functions of some bioactive compounds and the food sources in which they are found. To a better comprehension of all the mechanisms cited in this article, Fig. 3 shows which point of the SARS-CoV-2 infection pathway the main immunomodulatory polyphenols and micronutrients may act on.
Table 2.
Foods, bioactive compounds, their effects and other considerations
| Food | Bioactive compounds | Effects | Considerations |
|---|---|---|---|
| Açai Berry (Euterpe oleracea) | Flavonoids (pp. anthocyanins) |
Antioxidant Anti-inflammatory |
Increased antioxidant enzyme activity, vasodilator, and antidiabetic effect. Açai berry plays a role in the prevention and in the treatment of comorbidities that may aggravate COVID-19 symptoms [161, 162] |
| Citric Fruits | Polyphenols/vitamin C |
Antioxidant Immunomodulator |
Vitamin C improves chemotaxis, improves neutrophil phagocytic capacity and oxidative death, and supports lymphocyte proliferation and function. Polyphenols have an important antioxidant and anti-inflammatory function [50, 163] |
| Cocoa (Theobroma cacao) | Flavonoids (pp. Catechins) |
Antioxidant Anti-inflammatory Antiviral |
Cell protection against free radicals and unscheduled apoptosis. Evidence shows cocoa's protective action against some influenza strains [164, 165] |
| Garlic (Allium sativum L.) | Allicin and thiosulfinates |
Antibiotic Antifungal Antivirals Anti-inflammatory |
Protective action against the flu virus (in addition to other viruses such as cytomegalovirus and herpes) [166–168] |
| Ginger (Zingiber officinale Roscoe) | Gingerols |
Antioxidant Anti-inflammatory |
In addition to the anti-inflammatory property, there is evidence that indicates an improvement in respiratory function, shorter duration of mechanical ventilation and shorter intensive care unit hospitalization in patients with Acute Respiratory Distress Syndrome [169, 170] |
| Grapes and grapes-derived products | Resveratrol |
Antioxidant Anti-inflammatory |
In addition to the already known properties of resveratrol in chronic non-communicable diseases, evidence shows immunomodulatory functions and infectious disease prevention. The protective effect in vitro studies with coronavirus (less cell death, less cytotoxicity and greater cell viability after treatment with resveratrol). At higher doses, Resveratrol contributes to reducing viral replication [171, 172] |
| Kefir | Probiotics |
Antimicrobial Immunomodulator Anti-inflammatory |
Kefir is associated with a healthy microbiota (as a pathogen barrier). Some benefits, such as increased macrophage activity and cytokine production, are related to immunomodulatory effects [173, 174] |
| Linseed (Linum usitatissimum L.) and Linseed oil | Polyunsaturated fatty acids (Ω-3) |
Anti-inflammatory Immunomodulator |
Due to its fatty acid content, linseed has an anti-inflammatory effect (the increase of anti-inflammatory properties substances and the decrease in inflammatory cytokines) [175–177] |
| Propolis | Phenolic compounds (pp. flavonoids) |
Antimicrobial Antiviral Antifungal Antioxidant Anti-inflammatory |
Although there is no sufficient clinical studies, experimental and in vitro studies show propolis as a potentially protective factor against various infections and is related to the healing process [178–180] |
| Turmeric (Curcuma longa) | Curcuminoid pigments (Curcumin) |
Antivirals Anti-inflammatory Antioxidant |
Anti-inflammatory and antioxidant properties, as well as action against various viruses, bacteria, and fungi. Besides that, a study correlates curcumin analogs as a treatment of influenza [130, 181, 182] |
Fig. 3.
Immune response to SARS-CoV-2 and micronutrients and polyphenols participation in the various cells and cytokines involved in the defense mechanisms. Polyphenols 1 resveratrol, curcumin, EGCG, gingerol, epicatechins, catechins, quercetin, propolis, Polyphenols 2 resveratrol, curcumin, EGCG, quercetin, anthocyanins; Polyphenols 3 resveratrol, curcumin, catechin, quercetin, olive oil, chamomile extract, propolis; Polyphenol 4 curcumin; Polyphenol 5 EGCG; Polyphenol 6 propolis. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2; NK cell natural killer cell; Th 17 T helper type 17; IL-1 Interleukin 1; IL-6 Interleukin 6, IL-8 Interleukin 8; IL-21 Interleukin 21; TNF-β tumor necrosis factor β; MCP-1 monocyte chemoattractant protein-1, DPP4 dipeptidyl peptidase-4; S protein spike glycoprotein; TLR-3 tool-like receptor—3; TLR-4 tool-like receptor—4; TLR-7 tool-like receptor—7; TLR-9 tool-like receptor—s9; MyD88 myeloid differentiation factor-88; NF-kB nuclear factor kappa B; IRF interferon regulatory factors; ROS reactive oxygen species. Red lines refer to inhibitory effects. Green lines refer to activating effects
Immune system and elderly
Aging is a natural and complex process associated with uncountable human body physiological alterations, such as reductions in bone and muscle mass, in the basal metabolism rate (BMR), and total body water. Aging is also related to teeth loos, which can cause damage to the chewing, saliva production decrease, and dysphagia [131]. In this population, these changes also affect the immune system, a process characterized by immunosenescence, which can be defined as a reduced ability to respond to "foreign" antigens and to tolerate self-antigens. Thus, immunosenescence would be associated with a greater susceptibility to infections (including COVID-19), to cancer, to vaccination failure, and to autoimmune diseases [132].
Immunosenescence induces both the innate and adaptive IS modification and is associated with persistent low-grade inflammation. As the men have a more severe age-related immune function alteration than women, they suffer more from its effects [133]. Age-related decrease in phagocytosis, antigens presentation, in immune cells cytotoxic potential, lymphocyte number and function, are observed in this population. Besides that, these changes make the elderly more susceptible to infections, affect the ability to respond to pathogens and cause an exacerbation of the symptoms of these infections [134].
Decreased function of the thymus (an important lymphocyte maturation organ) and the loss of function and differentiation capacity of hematopoietic stem cells are some of them are some hypotheses that attempt to explain the aging-related immune function decline. It is also known that lifestyle during adulthood can directly influence this process: food, nutritional status, physical activity, social isolation, smoking, exposure to alcohol and drugs are factors that affect the degree of decline in the IS in the elderly [27]. The maintenance of an adequate nutritional status, a satisfactory supply of macro and micronutrients, ingestion of bioactive compounds, and the regular practice of physical activity can positively modulate the immune response of these individuals. Besides that, nutritional status can be highlighted as a vital condition for a healthy IS operation. Studies report that elderly people with protein-calorie malnutrition have a lower IR compared to those with adequate nutritional status [134].
In addition to protein-calorie malnutrition, commonly observed in aging, attention should be paid to the micronutrient supply of this a group. Although energy requirements to the elderly are lower, the micronutrients (vitamins and minerals) recommendation remains almost unchanged. Frequently, old people ingest less food than what was recommended, it can induce micronutrients deficiency and consequent impairment of IS and IR. Adequate dietary guidance and possible supplementation could be tools to prevent and/or reverse deficiencies of these nutrients [27].
Vitamin D deficiency, which is quite prevalent in the general population and especially in the elderly, induces impaired immune responses to influenza vaccination [135]. The literature also shows that vitamin D supplementation is associated with improving the immune response of patients with this vitamin deficiency [136]. Konijeti et al. [136] and Hemilä [137] demonstrated that vitamin E supplementation may be able to reduce the incidence of pneumonia in adult and elderly men [137]. Bouamama et al. [138] indicated that vitamins C and E supplementation improved the T lymphocytes response in the elderly and could contribute to the prevention of age-related immune system impairment.
In addition to a higher infection incidence, the elderly are more susceptible to prolonged infections, exacerbated symptoms, and complications. More than half of the elderly with a common cold develop a respiratory disease (e.g. pneumonia) and they have more than tenfold chances of death when compared to young adults. Since the beginning of the COVID-19 pandemic, advanced age has been highlighted as a risk factor for both susceptibilities to symptoms and to the infection outcome. In this way, death a common finding in individuals over 70 years and are associated with age-related physiological changes in IS [27, 139].
In Italy, approximately 87% of the first 2,000 cases of deaths were over 70 years old [140]. Another study compared the mortality rate between American elderly residents [mean 83 (range 51–100) years] and healthy workers and visitors [mean: 43 (range 21–79) years] of a long-term care institution that were positively diagnosed with SARS-CoV-2. The researchers found that 34% of elderly residents and 0% of workers die due to Covid-19. These results show that the disease affects the elderly more severely [140, 141]. Thus, special attention should be given to elderly individuals and to how to improve their immune system, especially those with other risk factors. In this scenario, adequate dietary planning that provides nutritional support within the current recommendations could contribute to a more competent IR, which can result in better chances of SARS-CoV-2 prevention and treatment. During more severe infection episodes, the elderly may have an increased requirement for these nutrients and it might be necessary to evaluate their supplementation.
Final considerations
The literature shows that some infected individuals have their sense of smell and taste affected [142] it is in turn associated with food intake reduction and lack of appetite. Based on that, the elaboration of the specific guidelines is interesting to help with a better understanding between nutrition and current COVID-19 pandemic treatment. For example, it is important to know which foods are better tolerated, respecting the individual's preference. Another example is to stimulate the consumption of harvest fruits and vegetables, once their flavor is more prominent. Besides that, other important nutritional strategies are to add natural spices in the preparation, to do memory exercise regarding the preparation that will be ingested and improving its acceptance, and to make the patient aware of the importance of their food for the correct IS function and the consequent response to COVID-19 [142].
Based on all information present in this review, in conclusion, a healthy, varied, and vegetables and fruits-based diet is important to ensure the IS balance and the consequent IR to SARS-CoV-2. It is worth mentioning the essential role of a qualified nutrition scientist to prescribe individualized guidance that considers a previous disease historic, nutritional status, and age as well.
Acknowledgements
We thank all the health professionals and researchers for all the support and care in the face of this pandemic. We also thank the Coordination of Superior Level Staff Improvement (CAPES—Brazil) for supporting graduate studies and providing us with scholarships to do research.
Author contributions
JASM and PRS: Conceptualization; investigation process; writing, review and editing; visualization; project administration. LM FC—Investigation process; writing, review and editing; visualization; project administration. JDM—Writing, review and editing; translation. GSR and RFM Conceptualization; investigation process; writing, review and editing; visualization; project administration, supervision.
Funding
This article did not receive any sources of funding.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Juliana Arruda de Souza Monnerat and Pedro Ribeiro de Souza contributed equally.
References
- 1.Organization WH (2020) WHO, National Health Commission of the People’s Republic of China https://covid19.who.int/. Accessed 31 Aug 2020
- 2.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for C Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 7.Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients. 2019 doi: 10.3390/nu11081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020 doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakovac H. COVID-19 and vitamin D-Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318(5):E589. doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panarese A, Shahini E. Letter: COVID-19, and vitamin D. Aliment Pharmacol Ther. 2020;51(10):993–995. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreou A, Trantza S, Filippou D, Sipsas N, Tsiodras S. COVID-19: The potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. Vivo. 2020;34(3 Suppl):1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24(1):133. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson DP, Lovegrove JA. Nutritional status of micronutrients as a possible and modifiable risk factor for COVID-19: a UK perspective. Br J Nutr. 2020 doi: 10.1017/S000711452000330X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cecere TE, Todd SM, Leroith T. Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it? Viruses. 2012;4(5):833–846. doi: 10.3390/v4050833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maggini S, Pierre A, Calder PC. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10(10):1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maggini SBS, Sorbara JP, Senatore G. Feeding the immune system: the role of micronutrients in restoring resistance to infections. Cab Rev Perspect Agric Vet Sci Nutr Nat Resour. 2018;3:1–21. doi: 10.1079/PAVSNNR20083098. [DOI] [Google Scholar]
- 29.Center MI (2016) Immunity in depth. Linus Pauling Institute. https://lpi.oregonstate.edu/mic/health-disease/immunity. Accessed 3 May 2020
- 30.Chang HK, Hou WS. Retinoic acid modulates interferon-gamma production by hepatic natural killer T cells via phosphatase 2A and the extracellular signal-regulated kinase pathway. J Interferon Cytokine Res. 2015;35(3):200–212. doi: 10.1089/jir.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol. 2011;85(16):8316–8327. doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34(3):435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blomhoff HK, Smeland EB, Erikstein B, Rasmussen AM, Skrede B, Skjonsberg C, Blomhoff R. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J Biol Chem. 1992;267(33):23988–23992. doi: 10.1016/S0021-9258(18)35934-9. [DOI] [PubMed] [Google Scholar]
- 34.Saeed FN, Muhammad; Ahmed, Rabia; Nadeem, muhammad kashif; Arshad, Muhammad Sajid; Ullah, Azmat, Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—a review. Food Agric Immunol. 2016;27:205–229. doi: 10.1080/09540105.2015.1079600. [DOI] [Google Scholar]
- 35.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(Suppl 1):S29–35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 36.Ueland PM, McCann A, Midttun O, Ulvik A. Inflammation, vitamin B6 and related pathways. Mol Asp Med. 2017;53:10–27. doi: 10.1016/j.mam.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Huang SC, Wei JC, Wu DJ, Huang YC. Vitamin B(6) supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur J Clin Nutr. 2010;64(9):1007–1013. doi: 10.1038/ejcn.2010.107. [DOI] [PubMed] [Google Scholar]
- 38.Calder P, Prescott S, Caplan M (2007) Scientific review: the role of nutrients in immune function of infants and young children. emerging evidence for long-chain polyunsaturated fatty acids. Mead Johnson Company, pp 1–35. http://eprints.soton.ac.uk/id/eprint/152657
- 39.Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol. 2015;93(12):1091–1096. doi: 10.1139/cjpp-2014-0460. [DOI] [PubMed] [Google Scholar]
- 40.Leon-Del-Rio A. Biotin in metabolism, gene expression, and human disease. J Inherit Metab Dis. 2019;42(4):647–654. doi: 10.1002/jimd.12073. [DOI] [PubMed] [Google Scholar]
- 41.Elahi A, Sabui S, Narasappa NN, Agrawal S, Lambrecht NW, Agrawal A, Said HM. Biotin deficiency Induces Th1- and Th17-mediated proinflammatory responses in human CD4(+) T lymphocytes via activation of the mTOR signaling pathway. J Immunol. 2018;200(8):2563–2570. doi: 10.4049/jimmunol.1701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal S, Agrawal A, Said HM. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol. 2016;311(3):C386–391. doi: 10.1152/ajpcell.00141.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erkurt MA, Aydogdu I, Dikilitas M, Kuku I, Kaya E, Bayraktar N, Ozhan O, Ozkan I, Sonmez A. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131–135. doi: 10.1159/000112967. [DOI] [PubMed] [Google Scholar]
- 44.Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T, Tamura T, Saitoh T, Kurabayshi H, Naruse T. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116(1):28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 46.Prentice S. They are what you eat: can nutritional factors during gestation and early infancy modulate the neonatal immune response? Front Immunol. 2017;8:1641. doi: 10.3389/fimmu.2017.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hemila H. Vitamin C and infections. Nutrients. 2017 doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Budi Haryanto TS, Wintergerst E, Maggini S. Multivitamin supplementation supports immune function and ameliorates conditions triggered by reduced air quality. Vitam Min. 2015;4(2):1–15. [Google Scholar]
- 49.Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2018;9:3160. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017 doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wishart K. Increased micronutrient requirements during physiologically demanding situations: review of the current evidence. Vitam Min. 2017;6(2):1–16. [Google Scholar]
- 52.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276(38):35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka H, Hruska KA, Seino Y, Malone JD, Nishii Y, Teitelbaum SL. Disassociation of the macrophage-maturational effects of vitamin D from respiratory burst priming. J Biol Chem. 1991;266(17):10888–10892. doi: 10.1016/S0021-9258(18)99102-7. [DOI] [PubMed] [Google Scholar]
- 54.Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018 doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7(4):3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Z, Li W. The roles of vitamin D and its analogs in inflammatory diseases. Curr Top Med Chem. 2016;16(11):1242–1261. doi: 10.2174/1568026615666150915111557. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol. 2004;34(4):1068–1076. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 59.Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160(11):5314–5319. [PubMed] [Google Scholar]
- 60.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 61.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58(4):563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 62.Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14(4):283–289. doi: 10.2174/1871530314666140922143950. [DOI] [PubMed] [Google Scholar]
- 63.Moriguchi S, Muraga M. Vitamin E and immunity. Vitam Horm. 2000;59:305–336. doi: 10.1016/s0083-6729(00)59011-6. [DOI] [PubMed] [Google Scholar]
- 64.Alpert PT. The role of vitamins and minerals on the immune system. Home Health Care Manag Pract. 2017;29(3):199–202. doi: 10.1177/1084822317713300. [DOI] [Google Scholar]
- 65.Besold AN, Culbertson EM, Culotta VC. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21(2):137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Institute of Medicine (US) Committee on Military Nutrition Research (1999) Military strategies for sustainment of nutrition and immune function in the field. National Academies Press (US), Washington (DC). 16, Trace Minerals, Immune Function, and Viral Evolution. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230971/ [PubMed]
- 67.Kelley DS, Daudu PA, Taylor PC, Mackey BE, Turnlund JR. Effects of low-copper diets on human immune response. Am J Clin Nutr. 1995;62(2):412–416. doi: 10.1093/ajcn/62.2.412. [DOI] [PubMed] [Google Scholar]
- 68.Percival SS. Copper and immunity. Am J Clin Nutr. 1998;67(5 Suppl):1064S–1068S. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- 69.Agoro R, Taleb M, Quesniaux VFJ, Mura C. Cell iron status influences macrophage polarization. PLoS ONE. 2018;13(5):e0196921. doi: 10.1371/journal.pone.0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassan TH, Badr MA, Karam NA, Zkaria M, El Saadany HF, Abdel Rahman DM, Shahbah DA, Al Morshedy SM, Fathy M, Esh AM, Selim AM. Impact of iron deficiency anemia on the function of the immune system in children. Medicine (Baltim) 2016;95(47):e5395. doi: 10.1097/MD.0000000000005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petrovic J, Stanic D, Dmitrasinovic G, Plecas-Solarovic B, Ignjatovic S, Batinic B, Popovic D, Pesic V. Magnesium supplementation diminishes peripheral blood lymphocyte DNA oxidative damage in athletes and sedentary young man. Oxid Med Cell Longev. 2016;2016:2019643. doi: 10.1155/2016/2019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bussiere FI, Mazur A, Fauquert JL, Labbe A, Rayssiguier Y, Tridon A. High magnesium concentration in vitro decreases human leukocyte activation. Magnes Res. 2002;15(1–2):43–48. [PubMed] [Google Scholar]
- 73.Baum MK, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher MA, Sauberlich H, Page JB. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15(5):370–374. doi: 10.1097/00042560-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 74.Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, Schneiderman N. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med. 2007;167(2):148–154. doi: 10.1001/archinte.167.2.148. [DOI] [PubMed] [Google Scholar]
- 75.Arthur JR, McKenzie RC, Beckett GJ. Selenium in the immune system. J Nutr. 2003;133(5 Suppl 1):1457S–1459S. doi: 10.1093/jn/133.5.1457S. [DOI] [PubMed] [Google Scholar]
- 76.Kitabayashi C, Fukada T, Kanamoto M, Ohashi W, Hojyo S, Atsumi T, Ueda N, Azuma I, Hirota H, Murakami M, Hirano T. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol. 2010;22(5):375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 77.Maywald M, Wang F, Rink L. Zinc supplementation plays a crucial role in T helper 9 differentiation in allogeneic immune reactions and non-activated T cells. J Trace Elem Med Biol. 2018;50:482–488. doi: 10.1016/j.jtemb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Fraker PJ, King LE, Laakko T, Vollmer TL. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130(5S Suppl):1399S–1406S. doi: 10.1093/jn/130.5.1399S. [DOI] [PubMed] [Google Scholar]
- 79.Jarosz M, Olbert M, Wyszogrodzka G, Mlyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-kappaB signaling. Inflammopharmacology. 2017;25(1):11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676–694. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wessels I, Rink L. Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D. J Nutr Biochem. 2020;77:108240. doi: 10.1016/j.jnutbio.2019.108240. [DOI] [PubMed] [Google Scholar]
- 82.Sheikh A, Shamsuzzaman S, Ahmad SM, Nasrin D, Nahar S, Alam MM, Al Tarique A, Begum YA, Qadri SS, Chowdhury MI, Saha A, Larson CP, Qadri F. Zinc influences innate immune responses in children with enterotoxigenic Escherichia coli-induced diarrhea. J Nutr. 2010;140(5):1049–1056. doi: 10.3945/jn.109.111492. [DOI] [PubMed] [Google Scholar]
- 83.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 84.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2 Suppl):447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 85.Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133(5 Suppl 1):1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 86.Roberfroid MB (2000) Prebiotics and probiotics: are they functional foods? Am J Clin Nutr 71 (6 Suppl):1682S–1687S; discussion 1688S–1690S. 10.1093/ajcn/71.6.1682S [DOI] [PubMed]
- 87.Medicine Io (2011) Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds) Dietary reference intakes for calcium and vitamin D. The national academies collection: reports funded by National Institutes of Health, Washington (DC). 10.17226/13050
- 88.Biesalski HK, Dragsted LO, Elmadfa I, Grossklaus R, Muller M, Schrenk D, Walter P, Weber P. Bioactive compounds: definition and assessment of activity. Nutrition. 2009;25(11–12):1202–1205. doi: 10.1016/j.nut.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 89.Recio MC, Andujar I, Rios JL. Anti-inflammatory agents from plants: progress and potential. Curr Med Chem. 2012;19(14):2088–2103. doi: 10.2174/092986712800229069. [DOI] [PubMed] [Google Scholar]
- 90.Somani SJ, Modi KP, Majumdar AS, Sadarani BN. Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytother Res. 2015;29(3):339–350. doi: 10.1002/ptr.5271. [DOI] [PubMed] [Google Scholar]
- 91.Karasawa K, Uzuhashi Y, Hirota M, Otani H. A matured fruit extract of date palm tree (Phoenix dactylifera L) stimulates the cellular immune system in mice. J Agric Food Chem. 2011;59(20):11287–11293. doi: 10.1021/jf2029225. [DOI] [PubMed] [Google Scholar]
- 92.John CM, Sandrasaigaran P, Tong CK, Adam A, Ramasamy R. Immunomodulatory activity of polyphenols derived from Cassia auriculata flowers in aged rats. Cell Immunol. 2011;271(2):474–479. doi: 10.1016/j.cellimm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 93.Capiralla H, Vingtdeux V, Venkatesh J, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Identification of potent small-molecule inhibitors of STAT3 with anti-inflammatory properties in RAW 264.7 macrophages. FEBS J. 2012;279(20):3791–3799. doi: 10.1111/j.1742-4658.2012.08739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leiherer A, Mundlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul Pharmacol. 2013;58(1–2):3–20. doi: 10.1016/j.vph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 95.Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, Hu M, Simms HH, Wang P. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34(7):1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 96.Marchiani A, Rozzo C, Fadda A, Delogu G, Ruzza P. Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem. 2014;21(2):204–222. doi: 10.2174/092986732102131206115810. [DOI] [PubMed] [Google Scholar]
- 97.Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19(11):2032–2046. [PubMed] [Google Scholar]
- 98.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28(12):1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bae J-S. Role of high mobility group box 1 in inflammatory disease: focus on sepsis. Arch Pharmacal Res. 2012;35:1511–1523. doi: 10.1007/s12272-012-0901-5. [DOI] [PubMed] [Google Scholar]
- 100.Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP. Recent advances on tea polyphenols. Front Biosci (Elite Ed) 2012;4:111–131. doi: 10.2741/363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Domitrovic R. The molecular basis for the pharmacological activity of anthocyans. Curr Med Chem. 2011;18(29):4454–4469. doi: 10.2174/092986711797287601. [DOI] [PubMed] [Google Scholar]
- 102.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 104.Boissier MC, Assier E, Biton J, Denys A, Falgarone G, Bessis N. Regulatory T cells (Treg) in rheumatoid arthritis. Jt Bone Spine. 2009;76(1):10–14. doi: 10.1016/j.jbspin.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 105.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Investig. 2004;114(10):1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J, Pae M, Meydani SN, Wu D. Green tea epigallocatechin-3-gallate modulates differentiation of naive CD4(+) T cells into specific lineage effector cells. J Mol Med (Berl) 2013;91(4):485–495. doi: 10.1007/s00109-012-0964-2. [DOI] [PubMed] [Google Scholar]
- 107.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 108.Deby-Dupont G, Mouithys-Mickalad A, Serteyn D, Lamy M, Deby C. Resveratrol and curcumin reduce the respiratory burst of Chlamydia-primed THP-1 cells. Biochem Biophys Res Commun. 2005;333(1):21–27. doi: 10.1016/j.bbrc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 109.Chow SE, Hshu YC, Wang JS. Chen JK (2007) Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 1985;102(4):1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 110.Petronio MS, Zeraik ML, Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18(3):2821–2839. doi: 10.3390/molecules18032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shen L, Ji HF. Insights into the inhibition of xanthine oxidase by curcumin. Bioorg Med Chem Lett. 2009;19(21):5990–5993. doi: 10.1016/j.bmcl.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 112.Aucamp J, Gaspar A, Hara Y, Apostolides Z. Inhibition of xanthine oxidase by catechins from tea (Camellia sinensis) Anticancer Res. 1997;17(6D):4381–4385. [PubMed] [Google Scholar]
- 113.Schmidt AP, Bohmer AE, Antunes C, Schallenberger C, Porciuncula LO, Elisabetsky E, Lara DR, Souza DO. Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: role of A1 adenosine receptors. Br J Pharmacol. 2009;156(1):163–172. doi: 10.1111/j.1476-5381.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen MT, Nguyen NT. Xanthine oxidase inhibitors from Vietnamese Blumea balsamifera L. Phytother Res. 2012;26(8):1178–1181. doi: 10.1002/ptr.3710. [DOI] [PubMed] [Google Scholar]
- 115.Braunlich M, Slimestad R, Wangensteen H, Brede C, Malterud KE, Barsett H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients. 2013;5(3):663–678. doi: 10.3390/nu5030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang XF, Li HQ, Shi L, Xue JY, Ruan BF, Zhu HL. Synthesis of resveratrol analogues, and evaluation of their cytotoxic and xanthine oxidase inhibitory activities. Chem Biodivers. 2008;5(4):636–642. doi: 10.1002/cbdv.200890059. [DOI] [PubMed] [Google Scholar]
- 117.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu AJ. Antagonism by bioactive polyphenols against inflammation: a systematic view. Inflamm Allergy Drug Targets. 2014;13(1):34–64. doi: 10.2174/1871528112666131119211002. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez R, Ballester I, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51(4):331–362. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 120.Drummond EM, Harbourne N, Marete E, Martyn D, Jacquier J, O'Riordan D, Gibney ER. Inhibition of proinflammatory biomarkers in THP1 macrophages by polyphenols derived from chamomile, meadowsweet and willow bark. Phytother Res. 2013;27(4):588–594. doi: 10.1002/ptr.4753. [DOI] [PubMed] [Google Scholar]
- 121.Fito M, Cladellas M, de la Torre R, Marti J, Munoz D, Schroder H, Alcantara M, Pujadas-Bastardes M, Marrugat J, Lopez-Sabater MC, Bruguera J, Covas MI, Investigators S. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr. 2008;62(4):570–574. doi: 10.1038/sj.ejcn.1602724. [DOI] [PubMed] [Google Scholar]
- 122.Comalada M, Ballester I, Bailon E, Sierra S, Xaus J, Galvez J, de Medina FS, Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem Pharmacol. 2006;72(8):1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 123.Crouvezier S, Powell B, Keir D, Yaqoob P. The effects of phenolic components of tea on the production of pro- and anti-inflammatory cytokines by human leukocytes in vitro. Cytokine. 2001;13(5):280–286. doi: 10.1006/cyto.2000.0837. [DOI] [PubMed] [Google Scholar]
- 124.Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung W, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521(1–3):9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Kim HH, Bae Y, Kim SH. Galangin attenuates mast cell-mediated allergic inflammation. Food Chem Toxicol. 2013;57:209–216. doi: 10.1016/j.fct.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 126.Mackenzie GG, Carrasquedo F, Delfino JM, Keen CL, Fraga CG, Oteiza PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18(1):167–169. doi: 10.1096/fj.03-0402fje. [DOI] [PubMed] [Google Scholar]
- 127.Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, Visioli F, Distante A, De Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23(4):622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 128.Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J Nutr. 2004;134(5):1039–1044. doi: 10.1093/jn/134.5.1039. [DOI] [PubMed] [Google Scholar]
- 129.Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10(1–6):55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Richart SM, Li YL, Mizushina Y, Chang YY, Chung TY, Chen GH, Tzen JT, Shia KS, Hsu WL. Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection. J Food Drug Anal. 2018;26(3):1015–1023. doi: 10.1016/j.jfda.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahan LK, Escott-stump S, Raymond JL. Krause: Alimentos, Nutrição e Dietoterapia. 13. Rio de Janeiro: Elsevier; 2013. p. 1228. [Google Scholar]
- 132.Leroux J, Morrison K, Rosenberg M. Prevalence and Predictors of Food Insecurity among Older People in Canada. Int J Environ Res Public Health. 2018 doi: 10.3390/ijerph15112511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fulop T, Dupuis G, Witkowski JM, Larbi A. The role of immunosenescence in the development of age-related diseases. Rev Invest Clin. 2016;68(2):84–91. [PubMed] [Google Scholar]
- 134.Maijo M, Clements SJ, Ivory K, Nicoletti C, Carding SR. Nutrition, diet and immunosenescence. Mech Ageing Dev. 2014;136–137:116–128. doi: 10.1016/j.mad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 135.Goncalves-Mendes N, Talvas J, Duale C, Guttmann A, Corbin V, Marceau G, Sapin V, Brachet P, Evrard B, Laurichesse H, Vasson MP. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Konijeti GG, Arora P, Boylan MR, Song Y, Huang S, Harrell F, Newton-Cheh C, O'Neill D, Korzenik J, Wang TJ, Chan AT. Vitamin D supplementation modulates T cell-mediated immunity in humans: results from a randomized control trial. J Clin Endocrinol Metab. 2016;101(2):533–538. doi: 10.1210/jc.2015-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hemila H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bouamama S, Merzouk H, Medjdoub A, Merzouk-Saidi A, Merzouk SA. Effects of exogenous vitamins A, C, and E and NADH supplementation on proliferation, cytokines release, and cell redox status of lymphocytes from healthy aged subjects. Appl Physiol Nutr Metab. 2017;42(6):579–587. doi: 10.1139/apnm-2016-0201. [DOI] [PubMed] [Google Scholar]
- 139.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 140.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 141.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, Lewis J, Baer A, Kawakami V, Lukoff MD, Ferro J, Brostrom-Smith C, Rea TD, Sayre MR, Riedo FX, Russell D, Hiatt B, Montgomery P, Rao AK, Chow EJ, Tobolowsky F, Hughes MJ, Bardossy AC, Oakley LP, Jacobs JR, Stone ND, Reddy SC, Jernigan JA, Honein MA, Clark TA, Duchin JS. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020 doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beltran-Corbellini A, Chico-Garcia JL, Martinez-Poles J, Rodriguez-Jorge F, Natera-Villalba E, Gomez-Corral J, Gomez-Lopez A, Monreal E, Parra-Diaz P, Cortes-Cuevas JL, Galan JC, Fragola-Arnau C, Porta-Etessam J, Masjuan J, Alonso-Canovas A. Acute-onset smell and taste disorders in the context of Covid-19: a pilot multicenter PCR-based case-control study. Eur J Neurol. 2020 doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tabela brasileira de composição de alimentos (2011) NEPA – UNICAMP, 4th edn. rev. e ampl. NEPA- UNICAMP, Campinas, p 161
- 144.Cozzolino SMF. Biodisponibilidade de Nutrientes. 5. Barueri: Manoele; 2016. p. 1443. [Google Scholar]
- 145.Meydani SN, Ribaya-Mercado JD, Russell RM, Sahyoun N, Morrow FD, Gershoff SN. Vitamin B-6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am J Clin Nutr. 1991;53(5):1275–1280. doi: 10.1093/ajcn/53.5.1275. [DOI] [PubMed] [Google Scholar]
- 146.Verbon EH, Trapet PL, Stringlis IA, Kruijs S, Bakker P, Pieterse CMJ. Iron and Immunity. Annu Rev Phytopathol. 2017;55:355–375. doi: 10.1146/annurev-phyto-080516-035537. [DOI] [PubMed] [Google Scholar]
- 147.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grober U, Schmidt J, Kisters K. Magnesium in Prevention and Therapy. Nutrients. 2015;7(9):8199–8226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Avery JC, Hoffmann PR. Selenium, Selenoproteins, and Immunity. Nutrients. 2018 doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, Selenoproteins and Viral Infection. Nutrients. 2019 doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of nutrition for development (BOND)-vitamin A review. J Nutr. 2016;146(9):1816S–1848S. doi: 10.3945/jn.115.229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mahalanabis D, Lahiri M, Paul D, Gupta S, Gupta A, Wahed MA, Khaled MA. Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J Clin Nutr. 2004;79(3):430–436. doi: 10.1093/ajcn/79.3.430. [DOI] [PubMed] [Google Scholar]
- 153.Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A. Vitamin C, aging and Alzheimer's disease. Nutrients. 2017 doi: 10.3390/nu9070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol (Bp) 2019;9(3):73–79. doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int J Mol Sci. 2018 doi: 10.3390/ijms19092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kakodkar P, Kaka N, Baig MN. A comprehensive literature review on the clinical presentation, and management of the pandemic Coronavirus disease 2019 (COVID-19) Cureus. 2020;12(4):e7560. doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 158.Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. 2018 doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maares M, Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 160.Bonaventura P, Benedetti G, Albarede F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14(4):277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 161.de Moura RS, Resende AC. Cardiovascular and metabolic effects of acai, an Amazon plant. J Cardiovasc Pharmacol. 2016;68(1):19–26. doi: 10.1097/FJC.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 162.Aranha LN, Silva MG, Uehara SK, Luiz RR, Nogueira Neto JF, Rosa G, Moraes de Oliveira GM. Effects of a hypoenergetic diet associated with acai (Euterpe oleracea Mart) pulp consumption on antioxidant status, oxidative stress and inflammatory biomarkers in overweight, dyslipidemic individuals. Clin Nutr. 2020;39(5):1464–1469. doi: 10.1016/j.clnu.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 163.Colunga Biancatelli RML, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18(2):99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 164.Kamei M, Nishimura H, Takahashi T, Takahashi N, Inokuchi K, Mato T, Takahashi K. Anti-influenza virus effects of cocoa. J Sci Food Agric. 2016;96(4):1150–1158. doi: 10.1002/jsfa.7197. [DOI] [PubMed] [Google Scholar]
- 165.Martins TF, Palomino OM, Alvarez-Cilleros D, Martin MA, Ramos S, Goya L. Cocoa flavanols protect human endothelial cells from oxidative stress. Plant Foods Hum Nutr. 2020 doi: 10.1007/s11130-020-00807-1. [DOI] [PubMed] [Google Scholar]
- 166.El-Saber Batiha G, Magdy Beshbishy A, Wasef LG. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 2020;12(3):872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhen H, Fang F, Ye DY, Shu SN, Zhou YF, Dong YS, Nie XC, Li G. Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate-early genes. Antiviral Res. 2006;72(1):68–74. doi: 10.1016/j.antiviral.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 168.Majewski M. Allium sativum: facts and myths regarding human health. Rocz Panstw Zakl Hig. 2014;65(1):1–8. [PubMed] [Google Scholar]
- 169.Anh NH, Kim SJ, Long NP, Min JE, Yoon YC, Lee EG, Kim M, Kim TJ, Yang YY, Son EY, Yoon SJ, Diem NC, Kim HM, Kwon SW. Ginger on human health: a comprehensive systematic review of 109 randomized controlled trials. Nutrients. 2020;12(1):57. doi: 10.3390/nu12010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kulkarni RA, Deshpande AR. Anti-inflammatory and antioxidant effect of ginger in tuberculosis. J Complement Integr Med. 2016;13(2):201–206. doi: 10.1515/jcim-2015-0032. [DOI] [PubMed] [Google Scholar]
- 171.Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019 doi: 10.3390/nu11050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rosa DD, Dias MMS, Grzeskowiak LM, Reis SA, Conceicao LL, Peluzio M. Milk kefir: nutritional, microbiological and health benefits. Nutr Res Rev. 2017;30(1):82–96. doi: 10.1017/S0954422416000275. [DOI] [PubMed] [Google Scholar]
- 174.Maciel FR, Punaro GR, Rodrigues AM, Bogsan CS, Rogero MM, Oliveira MN, Mouro MG, Higa EM. Immunomodulation and nitric oxide restoration by a probiotic and its activity in gut and peritoneal macrophages in diabetic rats. Clin Nutr. 2016;35(5):1066–1072. doi: 10.1016/j.clnu.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 175.Che L, Zhou Q, Liu Y, Hu L, Peng X, Wu C, Zhang R, Tang J, Wu F, Fang Z, Lin Y, Xu S, Feng B, Li J, Jiang P, Wu CD. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. 2019;10(12):8149–8160. doi: 10.1039/c9fo01877h. [DOI] [PubMed] [Google Scholar]
- 176.Hosomi K, Kiyono H, Kunisawa J. Fatty acid metabolism in the host and commensal bacteria for the control of intestinal immune responses and diseases. Gut Microbes. 2019 doi: 10.1080/19490976.2019.1612662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Caroprese M, Ciliberti MG, Albenzio M, Annicchiarico G, Sevi A. Dietary polyunsaturated fatty acids from flaxseed affect immune responses of dairy sheep around parturition. Vet Immunol Immunopathol. 2015;168(1–2):56–60. doi: 10.1016/j.vetimm.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 178.Takeda K, Nagamatsu K, Okumura K. A water-soluble derivative of propolis augments the cytotoxic activity of natural killer cells. J Ethnopharmacol. 2018;218:51–58. doi: 10.1016/j.jep.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 179.Al-Hariri M. Immune's-boosting agent: Immunomodulation potentials of propolis. J Fam Community Med. 2019;26(1):57–60. doi: 10.4103/jfcm.JFCM_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeitoun R, Najjar F, Wehbi B, Khalil A, Fayyad-Kazan M, Dagher-Hamalian C, Faour WH, El-Makhour Y. Chemical composition, antioxidant and anti-inflammatory activity evaluation of the Lebanese propolis extract. Curr Pharm Biotechnol. 2019;20(1):84–96. doi: 10.2174/1389201020666190206201241. [DOI] [PubMed] [Google Scholar]
- 181.Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 182.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]