Abstract
Aims
Amoxicillin (AMX)‐induced crystal nephropathy (AICN) is a rarely reported adverse drug reaction (ADR) but its increase has been recently reported in the Paris area. Our aim was to investigate the incidence, characteristics and outcome of AICN in France.
Methods
Retrospective analysis of all AICN cases reported to the French National Pharmacovigilance Database and the Marketing Authorization Holders Pharmacovigilance Database. AICN notification rate was compared to intravenous AMX and AMX–clavulanate sales.
Results
In total, 101 AICN cases were included. Intravenous AMX/AMX–clavulanate was prescribed as surgical prophylaxis (32 surgical patients) or to treat infection (69 medical patients). AKI KDIGO stage 3 was observed in 70 patients and 24/70 patients required renal replacement therapy and/or intensive care unit admission. The annual notification rate of AICN was increased by a factor of 13 since 2010 (6 [0;7] and 77 [24;111] cases per 100 000 patient‐years of exposure, before and after 2010 respectively; P < .001). In surgical patients, the increase in AICN has been reported since 2010 and was mainly related to inadequate AMX administration. In medical patients, the increase in AICN was observed since 2014. After 2014, medical patients were older (67 [42;77] vs 74 years [64;84] respectively; P < .05) and were treated more frequently for endocarditis (0/20 vs 15/49 respectively; P < .01). A contributing factor was observed or suspected in 62 patients.
Conclusion
AICN is a severe ADR that dramatically increased in France since 2010. Assessment of AICN contributing factors and AMX drug monitoring in patients receiving high dose of AMX could reduce the risk of AICN.
Keywords: acute kidney injury, adverse drug reaction, amoxicillin, crystals, renal replacement therapy
What is already known about this subject
Amoxicillin (AMX) induced crystal nephropathy (AICN) is a rarely reported adverse drug reaction
An increase in AICN has been recently reported in Paris area without evident contributing factors in most cases
What this study adds
After adjustment to AMX/AMX–clavulanate sales, this study confirmed the nationwide dramatic increase of AICN reported in France
In most AICN cases contributing factors related to AMX dose‐regimen/administration were identified
AICN may be responsible for life‐threatening complications and may require renal replacement therapy
1. INTRODUCTION
Amoxicillin (AMX) alone or in combination with clavulanate (AMX‐CLAV) is the most prescribed antimicrobial therapy worldwide. 1 Hypersensitivity reactions are the main mechanisms of AMX‐induced serious adverse drug reactions (ADRs). However, other mechanisms of toxicity, including AMX‐induced crystalluria, may be responsible for life‐threatening complications. 2
AMX‐induced crystalluria—i.e. the precipitation of crystals of AMX in urine—is a well‐known but rarely reported ADR. 3 , 4 While some patients remain asymptomatic, AMX‐induced crystalluria may be responsible for haematuria associated with acute kidney injury (AKI). 5 Amoxicillin‐induced crystal nephropathy (AICN) is related to the obstruction of the ureter and/or renal tubules by AMX crystals. 5 , 6 AICN onset may be responsible for life‐threatening complications and may require renal replacement therapy (RRT). 4 , 7 Although the oral route of AMX or AMX‐CLAV administration has been associated with AICN, most recently published AICN cases were associated with the intravenous route. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Several contributing factors for AICN have been suspected and include: high dose and/or too fast intravenous administration and/or administration of highly‐concentrated solution of AMX, acid urine pH and dehydration. 3 , 4 , 6
Recently, an increase in AICN associated with the prescription of intravenous AMX/AMX‐CLAV has been observed in the Paris area in France. It was first reported by a single hospital in Paris and further confirmed at the regional level by studying AICN cases reported to the regional centres of pharmacovigilance (RCPVs) of the Paris area. 4 , 11 In the latter study, only 1 case of AICN was reported from 1985 to 2010, while 44 cases were reported from 2010 to 2016. The most worrying point was that AICN occurred for 2/3 of cases in healthy patients undergoing scheduled surgery, without evident contributing factors in 2/3 of the cases. 4
However, those studies were limited to the Paris area and the annual notification rate of AICN was not compared to intravenous AMX/AMX‐CLAV sales, preventing from drawing any conclusion regarding the real increase of AICN in France. 4 , 11 Thus, the aim of this study was to investigate the annual notification rate, characteristics and outcome of AICN in France through 2 national complementary pharmacovigilance databases and to compare the trend in AICN annual notification rate to the trend in intravenous AMX/AMX‐CLAV sales in France.
2. MATERIALS AND METHODS
2.1. Source of data
To achieve an exhaustive epidemiological overview of AICN related to intravenous AMX/AMX‐CLAV use at a national level, we gathered data from 2 databases: the French National Pharmacovigilance Database (FNPD) and the Marketing Authorization Holders Pharmacovigilance Database (MAHPD). The FNPD was set up in 1985 to register all reports collected by the 31 French RCPVs. RCPVs record in the FNPD all ADRs spontaneously reported by health caregivers and, since June 2011, all ADRs reported by patients themselves. 12 The MAHPD records all worldwide ADRs reported spontaneously by patients or healthcare professionals to marketing authorization holders. 13 All databases are declared and approved by the French National Commission on Informatics and Liberty. As all cases were registered anonymously in databases, informed consent of patients was waived, according to the French law. 14
To compare the annual notification rate of AICN to FNPD/MAHPD with the evolution of intravenous AMX/AMX‐CLAV consumption in France, French health authorities requested marketing authorization holders to provide the annual sales of intravenous AMX/AMX‐CLAV in France. Four marketing authorization holders were involved. For the brand name, sales were available from 2001. For the generic formulations, sales were available since the launch of the generic formulations.
2.2. Case definition and selection
The diagnosis of AICN ultimately requires the detection of crystals in urine (by phase‐contrast microscopy at low and high magnifications) and their identification (by infrared spectroscopy or high‐performance liquid chromatography) on fresh urine that has to be analysed within 2 hours following urine collection. 15 , 16 As AMX crystal detection/identification is not routinely available in all French hospitals and is only performed on working days, it could not be a systematic inclusion criterion. 4 Consequently, the definition of AICN was based on clinical and biological findings and adapted from Vodovar et al. 4 AICN diagnosis was retained if cases fulfilled all the criteria of AICN diagnosis and none of exclusion criteria (Table 1). When potential nephrotoxic drugs were concomitantly administered, AKI causality assessment was performed using the Naranjo criteria. 17
TABLE 1.
Diagnosis criteria of amoxicillin‐induced crystal nephropathy adapted from Vodovar et al.4
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
|
Acute kidney injury defined according to the KDIGO criteria20 AND Microscopic or macroscopic haematuria confirmed by sediment urine analysis AND Temporal coincidence between AMX/AMX‐CLAV administration and AKI onset |
• concomitant administration of nephrotoxic drugs with a causality assessment equal to or greater than AMX/AMX‐CLAV therapy (according to Naranjo scale)17 • any underlying condition causing acute kidney injury and/or macroscopic haematuria (or red‐coloured urine by itself) (shock, sepsis, urinary tract infection, urological surgery, kidney disease, rhabdomyolysis, haemolysis, rifampicin treatment …) • fever, rash or liver enzyme abnormalities (suggesting hypersensitivity including acute interstitial nephritis) • purpura, arthralgia or bowel angina (suggesting amoxicillin induced leucocytoclastic vasculitis) |
AKI: acute kidney insufficiency; AMX: amoxicillin; AMX‐CLAV: amoxicillin–clavulanate; KDIGO: Kidney Disease Improving Global Outcomes
AICN cases reported to the FNPD and the MAPHD from January 1985 to June 2016 were reviewed by searching in the FNPD and MHPD the reports with the suspected active substance “amoxicillin” administered intravenously, combined with either “renal and urinary disorders” or “genitourinary tract disorders” (based on the High‐Level Group Term from the Medical Dictionary for Drug Regulatory Affairs). 18 One pharmacovigilance specialist (L.T.) and 1 critical care medicine and anaesthesiology physician (N.M.) reviewed cases and cases of disagreement were ultimately resolved by D.V., specialist in toxicology and critical care medicine.
2.3. Description of AICN cases
After selection of cases, the following data were collected: demographics (age, sex, weight, body mass index [BMI]), medical history, indication of AMX/AMX‐CLAV treatment (“medical” for treating active infection or “surgical” for surgical prophylaxis), AMX/AMX‐CLAV treatment characteristics (dose, concomitant drugs administered, AMX plasma concentration), AKI characteristics (baseline/maximal serum creatinine levels, baseline/minimal glomerular filtration rate [eGFR], urine sediment analysis/pH, AMX crystals identification in urine), outcome (intensive care unit [ICU] admission, need for RRT, kidney function recovery). eGFR was estimated using the Modification of Diet in Renal Disease equation. 19 AKI severity was assessed using the Kidney Disease Improving Global Outcomes (KDIGO) classification. 20 Kidney function recovery was defined by the return to baseline creatinine. In case of missing baseline serum creatinine, it was estimated using the Modification of Diet in Renal Disease back‐estimation formula assuming a baseline eGFR of 75 mL/min/1.73 m2. 20 AICN severity was assessed using the World Health Organization ADR terminology. 21 The following periods were also collected: AMX/AMX‐CLAV challenge to AICN onset, AICN onset to kidney function recovery.
2.4. Missing data
In case of missing data, a questionnaire was sent to the corresponding RCPV to obtain additional information. Moreover, 3 clinical wards were contacted by phone.
2.5. Statistical analysis
To compare the annual notification rate of AICN with the evolution of intravenous AMX/AMX‐CLAV consumption in France, the amount of intravenous AMX and AMX‐CLAV sold for a given year (g) was corrected using the intravenous AMX and AMX‐CLAV defined daily doses (DDD), which is the assumed average maintenance dose per day for a drug used for its main indication in adults. DDD was 1 g/d for intravenous AMX during the study period and is 3 g/d for intravenous AMX‐CLAV since 2005. 22 , 23 The intravenous AMX DDD changed in 2019 (3 g/d). However, as all reported cases of AICN occurred before 2019, we used the previous intravenous AMX DDD i.e. 1 g/d. DDD is usually multiplied by the average treatment duration to correct for differences in treatment duration. Intravenous AMX/AMX‐CLAV is prescribed in several indications with treatment duration varying from less than 1 day (surgical prophylaxis) to few weeks (osteoarticular infections, endocarditis). Moreover, intravenous AMX/AMX‐CLAV can be initially used for treating severe infections and then switched to AMX/AMX‐CLAV oral formulation. Therefore, we were unable to correct for differences in treatment duration the amount of intravenous AMX/AMX‐CLAV sold. To assess the annual consumptions of intravenous AMX/AMX‐CLAV comparable over time, we arbitrarily estimated the number of patient‐years of exposure by dividing the amount of intravenous AMX and/or AMX‐CLAV for a given year (g) by the AMX or AMX‐CLAV DDDs (g/d) multiplied by the number of days per year (365). 24 Consequently, we estimated the annual notification rate of AICN to FNPD/MAHPD per 100 000 patient‐years of exposure, as follows: [annual number of AICN cases for a given year × 100 000] / [estimated number of patient‐years of exposure].
Results were expressed as median and interquartile range (continuous variable) or number and percentage (non‐continuous variable). Median and percentage were compared using the Mann–Whitney test or the Fisher exact test, respectively. A P‐value < .05 was considered as significant. Statistical analysis was performed using XLSTAT 2019.
3. RESULTS
3.1. Patient's characteristics
In total, 101 AICN cases (age 64 years [48;77]; 32 males/69 females; sex‐ratio 0.46) were included in the study (Figure 1). The agreement between the 2 reviewers was excellent (κ 0.85) and 29 cases of disagreement were ultimately resolved (6 and 23 cases were included and excluded, respectively). The first case of AICN was reported in 1988 and most of cases were reported after 2010 (Figure 2). Intravenous AMX and/or AMX‐CLAV were prescribed to treat infection (further considered as medical patients) or as antimicrobial surgical prophylaxis (further considered as surgical patients) in 69 and 32 patients, respectively (Table 2).
FIGURE 1.
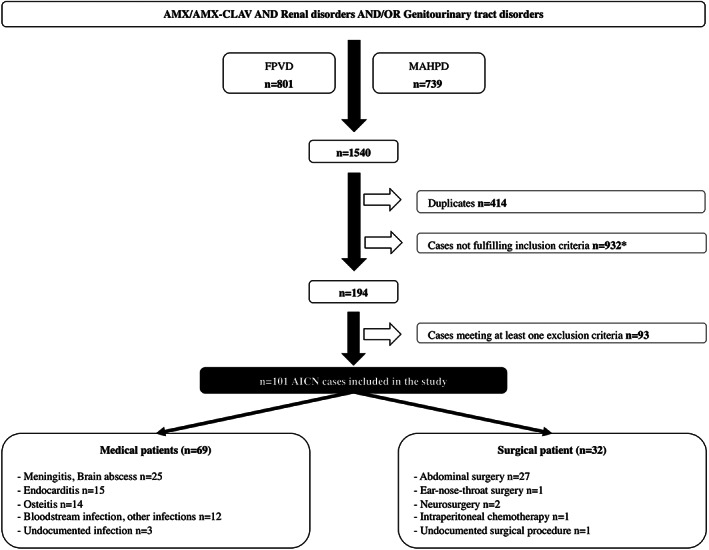
Process of AICN case selection. AMX: amoxicillin; CLAV: clavulanate; FNPD: French National Pharmacovigilance Database; MAHPD: marketing authorization holders pharmacovigilance databases; AICN: amoxicillin‐induced crystal nephropathy. *Including 10 cases of crystalluria without AKI or haematuria and 45 cases with AKI but without haematuria
FIGURE 2.
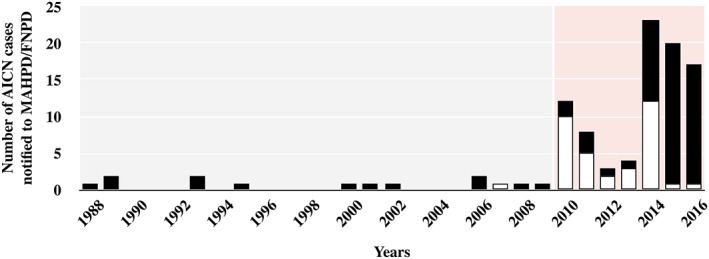
Trend in amoxicillin‐induced crystal nephropathy (AICN) notification to the French National Pharmacovigilance Database (FNPD) and marketing authorization holders pharmacovigilance databases (MAHPD). Black bar: amoxicillin; white bar: amoxicillin–clavulanate
TABLE 2.
Patient demographics, amoxicillin treatment characteristics, and AICN description (including AKI). AKI was staged using Kidney Disease Improving Global Outcomes criteria
| Total (n = 101) | Medical (n = 69) | Surgical (n = 32) | P value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Sex ratio, M/F (n = 101) | 0.46 | 0.56 | 0.28 | .2 |
| Age, years (n = 101) | 64 [48;77] | 73 [60;80] | 47 [34;64] | <.001 |
| Weight, kg (n = 73) | 72 [59;84] | 72 [60;83] | 70 [56;84] | .7 |
| BMI, kg/m2 (n = 62) | 26 [21;30] | 27 [24;30] | 26 [20;30] | .5 |
| Medical history (n = 101) | ||||
| • arterial hypertension | 29 (29) | 25 (36) | 4 (13) | <.05 |
| • diabetes mellitus | 14 (14) | 14 (20) | 0 (0) | <.01 |
| • CKD | 3 (4) | 3 (4) | 0 (0) | .5 |
| AMX/AMX‐CLAV treatment characteristics | ||||
| AMX formulation (n = 101) | ||||
| • AMX alone | 66 (66) | 65 (94) | 1 (3) | |
| • AMX‐CLAV alone | 31 (31) | 1(1) | 30 (94) | <.001 |
| • AMX and AMX‐CLAV in combination | 4 (4) | 3 (5) | 1 (3) | |
| AMX daily dose | ||||
| • g/d (n = 94) | 12[4;12] | 12 [12;15] | 2 [2;2] | <.001 |
| • mg/kg/d (n = 68) | 144 [57;189] | 180 [150;207] | 54 [36;66.5] | <.001 |
| AICN characteristics | ||||
| Baseline creatinine level, μmol/L (n = 65) | 68 [59;79] | 69 [59;85] | 67 [58;78] | .6 |
| Baseline creatinine eGFR, mL/min/1.73 m2 (n = 65) | 96 [76;113] | 90 [69;118] | 99 [78;110] | .6 |
| Maximum creatinine level, μmol/L (n = 84) | 385 [224;525] | 389 [244;545] | 357 [178;493] | .2 |
| Minimal creatinine eGFR, mL/min/1.73 m2 (n = 84) | 12 [9;21] | 11 [8;18] | 14 [9;30] | .1 |
| AKI stage according to KDIGO (n = 101) | ||||
| • stage 1 | 15 (15) | 7 (10) | 8 (25) | |
| • stage 2 | 16 (16) | 9 (13) | 7 (22) | <.05 |
| • stage 3 | 70 (70) | 53 (77) | 17 (53) | |
| Time from AMX/AMX‐CLAV | ||||
| • challenge to AICN onset, days (n = 100) | 3 [0.2;8.0] | 5.0 [3.0;11.0] | 0.1 [0.1;0.2] | <.001 |
| • withdrawal to kidney function recovery, days (n = 71) | 7 [4;11] | 10 [6;13] | 4 [2;8] | <.01 |
| Outcome | ||||
| ICU admission and/or renal replacement therapy | 24 (24) | 16 (23) | 8 (25) | 1.0 |
AKI: acute kidney insufficiency; AMX: amoxicillin; AMX‐CLAV: amoxicillin–clavulanate; BMI: body mass index; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; ICU: intensive care unit; KDIGO: Kidney Disease Improving Global Outcomes
Medical patients were older, more frequently had arterial hypertension and diabetes mellitus in comparison to surgical patients (Table 2; P < .05). The proportion of obese patients (BMI ≥ 30 kg/m2 n = 17/62) was similar between the medical and surgical group (9/31 and 8/31 respectively, P = .16). Three patients had previous chronic kidney disease (Table 2). Forty‐two patients concomitantly received potential nephrotoxic drugs (41/69 medical and 1/32 surgical patients; P < .05) with a causality assessment inferior to AMX/AMX‐CLAV therapy (according to Naranjo criteria). Causes of inferior causality assessment were identification of AMX crystals in urine, and/or long‐term and well‐tolerated potential nephrotoxic drug, and/or kidney function recovery despite the continuation of the potential nephrotoxic drug. Loop diuretics were concomitantly administered in 7/69 medical patients and bowel preparation in 15/32 surgical patients.
3.2. AMX/AMX‐CLAV treatment characteristics
AMX alone was more frequently prescribed in the medical group. Conversely, AMX‐CLAV was more frequently prescribed in the surgical group (Table 2; P < .001). The AMX daily dose was significantly higher in the medical group in comparison to the surgical group (Table 2; P < .001). In the medical group, the AMX/AMX‐CLAV daily dose was ≥12 g/d or 200 mg/kg/d in 31/60 cases. In 3 medical cases, the decrease of AMX daily dose resulted in kidney function recovery despite AMX continuation. In 3 other cases, AKI re‐occurred after AMX daily dose re‐increase/AMX re‐challenge, while AKI had previously recovered after AMX daily dose decrease/AMX withdrawal. AMX plasma concentration was available in 5 medical patients and was 224 mg/L [59;292] (therapeutic range: 20–40 mg/L). Intravenous AMX/AMX‐CLAV was administered (n = 4) or suspected to be administered (n = 12) faster than recommended by French guidelines (i.e. faster than 2 g in 30‐min infusion) in 50% of surgical patients. Overall, a contributing factor was observed or suspected in 62 patients.
3.3. Description of AICN
According to KDIGO criteria, 70 patients developed AKI stage 3, more frequently in the medical group (Table 2; P < .05). After AMX/AMX‐CLAV challenge, AICN occurred within 1 day in surgical patients while it occurred within 5 days in medical patients (Table 2; P < .001). AICN was confirmed by crystal analysis in the 31 cases in which it was analysed. Renal biopsy was performed in 7 patients and showed acute tubular necrosis (n = 6) and tubular vacuolization (n = 1). Urine pH was available in 9 patients and was 5 [5;6].
3.4. Outcome of AICN
All cases were serious according to the World Health Organization definition and 24 patients developed severe (AKI requiring RRT n = 19) and/or life‐threatening complications leading to ICU admission (n = 10). In all patients, kidney function recovered, usually within 7 days (Table 2). The delay between AMX/AMX‐CLAV withdrawal and kidney function recovery was 2.5‐fold longer in the medical group in comparison to the surgical group (Table 2; P < .01).
3.5. Trends in AICN compared to annual sales of AMX or AMX‐CLAV
The annual notification rate of AICN per 100 000 patient‐years of exposure (AMX/AMX‐CLAV) increased by a factor of 13 since 2010 (Figure 3a; 6 [0;7] and 77 [24;111] cases per 100 000 patient‐years of exposure, before and after 2010, respectively; P < .001) with 2 peaks of notification in 2010 and 2014. When AMX and AMX‐CLAV were considered separately, different patterns of AICN increase were observed. In patients receiving AMX‐CLAV (i.e. 31/32 surgical patients) the increase in AICN was noted since 2010 with 2 peaks of notification in 2010 and 2014 (Figure 3b; 0 [0;0] and 38 [14;137] cases per 100 000 patient‐years of exposure, before and after 2010 respectively; P < .001). No case of AICN was reported since 2015. In patients receiving AMX alone (i.e. 68/69 medical patients), the increase in AICN was noted since 2014 (11 [0;19] and 163 [113;190] cases per 100 000 patient‐years of exposure, before and after 2014 respectively; P < .01). Medical cases were reported before and after 2014, while 31/32 surgical cases—all reported by 3 anesthesiology departments in the same geographic area—were reported after 2010. In comparison to medical patients before 2014, medical patients after 2014 were older (67 [42;77] and 74 years [64;84] respectively; P < .05), were treated more frequently for endocarditis (0/20 and 15/49 respectively; P < .01). Intravenous AMX‐CLAV products from the 4 manufacturers were similarly involved in AICN cases.
FIGURE 3.
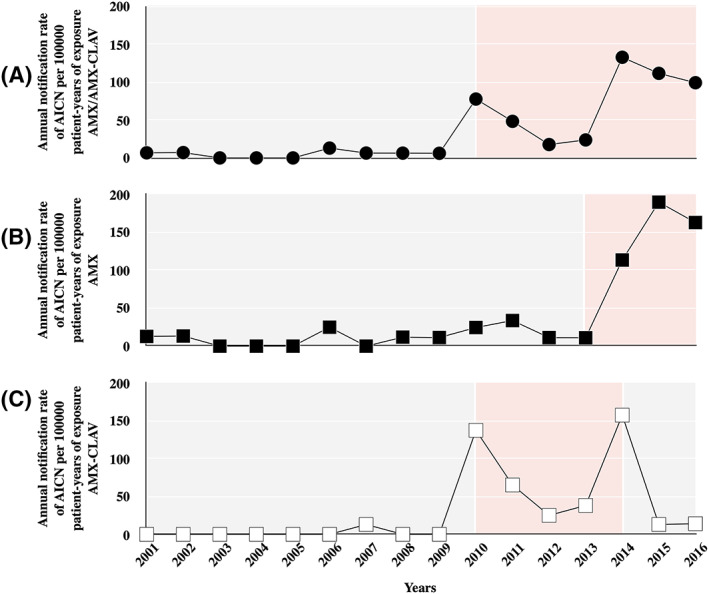
Annual notification rate of amoxicillin‐induced crystal nephropathy (AICN) per 100 000 patient‐years of exposure: (A) intravenous amoxicillin (AMX) and/or AMX–clavulanate (AMX‐CLAV; filled circle); (B) intravenous AMX (filled square); (C) intravenous AMX‐CLAV (open square)
4. DISCUSSION
Based on pharmacovigilance databases and after adjustment to AMX/AMX‐CLAV sales, this study confirmed the nationwide dramatic increase of AICN reported in France, since 2010 for surgical patients and since 2014 for medical patients. Only 1 surgical case of AICN was observed before 2010 and the annual notification rate of AICN per 100 000 patient‐years of exposure in medical patients was increased by a factor of 15 since 2014. This also reinforces the finding that AICN is a severe ADR, since all cases were serious, >2/3 of patients developed KDIGO stage 3 AKI and 1/4 of cases required renal replacement therapy and/or ICU admission.
In our previous study based on Parisian pharmacovigilance data, 2/3 of patients were surgical patients and it was concluded that AICN increased since 2010. 4 In the present study, medical cases outnumbered surgical cases and AICN in medical patients started increasing from 2014. Three anaesthesiology departments located in the Paris area reported almost all AICN surgical patients. Those departments had recently implemented the French guidelines on the prescription of antibiotics for surgical prophylaxis, and inadequate intravenous AMX/AMX‐CLAV administration was documented/suspected in almost half of these cases and related to inadequate local procedures. We could hypothesize that similar increase in AICN occurred in other departments but were not reported or that these guidelines were interpreted differently in other departments. Consequently, the proportion of surgical patients was overestimated in the regional study. 4 Moreover, Zeller et al. similarly reported the increase of AICN in medical patients between 2014–2015. 11 This increase of AICN in medical patients since 2014 is questionable and could be the consequence of information provided to health professionals regarding the increase of AICN surgical cases since 2010 (notification bias). However, publications on the increase of AICN in Paris area dated from 2016 and 2018, no publication/communication regarding the surgical AICN cases was made, no awareness campaign was launched during the study period and AICN in medical cases were reported throughout France. 4 , 11 Moreover, no major change in recommendations for treating infection was published in the meantime.
Surgical and medical patients were significantly different on demographics. Surgical patients were usually adults (only 1/4 were elderly) without major comorbidities. Conversely, almost all medical patients were elderly with significant comorbidities including arterial hypertension and diabetes mellitus. Moreover, medical patients developed more frequently severe AKI in comparison to surgical patients. In medical patients, previous undocumented kidney disease (despite normal eGFR) related to arterial hypertension/diabetes or concomitant medications might have worsened AKI. However, we could make other hypotheses. Before the onset of AICN, intravenous AMX/AMX‐CLAV was administered at higher dose and during a longer period in medical patients in comparison to surgical patients (12 vs 2 g/d and 3 vs 0.1 days, respectively). Moreover, in surgical patients AICN was usually diagnosed while intravenous AMX/AMX‐CLAV was already stopped, whereas in medical patients, we showed in a previous study, that intravenous AMX/AMX‐CLAV was discontinued within 2 days (IQR 1–3) after the onset of AICN. 4 Finally, diabetes may be a contributing factor, as it contributes to acidify urine pH and promotes AMX crystallization. 5 , 25
AICN contributing factors related to AMX dose‐regimen/administration were identified in both medical and surgical patients. High dose, rapid administration and/or administration of highly concentrated solution of AMX promote AICN onset. Most AMX is rapidly excreted unchanged in the urine and AMX renal excretion rate follows the plasma concentration curve (i.e. peak and then slow decrease). 3 , 5 Consequently, the urine AMX peak excretion is promoted by high AMX intravenous daily dose (promoting higher AMX plasma residual concentration and thus higher AMX plasma peak concentration after new administration of AMX) and/or high AMX administered dose per infusion and/or short duration of AMX infusion (high injected dose and fast injection promoting higher plasma peak concentrations). 4 In surgical patients, investigations have identified 3 intricate factors that could have directly contributed to AICN onset: the doubling of AMX‐CLAV prophylactic dose for surgery in obese patients in the new French guidelines for antibiotic prophylaxis during surgery published in 2010 (while AMX is poorly distributed in adipose tissue) and/or inadequate AMX‐CLAV dilution and/or duration of administration. 26 , 27 After probable change in administration protocol, no surgical case of AICN was reported since 2015. In medical patients, the daily dose of AMX was equal to or greater than to the maximal recommended AMX daily dose in half of patients. 28 We also constantly observed elevated AMX plasma residual concentrations when available. 29 Medical AICN patients after 2014 were 7 years older and more frequently treated for endocarditis. In this elderly population, AMX was apparently used without AMX dose adjustment, while AMX plasma clearance decreases in the elderly. 30 The increase in endocarditis was probably in relation with its increase in this population over the past decades. 31 , 32
In both surgical and medical patients, other factors may have also contributed to AICN onset. First, sepsis in medical patients and pre‐operative fasting or bowel preparation in the surgical patients may have been responsible for low urine output that increases AMX urine concentration and thus promotes AMX crystals formation. 5 Second, acid urinary pH decreases AMX solubility and may also promote AMX crystallization. 5 , 33 In our cohort, 50% of patients had a BMI ≥26 kg/m2 and 17% were obese while a strong association between increase in BMI and low urine pH has been demonstrated. 34 Moreover, 14% of patients had diabetes mellitus and 7% received loop diuretics while both are associated with acid urine pH. 25 , 35 Finally, the median urine pH measured in 9 patients was 5. Overall, acid urinary pH was measured or could be suspected in 42% of patients. Interestingly, only 3% of patients had previous CKD as previously reported. 4 CKD probably slows down AMX urine excretion, preventing AMX urine saturation and thus AICN onset.
Overall, in almost 2/3 of patients AICN contributing factors were observed/suspected. We assume that if 1 contributing factor could not be sufficient to induce AICN, their association may promote its onset in both groups. However, in 1/3 of cases (n = 39/101), no contributing factor was clearly identified. An underlying AMX quality default has been suspected. Although the active substance of AMX is manufactured in 3 different countries, all products from the 4 manufacturers were similarly involved and investigations on AMX manufacturing processes conducted by the Agence Nationale de Sécurité du Médicament (the health authority responsible for the quality of French drugs) in 2016 concluded an acceptable level of compliance with good manufacturing practices. 36 Consequently, a quality default specific to the product cannot be retained, even though a problem regarding a common raw material cannot be formally excluded.
Surprisingly, no similar AICN increase has been reported in other countries. Three case reports have been published since 2017 in European countries and none in the USA. 8 , 10 , 37 In 2 of the 3 case‐reports, AICN contributing factors were found (low urinary pH, dehydration). One explanation of this discrepancy could be that France recommends higher intravenous AMX dose regimen in comparison to other European countries. 38 Moreover, detection/identification of AMX crystals is not available in many countries, making the diagnosis of AICN difficult and probably leading to under‐diagnosis.
This study has several limitations. First, due to the retrospective design of the study, data were missing and contributing factors were mainly hypothesized rather than demonstrated. Thirty‐six baseline serum creatinine and 17 maximal serum creatinine were missing. All those patients had at least 2 serum creatinine level reaching the cut‐off increase of 26.5 μmol/L within 48 hours. Consequently, for the former, we possibly overestimated the severity of AKI while we possibly underestimated it for the latter. 39 Finally, in 13 patients, the exact time from AMX/AMX‐CLAV withdrawal to kidney function recovery was not exactly known. Second, we probably underestimated the real annual notification rate of AICN. Data were extracted from pharmacovigilance databases, which suffer from underreporting. We also probably overestimated the number of patient‐years of exposure and consequently underestimated the annual notification rate of AICN to FNPD/MAHPD per 100 000 patient‐years of exposure. Moreover, as AMX crystals identification was performed and identified in 1/3 of cases, we used stringent criteria for diagnosing AICN to avoid misdiagnosing. Forty‐five cases were excluded, while AICN was highly probable, but haematuria was absent or not documented. Ten additional cases were excluded despite AMX crystals identification. However, other contextual factors, including implementation of safety regulation, patient's reports and media coverage on safety issues, may have lead clinicians to more frequently report AICN during the study period. 40 Finally, we were not able to appreciate the consequences of AICN in term of cost and increase in length of hospital stay. However, as all cases were serious according to the World Health Organization definition, both these consequences could only be hypothesized.
In conclusion, we observed a dramatic increase of severe AICN cases in France since 2010 related to intravenous AMX administration. Most surgical cases were related to change in dose regimen protocol in obese patients, or in AMX administration. In medical patients, we identified that patients were older and were more frequently treated for endocarditis highlighting the need of AMX dose adaptation based on AMX therapeutic drug monitoring in these populations. We also identified, in both populations, factors (urine pH, hydration status) that could have contributed to AICN onset. The first step for avoiding AICN onset is to inform healthcare professionals on these contributing factors that need to be considered in case of intravenous AMX prescription. A warning was sent to all healthcare professionals in February 2018 by the Agence Nationale de Sécurité du Médicament and we plan to assess the impact of this warning on AICN annual notification rate in France. 39 Further investigations are required to better understand the contributing factors for AICN and its increase.
COMPETING INTERESTS
The authors have not disclosed any potential conflict of interest.
CONTRIBUTORS
L.T., N.M., C.L.B. and D.V. designed the study. L.T., C.L.B., T.T., J.M. and M.Z. collected data. L.T., N.M. and D.V. reviewed the cases. L.T. performed statistical analysis. L.T., N.M., C.L.B., E.L. and D.V. analysed the data and wrote the manuscript. All the authors reviewed the manuscript.
FUNDING
None.
ACKNOWLEDGEMENTS
This publication uses data collected from all Paris RCPVs that belong to the ANSM. The views expressed in this article are those of the authors and do not necessarily represent the ANSM position.
We are especially grateful to Pascal Auriche MD, from the Agence Nationale de Sécurité du Médicament, for gathering data from the FNPD database. We greatly appreciate Corinne Hooker MD, from Assistance Publique des Hôpitaux de Paris, Hôpitaux Universitaires Henri Mondor, Créteil, France for editorial assistance.
Thomas L, Le Beller C, Trenque T, et al. Amoxicillin‐induced crystal nephropathy: A nationwide French pharmacovigilance databases study. Br J Clin Pharmacol. 2020;86:2256–2265. 10.1111/bcp.14328
Principal Investigator: Dr Dominique Vodovar
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. World Health Organization . WHO report on surveillance of antibiotic consumption: 2016–2018 early implementation. https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf. Accessed January 21, 2020.
- 2. Salvo F, De Sarro A, Caputi AP, Polimeni G. Amoxicillin and amoxicillin plus clavulanate: a safety review. Expert Opin Drug Saf. 2009;8(1):111‐118. [DOI] [PubMed] [Google Scholar]
- 3. Sjövall J, Westerlund D, Alván G. Renal excretion of intravenously infused amoxycillin and ampicillin. Br J Clin Pharmacol. 1985;19(2):191‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vodovar D, Thomas L, Mongardon N, et al. Dramatic increase of amoxicillin‐induced crystal nephropathy found in a cohort study of French pharmacovigilance centers. Antimicrob Agents Chemother. 2018;62(3).e01630‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fogazzi GB, Cantù M, Saglimbeni L, Daudon M. Amoxycillin, a rare but possible cause of crystalluria. Nephrol Dial Transplant. 2003;18(1):212‐214. [DOI] [PubMed] [Google Scholar]
- 6. Rafat C, Haymann J‐P, Gaudry S, et al. The case: a crystal‐clear diagnosis: acute kidney injury in a patient with suspected meningoencephalitis. Diagnosis: amoxicillin‐induced crystal nephropathy. Kidney Int. 2014;86(5):1065‐1066. [DOI] [PubMed] [Google Scholar]
- 7. Hentzien M, Lambert D, Limelette A, et al. Macroscopic amoxicillin crystalluria. Lancet. 2015;385(9984):2296. [DOI] [PubMed] [Google Scholar]
- 8. Couto J, Dos Santos LP, Alves JC, López R, Maldonado C. Amoxicillin Crystalluria: A rare side‐effect of a commonly prescribed antibiotic. Eur J Case Rep Intern Med. 2017;4(10):000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geller RJ, Chevalier RL, Spyker DA. Acute amoxicillin nephrotoxicity following an overdose. J Toxicol Clin Toxicol. 1986;24(2):175‐182. [DOI] [PubMed] [Google Scholar]
- 10. Melero López D, Hidalgo Mayoral I, Delmiro Magdalena A. Crystalluria caused by amoxicillin. Rev Clin Esp. 2019. S0014‐2565(19)30109‐2. [DOI] [PubMed] [Google Scholar]
- 11. Zeller V, Puyraimond‐Zemmour D, Sené T, Lidove O, Meyssonnier V, Ziza J‐M. Amoxicillin Crystalluria, an emerging complication with an old and well‐known antibiotic. Antimicrob Agents Chemother. 2016;60(5):3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moulis G, Sommet A, Durrieu G, et al. Trends of reporting of'serious'vs. “non‐serious” adverse drug reactions over time: a study in the French PharmacoVigilance database. Br J Clin Pharmacol. 2012;74(1):201‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mammì M, Citraro R, Torcasio G, Cusato G, Palleria C, di Paola ED. Pharmacovigilance in pharmaceutical companies: an overview. J Pharmacol Pharmacother. 2013;4(Suppl 1):S33‐S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toulouse E, Masseguin C, Lafont B, et al. French legal approach to clinical research. Anaesth Crit Care Pain Med. 2018;37(6):607‐614. [DOI] [PubMed] [Google Scholar]
- 15. Verdesca S, Fogazzi GB, Garigali G, Messa P, Daudon M. Crystalluria: prevalence, different types of crystals and the role of infrared spectroscopy. Clin Chem Lab Med. 2011;49(3):515‐520. [DOI] [PubMed] [Google Scholar]
- 16. Lee TL, D'Arconte L, Brooks MA. High‐pressure liquid chromatographic determination of amoxicillin in urine. J Pharm Sci. 1979;68(4):454‐458. [DOI] [PubMed] [Google Scholar]
- 17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239‐245. [DOI] [PubMed] [Google Scholar]
- 18. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109‐117. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461‐470. [DOI] [PubMed] [Google Scholar]
- 20. Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1‐138. [Google Scholar]
- 21. World Health Organization . Safety of Medicines ‐ A Guide to Detecting and Reporting Adverse Drug Reactions ‐ Why Health Professionals Need to Take Action. https://apps.who.int/medicinedocs/en/d/Jh2992e/2.html#Jh2992e.2.
- 22. World Health Organization . J01CA04 ‐ WHOCC ‐ ATC/DDD Index.
- 23. World Health Organization . J01CR02 ‐ WHOCC ‐ ATC/DDD Index.
- 24. World Health Organization . Defined Daily Dose. https://www.whocc.no/ddd/definition_and_general_considera/. Accessed January 21, 2020.
- 25. Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J am Soc Nephrol. 2010;5(7):1277‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. French Society of Anaesthesia and Intensive Care (SFAR) . Antibioprophylaxie in surgery and interventionnelle medicine (adult patients) actualization 2010. Ann Fr Anesth Reanim. 2010;30(2):168‐190. [DOI] [PubMed] [Google Scholar]
- 27. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71‐87. [DOI] [PubMed] [Google Scholar]
- 28. European Comittee On Antimicrobial Susceptibility Testing (EUCAST) . Clinical breakpoints ‐ dosing of antimicrobial agents. http://www.eucast.org/clinical_breakpoints/.
- 29. European Comittee On Antimicrobial Susceptibility Testing (EUCAST) . Clinical breakpoints ‐ bacteria (v9.0). http://www.eucast.org/clinical_breakpoints/.
- 30. Pea F. Pharmacokinetics and drug metabolism of antibiotics in the elderly. Expert Opin Drug Metab Toxicol. 2018;14(10):1087‐1100. [DOI] [PubMed] [Google Scholar]
- 31. Forestier E, Fraisse T, Roubaud‐Baudron C, Selton‐Suty C, Pagani L. Managing infective endocarditis in the elderly: new issues for an old disease. Clin Interv Aging. 2016;11:1199‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Durante‐Mangoni E, Bradley S, Selton‐Suty C, et al. Current features of infective endocarditis in elderly patients: results of the international collaboration on endocarditis prospective cohort study. Arch Intern Med. 2008;168(19):2095‐2103. [DOI] [PubMed] [Google Scholar]
- 33. Moesch C, Rince M, Raby C, Leroux‐Robert C. Aminopenicillin crystalluria: identification by infrared spectrophotometry. Ann Biol Clin (Paris). 1985;43(3):227‐231. [PubMed] [Google Scholar]
- 34. Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams‐Huet B, Pak CYC. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65(4):1422‐1425. [DOI] [PubMed] [Google Scholar]
- 35. Kovacikova J, Winter C, Loffing‐Cueni D, et al. The connecting tubule is the main site of the furosemide‐induced urinary acidification by the vacuolar H+‐ATPase. Kidney Int. 2006;70(10):1706‐1716. [DOI] [PubMed] [Google Scholar]
- 36. Agence Nationale de Sécurité du Médicament et des produits de santé . Situation report on the active substance amoxicillin. https://ansm.sante.fr/var/ansm_site/storage/original/application/cae8a3e3e3ec6ef704b2ff963d02d310.pdf.
- 37. Guzzo G, Fogazzi GB, Cariello C, Barberini L‐E, Petignat P‐A, Teta D. The Friday evening case of acute kidney injury: a crystal dilemma. Clin Kidney J. 2018;11(4):450‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pulcini C. ESGAP AMOXDOSE working group. Amoxicillin dosing recommendations are very different in European countries: a cross‐sectional survey. Clin Microbiol Infect. 2017;23(6):414‐415. [DOI] [PubMed] [Google Scholar]
- 39. Kork F, Balzer F, Krannich A, et al. Back‐calculating baseline creatinine overestimates prevalence of acute kidney injury with poor sensitivity. Acta Physiol (Oxf). 2017;219(3):613‐624. [DOI] [PubMed] [Google Scholar]
- 40. Teng C, Reveles KR, Obodozie‐Ofoegbu OO, Frei CR. Clostridium difficile infection risk with important antibiotic classes: an analysis of the FDA adverse event reporting system. Int J Med Sci. 2019;16(5):630‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


