Abstract
Aims
This article sought to study the association between patterns of benzodiazepine (BZD) use and the risk of hip and forearm fractures in people aged 50 and 75 years or more.
Methods
In a representative cohort of the French National Health Insurance Fund of individuals aged 50 years or older (n = 106 437), we followed up BZD dispensing (reflecting their patterns of use) and the most frequent fall‐related fractures (hip and forearm) for 8 years. We used joint latent class models to simultaneously identify BZD dispensing trajectories and the risk of fractures in the entire cohort and in those 75 years or older). We used a survival model to estimate the adjusted hazard ratios (aHRs) between these trajectories and the risk of fractures.
Results
In the entire cohort, we identified 5 BZD trajectories: non‐users (76.7% of the cohort); occasional users (15.2%); decreasing users (2.6%); late increasing users (3.0%); and early increasing users (2.4%). Compared with non‐users, fracture risk was not increased in either occasional users (aHR = 0.99, 95% confidence interval [CI] 0.99–1.00) or in decreasing users (aHR = 0.90, 95% CI 0.74–1.08). It was significantly higher in early increasing users (aHR = 1.86, 95% CI 1.62–2.14) and in late increasing users (aHR = 1.39, 95% CI 1.15–1.60). We observed similar trajectories and risk levels in the people older than 75 years.
Conclusion
Occasional BZD use, which is compatible with current recommendations, was not associated with an excess risk of the most frequent fall‐related fractures in people older than 50 or 75 years.
Keywords: adverse drug reactions, benzodiazepines, drug utilization, pharmacoepidemiology
What is already known about this subject
Hip and forearm fractures, which are the most frequent fractures resulting from falls in older people, may decrease their autonomy and life expectancy.
Sedative–hypnotic and anxiolytic benzodiazepines are known to increase the risk of falls in older people.
Benzodiazepine use may follow various temporal patterns, including occasional use compatible with current guidelines.
What this study adds
No excess risk of hip and forearm fractures was found in occasional users of benzodiazepines either in a cohort of people aged 50 years or more, or in the group aged 75 years or more, compared with nonusers.
1. INTRODUCTION
Fractures are a frequent consequence of falls; in older people, they may lead to functional disability, decrease quality of life and autonomy, and even result in death. 1 , 2 Hip and forearm fractures are the most frequent types resulting from single or repeated falls; shoulder and ankle fractures are less frequent. 3 More than 87% of hip fractures are associated with falls by persons aged 65 years or more. 4 They are associated with a higher risk of mortality in elderly people 5 , 6 and a decrease in their life expectancy. 7
Strong evidence shows that use of sedative–hypnotic and anxiolytic benzodiazepines (BZD) increases the risk of falls or fractures in older people, 8 , 9 , 10 , 11 especially long‐acting BZD. 1 Three meta‐analyses exploring the association between drug use and falls in older people have reported concordant results, 12 , 13 , 14 with pooled odds ratios for the association between BZD use and falls ranging from 1.39 (95% confidence interval [CI] 1.24–1.54) 12 to 1.57 (95% CI 1.43–1.72). 14 Multiple mechanisms explain this increased risk: sedation, vertigo, reduced alertness, and balance or visual disorders. 15
Studies have also demonstrated that the risk of falls is higher at the very beginning of BZD treatment (24–120 hours after the first intake) 8 , 16 ; this risk is also higher in patients taking BZD with co‐prescribed psychotropic drugs such as antidepressants, antipsychotics or other hypnotic drugs than it is in those with a single BZD prescription and none of these co‐prescriptions, especially among older people. 10 , 13 , 14 The association between BZD use and fractures in older people has also been demonstrated. 17 Nevertheless, we have not found any studies addressing the risks of falls and fall‐related injuries or fractures in relation to temporal patterns of BZD use over the long term (several years). In 2 earlier cohort studies of hypnotic and anxiolytic BZD use over a period of 8 years or more, we found that BZD use follows various patterns of temporal dynamics. 18 , 19 Occasional use (periods of use lasting several weeks, from time to time) was the most frequent temporal pattern in BZD users. This use pattern is compatible with current guidelines regarding BZD use, which recommend occasional short‐term (4–12 weeks) use of BZD. 20 , 21 , 22 , 23 The question remains as to whether this use is safe, in terms of risks of falls.
This article sought to quantify the risk of the most frequent fall‐related fractures (of the hip and forearm) in people aged 50 and 75 years or more using hypnotic or anxiolytic BZD occasionally or briefly (occasional use), over a long period of time and compared with non‐users, after adjustment for a variety of clinical and medical characteristics.
2. METHODS
2.1. Data sources
The study used reimbursement data from the French National Healthcare Insurance Fund (NHIF). Data were anonymously extracted from the Echantillon Généraliste des Bénéficiaires database (EGB, or permanent sample of beneficiaries), with the authorisation of the French National Data Protection Authority. The EGB was created in 2005 by a national random sampling of 1/97th of the French population, stratified for age and sex. It records information about the health care consumption of insured individuals and includes data on reimbursement claims for drugs purchased at community pharmacies, classified with the Anatomical Therapeutic Chemical (ATC) index. It also includes information about long‐term illness (LTI) status; on request from the general practitioner, this status can be granted to patients with specifically authorised expensive diseases to exempt them from copayments.
This database is a permanent representative, and anonymised sample of persons affiliated with the 3 major NHIFs in France. 24 These funds cover 86% of the French general population and include salaried workers and their dependents, even after they have retired, lost their jobs, or became permanently disabled. People with very low incomes are also covered by this fund through special supplementary health insurance (CMU‐C) designed for those who could otherwise not afford it. Because data for those covered by the other 2 funds (agricultural workers and farmers, and the self‐employed, with their dependents) were only included in the EGB in 2011, we limited this study to salaried workers and their families. 24
The EGB is a dynamic cohort, updated every 3 months with registered births and foreign immigrants taking up employment in France (and their eligible dependents). It is linked to the French Medical Information System (PMSI), the hospital discharge database, which includes the diagnoses associated with all episodes of hospitalisation, coded according to the International Classification of Diseases, 10th revision (ICD‐10). The PMSI covers all French public and private hospitals, except military and psychiatric hospitals.
2.2. Study population
This cohort comprises the 106 437 persons aged 50 years or more included in the EGB on 1 January 2009, who had had no BZD dispensed in 2008 nor any history of hip or forearm fracture (defined below) that year. We followed them up until the day of any first hip or forearm fracture or until the end of the study period (31 December 2016). Those who died or withdrew from the insurance fund covered by the EGB during the follow‐up period were censored at the date of the event. BZD exposure (defined below) that began after the hip or forearm fracture during follow‐up was not used in the analysis to ensure that exposure preceded the event of interest.
2.3. Hip and forearm fractures
We studied hip and forearm fractures in this study, because they are the most frequent consequence of single or repeated falls, they almost always lead to hospital care, 3 and can be reliably identified through the PMSI. We defined a fracture as the first hospitalisation with an ICD‐10 diagnosis of S72.0, S72.1, S72.2 (hip fractures) or S52 (fractures of forearm) during the follow‐up period.
2.4. BZD exposure
Using the ATC information in the EGB, we collected all anxiolytic and hypnotic BZDs and their derivatives marketed in France and dispensed during the study period. Selected BZDs and their corresponding ATC codes are listed in Supplementary Table S1. Clonazepam and tetrazepam were excluded because they are not authorised in France for the treatment of anxiety.
As in our previous studies, which used trajectory analysis of hypnotic and anxiolytic BZD dispensing, 18 , 19 the study period was discretised into 3‐month intervals (max = 32 quarters for an uncensored person with no hip or forearm fracture during the 8‐year follow‐up). For each quarter, we built the binary variable BZD dispensed at least once (yes/no). Trajectories of BZD dispensing were estimated according to whether BZD was dispensed in each of these quarters (statistical units). BZD dispensing trajectories were considered proxies for BZD use trajectories.
The BZD trajectories identified were characterised according to the number and duration of BZD treatment episodes during the follow‐up period. Based on a published methodology, 18 , 19 , 25 the duration of each BZD treatment episode was estimated from information about the dates on which these drugs were dispensed. Because French pharmacies are not allowed to deliver more than 28 days' worth of a drug treatment, we defined discrete 28‐day periods after the first dispensing. Over each 28‐day period, we postulated that the cumulative duration of BZD treatment was 28 days if it was dispensed at least once, regardless of the number of medicine packages delivered. A treatment episode was considered either completed or discontinued if it was not renewed within 56 days after the last delivery (i.e. the 28‐day period started at delivery plus the following 28‐day period without a delivery). Repetition, during the follow‐up period, of these nonrenewals indicated occasional use and enabled it to be differentiated from continuous use.
2.5. Patients' characteristics
Patient characteristics included demographic information at inclusion (age and sex only). We then intentionally assessed a large number of variables associated with a risk of falls in the literature. 26 , 27 As we did for BZDs, we collected information about the other drugs dispensed during the follow‐up period and associated with the risks of falls 12 , 13 , 14 : antidepressants, antipsychotics, antiepileptics, oral antidiabetics, antihypertensive agents and drugs used in alcohol dependence (see Supplementary Table S2). Binary variables were built for each drug class: at least 1 delivery during the follow‐up period. Using a published methodology, 28 we also used hospital discharge data and data about to the LTI status available in the French social insurance system to identify persons with long‐term diseases also associated with the risks of falls or fractures (see Supplementary File S2): Parkinson disease, Alzheimer disease (or related dementias), epilepsy, psychiatric disorders, osteoporosis, age‐related macular degeneration, diabetes and cardiovascular diseases. 15 We also calculated a pharmacy‐based somatic multimorbidity score for each year of follow‐up. We assigned dispensed drugs to 14 chronic somatic conditions. A chronic condition was considered present when a person received at least 1 drug belonging to 1 of the 14 ATC groups in 1 year (see Supplementary Table S3). An individual chronic condition score was then calculated for each year of follow‐up as a weighted sum of the patient's chronic conditions identified by this procedure, as previously published. 18 , 19 , 29
2.6. Statistical analysis
To understand how the repeated marker data (BZD dispensing) and the risk of a fracture were linked, we used joint latent class models (JLCMs). This method considers the population of subjects as heterogeneous and assumes that it consists of several homogeneous latent subgroups of subjects (the latent classes) that share the same BZD dispensing trajectory and the same fracture risk. 30 , 31 JLCMs combine a class‐specific proportional hazard model for the time to first fracture with a class‐specific logistic model of the probability that BZDs are dispensed in each quarter (see Supplementary File S4). The survival model was adjusted for the other risk factors for falls and fractures: demographic information (age and sex), drugs dispensed, 26 , 27 the LTIs associated with falls listed above, and the somatic multimorbidity score 32 during the follow‐up period. To evaluate the risk of fractures in older people, we performed the analysis in the sample aged 50 years or older (also referred to as the entire cohort) and in the subsample of people aged 75 years or older (also referred to as the older cohort).
To evaluate the model's goodness‐of‐fit, we calculated individual class‐membership probabilities (posterior probabilities) with Bayes' theorem, as the probability of belonging to a latent class given the information collected. 33 Comparisons between observed and predicted trajectories were also checked to assess the quality of the classification.
The JLCM was estimated with the Fortran program HETMIXSURV. 34 Other statistical analyses were performed with SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Cohort characteristics
Of the 106 437 persons included in the entire cohort on 1 January 2009, 52.0% were women and 20.6% were older than 75 years. Their mean follow‐up lasted 7.3 years (standard deviation [SD] = 1.8, range 0.2–8.0) and 12.2% of them died during follow‐up. During this 8‐year period, 32.3% received at least 1 package of BZD (Table 1) at the pharmacy. In the older cohort, 35.0% died and 24.2% received at least 1 package of BZD (Table 1).
TABLE 1.
Study cohort characteristics (permanent sample of beneficiaries 2009–2016—n = 106 437)
| ≥50 years at inclusion n = 106 437 | ≥75 years at inclusion n = 21 852 | ||
|---|---|---|---|
| % a | % a | ||
| Sex | Women | 52.0 | 59.9 |
| Age at inclusion | 50–64 | 57.4 | |
| 65–74 | 22.1 | ||
| 75–84 | 15.2 | 73.9 | |
| ≥85 | 5.4 | 26.1 | |
| Death during follow‐up | 12.2 | 35.0 | |
| Mean duration of follow‐up (y), mean (SD) | 7.3 (1.8) | 6.0 (2.6) | |
| At least 1 benzodiazepine dispensed during follow‐up | 36.3 | 24.2 | |
| At least 1 antidepressant dispensed during follow‐up | 21.7 | 27.4 | |
| At least 1 antipsychotic dispensed during follow‐up | 4.8 | 9.2 | |
| At least 1 antiepileptic dispensed during follow‐up | 15.1 | 16.3 | |
| At least 1 oral antidiabetic dispensed during follow‐up | 17.7 | 19.4 | |
| At least 1 antihypertensive agent dispensed during follow‐up | 59.1 | 78.3 | |
| At least 1 drug used in alcohol dependence dispensed during follow‐up | 0.8 | 0.1 | |
| Parkinson's disease/dementia condition during follow‐up | 5.3 | 18.5 | |
| Osteoporosis during follow‐up | 2.4 | 40 | |
| Age‐related macular degeneration during follow‐up | 1.2 | 2.6 | |
| Diabetes during follow‐up | 16.5 | 20.3 | |
| Epilepsy during follow‐up | 1.2 | 1.7 | |
| Psychiatric condition during follow‐up | 2.2 | 1.9 | |
| Cardiovascular diseases during follow‐up | 31.1 | 54.8 | |
| Somatic multimorbidity score during follow‐up, mean (SD) | 0.4 (0.3) | 0.5 (0.3) |
Unless otherwise stated.
SD: standard deviation.
3.2. Incidence of fractures during follow‐up
During the follow‐up period, 5886 (5.5%) patients had an incident hip or forearm fracture. The incidence rate of fractures per 100 person‐years was significantly higher in women than in men (0.97 vs 0.54%; P < 0.001; Figure 1) and increased with age (0.32, 0.69, 1.96 and 4.68% in persons aged 50–64, 65–74, 75–84 and ≥ 85 years respectively).
FIGURE 1.
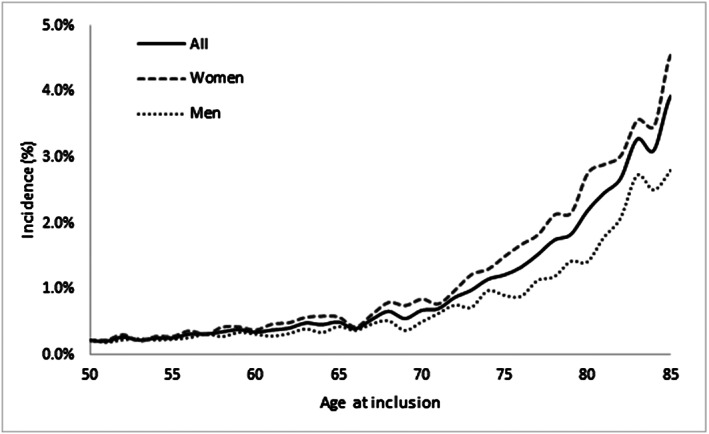
Fractures. Incidence rates per 100 persons‐years according to sex and age at inclusion (permanent sample of beneficiaries 2009–2016—n = 106 437)
3.3. Trajectories of BZD dispensing over time
Five different BZD trajectories were observed in the entire cohort over the 8‐year follow‐up period. Mean posterior class‐membership probabilities in each class exceeded 82%. Trajectories and their characteristics are presented in Figure 2 and Table 2. First, 76.7% of cohort members (n = 81 667) were non‐users with a permanent almost null probability of BZD dispensing (Trajectory 0).
FIGURE 2.
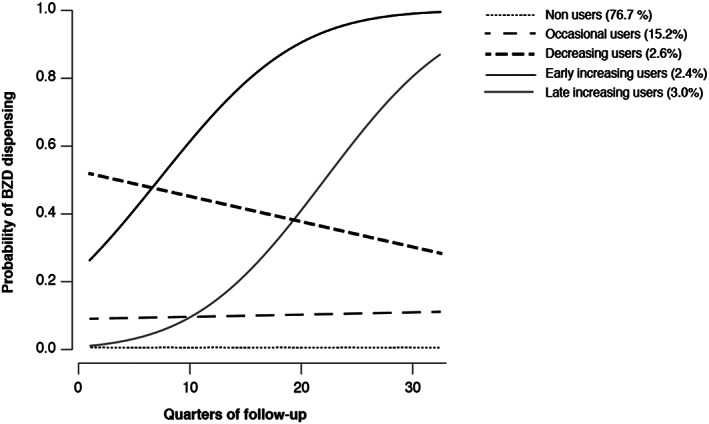
Predicted probabilities* of benzodiazepine (BZD) dispensing over the 8‐year follow‐up period for each of the 5 classes identified by the latent class mixed models (permanent sample of beneficiaries 2009–2016—n = 106 437). *: Probabilities of BZD dispensing were estimated for each quarter of follow‐up
TABLE 2.
Prevalence and characteristics of trajectories of benzodiazepine (BZD) dispensing (permanent sample of beneficiaries 2009–2016—n = 106 437)
| Trajectory 0 | Trajectory 1 | Trajectory 2 | Trajectory 3 | Trajectory 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non‐users | Occasional users | Decreasing users | Early increasing users | Late increasing users | |||||||
| ≥50 y at inclusion | ≥75 y at inclusion | ≥50 y at inclusion | ≥75 y at inclusion | ≥50 y at inclusion | ≥75 y at inclusion | ≥50 y at inclusion | ≥75 y at inclusion | ≥50 y at inclusion | ≥75 y at inclusion | ||
| Prevalence of trajectories according to age at inclusion and sex (%) | |||||||||||
| Women | 50–64 | 72.7 | 19.5 | 3.1 | 2.0 | 2.8 | |||||
| 65–74 | 73.5 | 17.0 | 3.1 | 3.0 | 3.5 | ||||||
| 75–84 | 73.5 | 74.5 | 14.9 | 14.3 | 3.1 | 2.9 | 3.9 | 3.4 | 4.7 | 4.9 | |
| >84 | 78.9 | 79.2 | 10.4 | 9.6 | 2.6 | 2.7 | 4.8 | 4.7 | 3.4 | 3.8 | |
| All | 73.4 | 75.9 | 17.6 | 12.9 | 3.1 | 2.9 | 2.7 | 3.8 | 3.3 | 4.6 | |
| Men | 50–64 | 80.9 | 13.0 | 2.0 | 1.8 | 2.5 | |||||
| 65–74 | 81.0 | 12.1 | 2.1 | 2.0 | 2.9 | ||||||
| 75–84 | 77.2 | 77.7 | 12.9 | 12.5 | 2.5 | 2.5 | 3.2 | 3.0 | 4.1 | 4.3 | |
| >84 | 78.5 | 78.9 | 12.2 | 10.7 | 2.5 | 2.4 | 3.8 | 4.3 | 3.0 | 3.7 | |
| All | 80.3 | 78.0 | 12.7 | 12.1 | 2.1 | 2.5 | 2.1 | 3.3 | 2.8 | 4.2 | |
| All | 50–64 | 76.8 | 16.3 | 2.5 | 1.9 | 2.6 | |||||
| 65–74 | 77.3 | 14.5 | 2.6 | 2.5 | 3.2 | ||||||
| 75–84 | 75.1 | 75.9 | 14.0 | 13.5 | 2.8 | 2.8 | 3.6 | 3.2 | 4.4 | 4.6 | |
| >84 | 78.8 | 79.1 | 11.0 | 9.9 | 2.6 | 2.6 | 4.5 | 4.6 | 3.2 | 3.8 | |
| All | 76.7 | 76.7 | 15.2 | 12.6 | 2.6 | 2.7 | 2.4 | 3.6 | 3.0 | 4.4 | |
| Death during follow‐up (%) | |||||||||||
| 11.4 | 34.3 | 12.1 | 33.45 | 15.0 | 37.7 | 27.0 | 47.51 | 19.4 | 38.0 | ||
| Proportion of quarters with at least 1 benzodiazepine dispensed during follow‐up a , mean (SD) | |||||||||||
| 0.6 (1.3) | 0.6 (1.5) | 12.0 (7.9) | 13.9 (7.5) | 44.7 (17.1) | 56.2 (22.0) | 67.0 (17.4) | 61.8 (20.0) | 30.8 (11.4) | 30.9 (14.0) | ||
| Total number of BZD treatment episodes during follow‐up, mean (SD) | |||||||||||
| 0.3 (0.7) | 0.3 (0.9) | 2.9 (1.7) | 2.8 (1.8) | 8.9 (4.4) | 8.0 (5.4) | 8.7 (6.5) | 6.3 (5.8) | 6.0 (3.7) | 5.0 (3.9) | ||
| Average duration of a BZD treatment episodes (days) b , mean (SD) | |||||||||||
| 51.5 (98.3) | 74.6 (138.2) | 45.1 (55.0) | 53.1 (70.3) | 68.7 (112.3) | 83.0 (142.2) | 149.6 (279.8) | 167.2 (298.7) | 91.3 (148.0) | 119.3 (191.1) | ||
| Somatic multimorbidity score during follow‐up, mean (SD) | |||||||||||
| 0.39 (0.26) | 0.46 (0.29) | 0.50 (0.25) | 0.57 (0.28) | 0.54 (0.25) | 0.57 (0.26) | 0.57 (0.26) | 0.60 (0.29) | 0.52 (0.24) | 0.55 (0.24) | ||
(Number of quarters with at least one BZD dispensed* 100).
Calculated in persons with at least one BZD treatment episode during the follow‐up period SD: standard deviation.
Then, 15.2% of the entire cohort members were occasional users (n = 16 224, 65.5% of BZD users) with, on average, at least 1 package of BZD dispensed in 12.0% of the quarters during follow‐up (Trajectory 1). Their prevalence decreased with age (16.3 vs 11.0% in persons aged 50–64 and 85 years or older, respectively; P < .001). The mean number of BZD treatment episodes during the follow‐up period was 2.9 (SD = 1.7). The mean duration of these episodes was 45.1 days (SD = 55; Table 2). In people aged 75 years or older, 12.6% were occasional users (n = 2747, 54.0% of the BZD users in the older cohort). Their mean number of BZD treatment episodes was 2.8 (SD = 1.8) and the mean duration of each episode was 53.1 days (SD = 70.3; Table 2).
Next (Trajectory 2), we found that 2.6% of the entire cohort (n = 2755, 11.1% of BZD users) and 2.7% of the older cohort (n = 592, 11.6% of BZD users in this cohort) were decreasing users with a probability of BZD dispensing decreasing during the follow‐up period. Their prevalence was relatively stable across the age groups (Table 2).
In Trajectory 3 (n = 2559, 2.4% of the entire cohort, 3.6% of the older cohort), the probability of BZD dispensing increased from the start of follow‐up onwards (early increasing users). In Trajectory 4 (n = 3232, 3.0% of the entire cohort and 4.4% of those in the older cohort), the probability of BZD dispensing was initially low and started to increase only after 2 years of follow‐up (late increasing users). Persons in trajectories 3 and 4 were considered chronic BZD users because they reached a stable level of use after their first BZD deliveries.
3.4. Associations between fractures and trajectories of BZD dispensing
Over the follow‐up period, the time‐to‐event analysis from the JLCM showed that the risk of hip and forearm fractures was similar in both occasional users, regardless of age, and non‐users (Table 3). The adjusted hazard ratio (aHR) was estimated at 0.99 (95% CI 0.99–1.00) for occasional users in the entire cohort and at 1.00 (95% CI 0.99–1.00) in the older cohort. The risk of fractures was also similar among decreasing users and non‐users, in both the entire and the older cohorts (Table 3).
TABLE 3.
Crude and adjusted hazard ratios of fractures according to trajectories of benzodiazepine (BZD) dispensing in people aged 50 years or older and those aged 75 years or older—joint latent class model estimates (permanent sample of beneficiaries 2009–2016—n = 106 437)
| ≥50 y at inclusion (n = 106 437) | ≥75 y at inclusion (n = 21 852) | |||
|---|---|---|---|---|
| Trajectories of BZD dispensing (ref. non‐users) | Crude hazard ratios with 95% CI | Adjusted hazard ratios a with 95% CI | Crude hazard ratios with 95% CI | Adjusted hazard ratios a with 95% CI |
| Occasional users | 0.91 0.83–0.99 | 0.99 0.99–1.00 | 1.00 0.99–1.00 | 1.00 0.99–1.00 |
| Decreasing users | 0.92 0.85–0.99 | 0.90 0.74–1.08 | 0.88 0.67–1.15 | 0.92 0.68–1.28 |
| Early increasing users | 3.35 2.96–3.79 | 1.86 1.62–2.14 | 2.08 1.73–2.50 | 1.87 1.53–2.27 |
| Late increasing users | 3.04 2.63–3.52 | 1.36 1.15–1.60 | 1.92 1.58–2.33 | 1.81 1.47–2.23 |
survival model adjusted on: age at inclusion, sex, drugs dispensed during follow‐up and long‐term diseases (see Supplementary Table S2), somatic multimorbidity score (see Supplementary Table S3).
CI: confidence interval.
In contrast, we found a significantly higher risk of fractures in chronic BZD users compared with non‐users: aHR = 1.86 (95% CI: 1.62–2.14) for early increasing users and aHR = 1.39 (95% CI 1.15–1.60) for late increasing users in the entire cohort (Table 3). This risk was also significantly higher for those aged 75 years or older (Table 3).
4. DISCUSSION
4.1. Major findings and comparison with literature
This is the first cohort study addressing the association between hypnotic or anxiolytic BZD use and the risk of the most frequent fall‐related fractures (hip and forearm) in people with occasional BZD use, assessed over 8 years. Occasional use was the most frequent trajectory, identified in 2/3 of BZD users in the entire cohort and half of those in the older cohort. We found no excess risk of hip and forearm fractures in these occasional users, compared with non‐users (76.7% of the entire cohort), both age groups. In contrast, the chronic BZD users (early and late increasing users) had a significantly higher risk of fractures than nonusers, in both age groups.
This is an important finding, given that occasional users account for most BZD users. It suggests that short, occasional BZD treatments, as recommended in guidelines, are acceptable in terms of the risk of the most frequent fall‐related fractures. 20 , 21 , 22 , 23 This finding has concrete implications for prescribers initiating a BZD treatment. They should have their patients understand from the outset that treatment duration should be limited in time and that it may be repeated if necessary, but only from time to time. However, physicians and patients should remain very cautious at the start of treatment, when the risk of falls is known to be highest. 8 , 16
The significant excess risk of fractures in elderly chronic BZD users is in line with the results of meta‐analyses that have found an overall excess risk of the same order of magnitude: HR from 1.39 (95% CI 1.24–1.54) 12 to 1.57 (95% CI 1.43–1.72). 14 It confirms the importance of deprescribing BZD in chronic users. 20 , 22 , 23 In this context, our finding that the decreasing BZD use trajectory (11.1% of all BZD users) was not associated with an increased risk of fractures is also important. However, patients' difficulties in stopping the use of anxiolytic or hypnotic BZD are well known. Overcoming these difficulties may require alternative management of anxiety and insomnia, such as temporary pharmacological substitution, psychological support 35 or educational intervention and support. 36 Patients and physicians should work together to apply and follow a withdrawal plan involving gradually decreasing doses. 37 , 38 Nevertheless, interventions aiming to improve quality of care are not always effective in reducing consumption of BZD in older people. 39 Our results suggest that achieving occasional BZD use may be a reasonable objective for chronic users, especially if zero use is unattainable.
The prevalence of long‐term BZD use in the literature ranges from 6 to 76%, due to heterogeneity of the definitions of long‐term use and data sources. 40 These prevalence rates are higher among the elderly. We found a similar trend for trajectories 3 and 4, defined by increasing BZD use with time: their prevalence also increased from younger categories to older ones, although this was not uniform for those 85 years or older. Any direct comparisons must be cautious, however, because the statistical methods of previous studies differ quite substantially from ours (Latent Class Analysis).
4.2. Strengths and limitations of the study
The NHIF databases made it possible to use a very large representative sample of insured persons to analyse BZD use trajectories over a long period of time (7.3 years on average) and their associated risks of fractures. The statistical method applied to model these trajectories avoided biases that can arise in separate analyses, since the individual trajectories of BZD use were truncated at the time of the first event. This truncation avoided a reverse causal bias that could arise from modifications of BZD treatment after fractures. Furthermore, our results for these trajectories are concordant with our previous study of those for BZD anxiolytic use in new users older than 50 years 19 and thus confirm the consistency of the method used to identify these use trajectories.
The study also has some limitations. While reimbursement data provide information about the delivery of drugs to patients with prescriptions, delivery does not necessarily mean use. This potential bias might have resulted in some overestimation of the prevalence of BZD use, especially in the occasional trajectory. The repeated purchase of BZDs by individuals classified as regular or chronic users suggests that most of them are indeed used. In the occasional trajectory, only 4% of cohort members had only a single package of BZDs dispensed during the follow‐up period. Most of the other patients in this trajectory had several deliveries (mean number = 3) of mean durations of 45 days. Again, this repeated purchase suggests that drugs were consumed. Moreover, using pharmacy dispensing data as a proxy for drug utilization is recognized as a robust method 41 , 42 and is often used to estimate BZD use among nationwide samples of the general population. 40 Some classification bias between trajectories resulting from the latent class model cannot be entirely excluded. However, the mean posterior class‐membership probabilities in each class exceed 82%, which indicates that discrimination between the classes was good; potential misclassification was thus limited. Moreover, descriptive statistics of the trajectories of anxiolytic or hypnotic use published in previous papers (including number and mean duration of treatment episodes) show clear differences between trajectories. 18 , 19
Although our analysis was adjusted for potential confounding factors (age, sex, comorbidities, other drug treatments), we cannot exclude some residual confounding. If it occurred, however, it should not have affected our results about occasional use. Finally, our results were based on hip and forearm fractures, which are the most frequent fall‐related fractures in the elderly. The frequency of these fractures was used as a proxy for the risk of falls but was not strictly identical to it and excluded other injuries that might result from falls but do not require hospitalisation, such as bruises and haematomas. However, the NHIF does not reliably record either such injuries or the cause of every fracture.
4.3. Implications for practice and research
The findings of this study reinforce the recommendations about the occasional use of BZD, limited to a maximum of 4–12 weeks. Such BZD use is acceptable in older people, as it does not appear to increase the risk of the most frequent severe fractures. These results also suggest that such a pattern of use may be a reasonable intermediary objective when attempting to reduce BZD use, since zero use may be unattainable for some patients. Doctors also need to review older people's medications frequently to prevent the gradual slip from occasional BZD use to inappropriate regular chronic use. However, further research is necessary to explore the association between occasional BZD use and the other side‐effects of these drugs, such as cognitive impairment, especially in older people.
ETHICAL STANDARDS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was also approved by the French Commission on Individual Data Protection and Public Liberties (Commission Nationale Informatique et Libertés). Patient information was anonymised and de‐identified prior to analysis.
COMPETING INTEREST
There are no competing interests to declare.
CONTRIBUTORS
P.V., S.C, H.V., M. T. and H.J.‐G. designed the study. S.C. and V.P. analysed the data. H.C. took the lead in writing the manuscript. All authors provided critical feedback and helped shaping the research, analysis and manuscript.
Supporting information
TABLE S1 List of selected benzodiazepines and related drugs according to Anatomical Therapeutic Chemical indexTABLE S2 List of adjustment variables with assigned International Classification of Diseases and Anatomical Therapeutic Chemical codesTABLE S3 List of chronic conditions and assigned Anatomical Therapeutic Chemical codes to calculate of the somatic multimorbidity scoreSUPPLEMENTARY FILE S4 Joint latent class models
ACKNOWLEDGEMENTS
We thank Ms Jo Ann Cahn and Mr Ray Cooke for revising our manuscript and the BJCP reviewers for their helpful comments.
Dr Verger reports grants from French National Agency for Medicines and Health Products Safety (grant number: 2014S029 ‐ http://ansm.sante.fr), during the conduct of the study. This funder had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Carrier H, Cortaredona S, Philipps V, et al. Long‐term risk of hip or forearm fractures in older occasional users of benzodiazepines. Br J Clin Pharmacol. 2020;86:2155–2164. 10.1111/bcp.14307
The authors confirm that the Principal Investigators for this paper are Prof. Hélène Verdoux and Dr Pierre Verger. Because this is a database study, no authors had direct (or indirect) clinical responsibility for any patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the French National Health Insurance Fund for Employees (CNAMTS). Restrictions apply to the availability of these data, which were used under license for this current study, and so are not publicly available. Data are available from the authors with permission of CNAMTS.
REFERENCES
- 1. Fuller GF. Falls in the elderly. Am Fam Physician. 2000;61:2159‐2168. 2173–4 [PubMed] [Google Scholar]
- 2. Guirguis‐Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;319(16):1705‐1716. 10.1001/jama.2017.21962 [DOI] [PubMed] [Google Scholar]
- 3. Court‐Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691‐697. 10.1016/j.injury.2006.04.130 [DOI] [PubMed] [Google Scholar]
- 4. Panula J, Pihlajamäki H, Mattila VM, et al. Mortality and cause of death in hip fracture patients aged 65 or older ‐ a population‐based study. BMC Musculoskelet Disord. 2011;12:105 10.1186/1471-2474-12-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haentjens P, Magaziner J, Colón‐Emeric CS, et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380‐390. 10.1059/0003-4819-152-6-201003160-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LeBlanc ES, Hillier TA, Pedula KL, et al. Hip fracture and increased short‐term but not long‐term mortality in healthy older women. Arch Intern Med. 2011;171(20):1831‐1837. 10.1001/archinternmed.2011.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J am Geriatr Soc. 2003;51(3):364‐370. 10.1046/j.1532-5415.2003.51110.x [DOI] [PubMed] [Google Scholar]
- 8. Díaz‐Gutiérrez MJ, Martínez‐Cengotitabengoa M, Sáez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17‐22. 10.1016/j.maturitas.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 9. Pariente A, Dartigues J‐F, Benichou J, Letenneur L, Moore N, Fourrier‐Réglat A. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61‐70. 10.2165/00002512-200825010-00007 [DOI] [PubMed] [Google Scholar]
- 10. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol. 2014;77(2):295‐301. 10.1111/j.1365-2125.2012.04418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waade RB, Molden E, Martinsen MI, Hermann M, Ranhoff AH. Psychotropics and weak opioid analgesics in plasma samples of older hip fracture patients ‐ detection frequencies and consistency with drug records: Psychotropics and weak opioids in plasma samples of hip fracture patients. Br J Clin Pharmacol. 2017;83(7):1397‐1404. 10.1111/bcp.13244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bloch F, Thibaud M, Dugué B, Brèque C, Rigaud AS, Kemoun G. Psychotropic drugs and falls in the elderly people: updated literature review and meta‐analysis. J Aging Health. 2011;23(2):329‐346. 10.1177/0898264310381277 [DOI] [PubMed] [Google Scholar]
- 13. Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta‐analysis: I. psychotropic drugs. J am Geriatr Soc. 1999;47:30‐39. 10.1111/j.1532-5415.1999.tb01898.x [DOI] [PubMed] [Google Scholar]
- 14. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952‐1960. 10.1001/archinternmed.2009.357 [DOI] [PubMed] [Google Scholar]
- 15. INSERM . Activité physique et prévention des chutes chez les personnes âgées. Paris: INSERM; 2015. [Google Scholar]
- 16. Berry SD, Lee Y, Cai S, Dore DD. Non‐benzodiazepine sleep medications and hip fractures in nursing home residents. JAMA Intern Med. 2013;173(9):754‐761. 10.1001/jamainternmed.2013.3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooft CSVD, Schoofs MWCJ, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276‐282. 10.1111/j.1365-2125.2008.03185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verger P, Cortaredona S, Jacqmin‐Gadda H, Tournier M, Verdoux H. Eight‐year follow‐up of hypnotic delivery by adults aged 50 and older from an insurance database. Sleep. 2017;40(11). 10.1093/sleep/zsx147 [DOI] [PubMed] [Google Scholar]
- 19. Verger P, Mrenda BM, Cortaredona S, Tournier M, Verdoux H. Trajectory analysis of anxiolytic dispensing over 10 years among new users aged 50 and older. Acta Psychiatr Scand. 2018;137(4):328‐341. 10.1111/acps.12858 [DOI] [PubMed] [Google Scholar]
- 20. HAS . Modalités d'arrêt des benzodiazépines et médicaments apparentés chez le patient âgé. 2007.
- 21. Mcintosh B, Clark M, Spry C. Benzodiazepines in older adults: a review of clinical effectiveness, cost‐effectiveness, and guidelines. Rapid response report: peer‐reviewed summary with critical appraisal 2011. [PubMed]
- 22. NICE . NICE clinical knowledge summaries: benzodiazepine and z‐drug withdrawal. 2013.
- 23. NHS West Essex . Guidelines for prescribing and withdrawing benzodiazepines and Z drugs. A resource for general practitioners. 2012.
- 24. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merlière Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58(4):286‐290. 10.1016/j.respe.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Verdoux H, Cougnard A, Thiébaut A, Tournier M. Impact of duration of antidepressant treatment on the risk of occurrence of a new sequence of antidepressant treatment. Pharmacopsychiatry. 2011;44(3):96‐101. 10.1055/s-0031-1271686 [DOI] [PubMed] [Google Scholar]
- 26. Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol a Biol Sci Med Sci. 2007;62:1172‐1181. [DOI] [PubMed] [Google Scholar]
- 27. Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication‐related falls in the elderly. Drugs Aging. 2012;29(5):359‐376. 10.2165/11599460-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 28. CNAM . Méthode générale de la cartographie des pathologies, version G5 (années 2012 à 2016). 2018. https://www.ameli.fr/fileadmin/user_upload/documents/Methode_medicale_Cartographie.pdf
- 29. Cortaredona S, Pambrun E, Verdoux H, Verger P. Comparison of pharmacy‐based and diagnosis‐based comorbidity measures from medical administrative data. Pharmacoepidemiol Drug Saf. 2017;26(4):402‐411. 10.1002/pds.4146 [DOI] [PubMed] [Google Scholar]
- 30. Lin H, Turnbull BW, McCulloch CE, Slate EH. Latent class models for joint analysis of longitudinal biomarker and event process data. J am Stat Assoc. 2002;97(457):53‐65. 10.1198/016214502753479220 [DOI] [Google Scholar]
- 31. Proust‐Lima C, Joly P, Dartigues J‐F, Jacqmin‐Gadda H. Joint modelling of multivariate longitudinal outcomes and a time‐to‐event: a nonlinear latent class approach. Comput Stat Data Anal. 2009;53(4):1142‐1154. 10.1016/j.csda.2008.10.017 [DOI] [Google Scholar]
- 32. Davies EA, O'Mahony MS. Adverse drug reactions in special populations – the elderly. Br J Clin Pharmacol. 2015;80(4):796‐807. 10.1111/bcp.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Proust‐Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw. 6;78(2):1‐56. 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 34. Proust‐Lima C. HETMIXSURV program. Institut de Santé Publique, d'Épidémiologie et de Développement, Inserm. 2018 http://etudes.isped.u-bordeaux2.fr/BIOSTATISTIQUE/HETMIXSURV/US-Biostats-HETMIXSURV.html
- 35. Reeve E, Ong M, Wu A, Jansen J, Petrovic M, Gnjidic D. A systematic review of interventions to deprescribe benzodiazepines and other hypnotics among older people. Eur J Clin Pharmacol. 2017;73(8):927‐935. 10.1007/s00228-017-2257-8 [DOI] [PubMed] [Google Scholar]
- 36. Guaiana G, Barbui C. Discontinuing benzodiazepines: best practices. Epidemiol Psychiatr Sci. 2016;25(3):214‐216. 10.1017/S2045796016000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parr JM, Kavanagh DJ, Young RM, McCafferty K. Views of general practitioners and benzodiazepine users on benzodiazepines: a qualitative analysis. Soc Sci Med. 2006;62(5):1237‐1249. 10.1016/j.socscimed.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 38. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014;78(4):738‐747. 10.1111/bcp.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Souto Barreto P, Lapeyre‐Mestre M, Cestac P, Vellas B, Rolland Y. Effects of a geriatric intervention aiming to improve quality care in nursing homes on benzodiazepine use and discontinuation. Br J Clin Pharmacol. 2016;81(4):759‐767. 10.1111/bcp.12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kurko TAT, Saastamoinen LK, Tähkäpää S, et al. Long‐term use of benzodiazepines: definitions, prevalence and usage patterns – a systematic review of register‐based studies. Eur Psychiatry. 2015;30(8):1037‐1047. 10.1016/j.eurpsy.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 41. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323‐337. 10.1016/j.jclinepi.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 42. Hallas J. Drug utilization statistics for individual‐level pharmacy dispensing data. Pharmacoepidemiol Drug Saf. 2005;14(7):455‐463. 10.1002/pds.1063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 List of selected benzodiazepines and related drugs according to Anatomical Therapeutic Chemical indexTABLE S2 List of adjustment variables with assigned International Classification of Diseases and Anatomical Therapeutic Chemical codesTABLE S3 List of chronic conditions and assigned Anatomical Therapeutic Chemical codes to calculate of the somatic multimorbidity scoreSUPPLEMENTARY FILE S4 Joint latent class models
Data Availability Statement
The data that support the findings of this study are available from the French National Health Insurance Fund for Employees (CNAMTS). Restrictions apply to the availability of these data, which were used under license for this current study, and so are not publicly available. Data are available from the authors with permission of CNAMTS.


