Abstract
Aims
The aim of this study was to investigate the effects of a single green tea (GT), administered concomitantly or 1 hour before nadolol intake on nadolol pharmacokinetics.
Methods
In a randomized 3‐phase crossover study, 11 healthy volunteers received an oral administration of nadolol with, or 1 hour after preingestion of brewed GT, or with water in a volume of 150 mL.
Results
Geometric mean ratio with 90% confidence interval for nadolol AUC0–48 was 0.371 (0.303–0.439) with concomitant GT. In addition, ingestion of GT 1 hour before nadolol administration resulted in a significant reduction of nadolol AUC0–48 with geometric mean ratio of 0.536 (0.406–0.665). There were no differences in time to maximal plasma concentration and renal clearance of nadolol among groups.
Conclusion
These results suggest that single concomitant ingestion of GT substantially decreases plasma concentrations of nadolol. Moreover, the reduction in nadolol bioavailability could persist for at least 1 hour after drinking a cup of GT.
Keywords: epigallocatechin gallate, food–drug interaction, green tea, nadolol, pharmacokinetics
What is already known about this subject
Repeated consumption of green tea causes a pronounced decrease in plasma concentrations of nadolol in humans presumably by inhibiting the intestinal absorption of nadolol.
Epigallocatechin gallate, a major catechin found in green tea, is suggested to be a key contributor to the pharmacokinetic interaction of green tea with nadolol.
What this study adds
Single concomitant ingestion of brewed green tea significantly decreases plasma concentrations of nadolol without changing its elimination half‐life and renal clearance in healthy volunteers.
Reduction in nadolol bioavailability could persist for at least 1 hour after drinking a cup of brewed green tea.
1. INTRODUCTION
Green tea (Camellia sinensis) has received considerable attention due to its potential beneficial health effects. 1 From the viewpoint of drug–food interactions, green tea catechins, flavonoids abundantly present in green tea leaves and infusion, are intriguing compounds because of their in vitro inhibitory potentials on several cytoplasmic or membrane proteins associated with drug metabolism and transport such as cytochromes P450 (CYPs), 2 , 3 efflux transporters such as P‐glycoprotein, 4 , 5 and influx transporters such as organic anion transporting polypeptides (OATP). 5 , 6 , 7 , 8 , 9
Previously, our group reported that green tea ingestion greatly decreased plasma concentrations of nadolol by 85%, a hydrophilic nonselective β‐adrenoceptor antagonist, after chronic consumption of green tea for 14 days in healthy subjects. 8 Moreover, we have revealed that, among various components of green tea, (–)‐epigallocatechin gallate (EGCG) is suggested to be a key contributor of this interaction. 9 A single concomitant administration of nadolol with an aqueous solution of EGCG‐concentrated green tea extract (GTE; at EGCG doses equivalent to approximately 50 or 150 mg) resulted in significant reductions in nadolol plasma concentrations (−40%) without altering terminal half‐life and renal clearance (CL R) of nadolol in healthy volunteers. However, it is still unknown whether a single ingestion of green tea affects nadolol pharmacokinetics.
After drinking green tea or an aqueous solution of GTE, EGCG reaches its maximal plasma concentration (t max) after around 1–2 hours. 8 , 9 , 10 Given that the mechanism underlying nadolol‐green tea and/or EGCG interaction could be attributed to the inhibition of absorptive transport of nadolol in the intestine, 8 , 9 it is possible that the interaction might be prevented or diminished by drinking green tea before (e.g. 1 h) oral administration of nadolol, since nadolol is taken up from the intestine for several hours with t max being approximately 3 hours. To avoid therapeutic inefficacy of nadolol, it is important to evaluate the duration of green tea–nadolol interaction after the ingestion. Therefore, the present study was undertaken to investigate whether a single concomitant or 1 hour before ingestion of brewed green tea affects nadolol pharmacokinetics in humans.
2. METHODS
2.1. Study design
The study protocol was approved by the ethics committee of the Fukushima Medical University, and was registered at UMIN‐CTR as number UMIN000019398. The study procedures were in accordance with the ethical standards of the Declaration of Helsinki. An open, randomized, single‐centre crossover study was carried out in 3 phases, separated by washout periods of at least 1 week. Twelve healthy volunteers (6 males, 6 females; aged 20–63 years; body mass index 18.9–33.1 kg m−2) were enrolled in the study after giving written informed consent. The volunteers were confirmed to be healthy by medical history, physical examination, and routine laboratory tests and to be nonsmokers before entering the study. A single oral dose of 30 mg nadolol (Nadic®, Sumitomo Dainippon Pharma, Osaka, Japan) was administered in the morning of the study day with 150 mL of concomitant brewed green tea, or 1 hour after 150 mL of brewed green tea or with 150 mL of concomitant water. Brewed green tea was prepared before ingestion in every trial according to previous studies 11 , 12 in order to achieve an EGCG concentration of approximately 100 mg dL−1. In brief, commercially available green tea leaves (3.0 g per 100 mL, Harada Tea Processing Co. Ltd., Shimada, Japan) were mixed with hot water (90°C) for 4 minutes with stirring 1 day before the clinical trial, and the brewed green tea was stored at 4°C until the subjects took it. Subjects were instructed to refrain from consuming green tea and fruit products including apple, grapefruit and orange juices for 7 days before the trial day. In addition, no medication was permitted during the study. Subjects fasted overnight before nadolol administration and had a standardized meal 1 and 4 hours after administration of nadolol. In each study period, 5 mL venous blood samples were obtained over 48 hours following nadolol administration. Blood samples were centrifuged for 10 minutes at approximately 2000 g. Urine was collected during periods of 0–4, 4–8, 8–24 and 24–48 hours after administration of nadolol. Plasma and urine samples were stored at −80°C until analysis. As pharmacodynamic tests, pulse rate (PR), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured after 10 minutes’ rest in a sitting position over 48 hours after nadolol administration with an automatic blood pressure monitor (HEM‐7051‐HP, Omron, Kyoto, Japan). The mean values for the pharmacodynamic parameters were calculated from triplicate measurements and given as percent changes from the individual baseline value. Twelve volunteers were required to determine a 25% change in the area under the plasma concentration–time curve (AUC) of nadolol with 80% power (α‐level 5%) on the basis of the previous studies. 8 , 13
2.2. Data analysis
Data are expressed as geometric means and 90% confidence interval unless stated otherwise. Pharmacokinetic analysis was performed using a noncompartmental model. AUC was determined by the trapezoidal method. CL R was calculated from the following equation: CL R = A e/AUC0–48, in which A e is the amount of nadolol excreted into urine up to 48 hours. The area under the time‐effect curve over 48 h (AUEC0–48) for PR, SBP and DBP was obtained using the trapezoidal method.
Pharmacokinetic and pharmacodynamic parameters including peak plasma concentration (C max), AUC0–8, AUC0–48, urinary excretion (A e), renal clearance (CL R) and AUEC for PR, SBP and DBP were analysed by repeated measures analysis of variance and posthoc testing was performed using the Dunnet's test for the comparison between groups using GraphPad Prism (ver. 6.07, GraphPad software, San Diego, CA, USA). The t max was compared using Dunn's multiple comparison test. Effects of green tea on pharmacokinetics of nadolol were accepted if the 90% confidence interval of the geometric mean ratios did not fall within the bioequivalence boundary of 0.8–1.25. The criterion for significance was P < .05.
3. RESULTS AND DISCUSSION
Eleven subjects completed this study with no adverse events, and 1 male participant withdrew from the study due to personal reasons. Mean ± standard deviation concentrations of 8 catechins and caffeine in the brewed green tea are shown in Table S1. Accordingly, doses (mg) of catechins and caffeine were estimated when subjects took 150 mL of the brewed green tea. Of note, subjects received approximately 166 mg of EGCG with a total catechin content of 539 ± 39 mg. The dose of EGCG was comparable to our previous study, in which EGCG was administered to healthy volunteers at a dose of 150 mg as aqueous solution of GTE. 9
Plasma concentration–time profiles and pharmacokinetic parameters of nadolol are summarized in Figures 1 and 2, and Table 1. Nadolol C max, AUC0–8, AUC0–48 and A e in the concomitant green tea phase were significantly decreased by 68% (P < .001), 63% (P < .001), 57% (P < .001), and 50% (P < .001), respectively, compared with the water phase. These data suggest that 1‐time co‐ingestion of brewed green tea substantially affects the pharmacokinetics of nadolol. No change was observed in CL R of nadolol between concomitant green tea and water phases, indicating that green tea modulates the intestinal absorption, but not the renal excretion, of nadolol. The individual value of AUC0–48 in water phase was highly correlated with the decrease in AUC0–48 in concomitant green tea phase (R 2 = .933, P < .001), indicating that individuals with higher nadolol AUC with water had greater decrease with green tea. In the previous studies, we have demonstrated that multiple ingestion of green tea or a single coadministration of EGCG significantly decreased plasma concentrations of nadolol in humans. 8 , 9 Nadolol has been found to be a substrate of OATP1A2, and green tea catechins such as EGCG potently inhibit OATP1A2‐mediated nadolol uptake. 6 , 8 , 9 Based on the assumption that OATP1A2 plays a role in the absorptive transport of nadolol in intestine, 14 , 15 it is suggested that the inhibition of OATP1A2 by catechins could lead to a reduction of intestinal absorption of nadolol. To gain insight into the effects of green tea on nadolol pharmacokinetics, the extent of green tea–nadolol interaction as indexed by % decrease in AUC of nadolol was plotted against estimated oral dose of EGCG in the different studies. As shown in Figure S1, the decrease in plasma exposure to nadolol appears to be dependent on the oral dose of EGCG. Under a hypothesis that green tea–nadolol interaction may be caused by the inhibition of carrier‐mediated uptake of nadolol in the intestine, the data were fitted by the hyperbolic equation with coefficient of determination of 0.79. It is estimated that a single coadministration of green tea, which contains approximately 130 mg of EGCG could halve the AUC of orally administered nadolol in humans.
FIGURE 1.
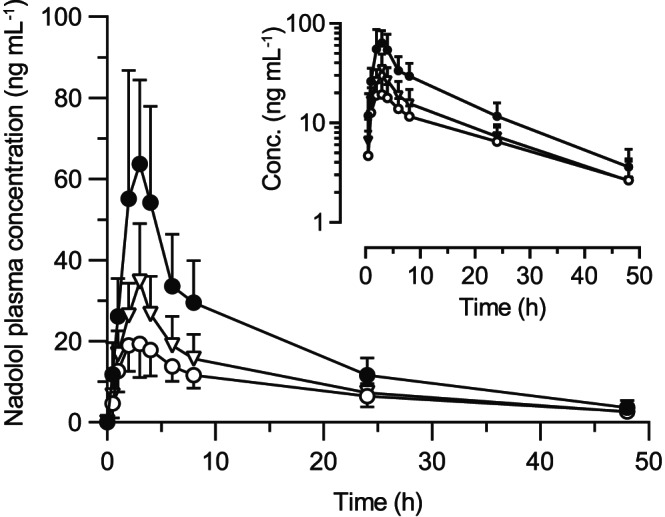
Plasma concentrations of nadolol after oral administration nadolol (30 mg) with 150 mL of concomitant brewed green tea (open circle), or 1 hour after 150 mL of brewed green tea (open triangle), or with 150 mL of concomitant water (closed circle) in 11 healthy volunteers. Data are expressed as mean ± standard deviation, and the insert depicts log‐plasma concentration of nadolol vs time profiles
FIGURE 2.
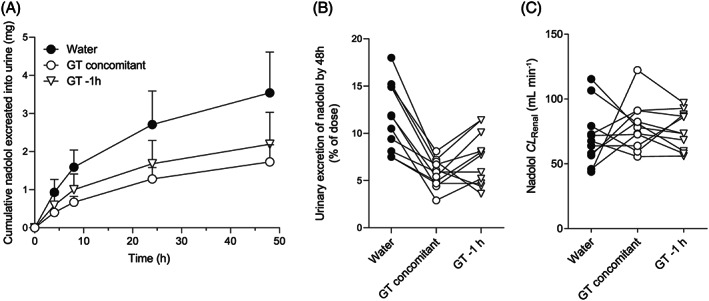
Urinary excretion of nadolol after oral administration nadolol (30 mg) with 150 mL of concomitant brewed green tea (open circle), or 1 hour after 150 mL of brewed green tea (open triangle), or with 150 mL of concomitant water (closed circle) in 11 healthy volunteers. (A) Mean cumulative amount of nadolol excreted into urine during 48 hours, (B) individual values of urinary excretion of nadolol by 48 hours, (C) individual values of renal clearance of nadolol. Data are expressed as mean ± standard deviation
TABLE 1.
Pharmacokinetic parameters of nadolol after administration of a single 30‐mg oral dose to healthy volunteers
| Water | Brewed green tea | |||||
|---|---|---|---|---|---|---|
| Concomitant | 1 hour before | |||||
| Geometric mean | CV | Geometric mean | CV | Geometric mean | CV | |
| C max (ng mL−1) | 69.3 | 0.33 | 22.0 | 0.36 | 32.2 | 0.41 |
| GMR (90% CI) | 0.318 | (0.255–0.382) | 0.465 | (0.328–0.601) | ||
| t max (h); median | 3.0 | (2.0–4.0) | 2.0 | (1.0–4.0) | 3.0 | (2.0–3.0) |
| AUC0–8 (h ng mL−1) | 306.7 | 0.35 | 113.8 | 0.26 | 162.5 | 0.31 |
| GMR (90% CI) | 0.371 | (0.303–0.439) | 0.530 | (0.402–0.657) | ||
| AUC0–48 (h ng mL−1) | 830.5 | 0.30 | 359.0 | 0.28 | 453.6 | 0.31 |
| GMR (90% CI) | 0.432 | (0.346–0.519) | 0.546 | (0.434–0.658) | ||
| A e (mg) | 3.4 | 0.30 | 1.7 | 0.26 | 2.0 | 0.38 |
| GMR (90% CI) | 0.491 | (0.417–0.565) | 0.601 | (0.481–0.722) | ||
| CL R (mL min−1) | 68.1 | 0.32 | 77.4 | 0.23 | 75.0 | 0.19 |
| GMR (90% CI) | 1.136 | (0.833–1.440) | 1.011 | (0.865–1.337) | ||
Nadolol (30 mg) was orally administered with 150 mL of concomitant brewed green tea, or 1 hour after 150 mL of brewed green tea, or with 150 mL of concomitant water to healthy volunteers (n = 11). The t max values are expressed as median (range). CV, coefficient of variance; C max, peak plasma concentration; GMR, geometric mean ratio; CI, confidence interval; t max, time to C max; AUC, area under the plasma concentration–time curve; A e, amount excreted unchanged into urine over 48 hours; CL R, renal clearance.
Interestingly, 1‐time ingestion of brewed green tea 1 hour before nadolol administration also resulted in significant reductions in C max, AUC0–8, AUC0–48 and A e of nadolol by 54% (P < .01), 47% (P < .01), 45% (P < .001) and 41% (P < .01), respectively, without changing CL R of nadolol (Table 1). The individual AUC0–48 value in water phase was correlated with the decrease in AUC0–48 (R 2 = .793, P < .001) due to green tea ingested 1 hour before nadolol. EGCG reaches its maximal plasma concentration around 1–2 hours after drinking green tea or an aqueous solution of GTE with a t 1/2 of about 1.6 hours. 8 , 9 , 10 In contrast, nadolol is taken up for several hours with t max being approximately 3 hours, thus posing the possibility that ingested green tea constituents could indirectly affect the intestinal absorption of nadolol. We have previously discussed that EGCG is unlikely to chemically bind to nadolol in aqueous solution. 9 These data suggest that, although the underlying mechanism is not fully elucidated, the functional modulation of green tea on intestinal absorption of nadolol, presumably mediated by OATP1A2, may be prolonged over 1 hour after ingestion.
As for the pharmacodynamic response to nadolol, changes in PR, SBP and DBP after nadolol administration with water or brewed green tea are shown as percent decrease from baseline in Figure S2. The mean baseline values with standard deviation of PR, SBP and DBP were 69 ± 8 beats/min, 120 ± 18 mmHg and 75 ± 12 mmHg, respectively, with no differences between phases. In water phase, nadolol lowered PR, SBP and DBP with the mean maximum decreases of 26, 15 and 20%, respectively. Although we did not find significant differences in the AUEC0–8 for PR, SBP and DBP between groups partly due to the small sample size, those pharmacodynamic responses to nadolol tended to decline with concomitant ingestion of brewed green tea. It has been reported that nadolol exerts dose‐related decreases of heart rate and SBP as indexed by double product, which is calculated by heart rate × SBP, 16 and therefore it is assumed that the decrease in plasma concentration of nadolol could result in the reduction of its β‐blocking effect. However, it should be noted that we only recorded the acute response to nadolol after single oral administration. Thus, we consider that it is difficult to evaluate from our study whether acute pharmacokinetic changes of nadolol by green tea may translate into long‐term antihypertensive effects. Another study limitation is that study participants of this study were all Japanese adults, and thus we cannot rule out the possibility of interethnic differences in the pharmacokinetic interaction between nadolol and green tea.
In conclusion, a single concomitant ingestion of brewed green tea markedly inhibited the intestinal absorption of nadolol and led to a substantial decrease in its systemic exposure. Moreover, drinking green tea 1 hour before nadolol administration had also a pronounced effect on disposition of nadolol. Although these results cannot necessarily be generalized to all other green tea formulations, it is recommended not to take green tea or catechin, particularly EGCG, supplement during nadolol treatment to reduce therapeutic failure.
COMPETING INTERESTS
The authors declare no competing interests.
CONTRIBUTORS
S.M., O.A., I.M., Y.S., M.F.F., H.Y. and K.S. designed the research; S. M, O.A., T.O., Y.O., H.O. and Y.S. performed the research; S.M., O.A., M.F.F. and K.S. analysed the data; S.M., I.M., Y.S., M.F.F., H.Y. and K.S. wrote the manuscript; all authors gave the final approval.
Supporting information
TABLE S1 Concentrations of catechins and caffeine in brewed green tea
FIGURE S1 Relationship between the extent of green tea vs nadolol interaction as indexed by % decrease in area under the plasma concentration–time curve (AUC) of nadolol and estimated oral dose of epigallocatechin gallate (EGCG). Data were obtained from 2 previous studies (Misaka et al., 2014; triangle) and (Abe et al., 2018; square), and the present study (circle). Results from the interaction with green tea or EGCG are expressed as closed or open symbols, respectively. The data were fitted to the hyperbolic equation % decrease in nadolol AUC = E max × D/(ID 50 + D), where E max represents the maximum extent of the interaction (assumed to be 100%), D is estimated oral dose of EGCG, and ID 50 is the dose needed to achieve a half‐maximum interaction.
FIGURE S2 Pharmacodynamic responses over 48 hours after nadolol administration, and area under the time–effect curve (AUEC0–8) of nadolol after oral administration with 150 mL of concomitant brewed green tea (open circle), or 1 hour after 150 mL of brewed green tea (open triangle), or with 150 mL of concomitant water (closed circle) in healthy volunteers (n = 11). Percent changes in pulse rate (a), systolic blood pressure (b), and diastolic blood pressure (c) from baseline are represented as the arithmetic means ± standard deviation.
DATA S1 Supporting information
ACKNOWLEDGEMENTS
The authors thank to Tsubasa Shinomiya and Kou Fujimaki for their excellent technical assistance. This study was partly supported by the Honjo International Scholarship Foundation (Food and Health Program 2015), Tokyo, Japan, and JSPS KAKENHI Grant Number JP17K17983.
APPENDIX A.
Determinations of drug concentration
Nadolol plasma and urinary concentrations were determined using ultra‐performance liquid chromatography (UPLC) with fluorometric detection (Waters, Milford, MA) according to our previous studies.1–3 Briefly, nadolol and the internal standard (IS, metoprolol) were extracted from plasma and urine by solid phase extraction using EVOLUTE CX (Biotage, Uppsala, Sweden) Chromatographic separation was archived using AQUITY BEH, C18, column (particle size 1.7 μm; column size 2.1 × 50 mm; Waters) at 40°C with mobile phase consisting of 20mM ammonium acetate (pH 9.0; A) and acetonitrile (B) with a flow rate of 0.50 mL min−1. The linear gradient condition of mobile phases was as follows: 0–5.0 minutes, 10–40% B; 5.0–7.0 minutes, 10% B. Nadolol and IS were detected with the excitation wavelength at 230 nm and emission at 300 nm. The limit of quantification of nadolol was 0.5 ng mL−1. The mean interday coefficients of variance for nadolol was 9.3%.
The concentrations of catechins in the green tea used in the clinical study were also determined by UPLC with UV detection (Waters) according to a previously described method.1,4 In brief, samples were injected into the UPLC system after 2‐fold dilution by 10mM ascorbic acid solution with 2mM catechol (IS), followed by filtrating through a 0.22‐μm filter membrane. The mobile phase consisted of 0.2% phosphoric acid (A) and acetonitrile (B), and chromatographic separation was performed on an AQCUITY Shield RP18 column (50 mm × 2.1 mm i.d., 1.7 μm; Waters). The gradient condition was set as follows: a linear increase from 2% B to 25% B over 6.5 minutes, followed by 3.5 minutes equilibration at 2% B. An UV detector was operated at a wavelength of 280 nm. The limits of quantification of caffeine and all catechins determined were 0.05 μM.
Misaka S, Abe O, Ono T, et al. Effects of single green tea ingestion on pharmacokinetics of nadolol in healthy volunteers. Br J Clin Pharmacol. 2020;86:2314–2318. 10.1111/bcp.14315
The authors confirm that the PI for this paper is Prof. Yayoi Shikama, MD, PhD and that she had direct clinical responsibility for participants.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin‐3‐gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat Res. 2001;479:197‐206. [DOI] [PubMed] [Google Scholar]
- 3. Misaka S, Kawabe K, Onoue S, et al. Effects of green tea catechins on cytochrome P450 2B6, 2C8, 2C19, 2D6 and 3A activities in human liver and intestinal microsomes. Drug Metab Pharmacokinet. 2013;28(3):244‐249. [DOI] [PubMed] [Google Scholar]
- 4. Jodoin J, Demeule M, Beliveau R. Inhibition of the multidrug resistance P‐glycoprotein activity by green tea polyphenols. Biochim Biophys Acta. 2002;1542:149‐159. [DOI] [PubMed] [Google Scholar]
- 5. Knop J, Misaka S, Singer K, et al. Inhibitory effects of green tea and (−)‐epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2‐K and P‐glycoprotein. PLoS ONE. 2015;10(10):1‐13. e0139370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion‐transporting polypeptides. Drug Metab Dispos. 2011;39:920‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y. Effects of herbal extracts on the function of human organic anion‐transporting polypeptide OATP‐B. Drug Metab Dispos. 2006;34(4):577‐582. [DOI] [PubMed] [Google Scholar]
- 8. Misaka S, Yatabe J, Müller F, et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin Pharmacol Ther. 2014;95(4):432‐438. [DOI] [PubMed] [Google Scholar]
- 9. Abe O, Ono T, Sato H, et al. Role of (−)‐epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur J Clin Pharmacol. 2018;74(6):775‐783. [DOI] [PubMed] [Google Scholar]
- 10. Masukawa Y, Matsui Y, Shimizu N, et al. Determination of green tea catechins in human plasma using liquid chromatography‐electrospray ionization mass spectrometry. J Chromatogr B. 2006;834(1‐2):26‐34. [DOI] [PubMed] [Google Scholar]
- 11. Mizukami Y, Sawai Y, Yamaguchi Y. Simultaneous analysis of catechins, gallic acid, strictinin, and purine alkaloids in green tea by using catechol as an internal standard. J Agric Food Chem. 2007;55:4957‐4964. [DOI] [PubMed] [Google Scholar]
- 12. Misaka S, Abe O, Sato H, et al. Lack of pharmacokinetic interaction between fluvastatin and green tea in healthy volunteers. Eur J Clin Pharmacol. 2018;74(5):601‐609. [DOI] [PubMed] [Google Scholar]
- 13. Misaka S, Miyazaki N, Yatabe MS, et al. Pharmacokinetic and pharmacodynamic interaction of nadolol with itraconazole, rifampicin and grapefruit juice in healthy volunteers. J Clin Pharmacol. 2013;53(7):738‐745. [DOI] [PubMed] [Google Scholar]
- 14. Couto N, Al‐Majdoub ZM, Gibson S, et al. Quantitative proteomics of clinically relevant drug‐metabolizing enzymes and drug transporters and their Intercorrelations in the human small intestine. Drug Metab Dispos. 2020;48(4):245‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362‐370. [DOI] [PubMed] [Google Scholar]
- 16. Duchin KL, Vukovich RA, Dennick LG, Groel JT, Willard DA. Effects of nadolol beta‐blockade on blood pressure in hypertension. Clin Pharmacol Ther. 1980;27(1):57‐63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Concentrations of catechins and caffeine in brewed green tea
FIGURE S1 Relationship between the extent of green tea vs nadolol interaction as indexed by % decrease in area under the plasma concentration–time curve (AUC) of nadolol and estimated oral dose of epigallocatechin gallate (EGCG). Data were obtained from 2 previous studies (Misaka et al., 2014; triangle) and (Abe et al., 2018; square), and the present study (circle). Results from the interaction with green tea or EGCG are expressed as closed or open symbols, respectively. The data were fitted to the hyperbolic equation % decrease in nadolol AUC = E max × D/(ID 50 + D), where E max represents the maximum extent of the interaction (assumed to be 100%), D is estimated oral dose of EGCG, and ID 50 is the dose needed to achieve a half‐maximum interaction.
FIGURE S2 Pharmacodynamic responses over 48 hours after nadolol administration, and area under the time–effect curve (AUEC0–8) of nadolol after oral administration with 150 mL of concomitant brewed green tea (open circle), or 1 hour after 150 mL of brewed green tea (open triangle), or with 150 mL of concomitant water (closed circle) in healthy volunteers (n = 11). Percent changes in pulse rate (a), systolic blood pressure (b), and diastolic blood pressure (c) from baseline are represented as the arithmetic means ± standard deviation.
DATA S1 Supporting information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


