Abstract
Chronic aortic regurgitation (AR) patients typically remain asymptomatic for a long time. Left ventricular mechanics, namely global longitudinal strain (GLS), has been associated with outcomes in AR patients. The authors conducted a systematic review to summarize and appraise GLS impact on mortality, the need for aortic valve replacement (AVR) and disease progression in AR patients. A literature search was performed using these key terms ‘aortic regurgitation’ and ‘longitudinal strain’ looking at all randomized and nonrandomized studies conducted on chronic aortic regurgitation. The search yielded six observational studies published from 2011 and 2018 with a total of 1571 patients with moderate to severe chronic AR. Only two studies included all-cause mortality as their endpoint. The other studies looked at the association between GLS with AVR and disease progression. The mean follow-up period was 4.2 years. We noted a great variability of clinical, methodological and/or statistical origin. Thus, meta-analytic portion of our study was limited. Despite a relevant heterogeneity, an impaired GLS was associated with adverse cardiac outcomes. Left ventricular GLS may offer incremental value in risk stratification and decision-making.
Key Words: aortic regurgitation, global longitudinal strain, outcomes, aortic valve replacement
Background
Chronic aortic regurgitation (AR) patients typically remain asymptomatic for a long time. In asymptomatic chronic severe AR, current European Society of Cardiology guidelines recommend as a Class I indication (Level of Evidence: B) to perform aortic valve replacement (AVR) when the left ventricular ejection fraction (LVEF) is <50%. AVR is a Class IIa indication (Level of Evidence: B) when severe left ventricular dilatation (left ventricle (LV) diastolic diameter >70 mm or LV systolic diameter >50 mm) develops in patients with a LVEF >50% (1). AVR is, therefore, the cornerstone to halt LV dysfunction. Patients with AR and reduced LVEF have higher mortality and heart failure risk (2). Changes in systolic function identify patients likely to develop symptoms and require AVR (3). Even 6–7 years after AVR, there is residually increased interstitial fibrosis that is associated with mortality (4, 5).
Global longitudinal strain (GLS) assessed with 2D speckle-tracking echocardiography (2D-STE), can be used to identify subclinical LV dysfunction in these patients (6, 7). Although there is no randomized study yet to indicate that surgery is better than conservative therapy in patients with chronic asymptomatic AR (8), it has been shown that even above-average values of GLS confer a poor prognosis (8, 9, 10).
This state-of-art systematic review assesses and critically summarizes studies to date on the impact of GLS on outcomes in patients with chronic moderate to severe AR.
Methods
Data sources and searches
Literature search was performed by one of the authors using the databases of MEDLINE, EMBASE, and the Cochrane Library, using the key terms ‘aortic regurgitation’ and ‘longitudinal strain’ from inception to November 2019. No language restrictions were selected. The bibliographies of all eligible studies were also screened for relevant reports. The selection of the papers to be included followed a three-step methodology: (1) reading of the title, (2) reading of the abstract, and (3) reading of the full text. In stages (1) and (2), efforts were made to aim to be more inclusive than exclusive. The full text of each pre-selected study was examined to verify the completeness of all inclusion criteria. Two of the authors assessed the eligibility of studies and disagreements were solved between the two authors. One author extracted the relevant data from eligible studies, which was then checked by another author.
Inclusion criteria
Studies were selected if they met the following criteria: (1) adult population (>18 years old), (2) asymptomatic or mild symptoms, (3) on conservative management, (4) patients with at least moderate chronic AR, (5) analyzed GLS with 2D-STE, and (6) evaluated symptom development, change in LV function, need for AVR, and/or all-cause mortality. The following exclusion criteria were used: case reports, case series and conference abstracts.
Outcomes
The main outcome of interest was all-cause mortality. Also, need for AVR and disease progression (symptoms and/or change in LV function) were analyzed.
Risk of bias assessment
A modified version of the Newcastle-Ottawa Quality Assessment Scale of cohort studies was used to assess the quality of included papers. Briefly, the scale appraises methodological quality in three domains: selection, comparability, and outcome. Studies score points for each subset domain and they are classified as high risk (1–3 points), intermediate risk (4–5 points), or low risk (6–9 points) of bias. Papers were included irrespective of the quality assessment score.
Data collection and statistical analysis
Review authors were not blinded to author, institution, journal, or results of a study for its assessment. For each paper, the following data were extracted: first author, publication year, country of origin, vendor used for echocardiography, reliability data, population studied, length of follow-up, LVEF and GLS. Hazard ratio (HR) and/or odds ratio (OR) were extracted directly from the studies along with 95% confidence intervals or P values. If these were not reported, other data, such as mean and standard deviation, for GLS in each group were recorded. Categorical data are expressed as a percentage and continuous variables as means ± standard deviation or medians with interquartile range. Wherever reported, we collected the results of the receiver operator curve (ROC) analysis as area under the curve (AUC), sensitivity and specificity and the estimated cutoff. Also, C-statistic and net reclassification indexes are reported where available. The data were then computerized in a dedicated database. A qualitative analysis of published data was performed, which is absolutely crucial when looking at observational studies. To avoid variability in reporting and interpretation, we used GLS percentages as negative values, regardless of whether absolute or negative integer were reported in the original study. In our report, a more negative value GLS is referred to a as better and a value closer to zero (less negative) is referred to worse.
Results
Search results
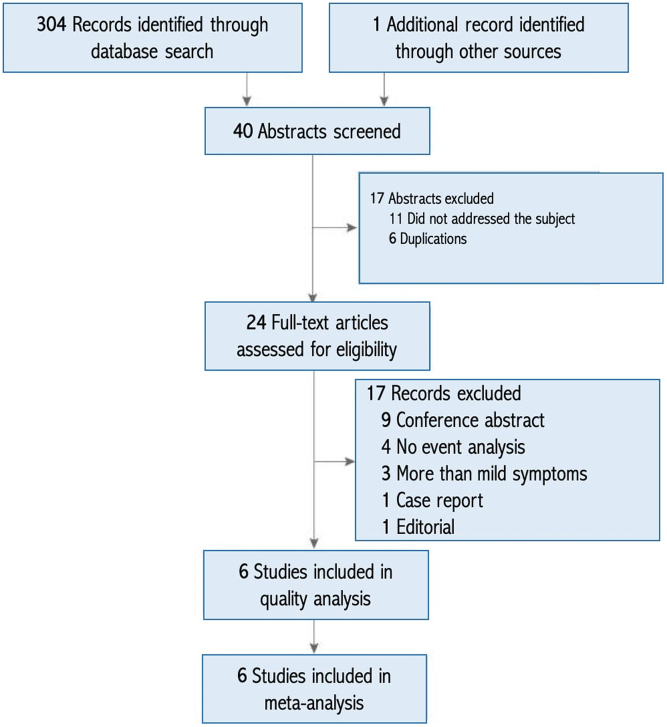
A total of 305 eligible titles were identified and screened (Fig. 1). Abstracts of 40 papers were judged for relevance. Upon reading the full texts, six papers were retained for analysis. All of the studies were observational and published between 2011 and 2018 (9, 10, 11, 12, 13, 14). In this report, we first describe studies’ characteristics, and then we elaborate on the outcomes.
Figure 1.
Flow chart of studies selection.
Description of included literature
Baseline characteristics of the studies are listed in Table 1. Half of them were prospective (10, 12, 13) and the others were retrospective (9, 11, 14). The search yielded no randomized controlled trial. Most studies were small, although one recruited 1063 patients (9). Worth to underline that four studies (11, 12, 13, 14) had sample sizes with less than 100 patients. Two papers were based on samples from the United States (9, 10), and one paper came from each of the following countries: the Netherlands (11), Denmark (12), Lithuania (13), and Korea (14).
Table 1.
Description of the studies included in the systematic review.
| Author, Year | Inclusion criteria | Exclusion criteria | Hardware | Follow-up period | Primary outcome | Secondary outcome |
|---|---|---|---|---|---|---|
| Software | ||||||
| Procedure | ||||||
| Alashi, 2018 (9) | ≥III chronic AR, LVEF ≥50% and iLVESD <2.5 cm/m2 | Other ≥ moderate valvular disease, hypertrophic cardiomyopathy, congenital disease, previous cardiac surgery, symptoms, CAD, technical issues | Philips Medical Systems; Siemens Medical Solutions; General Electric Velocity Vector Imaging (Syngo VVI, Siemens) 2D-STE |
Mean of 6.8 ± 3.0 years | All-cause mortality | Need of AVR |
| Ewe, 2015 (11) | NR | Acute AR, LVEF ≤50%, ≥ mild valvular disease, CAD, previous cardiac surgery, technical issues | General Electric (Vivid-7 and E9) EchoPAC version 110.0.0, GE-Vingmed 2D-STE |
Mean of 4.2 ± 3.2 years | Need of AVR | |
| Kusunose, 2014 (10) | Moderate-to-severe AR, LVEF >50%, LVEDD ≤70 mm, LVESD ≤50 mm, iLVESD ≤25 mm/m2 | NR | General Electric (Vivid 7 or Vivid 9); Philips (Sonos 5500 or iE33) Velocity Vector Imaging (Syngo VVI, Siemens) 2D-STE |
Mean of 2.5 ± 1.8 years | Need of AVR | |
| Olsen, 2011 (12) | Moderate-to-severe chronic AR | Previous cardiac surgery, other valvular disease, CAD, compromised LV function of known other reason than AR, atrial fibrillation. | General Electric (Vivid 7 and Vivid 7 Dimension) EchoPAC PC version 6.1.1 (GE-Vingmed) 2D-STE |
Mean of 1.6 ± 0.6 years | Development of symptoms or deterioration of LV size or function | |
| Park, 2015 (14) | Moderate-to-severe chronic AR | Other valvular heart disease, congenital heart disease, previous cardiac surgery | General Electric (Vivid 7) EchoPAC PC (GE-Vingmed) 2D-STE |
5.3 years | All-cause mortality | Need of AVR |
| Verseckaite, 2018 (13) | Moderate-to-severe chronic AR | Ovalve disease, symptoms or a history of CAD, LV wall motion abnormalities, atrial fibrillation, and left bundle branch block | General Electric (Vivid 7) EchoPAC PC version 112 (GE-Vingmed) 2D-STE |
Mean of 4.7 ± 2.6 years | Deterioration of the LVEF (≤50%) |
NR, not reported.
The search identified 1571 subjects from six studies. The definition of chronic AR varied among the authors. Four studies included only patients with chronic AR who were asymptomatic and with preserved LVEF (≥50%) (9, 10, 11, 13). One paper (12) included patients with at least moderate AR, but 6% had a NYHA≥II and mean LVEF was 58.2 ± 5.1%. In another sample (14), some patients had mild left ventricle dysfunction. Length of follow-up varied among studies from 1.6 ± 0.6 to 6.8 ± 3.0 years. Patients were middle-aged (48 ± 16 to 57 ± 13 years) and ≥ 50% were male. The prevalence of a bicuspid aortic valve varied from 36 to 60% (9, 10, 11, 13). LVEF was assessed by biplano Simpson’s method in all studies (9, 10, 11, 12, 13, 14). The mean LVEF ranged from 47.9 ± 12.1 to 61 ± 5%. Left ventricular end-systolic diameter varied from 34 ± 7 to 39 ± 5 mm (9, 10, 11, 13). The mean GLS ranged from 14.7 ± 4.5 to 19.5 ± 0.2%. Only one study (9) reported the Society of Thoracic Surgeons score, with a mean of 4.4 ± 5.0%. Studies mostly reported outcomes during conservative management, such as the time-to-AVR/disease progression. Reports on mortality analyzed the entire sample, including those who previously underwent AVR (9, 14). The ultrasound equipment varied between papers (Table 2), but most used equipment was from one vendor. LV peak systolic GLS measurements were obtained from images recorded in the apical four-chamber, two-chamber, and three-chamber views (9, 10, 11, 12, 13). In one study, only apical four-chamber views were used due to poor image quality (14). Two studies provided information on inter observer and/or intraobserver reproducibility, with intraclass correlation coefficients above 0.80 (9, 10). Quality appraisal of the included studies is reported in Table 3.
Table 2.
Results of studies number of events, mean LVEF and GLS and cardiac outcomes.
| Author, year | Sample discrimination | Patients (no.) | Age (mean ± s.d.) | LVEF (mean ± s.d.) | GLS (mean ± s.d.) | Hazard ratio/odds ratio | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | No Event | Event | No Event | Event | No Event | Event | No Event | ||||
| Alashi, 2017 (9) | All samplea | 146 | 917 | NR | NR | NR | NR | NR | NR | HR = 1.08 (1.03–1.18) | Death |
| Alashi, 2017 (9) | On conservative | 671 | 392 | 53 ± 16 | 54 ± 14 | 57 ± 4 | 57 ± 4 | 19.5 ± 2.0 | 19.5 ± 2.0 | NR | AVR |
| Ewe, 2015 (11) | On conservative | 26 | 23 | 55 ± 16 | 42 ± 15 | 61 ± 5 | 62 ± 5 | 15.7 ± 2.0 | 17.6 ± 2.7 | HR = 1.20 (1.01–1.44) | AVR |
| Olsen, 2011 (12) | On conservative | 8 | 27 | NR | NR | 57.6 ± 3.6 | 58.7 ± 5.4 | 16.3 ± 3.3 | 19.0 ± 2.6 | NR | AVR or symptoms |
| Olsen, 2011 (12) | AVR vs conservative | 29 | 35 | 57 ± 13 | 56 ± 14 | 50.3 ± 10.9 | 58.2 ± 5.1 | 14.0 ± 4.2 | 18.3 ± 2.9 | NR | AVR |
| Park, 2015 (14) | All sample* | 16 | 44 | 68.8 ± 12.3 | 50.7 ± 16.9 | 42.9 ± 13.4 | 49.8 ± 11.2 | 12.1 ± 3.7 | 15.7 ± 4.3 | HR = 1.3 (1.01–1.71) | Death |
| Park, 2015 (14) | AVR vs conservative | 38 | 22 | 51.8 ± 14.7 | 61.9 ± 20.8 | 49.2 ± 11.71 | 45.7 ± 12.7 | 14.6 ± 4.7 | 14.8 ± 4.1 | NR | AVR |
| Verseckaite, 2018 (13) | On conservative | 12 | 28 | 54 ± 13 | 42 ± 15 | 55 ± 3 | 59 ± 4 | 16.9 ± 2.5 | 20.1 ± 1.6 | OR = 2.58 (1.02–6.57) | Symptoms |
| Kusunose, 2014 (10) | On conservative | 50 | 109 | NR | NR | NR | NR | NR | NR | HR = 1.64 (1.19–2.26) | AVR |
aIncluding those submitted to AVR.
NR, not reported.
Table 3.
Quality assessment of the included papers based on Newcastle–Ottawa Quality Assessment Scale – Cohort Studies.
| Alashi, 2017 (9) | Ewe, 2015 (11) | Kusunose, 2014 (10) | Olsen, 2011 (12) | Park, 2015 (14) | Verseckaite, 2018 (13) | |
|---|---|---|---|---|---|---|
| Selection category | ||||||
| Representativeness of the cohort | * | * | * | |||
| Assessment of exposure reliability | * | * | ||||
| Completeness of collection of potential confounders | * | * | * | * | * | * |
| Demonstration that the outcome of interest not present at start of study (and/or sensitively analysis) | * | * | * | * | ||
| Outcome category | ||||||
| Assessment of outcome | * | * | * | * | * | * |
| Was follow-up long enough for outcomes to occur | * | * | * | * | * | |
| Adequacy of follow up of cohorts | * | * | * | * | * | * |
| Total | 4 | 6 | 7 | 4 | 4 | 6 |
Outcomes
Outcomes of the included papers are shown in Table 2. Only two studies reported all-cause mortality (9, 14). Although mortality was an endpoint for one other study, during follow-up period no death occurred (10). The others analyzed either the need for AVR or disease progression (symptoms and/or change in LV function). A total of 2593 patient-entries and a total of 985 events (death, AVR and/or disease progression) were analyzed. The meta-analytic portion of our study was limited by significant heterogeneity observed across studies that could not be explained by subgroup analyses or metaregressions. While attempting to meta-analyze the data, the I2 statistic, varied between 0.51 and 0.65. Also, the number of studies examining the primary endpoint was insufficient. Even though we tried to conduct the meta-analysis with extreme rigor, the results of highly heterogeneous studies may be less interpretable and useful.
GLS and all-cause mortality
One study (9) recruited the majority of the patients (1063 patients against 60 patients (14)). In Alashi et al. study (9) more than half of the cohort underwent AVR at a median of 42 days (3–122 days) from the baseline echocardiogram. Park et al. (14) reported that 13.2% of patients submitted to AVR did not have a proper surgical indication, while 81.8% were under conservative management despite having criteria for surgery. The most common causes of deferred surgery were patient’s refusal and withholding of physicians due to old age or poor condition.
Of the 1123 patients included, 151 were dead (13.4%). The incidence of all-cause mortality varied from 14% to 27% during a mean maximum follow-up of 6.8 ± 3.0 years (9, 14). The mean follow-up duration was longer in AVR group than the conservative treatment group (71 ± 30 months vs 40 ± 29 months, P < 0.001 (14)). Adjusted HR for mortality risk varied between 1.11 and 1.3 (9, 14). Addition of GLS to a clinical model provided incremental prognostic value. An increased C-statistic from 0.61 to 0.77 and an improved reclassification (continuous net reclassification improvement 0.24–0.23) were reported (9). Kaplan–Meier survival analysis showed a higher survival in patients with a better GLS. The estimated cutpoints for GLS varied from −12.5 to −19.5%. These different thresholds were chosen based on either ROC curve analysis (14) or the median value (9), respectively. The −12.5% cutoff was associated with an AUC of 0.74, a sensitivity of 69% and a specificity of 79% (14). Using quadratic spline, patients with a GLS better than approximately -19% had an excellent 5-year survival. Worth to underline is that the risk of death continuously increased when GLS worsened to <−19% and that patients with no surgery and a worse GLS worse had a higher long-term mortality. AVR seemed to blunt the impact of a worsening GLS on the risk of 5-year death (9). In contrast, in one study (14), GLS was more predictive of all-cause death in the AVR than in the conservative group (AUC 0.89 vs 0.68). Thus, despite a significant variation of cutoff values as well as on statistical analysis, there was a consistent finding of a worse strain being associated with increased all-cause mortality.
Need for AVR and disease progression
All the six studies examined the association between GLS with AVR and/or disease progression (9, 10, 11, 12, 13, 14). Most of the studies reported on the need of AVR (9, 10, 11, 14) and two on disease progression, either symptom development (12) or left ventricular dysfunction (LVEF < 50% (13)). A total of 1,606 patient-entries and 834 events were analyzed. The other half of the patients (48.1%) remained free of symptoms. The reported incidence of this endpoint, however, varied greatly from 9 to 63% (9, 10, 11, 12, 13, 14). Noteworthy to underscore that some patients underwent AVR for indications other than symptoms or LV dysfunction (9), were on conservative management despite an appropriate surgical indication or received AVR without a proper indication (14).
Globally, a worse GLS was evident in patients who later required AVR and/or developed symptoms. Adjusted HR varied from 1.20 to 2.58 (95% CI 1.01–6.57). Mean GLS for patients that needed AVR and/or disease progression varied between −16 ± 3.3 and −19.5 ± 2%, in contrast to those who remained free of AVR and symptoms (−17.6 ± 2.7 to −20.9 ± 2.2%). Only three studies examined the threshold value for GLS to predict AVR and/or disease progression. These different cutoffs were determined by ROC curve analysis. The optimal cutpoint varied between −17.4 and −19.3%. AUC’s varied between 0.70 and 0.89 with sensitivity ranging from 77 to 88% and specificity from 57 to 84% (11, 12, 13). A GLS cutpoint of −19.3% had the highest sensitivity to predict AVR with a negative predictive value of 100%. A GLS worse than −15.1% had the highest positive predicted value (75%). Under these assumptions, patients with a better GLS than −19.3% would be free from AVR and/or disease progression and three out four patients with a worse GLS than −15.1% will require AVR (11).
Discussion
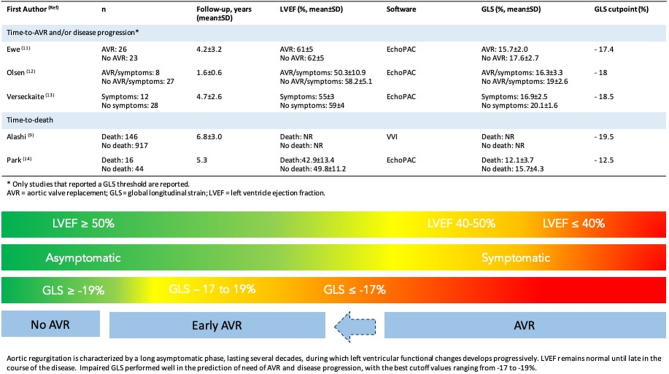
In our systematic review on chronic aortic regurgitation, we note significant heterogeneity. First, to mention is the variability in the clinical profile of the included patients. Second, most of the studies had a small sample size with the exception of one large study. Indeed, when the sample size is small, the confidence intervals are wide due to imprecision, which will often cause the pooled estimate to cross the null hypothesis. Third, different methodologies and the use of different vendors to analyze GLS. Last, the variable follow-up duration and variable definition of endpoints were present. This degree of heterogeneity poses interpretive challenges and make it impossible to conduct a meta-analysis. We present our results in the format of a systematic review. Despite all the shortcomings, we noted that in the majority of the studies, a worse GLS was associated with increased cardiac events. Chronic AR is an insidious disease with a clinically silent phase of variable duration followed by a relatively rapid decline. Echocardiographic deformation imaging may help to halt adverse cardiac outcomes in those with preserved LVEF and no symptoms. Based on our review, it might be reasonable to proceed to early AVR when GLS is worse than −19% (Fig. 2, central illustration).
Figure 2.
Central illustration: left ventricular GLS to predict outcomes in chronic aortic regurgitation.
Myocardial strain and LV systolic function
Strain has been used to gain a greater understanding of the pathophysiology of cardiac conditions (15). Currently, GLS is accepted as the parameter of myocardial deformation that is more reproducible and less susceptible to technical factors (16), and, therefore, the most accurate and sensitive parameter of early LV dysfunction (17). It has been proposed as a prognostic marker in patients with preserved ejection fraction (EF) (18) as it has emerged as a more sensitive index of LV systolic performance than EF itself (19). Nevertheless, both EF and strain are loading-dependent parameters (20). Reduced deformation despite preserved EF can be explained through geometric factors, and these confounders hamper the use of EF as an index of LV systolic function (21). GLS relation to myocardial fibrosis has been studied and it is documented that 70% of segments with late gadolinium enhancement have a GLS reduction (22). Also, wall stress is associated with myocardial strain (23).
Myocardial strain and valvular heart disease
Strain is being used to assess the effects of valvular disease on myocardial function (15). LVEF sensitivity for the detection of myocardial dysfunction is lower than previously stated and EF changes occur late, when cardiac damage is often irreversible. GLS was tested for the assessment of all valvular diseases and it has been associated with disease progression, the occurrence of heart failure, and impaired outcomes after surgery (17). In aortic stenosis patients with preserved EF, an impaired GLS was a predictor of reduced survival (24). In severe mitral regurgitation, GLS appeared to be a better predictor of cardiac events and mortality, regardless of LV dysfunction (25). In asymptomatic primary mitral regurgitation, the risk of death progressively increased as GLS worsened to ≥−21% (26). In moderate to severe aortic stenosis, a GLS ≥-12.1% was associated with poor survival (10). According to our review, GLS threshold for AR varied from −18 to −19% (9, 10, 11, 12, 13), although one study reported a value of −12.5% (14). This valvular heart disease GLS variability for asymptomatic patients could probably be explained by preload and afterload differences, and also mirrors LVEF variability. After 4.9 years of follow-up of 748 AR patients managed either medically or surgically, AVR was associated with better survival: baseline symptoms were the hallmark of mortality, even after AVR (27). These results suggest that it seems reasonable to make an early referral of asymptomatic AR patients without waiting for symptoms to develop or LV enlargement and/or dysfunction. Popovic et al. (8) argue the need for multimodal imaging follow-up of these patients in order to develop a risk profile and determine the best timing for AVR referral. Our review suggests that GLS enables early detection of subtle LV dysfunction in patients with chronic moderate to severe AR, making it a ‘rule in’ tool to trigger additional studies or to guide the timing of surgery.
Study limitations
Our primary endpoint was all-cause mortality because it is an objective assessment. However, most of the studies did not report mortality but, instead, the need for AVR and disease progression. Information on potential confounders was limited and inconsistent, and the small number of studies made impossible the use of metaregression to explore sources of heterogeneity between studies. Some authors argue that it is through the heterogeneity of studies in meta-analyses that the performance and variability of a test can be appreciated in different patient groups and sources of variation can be identified (28). Despite being reported that the variations in 2D-STE of GLS may be more subtle than are often portrayed (29), different vendor-specific hardware and software may introduce systematic differences among studies. Thus, we assumed that the reduced number of studies, the degree of clinical, methodological and statistical heterogeneity precluded the meta-analytic process. Our study is, therefore, a more comprehensive and analytical review of literature on the prognostic value of GLS in chronic AR. Notwithstanding, our systematic report made it possible to bring together relevant data on this topic and will help outline future studies.
Conclusion
The evidence indicates that GLS is useful in AR, with values worse than −17 to −19% being associated with poor cardiac outcomes. Larger prospective studies are needed to further define the role of GLS and better identify associated thresholds. A randomized controlled trial to test whether the identification of an imaging biomarker vs watchful waiting, in asymptomatic patients, would trigger earlier aortic valve replacement and translate in better outcomes is warranted.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
All authors have contributed to the manuscript. Rogério Teixeira and Diana de Campos have designed, elaborated the review and have written the paper. Carolina Saleiro, Ana Botelho and Lino Gonçalves provided critical reviews of the data and of the manuscript.
References
- 1.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz DR, et al 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal 2017. 38 2739–2791. ( 10.1093/eurheartj/ehx391) [DOI] [PubMed] [Google Scholar]
- 2.Chaliki HP, Mohty D, Avierinos JF, Scott CG, Schaff HV, Tajik AJ, Enriquez-Sarano M. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002. 106 2687–26. ( 10.1161/01.cir.0000038498.59829.38) [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation 1991. 84 1625–16. ( 10.1161/01.cir.84.4.1625) [DOI] [PubMed] [Google Scholar]
- 4.Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 1989. 79 744–7. ( 10.1161/01.cir.79.4.744) [DOI] [PubMed] [Google Scholar]
- 5.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. Journal of the American College of Cardiology 2010. 56 278–2. ( 10.1016/j.jacc.2009.12.074) [DOI] [PubMed] [Google Scholar]
- 6.Cameli M, Mondillo S, Righini FM, Lisi M, Dokollari A, Lindqvist P, Maccherini M, Henein M. Left ventricular deformation and myocardial fibrosis in patients with advanced heart failure requiring transplantation. Journal of Cardiac Failure 2016. 22 901–90. ( 10.1016/j.cardfail.2016.02.012) [DOI] [PubMed] [Google Scholar]
- 7.Katbeh A, Ondrus T, Barbato E, Galderisi M, Trimarco B, Van Camp G, Vanderheyden M, Penicka M. Imaging of myocardial fibrosis and its functional correlates in aortic stenosis: a review and clinical potential. Cardiology 2018. 141 141–149. ( 10.1159/000493164) [DOI] [PubMed] [Google Scholar]
- 8.Popović ZB, Desai MY, Griffin BP. Decision making with imaging in asymptomatic aortic regurgitation. JACC: Cardiovascular Imaging 2018. 11 1499–1. ( 10.1016/j.jcmg.2018.05.027) [DOI] [PubMed] [Google Scholar]
- 9.Alashi A, Mentias A, Abdallah A, Feng K, Gillinov AM, Rodriguez LL, Johnston DR, Svensson LG, Popovic ZB, Griffin BP, et al. Incremental prognostic utility of left ventricular global longitudinal strain in asymptomatic patients with significant chronic aortic regurgitation and preserved left ventricular ejection fraction. JACC: Cardiovascular Imaging 2018. 11 673–6. ( 10.1016/j.jcmg.2017.02.016) [DOI] [PubMed] [Google Scholar]
- 10.Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popović ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines incremental values of resting and exercise cardiac dysfunction. Circulation: Cardiovascular Imaging 2014. 7 352–3. ( 10.1161/CIRCIMAGING.113.001177) [DOI] [PubMed] [Google Scholar]
- 11.Ewe SH, Haeck ML, Ng AC, Witkowski TG, Auger D, Leong DP, Abate E, Marsan NA, Holman ER, Schalij MJ, et al. Detection of subtle left ventricular systolic dysfunction in patients with significant aortic regurgitation and preserved left ventricular ejection fraction: speckle tracking echocardiographic analysis. European Heart Journal Cardiovascular Imaging 2015. 16 992–9. ( 10.1093/ehjci/jev019) [DOI] [PubMed] [Google Scholar]
- 12.Olsen NT, Sogaard P, Larsson HB, Goetze JP, Jons C, Mogelvang R, Nielsen OW, Fritz-Hansen T. Speckle-tracking echocardiography for predicting outcome in chronic aortic regurgitation during conservative management and after surgery. JACC: Cardiovascular Imaging 2011. 4 223–2. ( 10.1016/j.jcmg.2010.11.016) [DOI] [PubMed] [Google Scholar]
- 13.Verseckaite R, Mizariene V, Montvilaite A, Auguste I, Bieseviciene M, Laukaitiene J, Jonkaitiene R, Jurkevicius R. The predictive value of left ventricular myocardium mechanics evaluation in asymptomatic patients with aortic regurgitation and preserved left ventricular ejection fraction. A long-term speckle-tracking echocardiographic study. Echocardiography 2018. 35 1277–12. ( 10.1111/echo.14030) [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Yang YA, Kim KY, Park SM, Kim HN, Kim JH, Jang SY, Hwan Bae MH, Lee JH, Yang DH. Left ventricular strain as predictor of chronic aortic regurgitation. Journal of Cardiovascular Ultrasound 2015. 23 78–85. ( 10.4250/jcu.2015.23.2.78) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorcsan J, Tanaka H. Echocardiographic assessment of myocardial strain. Journal of the American College of Cardiology 2011. 58 1401–1. ( 10.1016/j.jacc.2011.06.038) [DOI] [PubMed] [Google Scholar]
- 16.Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. International Journal of Cardiovascular Imaging 2009. 25 (Supplement 1) 9–22. ( 10.1007/s10554-008-9414-1) [DOI] [PubMed] [Google Scholar]
- 17.Zito C, Longobardo L, Citro R, Galderisi M, Oreto L, Carerj ML, Manganaro R, Cusmà-Piccione M, Todaro MC, Di Bella G, et al. Ten years of 2D longitudinal strain for early myocardial dysfunction detection: a clinical overview. BioMed Research International 2018. 2018 8979407 ( 10.1155/2018/8979407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentz RJ, Khouri MG. Longitudinal strain in heart failure with preserved ejection fraction: is there a role for prognostication? Circulation 2015. 132 368–3. ( 10.1161/CIRCULATIONAHA.115.017683) [DOI] [PubMed] [Google Scholar]
- 19.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014. 100 1673–16. ( 10.1136/heartjnl-2014-305538) [DOI] [PubMed] [Google Scholar]
- 20.Yip GW, Zhang Q, Xie JM, Liang YJ, Liu YM, Yan B, Lam YY, Yu CM. Resting global and regional left ventricular contractility in patients with heart failure and normal ejection fraction: insights from speckle-tracking echocardiography. Heart 2011. 97 287–2. ( 10.1136/hrt.2010.205815) [DOI] [PubMed] [Google Scholar]
- 21.Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T, Remme EW. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. Journal of the American College of Cardiology 2017. 70 942–9. ( 10.1016/j.jacc.2017.06.046) [DOI] [PubMed] [Google Scholar]
- 22.Spartera M, Damascelli A, Mozes F, De Cobelli F, La Canna G. Three-dimensional speckle tracking longitudinal strain is related to myocardial fibrosis determined by late-gadolinium enhancement. International Journal of Cardiovascular Imaging 2017. 33 1351–13. ( 10.1007/s10554-017-1115-1) [DOI] [PubMed] [Google Scholar]
- 23.Jayam M, Janosevic D, Kadiyala M, Cao JJ, Pollack S, Reichek N. Afterload excess and myocardial performance. Journal of Cardiovascular Magnetic Resonance 2013. 15 (Supplement1) 1–2.23324167 [Google Scholar]
- 24.Dahl JS, Magne J, Pellikka PA, Donal E, Marwick TH. Assessment of subclinical left ventricular dysfunction in aortic stenosis. JACC: Cardiovascular Imaging 2019. 12 163–1. ( 10.1016/j.jcmg.2018.08.040) [DOI] [PubMed] [Google Scholar]
- 25.Kim HM, Cho GY, Hwang IC, Choi HM, Park JB, Yoon YE, Kim HK. Myocardial strain in prediction of outcomes after surgery for severe mitral regurgitation. JACC: Cardiovascular Imaging 2018. 11 1235–12. ( 10.1016/j.jcmg.2018.03.016) [DOI] [PubMed] [Google Scholar]
- 26.Mentias A, Desai MY. Markers of increased risk in primary mitral regurgitation. Annals of Translational Medicine 2017. 5 338 ( 10.21037/atm.2017.04.08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang LT, Michelena HI, Scott CG, Enriquez-Sarano M, Pislaru SV, Schaff HV, Pellikka PA. Outcomes in chronic hemodynamically significant aortic regurgitation and limitations of current guidelines. Journal of the American College of Cardiology 2019. 73 1741–17. ( 10.1016/j.jacc.2019.01.024) [DOI] [PubMed] [Google Scholar]
- 28.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. Journal of the American Society of Echocardiography 2013. 26 185–1. ( 10.1016/j.echo.2012.10.008) [DOI] [PubMed] [Google Scholar]
- 29.Marwick TH. Will standardization make strain a standard measurement? Journal of the American Society of Echocardiography 2012. 25 1204–120. ( 10.1016/j.echo.2012.09.017) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a