Abstract
Background
Case reports describe incident sarcoidosis in persons with HIV (PWH). The association between HIV and risk of sarcoidosis, and differences in presentation in PWH, have not been systematically assessed.
Methods
Subjects were selected from the Veterans Aging Cohort Study (VACS), a longitudinal cohort study including veterans with HIV and matched uninfected veterans. This was a prospective observational analysis in which we evaluated both the incidence (via incidence rate ratio) and presentation and treatment (by comparison of rates of organ involvement and use of medications) of sarcoidosis in PWH compared with HIV-negative controls. We also assessed risk factors (via Cox regression) associated with the development of sarcoidosis including CD4 count and viral load trajectory.
Results
Of 1614 patients evaluated via chart review, 875 (54%) had prevalent sarcoidosis and 325 (20%) had confirmed incident sarcoidosis. Incident sarcoidosis occurred in 59 PWH and 266 uninfected. The incidence of sarcoidosis was lower in PWH than uninfected (incidence rate ratio [IRR], 0.61; 95% CI, 0.46–0.81) and especially low in patients with unsuppressed viremia (IRR, 0.04; 95% CI, 0.02–0.08) compared with uninfected). At diagnosis of sarcoidosis, the median CD4 count among PWH was 409 cells/mm3; 77% had HIV-1 RNA <500 copies/mL. No significant differences were observed between PWH and uninfected in terms of organ involvement, disease severity, or use of oral glucocorticoids.
Conclusions
HIV, particularly with persistent viremia, was associated with decreased risk of incident sarcoidosis; severity and treatment were similar between PWH and uninfected.
Keywords: CD4 T cell, granulomatous disorders, HIV, sarcoidosis
Sarcoidosis is a multisystem disorder characterized by the formation of granulomas, often in the mediastinal lymph nodes and lungs; however, it can involve nearly every organ [1–3]. Though the precise sequence of events that initiate sarcoidosis has not been established, it is widely believed that exposure to a triggering antigen results in polyclonal CD4+ T-cell activation and widespread granuloma formation [4].
Sarcoidosis and HIV infection are often considered antithetical; the former is driven by excess, the latter by deficiency, of CD4+ T cells. That these conditions can coexist simultaneously may seem paradoxical. However, there are case series and reports that describe incident sarcoidosis in HIV [5–7]. Investigation of comorbid HIV and sarcoidosis may provide insight into the biology of granulomatous diseases in patients with HIV (PWH), given that HIV is well known to disrupt granulomas, while sarcoidosis promotes the formation of granulomas [8].
The epidemiology, course, and manifestations of co-occurring sarcoidosis in PWH have not been rigorously evaluated with HIV-negative controls. In this analysis, our goal was to investigate the association between HIV infection and incidence of sarcoidosis using a large data set of PWH with matched HIV-negative controls. We focused on incident sarcoidosis in order to elucidate the role of viral load, viral load trajectory, and CD4 count on the viability of incipient granulomatous disease in sarcoidosis. This approach also allowed us to compare PWH and uninfected within a comparable window from the time of diagnosis of sarcoidosis. Our second aim was to evaluate differences in presentation, severity, and management between PWH and uninfected.
METHODS
Patient Consent Statement
Our cohort was developed as a subset of the Veterans Aging Cohort Study (VACS), a prospective, longitudinal, observational study of veterans with HIV and age-, sex-, race-, and site-matched HIV-negative controls [9, 10]. The institutional review boards associated with participating Veterans Administration sites and the study coordinating center approved the VACS with a waiver for informed consent.
Data Collection
We identified cases using a process similar to previously published cohorts [11, 12]. We used International Classification of Diseases (ICD) codes to identify potential cases and confirmed these via chart review. From the VACS cohort, all ICD-9 or ICD-10 sarcoidosis codes (ICD-9 135.x, ICD-10 D86.x) were extracted between 1997 and 2017. We reviewed the medical record of each patient with a code for sarcoidosis and determined whether there was (1) insufficient evidence of sarcoidosis, (2) prevalent sarcoidosis at the time of diagnostic code entry, or (3) incident sarcoidosis. Published criteria for cases of incident sarcoidosis were utilized [11, 12]. Inclusion required a compatible clinical presentation with exclusion of reasonable alternatives, compatible radiographic features if applicable, and tissue diagnosis (unless the only manifestation was hilar lymphadenopathy, ie, Scadding Criteria Stage I). Prevalent cases were required only to have physician documentation of a prior diagnosis. Patients with prevalent sarcoidosis at the time of the first ICD code, or who developed sarcoidosis before HIV diagnosis, were excluded.
Severity was evaluated using the Scadding criteria for chest radiography; the report of the chest x-ray closest to diagnosis was reviewed, as original images were not available for our review [13]. We also evaluated severity by comparing pulmonary function testing (PFT) results between groups; PFTs are routinely used for this purpose in evaluation of patients with sarcoidosis [14]. Organ involvement was assessed within the first year after diagnosis using the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) criteria for “at least probable” organ involvement [15]. See the Supplementary Methods for additional details regarding WASOG criteria, PFTs, and chart review methodology.
Statistical Analysis
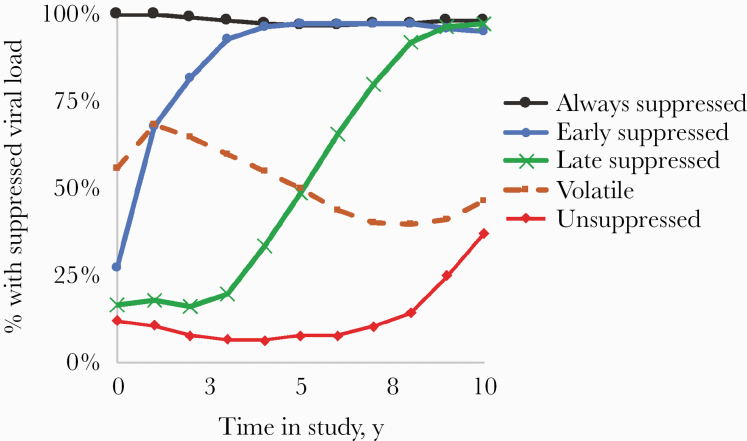
Measures of central tendency are reported as mean ± SD or median (interquartile range [IQR]). The chi-square test was used to evaluate differences in categorical variables between groups. Differences in continuous variables between groups were assessed using the t test for normally distributed variables and the Wilcoxon test for non–normally distributed variables. Observation started at the first ICD code for HIV in PWH or the first Department of Veterans Affairs (VA) visit for HIV-uninfected patients. Incidence of sarcoidosis in persons with and without HIV was determined by calculating a crude incidence rate (number of incident cases divided by person-time at risk in each category); confidence intervals were determined via generalized linear modeling using the Poisson distribution. To evaluate the association between viral suppression and incidence of sarcoidosis, we used trajectory analysis in SAS 9.4, with quadratic modeling of viral suppression over 10 years of follow-up. Viral suppression was defined as <500 copies/mL, due to variations in the lower limit of detection over time, with the highest viral suppression threshold at 500 copies/mL. The trajectory analysis identified 5 distinct viral load trajectories (Figure 1; Supplementary Methods). Cox time-to-event analysis was used to assess the age- and race-adjusted association between HIV status, viral suppression, and risk of sarcoidosis. Covariates in these models included CD4 count (assessed categorically, using cutoffs of <50, 50–199, 200–299, 300–349, 350–499, and >499) and viral load (suppressed vs unsuppressed) at baseline.
Figure 1.
Viral load trajectories identified by trajectory analysis. Lines depict the percentage of patients in each group with a suppressed viral load (<500 copies per mL) at any given time point in the first 10 years of follow-up.
We defined statistical significance as a 2-tailed P value <.05. Statistical analysis was performed in SAS, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Among 173 194 patients in the VACS, 1610 had a code for sarcoidosis. Characteristics of the cohort are provided in Table 1. From these 1610 patients, 884 cases of prevalent sarcoidosis and 325 cases of incident sarcoidosis were identified. The remaining patients (“ICD code only” in Table 1) had insufficient chart evidence of sarcoidosis. Nine patients developed HIV after diagnosis of sarcoidosis and were excluded from the incident group.
Table 1.
Baseline Characteristics of the Study Population
| PWH | Without HIV | |
|---|---|---|
| No. | 56 266 | 116 928 |
| Demographics | ||
| Age at study onset, y | 45 ± 9 | 44 ± 10 |
| Male sex, % | 97 | 97 |
| Race, % | ||
| White | 40 | 41 |
| Black | 48 | 46 |
| Hispanic | 8 | 8 |
| Other | 5 | 5 |
| Smoking status, % | ||
| Current | 56 | 51 |
| Former | 16 | 19 |
| Never | 28 | 30 |
| CD4 at first observation, % | ||
| ≥500 cells/mm3 | 33 | - |
| 350–499 cells/mm3 | 19 | - |
| 300–349 cells/mm3 | 7 | - |
| 100–299 cells/mm3 | 26 | - |
| 50–99 cells/mm3 | 6 | - |
| <50 cells/mm3 | 10 | - |
| HIV-1 RNA, % | ||
| ≤500 copies/mL | 41 | - |
| 500–10 000 copies/mL | 18 | - |
| ≥10 000 copies/mL | 42 | - |
| Sarcoidosis status, No. | ||
| No ICD code | 55 912 | 115 668 |
| ICD code present only | 354 | 1260 |
| Prevalent sarcoidosis | 163 | 721 |
| Incident sarcoidosis by chart review | 59 | 265 |
Percentages may add up to >100% due to rounding.
Abbreviations: ICD, International Classification of Diseases; PWH, people with HIV.
The study encompassed 2 338 089 person-years. The median follow-up among uninfected individuals (IQR) was 10.9 (4.8–17.1) years compared with 7.7 (3.0–14.6) years in PWH. The crude incidence rate of sarcoidosis was 1.6 per 10 000 person-years (95% CI, 1.9–2.4) in uninfected vs 0.9 per 10 000 person-years (95% CI, 0.7–1.2) in PWH. The incidence rate ratio for sarcoidosis in PWH compared with uninfected was 0.61 (95% CI, 0.46–0.81). Time-to-event analysis, adjusted for age and race, suggested that HIV infection was associated with decreased risk for incident sarcoidosis (hazard ratio [HR], 0.25; 95% CI, 0.06–1.02; P = .05; log-rank P = .09) (Supplementary Figure 1).
The characteristics of patients with incident sarcoidosis are described in Table 2. Among those with incident sarcoidosis, there were 59 (18%) PWH at diagnosis (Table 2). Though PWH and uninfected had similar demographic characteristics at study onset, incident sarcoidosis occurred more frequently in veterans of Black race; this was particularly true for PWH (79% of incident sarcoidosis in HIV occurred in Black individuals, while only 48% of PWH in the entire sample were of Black race).
Table 2.
Characteristics of Patients with Incident Sarcoidosis
| PWH | Without HIV | P Value | |
|---|---|---|---|
| No. | 59 | 265 | |
| Demographics | |||
| Age at diagnosis, y | 50 ± 9 | 47 ± 11 | .06 |
| Male sex, % | 100 | 93 | .03 |
| Race, % | .13 | ||
| White | 18 | 26 | |
| Black | 79 | 65 | |
| Hispanic | 2 | 7 | |
| Other | 2 | 2 | |
| Smoking status | .39 | ||
| Current | 32 | 33 | |
| Former | 21 | 14 | |
| Never | 47 | 53 | |
| CD4 closest to diagnosis, % | |||
| ≥500 cells/mm3 | 34 | - | |
| 350–499 cells/mm3 | 30 | - | |
| 300–349 cells/mm3 | 9 | - | |
| 100–299 cells/mm3 | 27 | - | |
| HIV-1 RNA closest to diagnosis, % | |||
| ≤500 copies/mL | 76 | - | |
| 500–10 000 copies/mL | 12 | - | |
| ≥10 000 copies/mL | 12 | - | |
| Stage of pulmonary involvement at diagnosis (Scadding criteria), % | .07 | ||
| 0 | 26 | 22 | |
| I | 32 | 36 | |
| II | 17 | 27 | |
| III | 19 | 14 | |
| IV | 6 | 1 | |
| Organ involvement in first year, % | |||
| Lung/mediastinum | 88 | 86 | .67 |
| Skin | 27 | 17 | .71 |
| Eye | 10 | 11 | .86 |
| Liver | 5 | 7 | .56 |
| CNS | 3 | 1 | .10 |
| Exocrine (eg, parotid gland) | 2 | 3 | .67 |
| Peripheral lymph node | 5 | 6 | .91 |
| Bone or bone marrow | 2 | 1 | .72 |
| Gut | 3 | 1 | .10 |
| Heart | 0 | 2 | .60 |
| Spleen | 3 | 4 | .89 |
| Kidney | 0 | 0.4 | >.99 |
| No. of involved organs in first year, % | .54 | ||
| 1 | 63 | 66 | |
| 2 | 27 | 23 | |
| 3 | 8 | 8 | |
| 4 | 2 | 0 | |
| Treatments in first year | |||
| Use of systemic corticosteroids, % | 34 | 35 | .84 |
| Total dose of corticosteroids, mg prednisone equivalents | 2195 | 1800 | .97 |
| Use of steroid-sparing immunosuppressants, % | 5 | 10 | .22 |
| Baseline PFTs | |||
| No. | 27 | 111 | |
| FVC, L | 3.3 (2.9–4.5) | 3.6 (3.2–4.4) | |
| FVC, % predicted | 76 (67–93) | 79 (69–89) | .73 |
| FEV1, L | 2.5 (2.3–3.5) | 2.8 (2.3–3.3) | |
| FEV1, % predicted | 75 (63–96) | 75 (67–87) | .85 |
| DLCO, mL/min/mmHg | 18 (14–22) | 21 (16–27) | |
| DLCO, % predicted | 64 (54–77) | 72 (56–82) | .34 |
| TLC, L | 5.5 (4.7–6.2) | 5.4 (4.7–6.1) | |
| TLC, % predicted | 80 (68–94) | 83 (73–94) | .47 |
PFT values assessed within 1 year of diagnosis of sarcoidosis.
Abbreviations: CNS, central nervous system; DLCO, diffusion limit for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PFT, pulmonary function test; PWH, people with HIV; TLC, total lung capacity.
Characteristics of HIV Disease at Time of Diagnosis
The median time from diagnosis of HIV to diagnosis of sarcoidosis (IQR) was 7.8 (3.9–15.9) years. The median CD4+ T-cell count near diagnosis (IQR) was 409 (268–535) cells/mm3, while the median CD8+ T-cell count (IQR) was 458 (206–792) cells/mm3. HIV RNA-1 before diagnosis was available in 76% of patients; of these patients, 34 (77%) had suppressed HIV RNA-1.
Among 59 PWH with sarcoidosis, 44 had CD4 data and 43 had viral load data available before diagnosis. Ten of 43 patients with viral loads measured had detectable viremia immediately before diagnosis of sarcoidosis. Six of 44 patients (14%) with CD4 data had counts <200 cells/mm3, and 10 (23%) had CD4 counts between 200 and 349 at the time of diagnosis of sarcoidosis. Further details regarding their presentation are found in the Supplementary Data.
Influence of Viral Suppression and Baseline CD4 Count on Incidence of Sarcoidosis
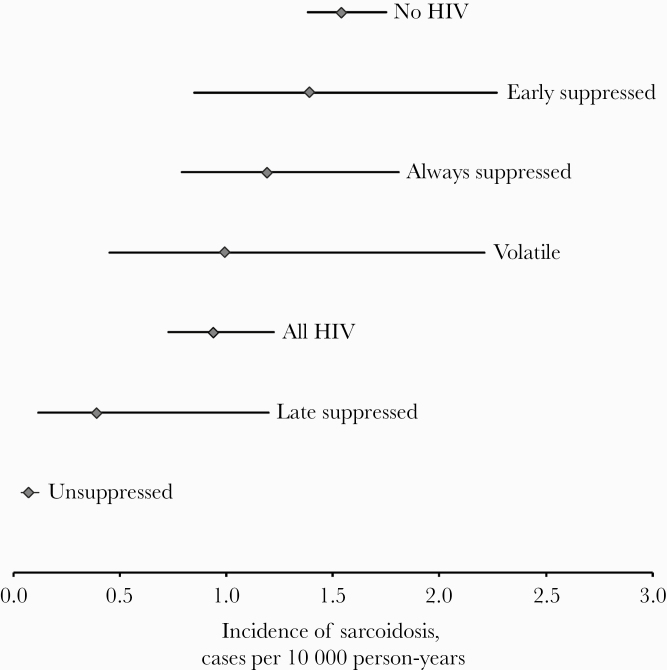
To assess the longitudinal impact of viremia on incidence of sarcoidosis, we used trajectory analysis. The trajectories identified are depicted in Figure 1. Point estimates of incidence among trajectory groups tended to increase with increasing viral suppression. However, the only subgroup in which the incidence of sarcoidosis differed in a statistically significant way from the overall HIV group was the “unsuppressed” group (Figure 2). Underscoring this point, the incidence rate ratio for sarcoidosis in uninfected patients compared with PWH with longitudinally unsuppressed viremia was 23 (95% CI, 13–41).
Figure 2.
Incidence of sarcoidosis in groups, defined by presence of HIV and viral load trajectory. Whiskers indicate 95% confidence intervals. Central dots indicate incidence rate. As the confidence intervals are calculated using a Poisson distribution, the size of the confidence intervals tends to increase with an increasing number of observed events.
To query the role of viral suppression vs CD4 count, we used 2 models, both of which incorporated CD4 count at initial observation (baseline). First, we assessed baseline (at beginning of observation, ie, first measurement in the electronic health record) measurements of CD4 count and viral load and incorporated both into a model. Race and age were included as covariates (Supplementary Table 1). Compared with a baseline CD4 count ≥500, a baseline CD4 count of 50–199 was associated with increased risk for sarcoidosis (HR, 2.8; 95% CI, 1.1–7.0; P = .03). Hazard ratios in this model included increased risk for sarcoidosis with a baseline CD4 count of 200–349 (HR, 2.4; 95% CI, 0.94–6.0; P = .07) and a decreased risk with baseline viremia (HR, 0.60; 95% CI, 0.32–1.15; P = .12); these findings did not reach statistical significance. Kaplan-Meier curves for CD4 groups with and without adjustment for age and race are found in Supplementary Figure 2.
To confirm these observations and assess the interplay between baseline CD4 count and viral load trajectory, we used a model incorporating baseline CD4 count (measured categorically) and longitudinal viral trajectory group (with unsuppressed group as referent) (Supplementary Table 2). In this model, we observed that lower baseline CD4 counts continued to be associated with increased risk for sarcoidosis. A CD4 count of 50–199 conferred an HR of 2.7 (95% CI, 1.1–6.7; P = .03). The group with baseline CD4 count between 200 and 349 had an HR of 2.1 (P = .10). All other CD4 groups did not differ significantly from the referent. Among longitudinal viral trajectory groups, none differed in a statistically significant manner from the unsuppressed group in this model; however, the early suppressed group had an HR of 2.54 (95% CI, 0.96–6.7; P = .06).
Organ Involvement and Severity
Pulmonary disease severity, as evaluated using Scadding criteria for chest radiography, was similar between PWH and uninfected (Table 2). The most frequently involved organ by WASOG criteria, in both groups, was the lung. There were no statistically significant differences in rates of involvement of any individual organ or number of organs involved by HIV status (Table 2).
PFTs at Diagnosis
Among 325 patients with incident sarcoidosis, PFTs were available in 46% of PWH and 42% of uninfected. There were no significant differences in percent-predicted values for FEV1, FVC, or TLC (Table 2). The median DLCO in PWH was 64% of predicted compared with 72% of uninfected, although this difference did not reach statistical significance. Among PWH with unsuppressed viremia at diagnosis, the median DLCO (IQR) was 60% predicted (44%–77%), compared with 64% predicted (55%–76%) among PWH with suppressed viremia.
Management of Sarcoidosis
Approximately one-third of patients both with and without HIV received systemic glucocorticoid therapy for sarcoidosis within the first year. We did not find differences between groups in terms of maximum daily prednisone dose or total dose prescribed over 1 year (Table 2). Five percent of PWH received a nonglucocorticoid immunosuppressant or immunomodulator, compared with 10% of patients without HIV. Of the 3 PWH receiving nonglucocorticoid therapy, 2 received hydroxychloroquine only and 1 received topical tacrolimus and hydroxychloroquine; all 3 had extrapulmonary involvement. Of 27 patients without HIV who received nonglucocorticoid therapy, drugs used included hydroxychloroquine (n = 12), methotrexate (n = 7), azathioprine (n = 4), mycophenolate mofetil (n = 2), topical tacrolimus (n = 2), cyclosporine (n = 1), and infliximab (n = 1). Extrapulmonary manifestations were present in 78% of these uninfected patients.
DISCUSSION
Our data support the intuitive notion that HIV is associated with lower likelihood of incident sarcoidosis. Exposure to high levels of HIV viremia over long periods of time was associated with reduced rates of incident sarcoidosis; our time-to-event data also suggest a role for CD4 counts modulating the risk of sarcoidosis. Most incident cases of sarcoidosis in HIV occurred in patients with CD4+ T-cell counts >350 and adequate viral suppression. This finding is not unexpected; if the function and number of CD4+ T cells is close to normal and there is adequate viral suppression, the ability to form functional granulomas is preserved, which is a prerequisite for development of sarcoidosis. Consistent with this observation, none of our incident cases had CD4+ T-cell counts <100. It is possible that critically low CD4+ T-cell counts preclude development of sarcoidosis. Similarly, HIV disrupts tumor necrosis factor–alpha signaling [16, 17]. It is therefore possible that the cellular signaling required to produce granulomas is hampered by high levels of HIV viremia. We also noted with interest a significant minority of patients with incident sarcoidosis and low CD4 or active viremia. Discussion of potential mechanisms for this phenomenon and the relationship of our findings to prior reports of sarcoidosis in PWH can be found in Supplementary Data.
HIV Viremia, CD4 Count, and Risk for Sarcoidosis
Our time-to-event analysis suggests that both viral load trajectory and CD4 counts modulated risk of incident sarcoidosis in PWH; the effect of CD4 count seemed at least as strong if not stronger than that of viral load. The finding that a lower CD4 count at baseline was associated with higher risk of sarcoidosis suggests that there may be an aspect of immune reconstitution in the pathogenesis of sarcoidosis in PWH. New sarcoidosis during antiretroviral therapy has been previously reported [18, 19]. Conventionally, immune reconstitution inflammatory syndrome (IRIS) occurs shortly after initiation of ART and is associated with a polyclonal recovery of CD4 T cells. As sarcoidosis in our population was diagnosed a median of 7.8 years after HIV, we would not suggest that sarcoidosis is part of a classical IRIS but rather that in PWH the process of immune reconstitution may be a risk factor for the later development of sarcoidosis [20].
Sarcoidosis in PWH vs Those Without HIV
Beyond the variability in incidence of sarcoidosis between PWH and uninfected, we found few differences in presentation between groups. Organ involvement in the year after diagnosis was similar between PWH and uninfected. Scadding pulmonary stage did not differ between PWH and uninfected. However, there was a statistically nonsignificant difference in DLCO between groups. Isolated decreased DLCO in PWH has been described previously [21]. In light of these results, we did not identify differences in severity of sarcoidosis in PWH vs uninfected; however, it is relevant to note that PWH with sarcoidosis may already have lower DLCO and decreased pulmonary function than uninfected, and thus may warrant closer monitoring.
Limitations
This analysis has several limitations. This was a retrospective, chart review–based study and therefore was susceptible to uncontrolled confounding. We report results from a population of veterans, the overwhelming majority of whom were male. PFT data were reported inconsistently between sites. Radiographic staging via computed tomography scan was not evaluated; it is possible that differences in anatomical disease severity at diagnosis were present but not visualized on plain film radiography. Our data are limited to the VA computerized health record; patients diagnosed with sarcoidosis outside of the VA, if not coded in the VA, would not have been detected. Finally, it is not possible, with the present analyses, to determine whether the driving force for decreased incidence of sarcoidosis in PWH is CD4 count vs the presence and chronicity of viremia (or a third variable). A future time-updated analysis incorporating longitudinal CD4 and viral load data would be valuable.
CONCLUSIONS
Incident sarcoidosis occurred at lower rates in PWH than in uninfected. Among PWH, both baseline CD4 count and the trajectory of viremia were associated with risk of incident sarcoidosis. Typically, new-onset sarcoidosis occurred in patients with higher CD4 counts and suppressed viral loads, although approximately one-fourth of incident sarcoidosis cases occurred in patients with either low CD4 counts or unsuppressed viremia. No differences in organ involvement or plain-radiographic severity were observed between PWH and patients without HIV. Further research should assess HIV-related differences in PFTs, HRCT severity, and histopathology.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We gratefully acknowledge the support of Dr. Janet P. Tate in the development of the trajectory analysis.
Financial support. This work was supported by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (grants U24 AA020794, U01 AA020790, and U10 AA013566); the National Heart, Lung and Blood Institute at the National Institutes of Health (Grant T35HL007649); in kind by the US Department of Veterans Affairs; and through travel support from the Rheumatology Research Foundation.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. This work has been presented in oral abstract form at the 2019 American College of Rheumatology Annual Meeting, Atlanta, Georgia [22].
References
- 1. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med 2014; 20:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmucci S, Torrisi SE, Caltabiano DC, et al. Clinical and radiological features of extra-pulmonary sarcoidosis: a pictorial essay. Insights Imaging 2016; 7:571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bargagli E, Prasse A. Sarcoidosis: a review for the internist. Intern Emerg Med 2018; 13:325–31. [DOI] [PubMed] [Google Scholar]
- 4. Grunewald J, Eklund A. Role of CD4+ T cells in sarcoidosis. Proc Am Thorac Soc 2007; 4:461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trevenzoli M, Cattelan AM, Marino F, et al. Sarcoidosis and HIV infection: a case report and a review of the literature. Postgrad Med J 2003; 79:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris DG, Jasmer RM, Huang L, et al. Sarcoidosis following HIV infection: evidence for CD4+ lymphocyte dependence. Chest 2003; 124:929–35. [DOI] [PubMed] [Google Scholar]
- 7. Almeida FA Jr, Sager JS, Eiger G. Coexistent sarcoidosis and HIV infection: an immunological paradox? J Infect 2006; 52:195–201. [DOI] [PubMed] [Google Scholar]
- 8. Diedrich CR, O’Hern J, Wilkinson RJ. HIV-1 and the Mycobacterium tuberculosis granuloma: a systematic review and meta-analysis. Tuberculosis (Edinb) 2016; 98:62–76. [DOI] [PubMed] [Google Scholar]
- 9. Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006; 44:S25–30. [DOI] [PubMed] [Google Scholar]
- 10. Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care 2006; 44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ungprasert P, Matteson EL, Crowson CS. Accuracy of diagnostic coding for sarcoidosis in electronic databases: a population-based study. Lung 2017; 195:713–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ungprasert P, Carmona EM, Utz JP, et al. Epidemiology of sarcoidosis 1946–2013: a population-based study. Mayo Clin Proc 2016; 91:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J 1961; 2:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ungprasert P, Ryu JH, Matteson EL. Clinical manifestations, diagnosis, and treatment of sarcoidosis. Mayo Clin Proc Innov Qual Outcomes 2019; 3:358–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Judson MA, Costabel U, Drent M, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31:19–27. [PubMed] [Google Scholar]
- 16. Kumar A, Coquard L, Herbein G. Targeting TNF-alpha in HIV-1 infection. Curr Drug Targets 2016; 17:15–22. [DOI] [PubMed] [Google Scholar]
- 17. DeSimone JA, Pomerantz RJ, Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med 2000; 133:447–54. [DOI] [PubMed] [Google Scholar]
- 18. Crothers K, Huang L. Pulmonary complications of immune reconstitution inflammatory syndromes in HIV-infected patients. Respirology 2009; 14:486–94. [DOI] [PubMed] [Google Scholar]
- 19. Foulon G, Wislez M, Naccache JM, et al. Sarcoidosis in HIV-infected patients in the era of highly active antiretroviral therapy. Clin Infect Dis 2004; 38:418–25. [DOI] [PubMed] [Google Scholar]
- 20. Walker NF, Scriven J, Meintjes G, Wilkinson RJ. Immune reconstitution inflammatory syndrome in HIV-infected patients. HIV AIDS (Auckl) 2015; 7:49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crothers K, McGinnis K, Kleerup E, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr 2013; 64:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanberg JS, Fraenkel L, Justice A. Epidemiology and presentation of sarcoidosis with and without HIV infection [abstract]. Arthritis Rheumatol 2019; 71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.