Abstract
Centrioles are subcellular organelles that were present in the last eukaryotic common ancestor, where the centriole’s ancestral role was to form cilia. Centrioles have maintained a remarkably conserved structure in eukaryotes that have cilia, while groups that lack cilia have lost their centrioles, highlighting the structure–function relationship that exists between the centriole and the cilium. In contrast, animal sperm cells, a ciliated cell, exhibit remarkable structural diversity in the centriole. Understanding how this structural diversity evolved may provide insight into centriole assembly and function, as well as their unique role in sperm. Here, we apply concepts used in the study of the evolution of animal morphology to gain insight into the evolution of centriole structure. We propose that centrioles with an atypical structure form because of changes in the timing of centriole assembly events, which can be described as centriolar “heterochrony.” Atypical centrioles of insects and mammals appear to have evolved through different types of heterochrony. Here, we discuss two particular types of heterochrony: neoteny and hypermorphosis. The centriole assembly of insect sperm cells exhibits the retention of “juvenile” centriole structure, which can be described as centriolar “neoteny.” Mammalian sperm cells have an extended centriole assembly program through the addition of novel steps such as centrosome reduction and centriole remodeling to form atypical centrioles, a form of centriole “hypermorphosis.” Overall, centriole heterochrony appears to be a common mechanism for the development of the atypical centriole during the evolution of centriole assembly of various animals’ sperm.
1.1. Introduction
Centrioles are present in most eukaryotic cell types and are essential for the development and physiology of humans and many animals. Because centrioles are so essential for life, they have been studied using multiple approaches in many in vitro and in vivo systems. Over the years, it has become evident that, while centriole structure and function are highly conserved, centrioles exhibit distinct and sometimes dramatic differences (Jana et al. 2018; Riparbelli et al. 2010). Many studies focus on the more universal aspects of centrioles to draw conclusions that are applicable across species because conservation suggests a similar underlying mechanism (Jana et al. 2016; Winey and O’Toole 2014; Sluder 2016). However, differences between centrioles are also significant for several reasons. First, some differences provide a unique opportunity to overcome a difficulty in investigating a process (e.g., the presence of the giant centriole cartwheel in some species was instrumental in elucidating its detailed structure) (Guichard et al. 2012). Second, understanding the differences can provide conceptual insight that would otherwise be hidden. For example, the observation that in some species centrioles with one symmetry can nucleate a centriole with a different symmetry, suggests that the preexisting centriole does not act as the template for centriole organization (Phillips 1967). Third, differences are commonly present and are essential for animal or tissue-specific function; impacting them can result in devastating pathologies. Fourth, differences provide a basis for tissue-specific therapeutics with minimal systemic side effects. Last, there are evolutionary reasons for differences—they are beneficial. For these reasons, in this chapter, we will focus on the diversity in centriole structure and how differently shaped centrioles evolved.
Here, to gain insight into centriole structural diversity, we take the approach best described by Theodosius Dobzhansky: “Nothing in Biology Makes Sense Except in the Light of Evolution” (Dobzhansky 1973). We will apply concepts from the study of animal development such as heterochrony, neoteny, and hypermorphosis to study the evolution of the centriole. We focus on sperm because, due to the postcopulatory sexual selection, it underwent rapid evolution, during which time the typical structure of the centriole changed in many species (Lupold and Pitnick 2018; Mordhorst et al. 2016). This chapter starts with background on heterochrony and centriole structure and function. We continue with describing two types of centriole changes: a neotenic change in insect proximal centrioles and a hypermorphotic change in mammalian distal centrioles. We will then discuss potential molecular mechanisms that may be essential to this evolutionary change. Finally, we propose that applying the concept of heterochrony, which was originally intended to explain organismal evolution, to organelle evolution is beneficial to understanding the molecular basis of heterochrony.
1.2. Heterochrony, Neoteny, and Hypermorphosis
A comparative biology approach is routinely used in the study of sperm where the sperm centriole mainly acts as a tool to determine the phylogenetic relationship between groups of animals (see for example Dias et al. 2015). Here, we borrow concepts from evolutionary developmental biology that are generally used to describe animal development, to explain changes in centriole assembly and structure. One general concept we focus on is heterochrony, a term originally coined by the nineteenth century German biologist Ernst Haeckel in the context of the theory of recapitulation. The modern premise of heterochrony, as explained by the twentieth century evolutionary biologist Stephen Jay Gould, is that the development of an organism (ontogeny) and the evolution of an organism (phylogeny) are related; and changes in the timing and the rate of developmental processes explain evolutionary change (Gould 1977; McNamara and McKinney 2005). For example, the developmental process for the formation of vertebrae may be happening quicker or slower resulting in a relatively longer or shorter spine in similar species (Keyte and Smith 2014). At its core, heterochrony provides an explanation for the differences observed in various species in terms of evolutionary change and timing of development. Other ideas that we do not discuss here are that the evolutionary change can be mediated by changing the location of a process (i.e., Heterotopy).
Heterochrony can be divided into two broad categories of changes (Smith 2002): (1) changes that result in a juvenile or simple shape in comparison to the ancestral shape and (2) changes that result in a more complex shape in comparison to the ancestral shape. Here we focus on a specific example for each category, known as neoteny and hypermorphosis. Neoteny is a decrease in the rate of development or a maturation arrest at an early stage. Hypermorphosis is an acceleration or extension of a preexisting process to accommodate additional steps.
The concept of neoteny has already been “borrowed” to describe a cellular process; the term “cellular neoteny” was used to describe the differentiation program that generates various neuronal and neuroendocrine cells. It was suggested that these cell types might represent different stages of differentiation by cells “arresting” along a linear development pathway, whose endpoint is a cholinergic sympathetic neuron (Anderson 1989). Here, we apply this concept to the subcellular level, which in our case is the alteration of the timing of centriole assembly events. We create distinct analogies between “animal” and “centriole,” “development of an animal” and “assembly of a centriole,” and “evolution of an animal” and “evolution of a centriole.” The centrioles of sperm cells are particularly suitable for this analysis because postmating sexual selection drove the rapid evolution of sperm, during which time centriole structure changed in many species (Mordhorst et al. 2016; Lupold and Pitnick 2018).
1.3. The Centriole and Cilium Structure–Function Relationship Restricts Centriole Diversity
Centrioles are barrel-shaped structures made of nine triplet microtubule blades that form a wall surrounding the centriole lumen (Fig. 1.1a–ii). Each blade is made up of three connected microtubules (named A, closest to the lumen, B, and C, furthest from the lumen) and therefore is referred to as triplet microtubules. Centrioles have two essential functions inside the cell (Bornens 2012). The centrioles form centrosomes, which are large microtubule-organizing centers in the cell; the resulting organized microtubules mediate cell division and intracellular transport. Centrioles are also responsible for the formation of cilia, which are hair-like organelles that are essential for cell motility as well as cell–cell communication. The centriole also provides a stable anchor for the cilium and centrosome after their formation when they perform their respective functions. A typical animal cell has two centrioles (Fig. 1.1a–i). These centrioles are different from each other in their age, structure, composition, and function. The older centriole (aka mother centriole) is structurally and compositionally mature, and it is functionally competent to form a centrosome or a cilium. The younger centriole (aka daughter centriole) is immature; thus, it is unable to build a centrosome or a cilium.
Fig. 1.1.
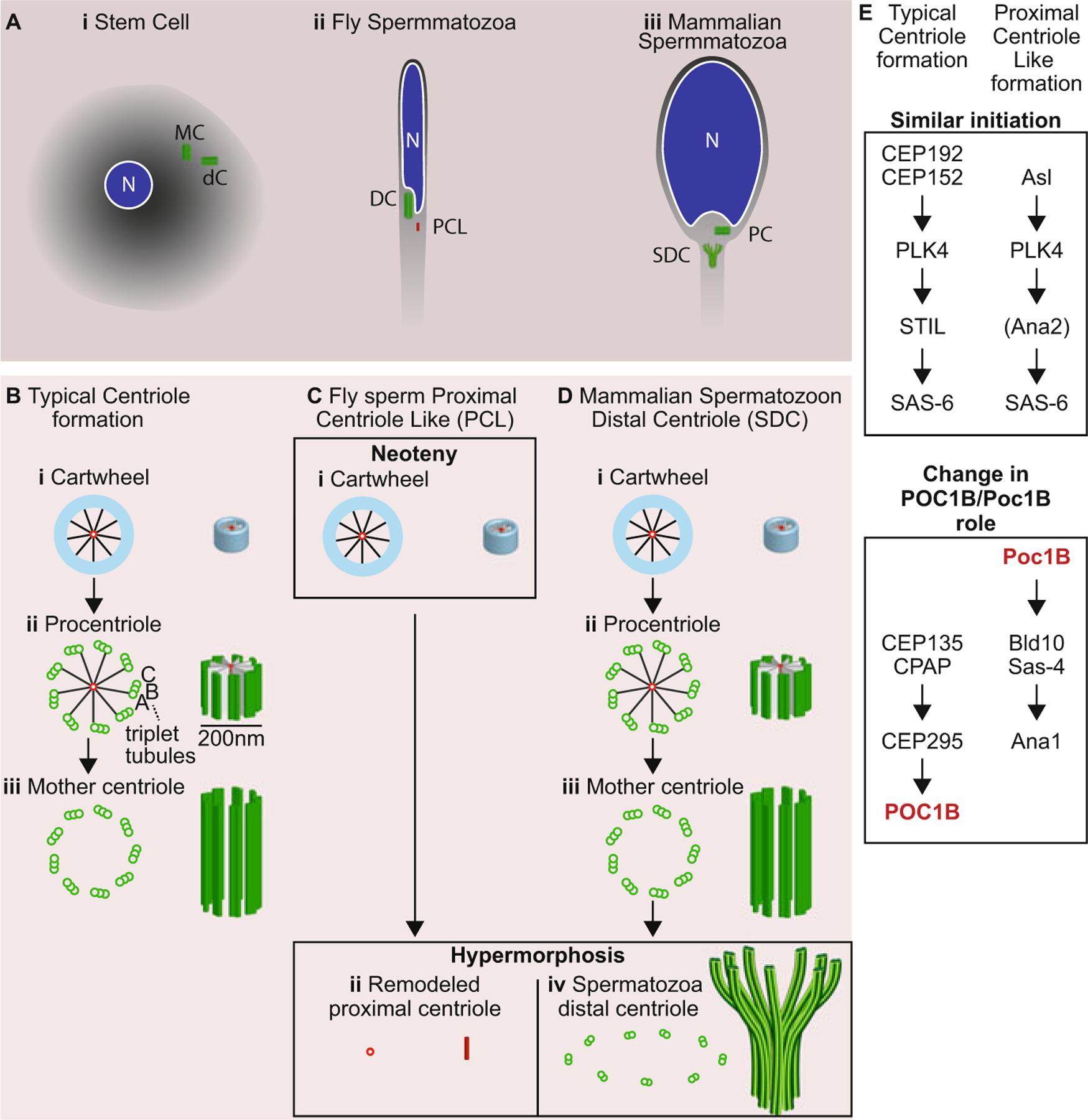
Model of centriole development in various animal groups. The centrioles are depicted via cross section at the centriole base and side view. (a) A model depicting the two centrioles in a stem cell (i), fly spermatozoon (ii), and non-rodent mammal spermatozoon (iii). N nucleus, MC mother centriole, dC daughter centriole, DC distal centriole, PCL proximal centriole like, SDC spermatozoon distal centriole, PC proximal centriole. (b–d) Models depicting the mechanism of a typical centriole formation in a stem cell (b), of an atypical centriole in fly sperm (c), and of an atypical centriole in mammalian sperm (d). (b) A typical centriole forms from a cartwheel made of a central tubule with spokes surrounded by an amorphous wall (i). Then, the procentriole develops a wall of nine singlet tubules, which grows to doublet tubules, and then triplet tubules (ii). Next, the procentriole elongates and loses its cartwheel (iii). (c) The neotenic sperm centriole of flies (the PCL) initially resembles the cartwheel stage and is made of a central tubule with spokes and an amorphous wall (i). Then, the neotenic centriole is remodeled, losing its amorphous wall (ii) in a hypermorphic step. (d) The hypermorphic sperm centriole of non-rodent mammals starts its formation like a typical centriole with a cartwheel (i), procentriole (ii), and a mature centriole (iii). Finally, the centriole is remodeled by splaying the microtubules in a hypermorphic step (iv). (e) The molecular pathway of human typical centriole formation (left column) and PCL formation (right column). Genes in the same row are orthologues to each other in humans and flies, except for Poc1B that changes position in the pathway. The figure shows that the same molecular pathway initiates the typical centriole and fly PCL, but Poc1B gains an earlier essential function in the formation of the PCL as compared to the human typical centriole pathway
Animal centrioles form centrosomes, and most animal cells require two centrosomes for normal mitosis (Nigg and Raff 2009; Bornens 2012). The centrosome nucleates and anchors asters of microtubules and determines the location of the mitotic spindle pole (Tang and Marshall 2012). When present, centrosomes are the dominant microtubule-organizing center in the cell. When centrosomes are normally absent, as in the oocyte, a self-assembly mechanism can mediate mitosis (Petry 2016). However, when centrosomes are abnormally absent, there is an increased rate of chromosome missegregation during mitosis (Poulton et al. 2014). An abnormal number of centrosomes can lead to mono- or multipolar spindles, which often results in cell death (Prosser and Pelletier 2017). An exception to this outcome occurs in cancer cells, which overcome the centrosome’s dominance by clustering the centrosomes in a bipolar spindle (Leber et al. 2010). However, asymmetric clustering of centrosomes can also cause chromosome missegregation (Cosenza et al. 2017). Altogether, mature centrosomes, and the centrioles within them, are microtubule organization centers whose precise number is essential for normal animal development.
Centriole number control is achieved through a two-part process: first, by regulating the number of newly assembled centrioles in the cell and, second, by precisely segregating centriole pairs, each made up of one old and one new, during cell division (Firat-Karalar and Stearns 2014). New centrioles are assembled in association with a preexisting (mature) centriole that serves as a platform to restrict centriole formation to one centriole per preexisting centriole per cell cycle. Many proteins that are key to centriole assembly have been identified, but the precise mechanism that assures that only a single new centriole forms near an old centriole is still under intensive investigation. However, it appears that centriole microtubules do not have an essential role in centriole duplication (Avidor-Reiss 2018). Altogether, having precisely two centrioles in a cell is essential for cellular function, animal viability, and reproductive success; the control of centriole formation requires a preexisting centriole, but centriolar microtubules are dispensable for the assembly of new centrioles or for centrosome function.
The ancestral role of cilia in eukaryotes is to produce cellular motility. This motility is generated by molecular machines known as dynein arms, which contain dynein motor proteins (Viswanadha et al. 2017). The dynein arms are permanently attached to each of the microtubule blades on one side and are transiently binding to a nearby microtubule blade to exert the force that produces motility. This force results in one microtubule blade sliding relative to the other microtubule blade. Each microtubule blade is made of two connected microtubules (called A and B) and are therefore referred to as doublet microtubules. There are nine doublets arranged in a circle, such that each of the nine microtubule doublets can slide against another doublet. This ninefold arrangement is conserved in animal evolution and found across many groups. These microtubules form the cilium skeleton that is named the axoneme, and they are the cilium’s most fundamental structural element. More details on cilium motility can be found in Downing and Sui (2007).
In addition to cell motility, cilia function as a cell receiver or antenna in cell signaling (Malicki and Johnson 2017). In many of these cases, the cilia are immotile and the dynein arms are missing. In order to be an efficient signaling device, the cilium is compartmentalized from the rest of the cell by a cilium gate and the cilium transport machinery allows entry of specific ciliary cargo. The cilium gate (aka transition zone in general or annulus in sperm cells) and the cilium transport machinery (aka intraflagellar transport) are built around and travel along the axoneme microtubules. The cilium gate connects the microtubule doublets and the ciliary membrane to form a barrier between both the cilioplasm and cytoplasm, and the cilium membrane and cell membrane. More details on cilium gate and cilium transport machinery can be found in Malicki and Avidor-Reiss (2014). The critical point to our discussion is that cilia mediate signals utilizing an axoneme made of microtubule doublets organize in ninefold symmetry.
During cilium formation, the centriolar microtubules extend to form the cilium microtubules. Therefore, the centriole’s microtubules dictate the symmetry of the axoneme microtubules, which are critical to the cilium’s motility and signaling function. Because the centriole’s structure has such an important role in axoneme structure, it makes sense that centriole structure is highly conserved throughout evolution.
Centriole assembly is conserved in protists, invertebrates, and vertebrates (Azimzadeh 2014). The new centriole initially forms as a cartwheel structure surrounded by electron dense material at the base of the preexisting centriole, near to the wall (Fig. 1.1b–i,ii). Next, microtubules are built around the cartwheel to create the procentriole. First, the A microtubules are formed and later the B and C microtubules. The completed procentriole structure is 200 nm long and 200 nm wide, including the wall made of nine microtubule triplets and a centriole lumen filled by the cartwheel. The formation of the cartwheel and procentriole usually happens in the early S phase of the cell cycle and is very rapid. The next step in centriole formation is the elongation of the centriole, which starts in the G2 phase of the cell cycle. In this stage, the microtubules of the centriole elongate to about 400–500 nm in length. The cartwheel does not elongate and is restricted to the base of the centriole. Finally, the cartwheel is eliminated from the centriole base and the distal lumen is formed, which has a distinct structure composed of rings and columns (Fig. 1.1b–iii). Altogether, centriole formation is a step-by-step process in which a cartwheel forms, then develops to become a procentriole, and further matures into a centriole.
1.4. Centriole Neoteny in Sperm Cells
During development, certain traits can be advantageous to a young animal, but those same traits become a detriment when the animal reaches maturity so they are replaced by adult features. Neoteny describes the inverse; it is a biological phenomenon where an adult animal retains juvenile features, presumably because those features remain advantageous (Gould 1977). The classic example of neoteny in an organism is the Ambystoma mexicanum, or axolotl, a species of salamander. Most salamanders start their life as a larva; at this stage, they live in water, and have external gills, and a caudal fin. They then develop into an adult form that lives on land and breathes air. However, unlike other salamanders, the adult axolotl retains some larval characteristics as it matures, it continues living in water, and has external gills, and a caudal fin (Rosenkilde and Ussing 1996).
Identifying neoteny in nature is useful because it provides insight into the type of evolutionary changes that led to the morphology of an animal and is likely linked to developmental genes. Here we propose that the term neoteny has a broader application and can be applied to subcellular structures that retain immature features in an otherwise mature subcellular system. We hypothesize that these structures may also exhibit neoteny by arresting early in certain specialized cells. We propose that the centriole found in insect sperm cells is a neotenic subcellular structure.
In most animals, round spermatids (haploid cells that differentiate to form spermatozoa) have two mature centrioles, named the distal centriole and the proximal centriole (Avidor-Reiss et al. 2015). However, insect spermatids for a long time were thought to have only one centriole, the distal centriole, which has the typical barrel-shaped structure with a microtubule wall. Recently, an early form of the procentriole was identified in the insect spermatid near the distal centriole (Khire et al. 2016; Blachon et al. 2014; Gottardo et al. 2015; Dallai et al. 2017; Fishman et al. 2017) (Fig. 1.1a–ii). This structurally immature form of sperm centriole was named the proximal centriole-like structure or PCL and may represent an example of subcellular neoteny; the structure maintains juvenile traits while the sperm itself matures from spermatid to spermatozoon. During spermatid differentiation, both the distal centriole and the PCL undergo remodeling that further modifies their structure (Fig. 1.1c). Both centrioles are deposited in the egg after fertilization and both function in zygotes like mature centrioles, which include nucleating new centrioles.
When neoteny is exhibited, it is thought that the halt of development is evolutionarily beneficial. In the case of humans, neoteny may provide more time to increase brain size after birth and more time to develop social skills (Skulachev et al. 2017; Bufill et al. 2011). The reason for sperm centriole neoteny is not yet clear, but it may be an advantage for sperm to have an immature centriole when competing with other sperm trying to fertilize the egg. The smaller size of the centriole does not deform the neck of the sperm, thus improving motility. Neoteny, like other evolutionary changes in development, is mainly thought to be a result of mutations in the regulation of genes that control development, but the precise mutations are not known. Similarly, the centriole neoteny that forms the PCL may be due to mutations in genes that control the development of centrioles in the sperm. One potential gene to mediate PCL neoteny is the gene poc1 (see Sect. 1.6).
1.5. Centriole Hypermorphosis in Sperm Cells
Adult animals exhibit certain traits that are characteristic of their maturation. Hypermorphosis is a biological phenomenon where development is extended, for example, by the addition of new developmental stages at the end of the ancestral development sequence. The common example of hypermorphosis is the enlargement of a body part relative to the rest of the body, such as the large antlers of reindeer or the large upper canine teeth of saber-toothed tigers. Interestingly, it was proposed that hypermorphosis may be a mechanism for the evolution of male weaponry (Kelly and Adams 2010). Similar to animal development, subcellular structures can also have developmental programs that reach a “mature” state, which then could be extended. Here, we propose that the centrioles found in mammalian sperm cells exhibit hypermorphosis.
In most animals, a spermatozoon has two centrioles, each with typical mature centrioles morphology (Avidor-Reiss et al. 2015). However, most mammalian spermatozoon only has one typical centriole, the proximal centriole. Recently, a distinctly shaped centriole was identified in the spermatozoon of non-rodent mammals (Fishman et al. 2018; Avidor-Reiss and Fishman 2018) (Fig. 1.1d–iv). This shape results from the remodeling of the distal centriole during spermatid differentiation. Both centrioles, the typical centriole and the atypical centriole, are deposited in the egg after fertilization, and both function in the zygote like mature centrioles, which includes forming centrosomes and nucleating new centrioles. A more moderate form of distal centriole remodeling is observed in insects (Khire et al. 2016; Dallai et al. 2018; Fishman et al. 2017). We propose that the alteration of the distal centriole’s structure is due to the addition of new developmental stages after the end of normal centriole maturation when the sperm is maturing from spermatid to spermatozoon and, therefore, is an example of centriolar hypermorphosis (Fig. 1.1c).
Sperm centriolar hypermorphosis can take several forms in various animal groups. Compared to other mammals, the rodent spermatozoon’s distal centriole is further modified, resulting in the apparent degeneration of the DC. Furthermore, the rodent spermatozoon’s proximal sperm centriole is also degenerated after it is fully formed (Simerly et al. 2016). Similarly, in insects, the neotenic proximal centriole, the PCL, undergoes further remodeling after its neotenic formation is finished, suggesting that the PCL is a product of two heterochronic processes: neoteny and hypermorphosis. Currently, it is unclear if the two processes evolved together, or one after the other.
1.6. The Genetic and Molecular Control of Centriole Heterochrony
In the last two decades, some progress has been made in understanding the molecular changes underlying heterochrony, but the complexity of studying whole animal development presents a major barrier to that progress (Keyte and Smith 2014). Centriole assembly is much simpler than animal development and may provide some insight into the understanding of the molecular basis of heterochrony. It would also be interesting to compare the molecular basis of heterochrony at a subcellular level and at the whole animal level to determine if there are general rules that affect developmental timing. Here, we suggest that the appearance of a neotenic centriole in flies is linked to a change in the essential function of the gene Protein of Centriole 1 (poc1), based on the comparison of the molecular pathways that form the PCL and the centriole.
Poc1 is a family of proteins that is evolutionarily conserved and is found throughout the eukaryotic tree of life suggesting it was present in the ancestral eukaryote that had a centriole (Hodges et al. 2010). Poc1 family members are found only in eukaryotes that have centrioles, pointing to its specific role in centriole biology. However, Poc1 members are absent in some eukaryotes, such as nematodes, indicating it is not one of the core essential centriole proteins. In vertebrates, the Poc1 family is made of two genes (POC1A and POC1B), in invertebrates the Poc1 family is made of one gene, poc1. In flies, the poc1 gene codes for two splice isoforms: Poc1A, which localizes to the typical centriole (the DC), and Poc1B, which localizes to the atypical centriole (the PCL) (Khire et al. 2016). Depletion of Poc1 proteins in human cells and fly sperm results in short centrioles that are unstable, hinting that Poc1 is essential after the initial formation of the procentriole (Keller et al. 2009; Pearson et al. 2009; Blachon et al. 2009). In fly sperm, Poc1 depletion also results in an abnormal looking PCL (Khire et al. 2015).
The placement of Poc1 proteins in the molecular pathway of centriole assembly was studied based on whether Poc1 was required or dispensable for the localization of other centriolar proteins to the centriole. In the centriole of human cells, the last steps in centriole assembly are Centrosomal Protein 135 (CEP135), which recruits Centrosomal Protein 295 (CEP295), which then recruits Protein of Centriole 1B (POC1B) (Chang et al. 2016) (Fig. 1.1e). In the fly PCL, the order of recruitment seems to be reversed; the fly ortholog gene of human POC1B (Poc1B) is essential for the recruitment of the fly CEP295 protein ortholog Anastral spindle 1 (Ana1) and the fly CEP135 protein ortholog Bald 10 (Bld10) (Fig. 1.1e) (Blachon et al. 2009). Together, these studies suggest that Poc1B gained a new essential early function in the centriole formation pathway in flies that is not observed in human typical centrioles. This new essential function may allow the cartwheel to be a stable structure and become the PCL, instead of being an intermediate structure that normally continues to develop into a stable centriole. To test this hypothesis, it would be critical to determine this new essential function more precisely.
One insight into the origin of Poc1’s essential function in the early centriole is its localization during early centriole formation. Poc1 is recruited to the procentriole and localizes to the cartwheel in Tetrahymena thermophila, a ciliated protozoan, although Poc1 does not appear to have an essential function at that stage (Pearson et al. 2009). Therefore, one possible scenario is that Poc1 was recruited to the cartwheel by an ancestral mechanism, and the nonessential function of Poc1 evolved to an essential function in the fly. The Poc1 recruitment mechanism and the molecular change that made Poc1 essential are currently unknown. Altogether, small perturbations in proteins already functioning in the centriole (possibly through the generation new splice isoforms) may be the mechanism of centriole heterochrony.
1.7. Conclusions
Heterochrony, neoteny, and hypermorphosis are useful concepts for the study of the evolution of centrioles and other subcellular structures. Here, using these terms enables us to describe the different types of changes that occur in the centriole assembly pathway resulting in the formation of an atypical centriole shape. This creates a conceptual framework to study the evolution of the centriole. The future challenge is to understand the genetic and molecular basis of centriole heterochrony. The molecular pathway that assembles centrioles is extensively studied in a variety of eukaryotes that are amenable for genetic analysis, including vertebrates, invertebrates, and protists. Therefore, in the future we should be able to draw the ancestral pathway of centriole assembly and the step-by-step evolutionary changes that produce a variety of diverse centriole forms.
Acknowledgements
We would like to thank Lilli Fishman for her assistance in preparing the manuscript. This work was supported by grant R03 HD087429 and R21 HD092700 from Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD).
Footnotes
Conflict of Interest The authors declare that they do not have any conflicts of interest.
Contributor Information
Tomer Avidor-Reiss, Department of Biological Sciences, University of Toledo, Toledo, OH, USA.
Katerina Turner, Department of Biological Sciences, University of Toledo, Toledo, OH, USA.
References
- Anderson DJ (1989) Cellular ‘neoteny’: a possible developmental basis for chromaffin cell plasticity. Trends Genet 5(6):174–178 [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss T (2018) Rapid evolution of sperm produces diverse centriole structures that reveal the most rudimentary structure needed for function. Cells 7(7):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Fishman EL (2018) It takes two (centrioles) to Tango. Reproduction. 10.1530/REP-18-0350 [DOI] [PMC free article] [PubMed]
- Avidor-Reiss T, Khire A, Fishman EL, Jo KH (2015) Atypical centrioles during sexual reproduction. Front Cell Dev Biol 3:21 10.3389/fcell.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J (2014) Exploring the evolutionary history of centrosomes. Philos Trans R Soc Lond B Biol Sci 369(1650). 10.1098/rstb.2013.0453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S, Cai X, Roberts KA, Yang K, Polyanovsky A, Church A, Avidor-Reiss T (2009) A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 182(1):133–144. 10.1534/genetics.109.101709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S, Khire A, Avidor-Reiss T (2014) The origin of the second centriole in the zygote of Drosophila melanogaster. Genetics 197(1):199–205. 10.1534/genetics.113.160523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M (2012) The centrosome in cells and organisms. Science 335(6067):422–426. 10.1126/science.1209037 [DOI] [PubMed] [Google Scholar]
- Bufill E, Agusti J, Blesa R (2011) Human neoteny revisited: the case of synaptic plasticity. Am J Hum Biol 23(6):729–739. 10.1002/ajhb.21225 [DOI] [PubMed] [Google Scholar]
- Chang CW, Hsu WB, Tsai JJ, Tang CJ, Tang TK (2016) CEP295 interacts with microtubules and is required for centriole elongation. J Cell Sci 129(13):2501–2513. 10.1242/jcs.186338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza MR, Cazzola A, Rossberg A, Schieber NL, Konotop G, Bausch E, Slynko A, Holland-Letz T, Raab MS, Dubash T, Glimm H, Poppelreuther S, Herold-Mende C, Schwab Y, Kramer A (2017) Asymmetric centriole numbers at spindle poles cause chromosome missegregation in cancer. Cell Rep 20(8):1906–1920. 10.1016/j.celrep.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Dallai R, Mercati D, Lino-Neto J, Dias G, Lupetti P (2017) Evidence of a procentriole during spermiogenesis in the coccinellid insect Adalia decempunctata (L): an ultrastructural study. Arthropod Struct Dev 46(6):815–823. 10.1016/j.asd.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Dallai R, Mercati D, Lino-Neto J, Dias G, Folly C, Lupetti P (2018) The peculiar structure of the flagellar axoneme in Coccinellidae (Insecta-Coleoptera). Arthropod Struct Dev. 10.1016/j.asd.2018.11.004 [DOI] [PubMed]
- Dias G, Lino-Neto J, Dallai R (2015) The sperm ultrastructure of Stictoleptura cordigera (Fussli, 1775) (Insecta, Coleoptera, Cerambycidae). Tissue Cell 47(1):73–77. 10.1016/j.tice.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Dobzhansky T (1973) Nothing in biology makes sense except in the light of evolution. Am Biol Teacher 35(3):125–129. 10.2307/4444260 [DOI] [Google Scholar]
- Downing KH, Sui H (2007) Structural insights into microtubule doublet interactions in axonemes. Curr Opin Struct Biol 17(2):253–259. 10.1016/j.sbi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- Firat-Karalar EN, Stearns T (2014) The centriole duplication cycle. Philos Trans R Soc Lond B Biol Sci 369(1650). 10.1098/rstb.2013.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman EL, Jo K, Ha A, Royfman R, Zinn A, Krishnamurthy M, Avidor-Reiss T (2017) Atypical centrioles are present in Tribolium sperm. Open Biol 7(3). 10.1098/rsob.160334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman EL, Jo K, Nguyen QPH, Kong D, Royfman R, Cekic AR, Khanal S, Miller AL, Simerly C, Schatten G, Loncarek J, Mennella V, Avidor-Reiss T (2018) A novel atypical sperm centriole is functional during human fertilization. Nat Commun 9(1):2210 10.1038/s41467-018-04678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo M, Callaini G, Riparbelli MG (2015) Structural characterization of procentrioles in Drosophila spermatids. Cytoskeleton (Hoboken) 72(11):576–584. 10.1002/cm.21260 [DOI] [PubMed] [Google Scholar]
- Gould SJ (1977) Ontogeny and phylogeny. Harvard University Press, Cambridge [Google Scholar]
- Guichard P, Desfosses A, Maheshwari A, Hachet V, Dietrich C, Brune A, Ishikawa T, Sachse C, Gonczy P (2012) Cartwheel architecture of Trichonympha basal body. Science 337(6094):553 10.1126/science.1222789 [DOI] [PubMed] [Google Scholar]
- Hodges ME, Scheumann N, Wickstead B, Langdale JA, Gull K (2010) Reconstructing the evolutionary history of the centriole from protein components. J Cell Sci 123(Pt 9):1407–1413. 10.1242/jcs.064873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana SC, Bettencourt-Dias M, Durand B, Megraw TL (2016) Drosophila melanogaster as a model for basal body research. Cilia 5:22 10.1186/s13630-016-0041-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana SC, Mendonca S, Machado P, Werner S, Rocha J, Pereira A, Maiato H, Bettencourt-Dias M (2018) Differential regulation of transition zone and centriole proteins contributes to ciliary base diversity. Nat Cell Biol 20(8):928–941. 10.1038/s41556-018-0132-1 [DOI] [PubMed] [Google Scholar]
- Keller LC, Geimer S, Romijn E, Yates J III, Zamora I, Marshall WF (2009) Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol Biol Cell 20(4):1150–1166. 10.1091/mbc.E08-06-0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CD, Adams DC (2010) Sexual selection, ontogenetic acceleration, and hypermorphosis generates male trimorphism in Wellington Tree Weta. Evol Biol 37(4):200–209. 10.1007/s11692-010-9096-1 [DOI] [Google Scholar]
- Keyte AL, Smith KK (2014) Heterochrony and developmental timing mechanisms: changing ontogenies in evolution. Semin Cell Dev Biol 34:99–107. 10.1016/j.semcdb.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khire A, Vizuet AA, Davila E, Avidor-Reiss T (2015) Asterless reduction during spermiogenesis is regulated by Plk4 and is essential for zygote development in Drosophila. Curr Biol 25 (22):2956–2963. 10.1016/j.cub.2015.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khire A, Jo KH, Kong D, Akhshi T, Blachon S, Cekic AR, Hynek S, Ha A, Loncarek J, Mennella V, Avidor-Reiss T (2016) Centriole remodeling during spermiogenesis in Drosophila. Curr Biol 26(23):3183–3189. 10.1016/j.cub.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B, Maier B, Fuchs F, Chi J, Riffel P, Anderhub S, Wagner L, Ho AD, Salisbury JL, Boutros M, Kramer A (2010) Proteins required for centrosome clustering in cancer cells. Sci Transl Med 2(33):33ra38 10.1126/scitranslmed.3000915 [DOI] [PubMed] [Google Scholar]
- Lupold S, Pitnick S (2018) Sperm form and function: what do we know about the role of sexual selection? Reproduction 155(5):R229–R243. 10.1530/REP-17-0536 [DOI] [PubMed] [Google Scholar]
- Malicki J, Avidor-Reiss T (2014) From the cytoplasm into the cilium: bon voyage. Organogenesis 10(1):138–157. 10.4161/org.29055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki JJ, Johnson CA (2017) The cilium: cellular antenna and central processing unit. Trends Cell Biol 27(2):126–140. 10.1016/j.tcb.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara KJ, McKinney ML (2005) Heterochrony, disparity, and macroevolution. Paleobiology 31(S2):17–26 [Google Scholar]
- Mordhorst BR, Wilson ML, Conant GC (2016) Some assembly required: evolutionary and systems perspectives on the mammalian reproductive system. Cell Tissue Res 363(1):267–278. 10.1007/s00441-015-2257-x [DOI] [PubMed] [Google Scholar]
- Nigg EA, Raff JW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139 (4):663–678. 10.1016/j.cell.2009.10.036. S0092-8674(09)01362-2 [pii] [DOI] [PubMed] [Google Scholar]
- Pearson CG, Osborn DP, Giddings TH Jr, Beales PL, Winey M (2009) Basal body stability and ciliogenesis requires the conserved component Poc1. J Cell Biol 187(6):905–920. 10.1083/jcb.200908019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S (2016) Mechanisms of mitotic spindle assembly. Annu Rev Biochem 85:659–683. 10.1146/annurev-biochem-060815-014528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DM (1967) Giant centriole formation in Sciara. J Cell Biol 33(1):73–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton JS, Cuningham JC, Peifer M (2014) Acentrosomal Drosophila epithelial cells exhibit abnormal cell division, leading to cell death and compensatory proliferation. Dev Cell 30 (6):731–745. 10.1016/j.devcel.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser SL, Pelletier L (2017) Mitotic spindle assembly in animal cells: a fine balancing act. Nat Rev Mol Cell Biol 18(3):187–201. 10.1038/nrm.2016.162 [DOI] [PubMed] [Google Scholar]
- Riparbelli MG, Dallai R, Callaini G (2010) The insect centriole: a land of discovery. Tissue Cell 42 (2):69–80. 10.1016/j.tice.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Rosenkilde P, Ussing AP (1996) What mechanisms control neoteny and regulate induced metamorphosis in urodeles? Int J Dev Biol 40(4):665–673 [PubMed] [Google Scholar]
- Simerly C, Castro C, Hartnett C, Lin CC, Sukhwani M, Orwig K, Schatten G (2016) Post-testicular sperm maturation: centriole pairs, found in upper epididymis, are destroyed prior to sperm’s release at ejaculation. Sci Rep 6:31816 10.1038/srep31816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP, Holtze S, Vyssokikh MY, Bakeeva LE, Skulachev MV, Markov AV, Hildebrandt TB, Sadovnichii VA (2017) Neoteny, prolongation of youth: from naked mole rats to “naked apes” (humans). Physiol Rev 97(2):699–720. 10.1152/physrev.00040.2015 [DOI] [PubMed] [Google Scholar]
- Sluder G (2016) Using sea urchin gametes and zygotes to investigate centrosome duplication. Cilia 5(1):20 10.1186/s13630-016-0043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KK (2002) Sequence heterochrony and the evolution of development. J Morphol 252 (1):82–97 [DOI] [PubMed] [Google Scholar]
- Tang N, Marshall WF (2012) Centrosome positioning in vertebrate development. J Cell Sci 125 (Pt 21):4951–4961. 10.1242/jcs.038083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanadha R, Sale WS, Porter ME (2017) Ciliary motility: regulation of axonemal dynein motors. Cold Spring Harb Perspect Biol 9(8). 10.1101/cshperspect.a018325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, O’Toole E (2014) Centriole structure. Philos Trans R Soc Lond B Biol Sci 369(1650). 10.1098/rstb.2013.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]


