Abstract
Contrast-induced nephropathy (CIN) or contrast-induced acute kidney injury (CI-AKI) is an iatrogenic acute kidney injury observed after intravascular administration of contrast media for intravascular diagnostic procedures or therapeutic angiographic intervention. High risk patients including those with chronic kidney disease (CKD), diabetes mellitus with impaired renal function, congestive heart failure, intraarterial intervention, higher volume of contrast, volume depletion, old age, multiple myeloma, hypertension, and hyperuricemia had increased prevalence of CIN. Although CIN is reversible by itself, some patients suffer this condition without renal recovery leading to CKD or even end-stage renal disease which required long term renal replacement therapy. In addition, both CIN and CKD have been associated with increasing of mortality. Three pathophysiological mechanisms have been proposed including direct tubular toxicity, intrarenal vasoconstriction, and excessive production of reactive oxygen species (ROS), all of which lead to impaired renal function. Reports from basic and clinical studies showing potential preventive strategies for CIN pathophysiology including low- or iso-osmolar contrast media are summarized and discussed. In addition, reports on pharmacological interventions to reduce ROS and attenuate CIN are summarized, highlighting potential for use in clinical practice. Understanding this contributory mechanism could pave ways to improve therapeutic strategies in combating CIN.
Keyword: Contrast-induced nephropathy, Oxidative stress, Mitochondria, Prevention, Statin
Introduction
Contrast-induced nephropathy (CIN) or contrast-induced acute kidney injury (CI-AKI) is an iatrogenic acute kidney injury (AKI) observed after intravascular administration of contrast media (CM) for diagnostic procedures or therapeutic angiographic interventions [1–4]. Chemical hypersensitivity has also been reported as another side effect of CM [5]. According to the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines for AKI, a serum creatinine (Cr) increase of at least 0.3 mg/dL (or 26.5 µmol/L) over the baseline value within 48 h after exposure to CM, or an increase greater than 1.5 times over the baseline value within 7 days after exposure to CM, or a urinary volume of less than 0.5 mL/kg/h for at least 6 h after exposure, are the definition of this condition [6]. Incidence of CIN has been reported in 1–25% of cases of hospital-acquired AKI, and is the third common cause of acute tubular necrosis in hospitalized patients leading to prolonged hospitalization [1]. In the general population, the CIN incidence is 1–2% [7]. Although CIN can be reversible, up to 15% of the patients may need temporary dialysis [8]. In patients without renal recovery, CKD can develop 4% progressing to end-stage renal disease (ESRD) [9]. The mortality rate of CIN varies from 3.8 to 64% [10, 11]. Patients with high risk of developing CIN include chronic kidney disease (CKD) and diabetes mellitus (DM) with impaired renal function. Other associated risks include congestive heart failure, volume depletion, old age, hypertension, and hyperuricemia increasing CIN prevalence by up to 25% [7].
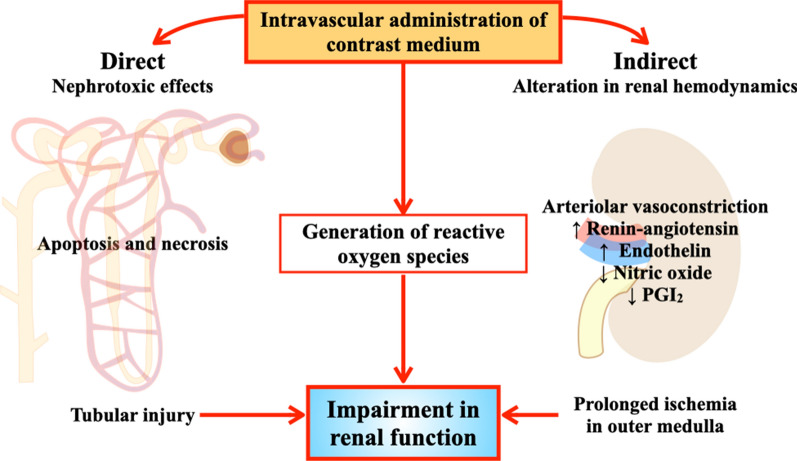
The pathophysiological mechanisms of CIN have not been completely elucidated. Currently, several mechanisms including direct effect, indirect effect, and generation of reactive oxygen species (ROS) have been proposed (Fig. 1). In direct effects, CM with high osmolality can directly cause cytotoxicity in nephrons including renal tubular epithelial cells and endothelial cells, leading to mitochondrial dysfunction, cellular apoptosis or necrosis and interstitial inflammation [12]. In indirect effects, CM can alter renal hemodynamics, leading to intrarenal vasoconstriction contributing to medullary hypoxia [12]. Regarding ROS generation, CM can either cause excessive ROS production or reduce antioxidant enzyme activity, resulting in increased oxidative stress and leading to impaired renal function [13]. In addition, medullary hypoxia also leads to enhanced ROS formation, resulting in mitochondrial oxidative stress and mitochondrial dysfunction [13]. Overall, it can be seen that mitochondrial function and oxidative stress play important roles in the pathophysiology of CIN [13]. Therefore, strategies that reduce oxidative stress as well as protecting mitochondrial dysfunction are potential targets for CIN prevention.
Fig. 1.
Pathophysiology of CIN. Pathogenesis of CIN consists of 3 mechanisms; direct effect, indirect effect, and generation of ROS. Direct effects include, direct cytotoxicity of CM to nephron leading to cellular apoptosis or necrosis and tubular injury. Indirect effects are that CM could alter renal hemodynamics, leading to intrarenal vasoconstriction, contributing to medullary hypoxia. This mechanism is mediated by the increase in vasoconstrictive mediators including renin, angiotensin II, and endothelin along with the decreasing of vasodilatory mediators including nitric oxide and PGI2. Lastly, CM can generate ROS and also reduce antioxidant enzyme activity as a result of various complex mechanisms which result in oxidative stress, leading to progression of impaired renal function. CIN, contrast-induced nephropathy; CM, contrast media; PGI2, prostaglandin I2; ROS, reactive oxygen species
KDIGO clinical practice guidelines for AKI state there is no definitive treatment available for established CIN [6]. Thus, the prevention of CIN is the best option. This review aims to comprehensively summarize the available in vitro, in vivo, and clinical reports regarding the pathophysiologic roles of mitochondria and ROS in CIN. Reports on pharmacological interventions to prevent CIN by targeting ROS and mitochondria are also presented and discussed with their potential for clinical use in in the future.
Searching methodology and selection criteria
A comprehensive search of the literature was performed using PubMed covering the period from database inception to September 2019. The search for literature included only articles written in English. An article was rejected if it was clearly a letter or case report. The search used the following keywords: contrast-induced nephropathy; oxidative stress; mitochondria; prevention; and statin either in the title, abstracts, or in the text. The relevance of the subject and eligibility of all publications detected was further evaluated, and data were then extracted from relevant papers to be included in this comprehensive review.
Pathogenesis of CIN via ROS generation: reports from in vitro studies
In vitro studies offer the unique opportunity to evaluate the activation of intracellular signaling pathways involved in cellular apoptosis or necrosis, which could pave ways for developing specific therapies to be used in in vivo and clinical studies. However, a major shortcoming of preclinical models of CIN relates to the fact that contrast administration alone does not cause AKI in animals. Multiple stressors are required to be utilized concomitantly to inflict CIN; such as inhibition of nitric oxide (NO), dehydration, and use of prostaglandin inhibitor. A summary of findings from in vitro reports is shown in Table 1.
Table 1.
Roles of oxidative stress in the pathogenesis of contrast-induced nephropathy: reports from in vitro studies
| Models | Methods (drug/dose/route/duration) | Major findings | Interpretations | References | ||
|---|---|---|---|---|---|---|
| Oxidative stress | Apoptosis | Histopathology | ||||
| HK-2 cells | Iohexol/50, 100, 200 mg I/mL/6 h | ↑ LDH cell injury in dose-dependent manner |
↓ MTT cell viability (100 and 200 mg I/mL) ↑ annexin V-positive cells ↑ mRNA expression of intracellular Nox4 and p22phox |
Nuclear fragmentation Organelle reduction Mitochondrial vacuolar degeneration |
Iohexol upregulated expression of Nox4 and p22phox, induced cell injury, leading to CIN | [16] |
| Renal cortical slices isolated from male Fischer 344 (F344) rats |
Diatrizoic acid/0, 9.25, 18.5, 37, 74, 111 mg I/mL/60–120 min Iothalamic acid/0, 9.25, 18.5, 37, 74, 111 mg I/mL/60–120 min |
↑ LDH leakage in a dose-dependent manner ↔ cellular total GSH ↔ %GSSG |
– | – | Diatrizoic acid and iothalamic acid at clinically relevant concentrations caused damage to renal cortical slices | [18] |
| Human embryonic kidney 293 T cells |
Diatriazoate meglumine/11.1 mg I/mL/1, 2, 4, 6 h Iothalamate meglumine/11.1 mg I/mL/1, 2, 4, 6 h Iohexol/11.1 mg I/mL/1, 2, 4, 6 h Iodixanol/11.1 mg I/mL/1, 2, 4, 6 h |
– |
↑ ATF2 mRNA expression in a time-dependent manner (diatrizoate, iodixanol and iothalamate) ↑ phosphorylation of Thr69/71 of ATF2 in a time-dependent manner (diatrizoate and iothalamate) ↑ phosphorylation of JNK1 and JNK2 in a time-dependent manner (iodixanol, diatrizoate and iothalamate) ↑ cleaved caspase-3 (diatrizoate) ↓ cell viability (diatrizoate and siRNA transfection for ATFz2) |
– | Iodinated CM, except iohexol, activated JNK/ATF2 signaling pathways, and diatrizoate caused apoptosis in kidney cells | [19] |
| HK-2 cells | Iohexol + Nox4 siRNA |
↑ Nox2 and Nox4 mRNA expression ↑ ROS production ↓ ROS production (Nox4 siRNA) ↓ GPx and SOD (Nox4 siRNA) |
↑ caspase 3/7 activity ↓ caspase 3/7 activity (Nox4 siRNA) ↓ MTT and ATP cell viability ↑ MTT and ATP cell viability (Nox4 siRNA) ↑ MAPK pathways (phospho-p38, JNK and ERK pathways) ↓ phospho-p38, JNK and ERK (Nox4 siRNA) ↑ Bax ↓ Bax (Nox4 siRNA) |
– | CM increased ROS production by triggering induction of MAPKs, especially p38 via upregulation of Nox4 | [17] |
| NRK-52E rodent tubular cells | Iohexol/100 mg/mL/3 h + SIRT1 siRNA |
↓ SIRT1 ↓↓ SIRT1 in siRNA |
↓ MTS cell viability ↓↓ MTS cell viabilitzy in SIRT1 siRNA |
– | Iohexol decreased cell viability by downregulation of SIRT1 | [21] |
ATF2, activating transcriptional factor 2; Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma 2; CIN, contrast-induced nephropathy; CM, contrast media; ERK, extracellular signal-regulated kinase; GPx, glutathione peroxidase; GSH, glutathione; GSSG, glutathione disulfide; HK-2 cells, human proximal tubular epithelial cells; HO-1, heme oxygenase 1; HSA-Trx, recombinant human serum albumin-Thioredoxin-1 fusion protein; IV, intravenously; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; MAPKs, mitogen-activated protein kinases; MESNA, sodium-2-mercaptoethane sulphonate; MTS, 5-(3-carboxymethoxyphenyl)-2H-tetrazolium inner salt; MTT, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide; NADPH, nicotinamide adenine dinucleotide phosphate; Nox4, NADPH oxidases; NQO-1, NAD(P)H: quinone oxidoreductase 1; Nrf-2, nuclear factor erythroid 2-related factor 2; p22phox, p22 phagocyte B-cytochrome; PGC-1α, peroxisome proliferator-activated receptor-γ co-activator 1α; ROS, reactive oxygen species; siRNA, short interfering ribonucleic acid; SIRT1, sirtuin 1; SOD, superoxide dismutase
Roles of mitogen-activated protein kinase (MAPK) pathways in CIN
ROS induced by CM could activate the MAPK signaling pathway through 4 cascades, including extracellular signal-related kinases (ERK) 1 and 2, c-JUN N-terminal kinase (JNK) 1, 2, and 3, p38-MAPK, and ERK5 [14]. These pathways contribute to the activation of caspase-9 and caspase-3, thus inducing apoptosis [15]. In HK-2 cells, CM increased cell injury and decreased cell viability, leading to severe mitochondrial vacuolar degeneration and nucleus fragmentation [16]. CM also increased ROS production via upregulation of nicotinamide adenine dinucleotide phosphate oxidase 2 (Nox2), Nox4 and p22phox, [16] and triggered apoptosis via induction of caspase 3/7 activity, MAPK pathways (including p38, JNK and ERK pathways), and B-cell lymphoma 2-associated X protein (Bax) expression [17]. Transfection of Nox4 short interfering ribonucleic acid (siRNA) caused a reduction in ROS production and apoptosis [17]. These findings indicated that both Nox and MAPK pathways are involved in the CM-induced ROS production.
Different types of high-osmolar CM were studied in renal cortical cells isolated from male Fischer 344 rats (Table 1). CM was shown to induce renal cell injury in a dose-dependent manner regardless of type of high-osmolar CM [18]. In human embryonic kidney 293 T cells, CM activated JNK/activating transcriptional factor 2 (ATF2) signaling pathways and decreased cell viability. Transfection with ATF2 siRNA caused reduced apoptosis in those CM-treated cells [19]. These findings indicated that JNK/ATF2 pathways are involved in CM-induced ROS production (Table 1).
Roles of silent information regulator 1 (SIRT1) in CIN
SIRT1 is a histone deacetylase of nicotinamide adenine dinucleotide (NAD+), which mainly exists in the nucleus [20]. In NRK-52E rodent tubular cells, CM caused oxidative stress and decreased cell viability by downregulation of SIRT1 [21]. Transfection with SIRT1 siRNA resulted in increased apoptosis in these cells treated with CM [21]. These findings indicated that CM downregulated SIRT1, leading to increased cell apoptosis (Table 1).
Pathogenesis of CIN via ROS generation: reports from in vivo studies
Consistent with in vitro reports, data from in vivo studies demonstrated that CM increased ROS levels and apoptosis, leading to impaired renal function [19]. A summary of these in vivo reports is shown in Table 2.
Table 2.
Roles of oxidative stress in pathogenesis of contrast-induced nephropathy: reports from in vivo studies
| Animals | Models | Major findings | Interpretations | References | ||||
|---|---|---|---|---|---|---|---|---|
| Renal function | Oxidative stress | Inflammatory markers | Apoptosis | Histopathology | ||||
| Male BALB/c mice | Restricted water 24 h treated with iodixanol/IV/24 h |
↑ Cr ↑ BUN ↑ urinary NAG ↓ RBF |
↑ ROS ↑ 8-OHdG-positive cells ↓ SOD-1 ↔ SOD-2 |
↑ phospho- NF-kB p65 ↑ TNF-α ↑ IL-6 ↑ iNOS-positive cells |
↑ ROCK-2 protein ↑ p-MYPT1 and p-MYPT1/MYPT ratio ↑ TUNEL-positive cells ↑ cleaved caspase-3 ↑ Bax ↓ p-Akt/total Akt ratio |
Moderate tubular injury with tubular degeneration Loss of brush border membranes Formation of cast Vacuolization of tubular epithelial cells Dilation of tubules |
Iodixanol increased ROCK-2 activity, contributing to increased NF-kB transcriptional activity, oxidative stress, inflammation and apoptosis, leading to impaired renal function | [23] |
| Male C57BL/6 J mice | L-NAME/IP + indomethacin/IP treated with iohexol/IP/1 h | ↑ Cr |
↓ SIRT1 ↑↑ PGC-1α expression ↑ phosphor-Ser256 FoxO1 expression ↓ SOD2 ↑ MDA |
– |
↑ TUNEL-positive cells ↑ cleaved caspase-3 |
Tubular vacuolization Disruption of tubular structures in outer medulla ↑ macrophage infiltration |
Iohexol upregulated SIRT1-PGC-1α-FoxO1 signaling mediated oxidative stress, apoptosis, leading to impaired renal function | [21] |
| Male Sprague–Dawley rats | Dehydration 48 h treated with iohexol/IV/24 h |
↑ Cr ↑ BUN |
↑ 8-OHdG-positive cells ↑ MDA |
– |
↑ TUNEL-positive cells ↑ Nrf-2-positive cells ↑ p-Akt/Akt ↑ nuclear-Nrf-2 ↑ HO-1/Actin |
Severe tubular detachment Foamy degeneration of tubular cells |
CM upregulated PI3K/Akt/Nrf-2 pathway, leading to increased oxidative stress and apoptosis, leading to impaired renal function | [26] |
| Adult Sprague Dawley rats | Indomethacin/IV + L-NAME/IV treated with ioversol/IV/72 h |
↑ Cr ↑ BUN |
↑ MDA ↓ SOD |
– |
↑ Nrf-2/HO-1-positive cells ↑ HO-1-positive cells ↑ Nrf-2, NQO-1 and HO-1 gene expression ↑ Nrf-2 nuclear translocation ↑ HO-1 and NQO-1 protein levels |
Tubular necrosis Hemorrhagic casts |
Nrf-2/HO-1 pathway regulated adaptive cytoprotective responses to counteract tissue damage, oxidative stress and apoptosis caused by CM | [27] |
| Male Sabra rats (Wistar-derived colony) | Low sodium diet 7 d + indomethacin/IV treated with iothalamate/IV |
↑ Cr ↓ CrCl |
↑ O2− production |
↓ eNOS ↑ iNOS |
↑ HO-1 protein ↑ renal heme ↑ caspase-3 ↑ caspase-9 ↑ Bax ↓ Bcl-2 |
– | Increased level of HO-1 are protective against AKI due to CM exposure | [28] |
| Male C57BL/6 mice | Water deprivation 16 h + indomethacin/IP + L-NAME/IP treated with iohexol/24 h |
↑ BUN ↔ Cr ↑ KIM-1-positive cells |
↔ SOD ↔ Nox4 ↔ Nox1 ↑ Nox2 ↑ 8-OHdG-positive cells |
– |
↑ phospho-p38/p38 ↑ phospho-pJNK/pJNK ↑ phospho-ERK/ERK ↑ Bax ↓ Bcl-2 ↑ TUNEL-positive cells |
Tubular epithelial cell shedding Basement membrane nudity Vacuolar degeneration of tubular epithelial cells Protein casts Tubular dilation Loss of tubular brush borders Necrosis of partial tubular epithelial cells ↑ tubular pathological scores |
The Nox4/Nox2 axis was involved in the amplification of ROS production, apoptosis and CIN progression, leading to impaired renal function | [17] |
| Male Wistar rats | Diatrizoate/no dose provided/IV/1, 24 h | – | – | – | ↑ TUNEL-positive cells | – | Diatrizoate caused apoptosis, leading to impaired renal function | [19] |
AKI, acute kidney injury; Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma 2; CAG, coronary angiography; CIN, contrast-induced nephropathy; CM, contrast media; Cr, creatinine; CrCl, creatinine clearance; DNA, deoxyribonucleic acid; eNOS, endothelial nitric oxide; HO-1, heme oxygenase 1; iNOS, inducible nitric oxide synthase; KIM-1, kidney injury molecule-1; L-NAME, Nω-nitro-L-arginine methyl ester; MDA, malondialdehyde; MESNA, sodium-2-mercaptoethane sulphonate; NADPH, nicotinamide adenine dinucleotide phosphate; NF-kB, nuclear factor-kB; NO, nitric oxide; Nox4, NADPH oxidases; NQO-1, NAD(P)H: quinone oxidoreductase 1; Nrf-2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; TUNEL, terminal deoxynucleotide transferase dUTP nick end labeling; 8-OHdG, 8-hydroxy-2′-deoxyguanosine
Roles of MAPK and SIRT1 in CIN
In mice, CM administration activated the Nox4/Nox2 axis, resulting in increased ROS production, and involving the MAPK pathway (including p38, JNK and ERK pathways) resulting in apoptosis, leading to impaired renal function [17]. CM administration also downregulated SIRT1 and upregulated peroxisome proliferator-activated receptor gamma-assisted activating factor-1α-Forkhead-box transcription factor 1 (PGC-1α-FoxO1) signaling mediated oxidative stress and apoptosis, leading to impaired renal function (Table 2) [21].
Roles of Rho/Rho-kinase (Rho/ROCK) pathway in CIN
The Rho/ROCK pathway is an important regulator in vascular smooth muscle cell contraction, cell migration, proliferation and differentiation [22]. Administration of CM in mice increased Rho/ROCK pathway activity, contributing to increased nuclear factor-kB (NF-kB) transcriptional activity, oxidative stress, inflammation and apoptosis, finally resulting in impaired renal function (Table 2) [23].
Roles of nuclear factor erythroid 2-related factor 2/heme oxygenase 1 (Nrf-2/HO-1) pathway in CIN
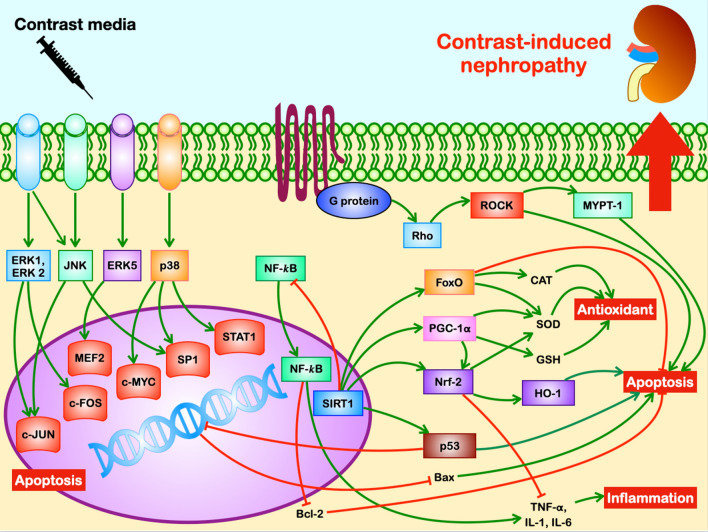
The Nrf-2/HO-1 pathway is involved in many functions including mitochondrial oxidative stress, autophagy, and programmed cell death [24]. Nrf-2, when translocated into the nucleus, stimulates transcription of genes that encode detoxifying and antioxidant enzymes, contributing to cellular protection by reducing oxidative stress [25]. In CM-treated rats, the Nrf-2/HO-1 pathway was upregulated to develop adaptive cytoprotective responses to counteract tissue damage, increased oxidative stress and apoptosis caused by CM (Table 2) [26–28]. Fig. 2 illustrates the mechanisms involved in the pathogenesis of CIN from in vitro and in vivo reports.
Fig. 2.
Mechanism of CIN via complex pathways of ROS from in vitro and in vivo studies. Contrast media can generate ROS especially in high risk patients such as DM and CKD through 4 major pathways: (1) MAPK pathway including ERK, JNK and p38; (2) SIRT1 pathway including SIRT1, FoxO, NF-kB, PGC-1 and p53; (3) Rho/ROCK pathway including MYPT-1 and NF-kB; (4) Nrf-2/HO-1 pathway including Nrf-2, NQO1, GSH and HO-1. CIN, contrast-induced nephropathy; CKD, chronic kidney disease; DM, diabetes mellitus; ERK, extracellular signal-related kinases; FoxO, Forkhead-box transcription factor; GSH, glutathione; JNK, c-JUN N-terminal kinase; MAPK, mitogen-activated protein kinase; MYPT-1, myosin-phosphatase target unit; NF-kB, nuclear factor-kB; NQO1, nicotinamide adenine dinucleotide phosphate quinone oxidoreductase 1; Nrf-2/HO-1, nuclear factor erythroid 2-related factor 2/heme oxygenase 1; PGC-1, peroxisome proliferator-activated receptor gamma-assisted activating factor-1; ROCK, rho-kinase; ROS, reactive oxygen species; SIRT1, silent information regulator 1
Interventions targeting ROS for CIN prevention: evidence from in vitro reports
In HK-2 cells [16] and MDCK cells, [29] atorvastatin attenuated CM-induced cytotoxicity through the downregulation of Nox4 and p22phox, and activation of MAPK pathways via JNK and tumor suppressor p53 activation [16, 29]. GKT137831, a specific Nox1/4 inhibitor, also decreased Nox2 expression, leading to decreased ROS production, and reduced apoptosis via decreasing caspase 3/7 activity and Bax along with activating the phosphorylation of p38, JNK and ERK [17].
Resveratrol, a known SIRT1 activator, was shown to increase SIRT1, PGC-1α expression, and superoxide dismutase 2 (SOD2), and increased cell viability in NRK-52E rodent tubular cells [21]. These findings indicated that resveratrol attenuated CM-induced nephrotoxicity via activating SIRT1-PGC-1α-FoxO1 signaling, leading to reduced oxidative stress and apoptosis [21]. In addition, Sulforaphane, an Nrf-2 activator, decreased ROS production and increased cell viability in HK-2 cells [27]. These reports are summarized in Table 3 and Fig. 3.
Table 3.
Interventions to attenuate oxidative stress in contrast-induced nephropathy: reports from in vitro studies
| Models | Methods (drug/dose/route/duration) | Major findings | Interpretations | References | ||
|---|---|---|---|---|---|---|
| Oxidative stress | Apoptosis | Histopathology | ||||
| HK-2 cells treated with iohexol/200 mg I/mL/6 h | Atorvastatin/1, 20, 40 µM/2 h prior to iohexol | – |
↑ MTT cell viability ↓ annexin V-positive cells ↓ mRNA expression of intracellular NOX4 and p22phox All effects by atorvastatin 40 µM |
↓ nuclear fragmentation ↓ organelle reduction ↓ mitochondrial vacuolar degeneration |
Atorvastatin attenuated iohexol-induced cytotoxicity through downregulation of NOX4 and p22phox | [16] |
| MDCK cells & HK-2 cells treated with iodixanol/200 mg/mL/3 h | Atorvastatin/0.2 µmol/L/12 h prior to iodixanol | – |
↑ MTS cell viability ↓ caspase-3 ↓ JNK ↓ p53 phosphorylation |
– | Pretreatment with atorvastatin reduced contrast-induced JNK activation, leading to apoptosis | [29] |
| HK-2 cells treated with iohexol 150 mg I/mL/12 h | Specific Nox1/4 inhibitor (GKT137831)/20 µg/mL/30 min prior to iohexol |
↑ Nox2 and Nox4 mRNA expression ↓ ROS production |
↓ caspase 3/7 activity ↑ MTT and ATPlite cell viability ↓ MAPK pathways (phospho-p38, JNK and ERK pathways) ↓ Bax |
– | Inhibition of Nox4 activity attenuated CIN | [17] |
| NRK-52E rodent tubular cells treated with iohexol/100 mg/mL/3 h | Resveratrol/10, 50 µmol/24 h prior to iohexol |
↑ SIRT1 ↑ PGC-1α expression ↑ SOD2 |
↑ MTT cell viability | – | Resveratrol attenuated iohexol-induced nephrotoxicity via activating SIRT1-PGC-1α-FoxO1 signaling, leading to reduced oxidative stress and apoptosis | [21] |
| HK-2 cells treated with ioversol/50 mg/mL/24 h | Sulforaphane (Nrf-2 activator)/5 µmol/L/30 min prior to ioversol | ↓ ROS production |
↑ MTT cell viability ↑ Nrf-2, NQO-1 and HO-1 gene expression ↔ MTT cell viability in Nrf-2 siRNA |
– | Renoprotective effect of sulforaphane in ioversol-induced nephrotoxicity was associated with Nrf-2/HO-1 pathway | [27] |
| HK-2 cells treated with H2O2/250 µM/L/3, 24 h | HSA-Trx/0.1, 0.5, 1, 5, 10 µmol/L/1 h prior to H2O2 | ↓ ROS production in a dose-dependent manner | ↓ WST-8-positive cells in a dose-dependent manner | – | HSA-Trx attenuated oxidative stress and inflammation in CIN | [37] |
| HK-2 cells treated with H2O2/500 µmol/L/24 h | Magnolin/10, 40 µg/mL/prior to H2O2 | ↓ ROS |
↓ caspase-3 ↑ Bcl-2 |
– | Magnolin attenuated oxidative stress and apoptosis | [49] |
| HK-2 cells treated with H2O2/250 mM/3, 24 h |
Salvianolic acid B/50 µM/1 h prior to H2O2 Wortmannin (PI3K inhibitor)/10 µM/1 h prior to H2O2 |
↓ ROS production |
↑ MTT cell viability ↑ CCK-8 cell viability ↑ p-Akt and nuclear-Nrf-2 expression (salvianolic acid B) ↓ p-Akt and nuclear-Nrf-2 expression (wortmannin) |
– | Salvianolic acid B attenuated oxidative stress and provided cell protection via PI3K/Akt/Nrf-2 pathway | [26] |
ATF2, activating transcriptional factor 2; Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma 2; CIN, contrast-induced nephropathy; ERK, extracellular signal-regulated kinase; GPx, glutathione peroxidase; GSH, glutathione; GSSG, glutathione disulfide; HK-2 cells, human embryonic proximal tubular epithelial cells; HO-1, heme oxygenase 1; HSA-Trx, recombinant human serum albumin-Thioredoxin-1 fusion protein; IV, intravenously; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; MAPKs, mitogen-activated protein kinases; MDCK cells, Madin Darby distal nonhuman tubular epithelial cells; MESNA, sodium-2-mercaptoethane sulphonate; MTS, 5-(3-carboxymethoxyphenyl)-2H-tetrazolium inner salt; MTT, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide; NADPH, nicotinamide adenine dinucleotide phosphate; Nox4, NADPH oxidases; NQO-1, NAD(P)H: quinone oxidoreductase 1; Nrf-2, nuclear factor erythroid 2-related factor 2; p22phox, p22 phagocyte B-cytochrome; PGC-1α, peroxisome proliferator-activated receptor-γ co-activator 1α; ROS, reactive oxygen species; siRNA, short interfering ribonucleic acid; SIRT1, sirtuin 1; SOD, superoxide dismutase
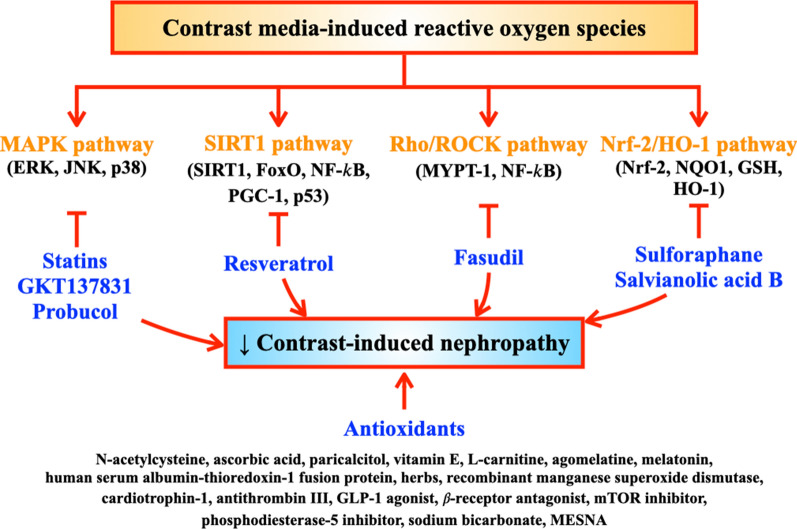
Fig. 3.
Intervention to reduce ROS for the prevention of CIN: evidence from in vitro, in vivo and clinical studies. In response to the mechanisms involved in ROS production in CIN, interventions to reduce ROS via complex pathways are illustrated. The MAPK pathway was inhibited by statins, GKT137831 and probucol. The SIRT1 pathway was inhibited by resveratrol. Rho/ROCK pathway was inhibited by fasudil. The Nrf-2/HO-1 pathway was inhibited by sulforaphane and salvianolic acid B. Antioxidant agents reported to exert benefits in CIN prevention have also been shown in this figure. CIN, contrast-induced nephropathy; GLP-1, glucagon-like peptide-1; MAPK, mitogen-activated protein kinase; MESNA, sodium-2-mercaptoethane sulphonate; mTOR, mammalian target of rapamycin; Nrf-2/HO-1, nuclear factor erythroid 2-related factor 2/heme oxygenase 1; ROCK, rho-kinase; ROS, reactive oxygen species; SIRT1, silent information regulator
Interventions targeting ROS for CIN prevention: evidence from in vivo reports
Interventions targeting MAPK, SIRT1, Rho/ROCK and Nrf-2/HO-1 pathways
In mice, GKT137831 could ameliorate oxidative stress via increased SOD and decreased Nox2, reducing apoptosis through the phosphorylation of p38, JNK, ERK, thus resulting in improved renal function [17]. Resveratrol was shown to attenuate CIN in rats via increasing SIRT1, PGC-1α, and SOD2, and decreasing phosphorylation of Ser256 FoxO1 expression, leading to a reduction in oxidative stress, apoptosis, improving renal function [21].
Fasudil, a Rho kinase inhibitor, was shown to decrease ROS and increase SOD-1, and reduced Inflammation via the reduction of NF-kB p65, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [23]. Moreover, apoptosis was decreased via a reduction in cleaved caspase-3 and Bax, together with increased B-cell lymphoma-2 (Bcl-2) and p-Akt/total Akt ratio. These benefits on ROS and apoptosis attenuation led to improved renal function [23]. Similarly, sulforaphane was shown to exert CIN protection in rats via the Nrf-2/HO-1 pathway, resulting in reduced renal damage and improved Cr [27]. Salvianolic acid B, a component of Danshen (Salvia miltiorrhiza root), attenuated CIN in rats via decreasing malondialdehyde (MDA) and increasing Nrf-2-positive cells, p-Akt/Akt, Nrf-2/Histone H3, and HO-1/Actin, with antioxidative effects through PI3K/Akt/Nrf2 pathway, leading to improved renal function [26]. Table 4 and Fig. 3 show a summary of these reports.
Table 4.
Interventions to attenuate oxidative stress in contrast-induced nephropathy: reports from in vivo studies
| Animals | Models | Intervention (drug/dose/route/duration) | Major findings | Inter-pretations | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Renal function | Oxidative stress | Inflammatory markers | Apoptosis | Histo-pathology | |||||
| Streptozotocin-induced diabetes in male Wistar rats | Indomethacin/IV + L-NAME/IV + amidotriazoate meglumine | Rosuvastatin/10 mg/kg/day/po/OD/5 day prior to amidotriazoate meglumine |
↓ Cr ↑ CrCl ↓ urine microprotein |
↓ kidney TBARS ↓ serum MDA ↓ serum PCC ↑ serum thiol |
↑ kidney nitrite ↓ IL-6 ↓ TNF-α |
↓ TUNEL-positive cells ↓ expression of phospho-p38 ↓ cleaved caspase-3 ↓ Bax/Bcl-2 ratio |
↓ histological scores | Rosuvastatin attenuated CIN by modulation of NO, inflammatory responses, oxidative stress and apoptotic processes, leading to improved renal function | [30] |
| Adult Sprague Dawley rats | Water deprivation 24 h treated with 25% glycerol/IM + iohexol | Simvastatin/15, 30, 60 mg/kg/po/24 h prior to iohexol/4 d |
↓ Cr in a dose-dependent manner ↓ BUN in a dose-dependent manner |
↓ kidney TBARS ↑ GSH |
↓ MPO ↑ NO |
– | ↓ tubular dilatation, tubular vacuolation, and tubular necrosis in a dose dependent manner | Simvastatin prevented CIN and structural changes in kidney via a reduction of oxidative stress and inflammation, leading to improved renal function | [32] |
| Male Sprague–Dawley rats | Water deprivation 72 h + furosemide 10 mg/kg/IM treated with iohexol |
Rosuvastatin/10 mg/kg/day/po/OD/3 day before and 4 h after iohexol Simvastatin/80 mg/kg/day/po/OD/3 day before and 4 h after iohexol Atorvastatin/20 mg/kg/day/po/OD/3 day before and 4 h after iohexol |
↓ Cr by atorvastatin and rosuvastatin |
↓ kidney TBARS ↓ serum MDA ↑ serum thiol |
↓ IL-6 ↓ MCP-1 ↓ TNF-α Most effective in rosuvastatin > atorvastatin ↑ NO by atorvastatin |
↓ TUNEL-positive cells ↓ Bax/Bcl-2 ratio Most effective in atorvastatin > rosuvastatin |
↓ tubular necrosis and medullary congestion by atorvastatin and rosuvastatin |
Atorvastatin and rosuvastatin prevented CIN and reduced oxidative stress In addition, atorvastatin was most effective in attenuating NO system dysfunction and cell apoptosis, whereas rosuvastatin was most effective in reduction of inflammation, leading to improved renal function |
[31] |
| Male Sprague–Dawley rats | Dehydration 3 day treated with furosemide/IM + iohexol/IV |
Xuezhikang/2,400 mg/kg/day/po/3 day prior to iohexol Atorvastatin/20 mg/kg/day/po/3 day prior to iohexol |
↓ Cr ↓ BUN |
↓ renal MDA ↑ GSH |
↓ TNF-α ↓ IL-6 ↑ kidney total NO (nitrite/nitrate) |
↓ TUNEL-positive cells ↑ Bcl-2/Bax ratio by xuezhikang |
↓ tubular necrosis and medullary congestion ↓ medullary damage scores |
Xuezhikang and atorvastatin shared similar effect on iohexol-induced CIN, leading to improved renal function | [33] |
| Female albino Wistar rats | Water deprivation 24 h + 25% glycerol/IM treated with iohexol/IV | Agomelatine/20, 40 mg/kg/po/OD/24 h before and 4 day after iohexol |
↓ Cr ↓ BUN |
↑ SOD ↑ GSH ↓ MDA |
↓ TNF-α ↓ NF-kB ↓ IL-6 mRNA expression |
– | ↓ hyaline and hemorrhagic casts & tubular necrosis | Agomelatine provided nephroprotective, antioxidant and anti-inflammatory effects against CIN in rats, leading to improved renal function | [42] |
| Adult male Sprague–Dawley rats | Dehydration 24 h + furosemide/IM + indomethacin/IP treated with iomeprol | Melatonin/10 mg/kg/IP/15 min prior to ± 24 h after iomeprol |
↓ Cr ↑ CrCl ↓ FENa All effects by pre- and post-treatment |
– | – | – | – | Melatonin prevented and attenuated CIN in rats with pre- & post-treatment, leading to improved renal function | [43] |
| Male Sprague–Dawley rats | Streptozotocin-induced diabetes treated with iohexol/IV | Melatonin/20 mg/kg/day/IP/OD/7 day prior to iohexol | ↓ Cr |
↓ MDA ↑ SOD ↑ GSH ↓ CAT |
↓ MPO ↓ IL-6 ↓ IL-33 |
– |
↓ apoptosis ↓ necrotic changes ↓ glucogenic vacuolization ↓ inflammatory cell infiltration |
Melatonin provides functional and histologic protection against CIN via inhibiting of IL-33, leading to improved renal function | [44] |
| Male Sprague–Dawley rats | Indomethacin/IV + L-NAME/IV treated with ioversol/IV | HSA-Trx/30 mg/kg/IV/1 h prior to ioversol |
↓ Cr ↓ BUN ↓ urinary NAG ↑ CrCl |
↓ 8-OHdG-positive cells ↓ MDA |
– | ↓ TUNEL-positive cells | ↓ renal tubular injuries | Administration of single dose of HSA-Trx before induction of CIN exerted renoprotective effects in CIN rat model, leading to improved renal function | [37] |
| Adult male Sprague Dawley rats | Indomethacin/IV + L-NAME/IV treated with iopromide/IV | Vitamin E/250, 500 mg/kg/day/po/5 day prior to iopromide | ↓ Cr |
↓ MDA ↑ TAC ↑ SOD in a dose-dependent manner |
– | – |
↓ severity of proximal tubular epithelial cells necrosis and proteinaceous cast ↓ peritubular capillary congestion ↓ interstitial edema |
Vitamin E prevented CIN through its antioxidant activity, leading to improved renal function | [35] |
| Male Sprague–Dawley rats | Indomethacin 10 mg/kg + L-NAME + ioversol/IV | Antithrombin III/500 µg/kg/IV/30 min before or after ioversol |
↓ Cr ↓ BUN ↑ renal cortical blood supply ↓ intrarenal resistance index |
↓ MDA ↑ SOD |
↓ TNF-α ↓ MCP-1 ↓ ICAM-1 expression ↓ F4/80-positive cells infiltration |
↓ cleaved caspase-3 expression ↑ Bcl-2 |
↓ renal tubular detachment ↓ brush border loss ↓ necrosis of tubular cells |
Antithrombin III prevented and attenuated CIN through inhibiting inflammation, oxidative stress, apoptosis and improving RBF, leading to improved renal function | [53] |
| Male Sprague–Dawley rats | Dehydration 72 h treated with iopamidol/IV | Astragaloside IV/20 mg/kg/po/OD/7 day prior to iopamidol |
↓ Cr ↓ BUN ↓ cystatin C ↓ NGAL ↓ uKIM-1 |
↓ MDA ↑ CAT ↑ SOD ↓ serum, urinary and renal 8-OHdG |
– |
↓ TUNEL-positive cells ↓ caspase-3 activity ↓ cleaved caspase-3 protein expression ↓ Bax protein and mRNA expressions ↑ Bcl-2 protein and mRNA expressions ↓ p38 MAPK phosphorylation |
↓ tubular injuries | Astragaloside IV prevented AKI through inhibition of oxidative stress and apoptosis pathways, leading to improved renal function | [45] |
| Male Wistar rats | Gentamicin/IP/6 day treated with gastrographin/IV | Cardiotrophin-1/100 µg/kg/day/IV/24 h prior to and 4 day after gastrographin |
↓ Cr ↓ BUN ↑ CrCl ↑ inulin clearance ↑ RBF ↓ RVR ↓ proteinuria ↓ albuminuria ↓ NAG ↓ uKIM-1 ↓ PAI-1 |
↓ MDA | – | ↓ cleaved caspase-3-positive cells |
↓ tubular necrosis in cortex ↓ tubular obstruction with hyaline material in medulla ↓ Ki-67-positive proliferating cells |
Cardiotrophin-1 prevented CIN through a reduction of oxidative stress, leading to improved renal function | [54] |
| Male albino Wistar rats | Water deprivation 24 h + 25% glycerol/IM treated with iohexol/IV | L-carnitine/200, 400 mg/kg/IP/24 h prior to iohexol |
↓ Cr ↓ BUN |
↑ SOD ↑ GSH ↓ MDA by L-carnitine 400 mg/kg |
↓ TNF-α ↓ TGF-1β expression ↓ IL-1β mRNA expression ↓ TNF-α and NF-kB-positive cells |
↓ caspase-3 mRNA expression |
↓ hyaline and hemorrhagic casts ↓ tubular necrosis in cortical segments of proximal tubules |
L-carnitine protected against CIN via a reduction of oxidative stress, inflammation and apoptosis in rats, leading to improved renal function | [36] |
| Male Wistar-albino rats | Dehydration 24 h + furosemide/IM + indomethacin/IP treated with iomeprol/IV | Curcumin/200 mg/kg/day/po/5 day prior to & 5 day after iomeprol |
↓ Cr ↓ BUN |
↑ SOD ↑ CAT ↑ GSH ↑ GSH-Px ↓ MDA |
↓ iNOS-specific-positive cells |
↓ LC3/B-specific-positive cells ↓ cleaved caspase 3-specific-positive cells |
↓ necrotic and degenerative changes ↓ intertubular hemorrhage |
Curcumin attenuated inflammation and apoptosis in CIN, leading to improved renal function | [46] |
| Male BALB/c mice | Restrict water 24 h treated with iodixanol/IV | Fasudil/3, 10 mg/kg/IV/12, 2 h prior to and 4 h after iodixanol |
↓ Cr ↓ BUN ↓ urinary NAG ↑ RBF ↑ renal vasodilation All effects by 10 mg/kg |
↓ ROS in a dose-dependent manner ↓ 8-OHdG-positive cells in a dose-dependent manner ↑ SOD-1 ↔ SOD-2 |
↓ phospho-NF-kB p65 ↓ IL-6 ↓ TNF-α ↓ iNOS-positive cells (10 mg/kg) |
↓ ROCK-2 protein ↓ p-MYPT1 and p-MYPT1/MYPT1 ratio ↓ TUNEL-positive cells ↓ cleaved caspase-3 ↓ Bax ↑ Bcl-2 ↑ p-Akt/total Akt ratio All effects by 10 mg/kg |
↓ tubular injury ↓ formation of cast All effects by 10 mg/kg |
Fasudil exerted renoprotective effects by suppressing inflammation, apoptosis and oxidative stress via inhibiting Rho/ROCK pathway and ameliorating hemodynamic disturbances, leading to improved renal function | [23] |
| Streptozotocin-induced diabetes in male Sprague–Dawley rats | Treated with diatrizoate meglumine/IV | Exendin-4/25 nmol/kg/SC/10 day prior to diatrizoate/11 d |
↓ Cr ↓ BUN ↓ urinary albumin excretion ↑ CrCl |
↓ MDA ↓ ET-1 ↑ GSH ↑ SOD |
↑ nitrate ↑ eNOS |
↓ caspase-3 expression |
↓ edema ↓ tubular vacuolization ↓ hemorrhage |
Pretreatment with exendin-4 ameliorated CIN effects independent of glycemic state, leading to improved renal function | [55] |
| Female Sprague–Dawley rats | Water deprivation 24 h + diatrizoate/IV | Grape seed proanthocyanidin/100 mg/kg (1 cm3)/po/6 day prior to diatrizoate/5 d |
↓ Cr ↓ BUN |
↓ MDA ↓ TOS ↓ OSI |
– | ↓ TUNEL-positive cells |
↓ perivascular edema ↓ vascular congestion ↓ tubular vacuoles ↓ renal injury score |
Proanthocyanidin attenuated CIN by reducing oxidative damage and apoptosis, leading to improved renal function | [47] |
| Male Wistar albino rats | 24-h dehydration + furosemide/IM + indomethacin/IP treated with iomeprol/IV | Lycopene/4 mg/kg/day/po/5 day prior to and 5 day after iomeprol |
↓ Cr ↓ BUN |
↑ SOD ↑ CAT ↑ GSH ↑ GSH-Px ↓ MDA |
↓ iNOS-specific-positive cells |
↓ LC3/B-specific positive cells ↓ cleaved caspase 3-specific positive cells |
↓ number of infiltrated inflammatory cells and necrotic degenerative changes | Lycopene prevented and attenuated inflammation, autophagy and apoptosis in CIN rats, leading to improved renal function | [48] |
| Male Sprague–Dawley rats | Indomethacin/IV + L-NAME treated with ioversol/IV | Magnolin/1 mg/kg/SC/15 min prior to ioversol |
↓ Cr ↓ BUN ↓ serum NGAL ↓ uKIM-1 |
↓ MDA ↑ SOD |
– |
↓ TUNEL-positive cells ↓ caspase-3 activity ↑ Bcl-2 expression |
↓ renal tubular injury scores | Magnolin attenuated CIN in rats through reducing oxidative stress and apoptosis, leading to improved renal function | [49] |
| Male Sprague–Dawley rats | Deprived of water 3 d + indomethacin/IV treated with diatrizoate | Recombinant manganese SOD/15 µg/kg/IP/4 h prior to diatrizoate | ↑ GFR |
↑ SOD ↓ intrarenal superoxide anion (O2−) ↓ ROS production |
– | – |
↓ tubular necrosis ↓ proteinaceous casts |
Recombinant manganese SOD reduced oxidative stress, thus preventing CIN, leading to improved renal function | [41] |
| Adult male Wistar rats | Meglumine ioxaglate/IV |
NAC/150 mg/kg/day/IP/6 h before and 6 h after ioxaglate Ozone (5%O3 – 95%O2)/1 mg/kg/IP/6 h prior to and 6 h after or 5 day after ioxaglate |
↓ Cr (NAC) ↓ NGAL |
↑ TAC by ozone ↓ PCC |
– | – |
↓ renal tubular injury ↓ hemorrhage |
NAC and ozone treatment prevented and attenuated CIN via a reduction of oxidative stress, leading to improved renal function | [39] |
| Wistar albino rats | Water deprivation 72 h treated with diatrizoate meglumine/IV | Nebivolol/2 mg/kg/day/po/3 day prior to and 2 day after diatrizoate |
↔ Cr ↔ CrCl ↔ BUN ↓ urine microprotein |
↓ serum PCC ↓ kidney TBARS ↓ MDA ↑ serum thiol |
↑ kidney nitrite levels | – |
↓ tubular necrosis ↓ proteinaceous casts ↔ medullary congestion |
Nebivolol attenuated either systemic or renal oxidative stress and increased either nitrite production or restored pathology, leading to improved renal function | [56] |
| Male Wistar albino rats | Indomethacin/IV + L-NAME/IV treated with amidotrizoate meglumine/IV | Paricalcitol/0.4 µg/kg/day/IP/3 day prior to and 2 day after amidotrizoate |
↓ Cr ↑ CrCl ↓ FENa |
↓ MDA ↓ kidney TBARSs |
↓ VEGF score | – |
↓ tubular necrosis ↓ proteinaceous casts ↓ medullary congestion |
Paricalcitol reduced unfavourable histopathology of CIN via antioxidant effects, leading to improved renal function | [38] |
| Male Sprague Dawley rats | Indomethacin/IV + L-NAME/IV treated with iopromide/IV | Phyllanthus emblica extract/125, 250, 500 mg/kg/day/po/5 day prior to iopromide |
↓ Cr (250, 500 mg/kg/d) ↓ BUN |
↓ MDA (250, 500 mg/kg/d) ↑ TAC (250, 500 mg/kg/d) ↑ SOD ↑ CAT |
– | – |
↓ tubular necrosis ↓ proteinaceous cast formation ↓ peritubular capillary congestion ↓ interstitial edema All changes by 250, 500 mg/kg/d |
Phyllanthus emblica extract exerted renoprotective effects of CIN in rat, leading to improved renal function | [50] |
| Streptozotocin-induced diabetes in male Sprague–Dawley rats | Treated with diatrizoate/IV | 5% Probucol/500 mg/kg/po/14 day prior to diatrizoate |
↓ Cr ↑ CrCl |
– | – |
↑ p-ERK1/2 ↓ p-JNK ↑ Bcl-2 ↓ Bax ↓ caspase-3 |
↓ vacuolar degeneration of renal tubular cells ↑ dilatation of lumen ↓ renal tubular injury score |
Probucol exerted protective effects on CIN in diabetic rats via inhibition of renal cell apoptosis, leading to improved renal function | [34] |
| Male Sprague–Dawley rats | Iohexol/IP | Rapamycin/2, 5 mg/kg/IP/7 day prior to iohexol | ↓ Cr in a dose-dependent manner |
↓ MDA in a dose-dependent manner ↓ CAT in a dose-dependent manner |
– |
↑ LC3II/I ↑ Beclin-1 ↑ Pink1 ↓ P62 ↑ ∆ψm in a dose-dependent manner ↓ cytosolic/mitochondrial Cyt c in a dose-dependent manner ↑ TOMM20-stained mitochondria in a dose-dependent manner ↑ LC3-stained autophagosomes ↑ LAMP2-stained lysosomes ↓ renal tubular epithelial cell apoptosis in a dose-dependent manner |
↓ renal tubular necrosis in a dose-dependent manner | Rapamycin exerted renoprotective effects against CIN via suppressing mitochondrial injury and oxidative stress, mitophagy and apoptosis, leading to improved renal function | [62] |
| Male C57BL/6 J mice | L-NAME/IP + indomethacin/IP treated with iohexol/IP | Resveratrol/30 mg/kg/IP/simultaneously with iohexol | ↓ Cr |
↑ SIRT1 ↑ PGC-1α expression ↓ phosphor-Ser256 FoxO1 expression ↑ SOD2 ↓ MDA |
– |
↓ TUNEL-positive cells ↓ cleaved caspase-3 |
↓ severity score for tubular vacuolization ↓ disruption of tubular structures ↓ macrophage infiltration |
Resveratrol attenuated CIN via a reduction of oxidative stress and apoptosis, leading to improved renal function | [21] |
| Wistar rats | Indomethacin/IV + L-NAME/IV treated with diatrizoate meglumine/IV |
NAC/100 mg/kg/po/7 day prior to diatrizoate Salidroside/20 mg/kg/IP/7 day prior to diatrizoate |
↓ Cr ↓ BUN ↓ NAG ↓ 24-h urinary protein |
↑ SOD ↓ MDA ↓ angiotensin II ↓ 8-OHdG |
↑ NO ↑ eNOS mRNA and protein ↑ NOS activity |
– |
↓ disintegrated and shed brush border of tubular epithelial cells ↓ vacuolar degeneration ↓ cell debris and protein cast in tubular lumen ↓ focal interstitial edema and inflammatory cell infiltration |
Salidroside or NAC prevented CIN via a reduction of oxidative stress, leading to improved renal function | [40] |
| Male Sprague–Dawley rats | Dehydration 48 h treated with iohexol/IV |
Salvianolic acid B/50 mg/kg/IV/5 min prior to iohexol Wortmannin (PI3K inhibitor)/15 µg/kg/IV/5 min prior to iohexol Sulforaphane (Nrf-2 activator)/10 mg/kg/IV/5 min prior to iohexol |
↓ Cr (salvianolic acid and sulforaphane) ↓ BUN (salvianolic acid) ↑ Cr (wortmannin) |
↓ 8-OHdG-positive cells (salvianolic acid and sulforaphane) ↔ 8-OHdG-positive cells (wortmannin) ↓ MDA (salvianolic acid and sulforaphane) ↔ MDA (wortmannin) |
– |
↓ TUNEL-positive cells (salvianolic acid and sulforaphane) ↑ TUNEL-positive cells (wortmannin) ↑ Nrf-2-positive cells (salvianolic acid and sulforaphane) ↔ Nrf-2-positive cells (wortmannin) ↑ p-Akt/Akt (salvianolic acid) ↔ p-Akt/Akt (sulforaphane) ↓ p-Akt/Akt (wortmannin) ↑ Nrf-2/Histone H3 (salvianolic acid and sulforaphane) ↓ Nrf-2/Histone H3 (wortmannin) ↑ HO-1/Actin (salvianolic acid and sulforaphane) ↓ HO-1/Actin (wortmannin) |
↓ histological scores (tubular epithelium degeneration) (salvianolic acid B and sulforaphane) ↑ histological scores (wortmannin) |
Salvianolic acid B exerted renoprotection and antioxidative effects through PI3K/Akt/Nrf2 pathway, leading to improved renal function | [26] |
| Male Sprague–Dawley rats | Gentamicin/SC + iothalamate meglumine/IV | Sesame oil/0.5 ml/kg/po/1 h prior to iothalamate |
↓ Cr ↓ BUN |
↓ MDA ↓ renal hydroxyl radicals ↓ renal superoxide anion generation |
↓ MPO ↓ renal nitrite/nitrate level ↓ iNOS expression |
– |
↓ inflammatory cell infiltration ↓ tubular dilation ↓ congestion in tubules |
Sesame oil prevented CIN via inhibiting oxidative stress in rats, leading to improved renal function | [51] |
| Male Wistar rats | 24-h water deprivation + L-NAME/IP + indomethacin/IP treated with iohexol/IV | Sildenafil citrate/50 mg/kg/day/po/5 day prior to and 2 day after iohexol |
↓ Cr ↑ GFR ↑ RPF ↑ RBF ↓ RVR ↓ BUN ↓ proteinuria |
↓ intracellular O2− ↓ H2O2 |
– | – | – | Sildenafil prevented CIN through vasodilator and antioxidant activity, leading to improved renal function | [58] |
| Male Wistar rats | 12-h dehydration + L-NAME/IP + indomethacin/IP treated with iopromide/IV |
Sildenafil/10 mg/kg/day/po/7 day prior to iopromide Taladafil/5 mg/kg/day/po/7 day prior to iopromide NAC/100 mg/kg/day/po/7 day prior to iopromide |
↓ Cr ↓ BUN |
– | – | – |
↓ hydropic changes of renal tubules ↓ Bowman space with lobulated glomerulus ↓ alteration of macula densa |
Sildenafil and taladafil prevented CIN-related structural kidney damage and superior to NAC | [59] |
| Male Wistar rats | 12-h dehydration + L-NAME/IP + indomethacin/IP treated with iopromide/IV |
Sildenafil/10 mg/kg/day/po/7 day prior to iopromide Taladafil/5 mg/kg/day/po/7 day prior to iopromide NAC/100 mg/kg/day/po/7 day prior to iopromide |
↓ Cr ↓ BUN |
↑ TAC ↑ GSH ↑ CAT ↓ PCC ↓ TBARS |
– | – | – | Sildenafil and taladafil prevented CIN through antioxidant activity | [60] |
| Adult male Swiss mice | Overnight water deprivation + L-NAME/IP + indomethacin/IP treated with ioversol/IP |
NAC/200 mg/kg/po/5 day prior to ioversol Silymarin/50, 200, 300 mg/kg/po/5 day prior to ioversol |
↓ Cr in a dose-dependent manner (silymarin) ↓ BUN in a dose-dependent manner ( silymarin) ↓ cystatin C in a dose-dependent manner (silymarin) |
↓ intracellular superoxide (O2−) ↓ H2O2 ↓ OH−/ONOO− ↓ advanced oxidation protein products in plasma (silymarin 300 mg) |
– |
↓ DNA damage (silymarin 300 mg) ↓ annexin V-positive cells |
↓ shrunken glomerular tuft ↓ loss of structural cohesion with atypical podocytes ↓ loss of nuclei ↓ tubular dilation with luminal congestion ↓ tubular epithelial cell vacuolization ↓ tubular shedding ↓ tubulo-interstitial lesions |
Silymarin decreased systemic and renal oxidative damage, preserving renal function, morphological architectures antigenotoxic and antiapoptotic activities under exposure to radiocontrast agent in mice, leading to improved renal function | [52] |
| Adult Wistar Albino rats | Iodixanol/IV | Sphingosylphosphorylcholine/2, 10 µM/IP/3 day after iodixanol |
↔ Cr ↓ BUN |
↑ SOD ↓ MDA |
↓ NO ↓ iNOS-positive cells |
↓ TUNEL-positive cells |
↓ widespread loss of brush border ↓ denudation of tubular cells ↓ tubule dilatation ↓ intratubular obstruction by granular casts |
Sphingosyl-phosphoryl-choline reduced CIN via preventing oxidative stress and apoptosis, leading to improved renal function | [63] |
| Adult Sprague Dawley rats | Indomethacin/IV + L-NAME/IV treated with ioversol/IV | Sulforaphane/5 mg/kg/po/5 day prior to ioversol |
↓ Cr ↓ BUN |
↓ MDA ↑ SOD |
– |
↑ Nrf-2, NQO-1 and HO-1 gene expression ↑ Nrf-2 nuclear translocation ↑ HO-1 and NQO-1 protein levels |
↓ tubular necrosis ↓ hemorrhagic casts |
Sulforaphane ameliorated CIN via Nrf-2/HO-1 pathway, leading to improved renal function | [27] |
| Male C57BL/6 mice | Water deprivation 16 h + indomethacin/IP + L-NAME/IP treated with iohexol | GKT137831 (Nox1/4 inhibitor)/40 mg/kg/po/5 day prior to iohexol |
↔ Cr ↓ BUN ↓ KIM-1-positive cells |
↑ SOD ↔ Nox4 ↔ Nox1 ↓ Nox2 ↓ 8-OHdG-positive cells |
– |
↓ phospho-p38/p38 ↓ phospho-pJNK/pJNK ↓ phospho-ERK/ERK ↓ Bax ↑ Bcl-2 ↓ TUNEL-positive cells |
↔ tubular epithelial cell degeneration ↓ basement membrane nudity ↓ vacuolar degeneration of tubular epithelial cells ↓ protein casts ↓ tubular dilation ↓ loss of tubular brush borders ↓ necrosis of partial tubular epithelial cells ↓ tubular pathological scores |
Inhibition of Nox1/4 prevented CIN via a reduction of oxidative stress and apoptosis, leading to improved renal function | [17] |
| Male Wistar albino rats | Dehydration 3 day treated with diatrizoate/IV |
Carvedilol/2 mg/kg/po/3 day prior to diatrizoate Nebivolol/2 mg/kg/po/3 day prior to diatrizoate |
↔ Cr ↔ BUN |
↓ MDA ↑ TAC ↔ SOD |
– | – |
↓ interstitial inflammation ↓ tubular degeneration ↓ tubular dilatation |
Both carvedilol and nebivolol attenuated oxidative stress but did not improve renal function | [57] |
| Female Wistar albino rats | Furosemide/SC + deprived of water for 24 h treated with iothalamate sodium/IV | 8.4% NaHCO3/1 mL/IV/3 h prior to iothalamate |
↔ Cr ↔ CrCl |
↔ MDA ↓ GSH |
↔ MPO ↔ NO |
– | ↓ % of tubular injury | Urinary alkalinization before IV contrast protected morphological change protection in rats but did not improve renal function | [64] |
AKI, acute kidney injury; Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma 2; BUN, blood urea nitrogen; CAT, catalase; CIN, contrast-induced nephropathy; CM, contrast media; Cr, creatinine; CrCl, creatinine clearance; Cyt c, cytochrome c; eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; FENa, fractional excretion of sodium; GFR, glomerular filtration rate; GSH, glutathione; GSH-Px, glutathione peroxidase; HO-1, heme oxygenase-1; HSA-Trx, recombinant human serum albumin-Thioredoxin-1 fusion protein; ICAM-1; intercellular cell adhesion molecule 1; IL, interleukin; iNOS, inducible nitric oxide synthase; IP, intraperitoneally; IV, intravenously; LC3, light-chain 3; L-NAME, Nω-nitro-L-arginine methyl ester; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein-1; MDA, malondialdehyde; MPO, myeloperoxidase; mRNA, messenger ribonucleic acid; MYPT-1, myosin light-chain phosphatase; NAC, N-acetylcysteine; NADPH, nicotinamide adenine dinucleotide phosphate; NAG, N-acetyl-β-glucosaminidase; NF-kB, nuclear factor-kB; NGAL, neutrophil gelatinase-associated lipocalin; NO, nitric oxide; Nrf-2, Nuclear factor erythroid-derived 2-like 2; OSI, oxidative stress index; PAI-1, plasminogen activator inhibitor 1; PCC, protein carbonyl content; PCR, polymerase chain reaction; PGC-1α, peroxisome proliferator-activated receptor-γ co-activator 1α; Pink1, PTEN-induced putative kinase; RBF, renal blood flow; ROCK-2, Rho kinase 2; RPF, renal plasma flow; RNA, ribonucleic acid; RVR, renal vascular resistance; SC, subcutaneously; SIRT1, sirtuin 1; SOD, superoxide dismutase; TAC, total antioxidant capacity; TBARS, thiobarbituric acid-reacting substances; TGF-1β, transforming growth factor-1β; TNF-α, tumor necrosis factor-α; TOS, total oxidant system; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; uKIM-1, urinary kidney injury molecule-1; VEGF, vascular endothelial growth factor; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; ∆ψm, Mitochondrial membrane potential
Lipid-lowering agents as interventions to reduce CIN
Lipid-lowering agents including rosuvastatin, [30, 31] simvastatin, [31, 32] atorvastatin, [31, 33] xuezhikang (containing lovastatin), [33] and probucol [34] were investigated as potential pharmacological interventions in CIN animal models. These interventions appeared to effectively attenuate CIN as indicated by decreased level of kidney thiobarbiturates (TBARS), serum or renal MDA, serum protein carbonyl content (PCC), and increased serum thiol and glutathione (GSH) [29–34]. Inflammatory markers were also ameliorated as indicated by reduced IL-6, TNF-α, monocyte chemotactic protein-1 (MCP-1), myeloperoxidase (MPO), and increased NO [29–34]. The apoptotic markers were also reduced [29–34]. Furthermore, an appearance of unfavorable histological findings was decreased in an ischemic-reperfusion injury model [29–34]. These findings suggested that statins and probucol could attenuate CIN by modulation of NO, inflammatory responses, oxidative stress and apoptotic processes, leading to improved renal function [29–34]. A summary of these reports on the effects of lipid lowering agents on the protection of CIN is shown in Table 4. There are few clinical studies in this area so these statins are not recommended in the guidelines for CIN prevention.
Antioxidants as interventions to reduce CIN
Many antioxidants; such as vitamin E, [35] L-carnitine, [36] human serum albumin-thioredoxin-1 fusion protein (HSA-Trx), [37] paricalcitol, [38] N-acetylcysteine (NAC), [39, 40] recombinant manganese SOD (rMnSOD), [41] and agomelatine and melatonin; [42–44] were investigated for their potential effects to prevent CIN in rat models. All of the studies demonstrated the renoprotective effect by attenuating serum Cr and renal histological damage through their antioxidant activities (Table 4). Both inflammatory process and apoptosis were decreased following antioxidant treatments [35–38, 42–44].
Active component of herbs; such as astragaloside, [45] curcumin, [46] grape seed proanthocyanidin, [47] lycopene, [48] magnolin (major active ingredient of herb Magnolia fargesii), [49] Phyllanthus emblica extract, [50] salidroside, [40] sesame oil, [51] and silymarin, [52] were investigated in CIN in rats (Table 4). All studies demonstrated their benefits in attenuating CIN and AKI biomarkers such as cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), and urine kidney injury molecule-1 (KIM-1), due to reduced oxidative stress and apoptosis.
Other agents such as cardiotrophin-1 and antithrombin III, [53, 54] exendin-4, [55] β-receptor antagonist, [56, 57] phosphodiesterase-5 inhibitor, [58–61] an mTOR inhibitor, [62] exogenous sphingosylphosphorylcholine, [63] and sodium bicarbonate; [64] have been investigated in CIN models (Table 4 and Fig. 3). They all effectively reduced oxidative stress, inflammation and apoptosis, with improved renal histopathology. These findings suggested that these pharmacological interventions prevented CIN through a reduction in oxidative stress, inflammation and apoptosis, leading to improved renal function in rats.
Pharmacological interventions to reduce CIN: evidence from clinical reports
Effects of statins on the prevention of CIN
Statins have been shown to exert renoprotective effects in CIN via inhibition of uptake of contrast into renal tubular cells, attenuation of endothelial dysfunction and oxidative stress, anti-inflammation, anti-proliferation of mesangial cells, and protection of podocytes [9]. Clinical studies of statins on the prevention of CIN are summarized in Table 5 and Fig. 3.
Table 5.
The effects of statins on the prevention of contrast-induced nephropathy: reports from clinical studies
| Study type | Models | Intervention (drug/dose/route/duration) | Major findings | Interpretations | References | |
|---|---|---|---|---|---|---|
| Renal function | Oxidative stress/inflammatory markers | |||||
| Single-center, double-blind randomized placebo-controlled clinical trial |
Age 55–75 years with DM or CKD (Cr > 1.5 mg/dL or GFR 15–60 mL/min/1.73 m2) undergoing elective angiography NAC 1200 mg/po/bid/1 day prior to and until 4 h after angiography treated with nonionic iso-osmolar CM |
Atorvastatin/80 mg/day/po/48 h prior to angiography (n = 110) vs. Placebo (n = 110) |
↓ CIN 24 h after angiography ↔ CIN at 48 h after angiography ↔ Cr |
– | Short-term pretreatment with atorvastatin 80 mg along with high-dose NAC decreased incidence of CIN in high-risk patients undergoing angiography | [66] |
| Prospective, double-blind, randomized, two-arm, parallel group, controlled, clinical trial | Age 18–65 years with Cr 1–1.5 mg/dL or eGFR > 60 mL/min/1.73 m2 and controlled DM or hypertension undergoing CAG |
Atorvastatin/80 mg/po + NAC/1200 mg/po/OD/3 day prior to and 2 day after angiography (n = 80) vs NAC 1200 mg/po/OD 3 day prior to and 2 day after angiography (n = 80) |
Atorvastatin ↓ CIN ↓ mean change in Cr Lesser ↓ eGFR No required dialysis |
– | Short-term high-dose atorvastatin along with NAC was effective in prevention of CIN in high risk patients | [67] |
| Randomized, multicenter, prospective, double-blind clinical trial | Statin-naïve NSTE-ACS undergoing invasive strategy PCI treated with iobitridol | Atorvastatin/80 mg/po/12 h prior to PCI + 40 mg/po/2 h prior to PCI (n = 120) vs. Placebo (n = 121) |
↓ CIN ↓ Cr ↓ CrCl change ↓ hospital stay |
↓ CRP | Short-term pretreatment with high-dose atorvastatin prevented CIN via anti-inflammatory effects, and shortened hospital stay in patients with ACS undergoing PCI | [68] |
| Randomized controlled study | Statin-naïve acute STEMI undergoing emergency PCI treated with non-ionic contrast | Atorvastatin/80 mg/po/prior to PCI (n = 78) vs. Placebo (n = 83) |
↓ CIN ↓ Cr ↓ cystatin C |
– | Short-term pretreatment with high-dose atorvastatin prevented CIN and protected renal function in patients with acute STEMI undergoing emergency PCI | [69] |
| Prospective, randomized trial |
Patients undergoing CAG NAC 600 mg/po/bid/prior to procedure treated with iopamidol |
Atorvastatin/80 mg/po/bid/prior to procedure + 80 mg/po/OD/2 day after procedure (n = 60) vs No atorvastatin (n = 70) |
↔ CIN ↓ Cr ↑ eGFR ↑ Cr change |
– | Short-term atorvastatin protected CIN in patients undergoing CAG | [70] |
| Randomized trial |
CKD (eGFR < 60 mL/min/1.73 m2) scheduled for elective CAG or PCI NAC/1200 mg/po/bid/1 day prior to and day of administration of CM treated with iodixanol |
Atorvastatin/80 mg/po/24 h prior to iodixanol (n = 202) vs No atorvastatin (n = 208) |
↓ CIN ↓ Cr |
– | Single high loading dose of atorvastatin administered 24 h before CM exposure was effective in reducing rate of CIN | [29] |
| Randomized, double-blind, controlled trial | Patients with normal renal function (Cr ≤ 1.5 mg/dL) undergoing elective CTA treated with iopromide | Atorvastatin/80 mg/po/24 h prior to and 48 h after CM (n = 115) vs. Placebo (n = 121) |
↔ CIN ↓ Cr |
– | Short-term treatment with high dose atorvastatin was effective in reduction of Cr level after CM injection in patients undergoing CTA | [71] |
| Randomized trial | Patients undergoing CAG |
Atorvastatin/10 mg/po/24 h prior to procedure (n = 100) vs Atorvastatin/80 mg/po/24 h prior to procedure (n = 50) |
↓ β2M ↓ urine NAG/Cr ↑ CrCl All effects by 80 mg > 10 mg |
– | Short-term pretreatment with high-dose atorvastatin was superior than low dose on attenuating CIN | [72] |
| Randomized trial | STEMI undergoing primary PCI treated with iopromide |
Atorvastatin/80 mg/po/prior to procedure (n = 98) vs Rosuvastatin/40 mg/po/prior to procedure (n = 94) |
↔ CIN ↔ Cr ↔ eGFR ↔ Cr change |
– | Short-term pretreatment with atorvastatin or rosuvastatin had similar efficacy in preventing CIN in patients with STEMI undergoing primary PCI | [114] |
| Prospective, randomized and non-randomized controlled trial | Patients undergoing elective CAG treated with iohexol |
Short-term atorvastatin 40 mg/po/3 day prior to and 2 day after CAG (n = 80) No statin (n = 80) Chronic statin therapy/po/at least 1 mo (n = 80) Atorvastatin/10–40 mg/day/po (n = 57) Simvastatin/10–40 mg/day/po (n = 12) Pravastatin/10–20 mg/day/po (n = 6) Rosuvastatin/10 mg/day/po (n = 3) Fluvastatin/80 mg/day/po (n = 2) |
↓ Cr (atorvastatin and chronic statin therapy) ↑ GFR (atorvastatin and chronic statin therapy) ↓ cystatin C (chronic statin therapy) ↔ Cr, cystatin C and GFR between short term atorvastatin and chronic statin therapy |
– | Short-term and long-term use of atorvastatin had renoprotective effects in low-risk patients undergoing elective CAG | [73] |
| Observational study | ACS undergoing PCI treated with iopamiron |
Simvastatin/40 mg/po/OD/6 months after PCI (n = 128) vs Atorvastatin/20 mg/po/OD/6 months after PCI (n = 143) |
↔ Cr ↔ eGFR |
– | Simvastatin and atorvastatin were similar renoprotective effects for 6 months after PCI | [115] |
| Prospective, audited, multicenter regional registry | Patients undergoing PCI |
Pre-statin/po (n = 10,831) vs No pre-statin (n = 18,040) |
↓ CIN ↓ % of peak Cr ≥ 1.5 mg/dL ↓ nephropathy requiring dialysis |
– | Initiating statin therapy before PCI reduced risk of CIN | [65] |
| Prospective randomized placebo-controlled trial | Patients undergoing CAG treated with iodixanol | Simvastatin/80 mg/day/po/48 h prior to CAG (n = 98) vs. Placebo (n = 96) |
↔ GFR in first 24 h ↓ eGFR reduction after 48 h |
– | Prophylactic administration of simvastatin reduced CIN | [76] |
| Prospective, randomized, controlled, multicenter clinical trial | Age 18–75 years with type 2 DM and CKD stage 2–3 undergoing CAG ± PCI treated with iodixanol | Rosuvastatin/10 mg/po/2 day prior to and up to 3 day after procedure (n = 1498) vs No rosuvastatin (n = 1500) | ↓ CIN | ↓ hsCRP | Short-term rosuvastatin reduced CIN in patients with type 2 DM and CKD undergoing arterial CM injection | [74] |
| Prospective, randomized trial |
Statin-naïve NSTE-ACS patients scheduled for early invasive PCI NAC 1200 mg/po/bid/1 day prior to and 1 day after angiography treated with iodixanol |
Rosuvastatin/40 mg/po/prior PCI + 20 mg/po/after PCI (n = 252) vs No rosuvastatin (n = 252) |
↓ CIN | – | Short-term high-dose rosuvastatin reduced CIN in statin-naïve NSTE-ACS patients undergoing early invasive PCI | [75] |
| Randomized trial | ACS undergoing elective PCI treated with iodixanol |
Simvastatin/20 mg/po/1 day prior to PCI (n = 115) vs Simvastatin/80 mg/po/1 day prior to PCI (n = 113) |
↓ CIN ↓ Cr (80 mg) ↑ CrCl (80 mg) |
↓ hsCRP ↓ P-selectin ↓ intercellular adhesion molecule-1 |
Short-term pretreatment with simvastatin 80 mg before PCI decreased CIN compared with simvastatin 20 mg | [77] |
| Prospective, single-center, randomized, placebo-controlled trial |
CKD (CrCl < 60 mL/min) undergoing elective CAG ± PCI NAC 1200 mg/po/bid/1 day prior to and 1 day after procedure treated with iodixanol |
Atorvastatin/80 mg/po/48 h prior to and 48 h after CM (n = 152) vs. Placebo (n = 152) |
↔ CIN ↔ Cr ↔ persistent kidney injury |
– | Short-term administration of high-dose atorvastatin before and after contrast exposure, in addition to oral NAC, did not decrease CIN occurrence in patients with pre-existing CKD | [79] |
| Prospective, randomized, double-blind, placebo-controlled, 2-center trial | CKD (CrCl ≤ 60 ml/min ± SCr ≥ 1.1 mg/dl) undergoing CAG | Simvastatin/40 mg/po/every 12 h evening prior to up to morning after procedure (n = 124) vs. Placebo (n = 123) |
↔ CIN ↔ Cr ↔ length of hospital stays or 1- and 6-mo |
– | Short-term pretreatment with high-dose simvastatin did not prevent CIN in patients with CKD undergoing CAG | [78] |
| Prospective cohort | CAD ± CKD undergoing CAG |
Atorvastatin/10–40 mg/po (n = 1219) vs Rosuvastatin/5–40 mg/po (n = 635) |
↔ CIN between 2 groups High plasma atorvastatin or rosuvastatin in CIN subgroups |
– | High plasma atorvastatin or rosuvastatin increased risk of CIN | [81] |
| Retrospective study | Age > 18 years undergoing non-emergent PCI |
Statins before PCI (n = 239) Atorvastatin/10–80 mg/po (n = 89) Simvastatin/10–80 mg/po (n = 74) Pravastatin/10–40 mg/po (n = 53) Lovastatin/20–40 mg/po (n = 13) Rosuvastatin/5–20 mg/po (n = 9) Fluvastatin/po (n = 1) No statin before PCI (n = 114) |
↑ CIN | – | Statin use before non-emergent PCI increased incidence of CIN | [80] |
ACS, acute coronary syndrome; β2M, β2-microglobulin; CAD, coronary artery disease; CAG, coronary angiography; CIN, contrast-induced nephropathy; CKD, chronic kidney disease; CM, contrast media; Cr, creatinine; CrCl, creatinine clearance; CRP, C-reactive protein; CTA, computed tomography angiography; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; NAC, N-acetylcysteine; NAG, NAG, N-acetyl-β-glucosaminidase; NSTE-ACS, non-ST-elevated acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction
In a retrospective study of 29,409 patients undergoing percutaneous coronary intervention (PCI), initiating statin therapy before PCI reduced risk of CIN [65]. Many randomized-controlled trials of atorvastatin for CIN prevention were done in patients undergoing coronary angiography (CAG). In high risk patients, short-term pretreatment with high-dose atorvastatin decreased incidence of CIN [29, 66–73], reducing C-reactive protein (CRP) [68].
The largest randomized-controlled trial in rosuvastatin was done in 2,998 patients with type 2 DM and CKD who underwent coronary or peripheral angiography, receiving either pre and post-intervention rosuvastatin or standard care. The rosuvastatin-treated group had lower incidence of CIN and high-sensitivity CRP (hsCRP) [74]. In the PRATO-ACS trial, the incidence of CIN in non-ST elevated ACS patients undergoing CAG who receive rosuvastatin in statin-naïve patients was lower than in control group [75]. With simvastatin, the prospective randomized-controlled trials in patients undergoing CAG demonstrated that short-term pretreatment of high-dose simvastatin reduced the incidence of CIN [76, 77]. Simvastatin also reduced inflammation by decreasing hsCRP, P-selectin, and intracellular cell adhesion molecule 1 (ICAM-1) [77].
Despite these promising findings, inconsistent reports exist. The PROMISS trial failed to show a difference between simvastatin and placebo with respect to a primary end point based on the mean peak increase in plasma Cr within 48 h after CAG in patients with CKD [78]. Also, another randomized-controlled trial demonstrated that short-term administration of high-dose atorvastatin with oral NAC did not decrease incidence of CIN in pre-existing CKD patients, [79] and a retrospective study, statin given before non-emergent PCI increased the incidence of CIN [80]. Similarly, a prospective cohort study in patients with or without CKD undergoing CAG demonstrated that high plasma atorvastatin or rosuvastatin was associated with increased CIN risk [81]. Therefore, currently statins are not recommended in the guidelines for CIN prevention.
Effects of antioxidants on the prevention of CIN
Many clinical trials have investigated the effects of various antioxidants on the prevention of CIN. These include NAC, ascorbic acid, sodium bicarbonate, sodium-2-mercaptoethane sulphonate (MESNA), and nebivolol. A summary of these reports is shown in Table 6 and Fig. 3.
Table 6.
The effects of non-statin on the prevention of contrast-induced nephropathy: reports from clinical studies
| Study type | Models | Intervention (drug/dose/route/duration) | Major findings | Interpretations | References | |
|---|---|---|---|---|---|---|
| Renal function | Oxidative stress/inflammatory markers | |||||
| Randomized, double-blind, placebo-controlled trial | Patients with Cr ≥ 1.2 mg/dL undergoing clinically driven, nonemergent CAG or PCI treated with nonionic, low- or iso-osmolar contrast | Ascorbic acid/3 g/po/2 h prior to procedure + 2 g/po/night and morning after procedure (n = 118) vs. Placebo (n = 113) |
↓ CIN ↓ Cr ↓ CrCl changes ↔ BUN |
– | Ascorbic acid prevented CIN after coronary imaging procedures in patients with pre-existing renal dysfunction | [110] |
| Prospective randomized-controlled trial | Patients with Cr > 1.2 mg/dL or CrCl < 50 mL/min underwent elective CT treated with iopromide | NAC/600 mg/po/bid/1 day prior to and after CT (n = 41) vs. Placebo (n = 42) |
↓ CIN ↓ Cr changes at 48 h after CT |
– | Short-term pretreatment with NAC prevented CIN | [82] |
| Prospective randomized-controlled trial | Patients with Cr > 1.2 mg/dL or CrCl < 70 mL/min underwent elective CAG ± PCI treated with iopromide |
NAC/600 mg/po/bid/1 day prior to and after CAG (n = 92) No NAC (n = 91) |
↔ CIN ↔ Cr changes at 48 h after CAG ↓ Cr changes at 48 h after CAG by using small volume of CM |
– | Short-term NAC prevented CIN in patients with CKD and using small volume of CM | [83] |
| Randomized, double-blind, placebo-controlled trial | Patients with Cr ≥ 1.4 mg/dL or CrCl < 50 mL/min underwent elective CAG treated with ioxilan | NAC/600 mg/po/bid/1 dose prior to and 3 doses after CAG (n = 25) vs. Placebo (n = 29) |
↓ CIN ↓ Cr at 48 h after CAG ↓ Cr changes |
– | Short-term NAC reduced risk of CIN in patients with CKD | [84] |
| Prospective randomized, double-blind study | Patients with Cr > 106 µmol/L underwent elective CAG treated with non-ionic, low osmolar iodine | NAC/1,000 mg/po/bid/24 h prior to and 24 h after CAG (n = 24) vs. Placebo (n = 25) | ↓ CrCl changes at 24 and 96 h after CAG |
↑ urinary NO ↔ urinary F2-isoprostanes |
Short-term NAC prevented CIN in patients with CKD undergoing CAG via increasing NO production | [85] |
| Prospective, randomized, double-blind, placebo-controlled trial | Patients with Cr > 1.2 mg/dL or CrCl < 60 mL/min underwent elective CAG ± PCI treated with iopamidol | NAC/600 mg/po/bid/1 day prior to and after procedure (n = 102) vs. Placebo (n = 98) |
↓ CIN ↓ Cr at 48 h after procedure ↑ CrCl |
– | Short-term NAC prevented CIN in patients with moderate CKD after CAG | [86] |
| Prospective randomized trial | Patients with Cr ≥ 1.5 mg/dL underwent CAG treated with iopromide or ioxilan | NAC/600 mg/po/bid/after randomization, 4 h later and every 12 h after CAG total 5 doses (n = 21) vs. Placebo (n = 22) |
↓ CIN ↓ Cr changes at 48 and 72 h after CAG |
– | Short-term NAC reduced CIN in patients with mild to moderate renal impairment undergoing CAG | [87] |
| Prospective randomized trial | Patients with Cr > 1.8 mg/dL (males), > 1.6 mg/dL (females), or CrCl < 50 mL/min underwent CAG ± PCI | NAC/1000 mg/po/bid/1 h prior to and 4 h after procedure (n = 36) vs. Placebo (n = 44) |
↔ CIN ↓ Cr changes at 48 h |
– | Short-term high-dose NAC prevented the rise of Cr 48 h after CAG/PCI and might prevent CIN | [88] |
| Prospective randomized-controlled trial | Patients with Cr > 2.0 mg/dL and < 6.0 mg/dL or CrCl < 40 mL/min and > 8 mL/min underwent CAG treated with iopamiro | NAC/400 mg/po/bid/1 day prior to and after CAG (n = 60) vs. Placebo (n = 61) |
↓ Cr ↓ Cr changes at 48 h |
– | Short-term NAC protected CIN in patients with CKD undergoing CAG | [89] |
| Prospective randomized-controlled trial | Patients with eGFR 30–60 mL/min/1.73 m2 underwent CAG treated with ioversol |
NAC/600 mg/po/bid/1 day prior to and after CAG (n = 73) vs NAC/600 mg/po/bid/1 day prior to and after CAG + theophylline/200 mg/po/bid/1 day prior to and after CAG (n = 72) vs No NAC (n = 72) |
↓ CIN (NAC + theophylline) ↓ Cr at 48 h after CM (NAC + theophylline) |
– | Short-term NAC along with theophylline prevented CIN in patients with eGFR 30–60 mL/min/1.73 m2 | [90] |
| Double-blind, placebo-controlled, randomized study | Age 18–80 years with Cr 1.4–5.0 mg/dL and CrCl < 70 mL/min/1.73 m2 scheduled for elective CAG treated with iopamidol | NAC/600 mg/po/bid/2 day prior to and 2 day after angiography (n = 13) vs. Placebo (n = 11) |
↑ CrCl ↓ α-GST |
↔ urinary 15-isoprostane F2t | Short-term NAC treatment was associated with suppression of oxidative stress-mediated proximal tubular injury | [91] |
| Prospective randomized-controlled trial | Patients with Cr > 1.36 mg/dL or CrCl < 50 mL/min underwent CAG or PCI treated with iodixanol |
NAC/150 mg/kg/IV/30 min prior to CM + NAC/50 mg/kg/IV/4 h after CM (n = 41) vs No NAC (n = 39) |
↓ CIN ↓ Cr at 48 and 96 h after CM |
– | Short-term IV NAC prevented CIN | [107] |
| Single center, Prospective, single-blind, placebo-controlled, randomized controlled trial | STEMI undergoing primary PCI treated with iopromide | NAC/1200 mg/day/IV/bid/bolus prior to and up to 48 h after PCI (n = 126) vs. Placebo (n = 125) |
↔ CIN ↔ Cr ↔ CrCl |
↓ activated oxygen protein products at day 1–2 ↓ oxidized LDL at day 1–3 |
High-dose IV NAC reduced oxidative stress after reperfusion of MI but not provided additional clinical benefit to nephropathy | [108] |
| Randomized, placebo-controlled, double blind trial | Age > 18 years with Cr ≥ 1.2 mg/dL or CrCl < 50 mL/min underwent CAG treated with iomeperole |
NAC/600 mg/po/bid/1 day prior to and after CAG (n = 19) vs Zinc/60 mg/po/1 day prior to CAG (n = 18) vs. Placebo (n = 17) |
↔ CIN ↔ Cr ↓ cystatin C |
– | Short-term NAC and zinc did not prevent CIN but NAC had renoprotective effect by reducing cystatin C | [92] |
| Double-blind, placebo and comparator-drug-controlled, randomized trial | eGFR 15–44.9 mL/min/1.73 m2 or 45–59.9 mL/min/1.73 m2 in DM underwent CAG or noncoronary angiography | NAC/1200 mg/po/bid/1 h prior to, 1 h, and 4 day after angiography (n = 2495) vs. Placebo (n = 2498) |
↔ CIN ↔ Cr at 90–104 day after angiography |
– | Oral NAC did not prevent CIN | [93] |
| Pragmatic randomized-controlled trial | Patients with at least 1 risk factor for CIN (age > 70 years, Cr > 1.5 mg/dL, DM, CHF, LVEF < 0.45, hypotension) underwent coronary or peripheral arterial diagnostic intravascular angiography or percutaneous intervention | NAC/600 mg/po/bid/1 day prior to and after procedure (n = 1172) vs. Placebo (n = 1136) |
↔ CIN ↔ Cr |
– | Short-term NAC did not reduce the risk of CIN | [94] |
| Randomized prospective study | Patients with Cr ≥ 1.6 mg/dL or CrCl ≤ 60 mL/min underwent PCI treated with low-osmolality nonionic CM |
NAC/600 mg/po/bid/1 day prior to and after procedure (n = 45) vs Fenoldopam/0.1 µg/kg/min/IV/4 h prior to and 4 h after procedure (n = 38) vs No NAC or fenoldopam (n = 40) |
↔ CIN ↔ Cr changes at 24 and 48 h after procedure |
– | Short-term NAC or fenoldopam did not prevent CIN in patients with CKD | [95] |
| Prospective, double-blind, placebo-controlled, randomized clinical trial | Age > 18 years with DM and Cr ≥ 1.5 mg/dL for men and ≥ 1.4 mg/dL for women underwent elective CAG treated with iohexol or iodixanol or diatrizoate meglumine | NAC/600 mg/po/bid/24 h prior to and after procedure (n = 45) vs. Placebo (n = 45) |
↔ CIN ↔ Cr changes at 48 after CAG ↔ BUN changes at 48 after CAG ↔ CrCl changes at 48 after CAG |
– | Short-term NAC did not prevent CIN in patients with DM and CKD | [96] |
| Prospective randomized-controlled trial | Patients with Cr > 1.2 mg/dL or CrCl < 50 mL underwent elective CAG treated with iodixanol |
NAC/600 mg/po/bid/1 day prior to and after CAG (n = 73) No NAC (n = 106) |
↔ CIN ↔ Cr changes at 48 h after CAG |
– | Short-term NAC did not prevent CIN in patients with CKD | [97] |
| Randomized-controlled trial | Patients with Cr > 1.7 mg/dL underwent CAG treated with iohexol | NAC/1200 mg/po/1 h prior to and 3 h after CAG (n = 38) vs. Placebo (n = 41) |
↔ CIN ↔ Cr changes at 48 h after CAG |
– | Short-term NAC did not prevent CIN after CAG | [98] |
| Prospective, randomized clinical study | Age ≥ 18 years with CrCl < 55 ml/min underwent elective coronary ± peripheral angiography treated with iodixanol | NAC/600 mg/po/bid/1 day prior to and after procedure (n = 99) vs. Placebo (n = 101) | ↔ CIN | – | Short-term NAC did not prevent CIN | [99] |
| Prospective, open-label, randomized, controlled trial | Patients with Cr 1.69–4.52 mg/dL underwent elective CAG or PCI treated with iopromide | NAC/400 mg/po/tid/1 day prior to and after procedure (n = 46) vs No NAC (n = 45) |
↔ CIN ↔ Cr changes at 48 h after procedure ↔ eGFR changes at 48 h after procedure |
– | Short-term NAC did not prevent CIN in patients with moderate to severe renal insufficiency undergoing CAG or PCI | [100] |
| Multicenter, randomized, double-blind, placebo-controlled clinical trial | Diabetic patients with Cr ≥ 106.08 µmol/L or CrCl < 50 mL/min underwent elective CAG or PCI treated with ioxaglate | NAC/600 mg/po/bid/1 day prior to and after procedure (n = 77) vs. Placebo (n = 79) |
↔ CIN ↔ Cr changes at 48 h after procedure ↔ CrCl changes at 48 h after procedure |
– | Short-term NAC did not prevent CIN in patients undergoing cardiac catheterization | [101] |
| Prospective, randomized, double-blind placebo-controlled trial | Patients with Cr ≥ 1.5 mg/dL or CrCl < 50 mL/min underwent CAG treated with iopamidol | NAC/600 mg/po/tid/24 h prior to and after procedure (n = 41) vs. Placebo (n = 39) | ↔ CIN | – | Short-term NAC did not prevent CIN in CKD patients undergoing CAG | [102] |
| Prospective, randomized, double-blind, placebo-controlled trial | Age ≥ 19 years with Cr > 1.2 mg/dL and CrCl < 50 mL/min underwent elective CAG ± PCI treated with iopamidol | NAC/1,500 mg/po/1 day prior to and every 12 h after procedure for 4 doses (n = 49) vs. Placebo (n = 47) |
↔ CIN ↔ Cr ↔ BUN |
– | Short-term NAC did not prevent CIN in patients with CKD undergoing elective CAG | [103] |
| Prospective, randomized, single-blinded, single-center clinical trial | Age > 18 years with eGFR > 30 mL/min/1.73 m2 underwent elective CAG or PCI treated with iopromide |
NAC/600 mg/po/bid/24 h prior to and after procedure (n = 157) vs NaHCO3/1.5 mL/kg/h/IV/6 h prior to and 6 h after procedure (n = 159) vs. NAC/600 mg/po/bid/24 h prior to and after procedure + NaHCO3/1.5 mL/kg/h/IV/6 h prior to and 6 h after procedure (n = 150) vs No NAC or NaHCO3 (n = 161) |
↔ CIN | – | NAC and NaHCO3 did not reduce incidence of CIN | [104] |
| Single-center prospective controlled trial | Patients with Cr > 1.2 mg/dL underwent CAG or PCI treated with ioxaglate |
NAC/600 mg/po/bid/1 day prior to and after procedure (n = 88) vs NaHCO3/1 mL/kg/h/IV/6 h prior to and 6 h after procedure (n = 88) vs No NAC or NaHCO3 (n = 88) |
↓ CIN (NaHCO3 > NAC > No NAC or NaHCO3) ↓ CrCl (NaHCO3 > NAC = No NAC or NaHCO3) |
– | NaHCO3 protected CIN better than NAC and standard treatment | [105] |
| Prospective randomized trial | Patients with CrCl > 30 mL/min/1.73 m2 underwent CAG ± PCI treated with iopromide | NAC/1200 mg/IV/12 h prior to and after procedure (n = 53) vs. Placebo (n = 51) |
↔ CIN ↔ CrCl |
– | Short-term IV NAC did not prevent CIN in patients with normal, mild and moderate CKD undergoing coronary procedure | [109] |
| Single center, prospective, randomized study | CAD with Cr ≥ 1.5 mg/dL ± CrCl < 60 mL/min) who underwent elective CAG treated with iomeprol |
NAC/704 mg/po/bid/1 day prior to and up to 2 day after CAG (n = 7) vs GSH/100 mg/min/IV/30 min prior to CAG (n = 7) vs Control group (n = 7) |
↔ CIN |
↑ LOOHs at 2 h after CAG (control > NAC > GSH) ↓ serum GSH at 2 h after CAG (NAC > control > GSH) |
GSH protected kidney against CM-induced oxidative stress more effectively than oral administration of NAC before CAG | [106] |
| Randomized trial | Age > 18 years underwent elective or emergent CAG |
NaHCO3 (166 mEq/L)/3 mL/kg/h/IV/1 h prior to CAG + 1 mL/kg/h/IV/6 h after CAG (n = 50) vs NaHCO3 (166 mEq/L)/3 ml/kg/h/IV/6 h prior to CAG + 1 mL/kg/h/IV/6 h after CAG (n = 50) |
↑ Cr and ↓ eGFR 48 h post-intervention (short regimen) ↔ Cr and↔ eGFR 48 h post-intervention (long regimen) ↓ serum K |
– | Long-term regimen of bicarbonate supplementation was more effective strategy to prevent CIN than short regimen | [111] |
| Cross-sectional case–control study | CAD with at least 1 risk factor for CIN (DM, advanced age, reduced GFR, anemia) undergoing CAG |
Nebivolol/po/at least 1 mo (n = 45) vs No nebivolol (n = 114) |
↔ CIN ↔ Cr, eGFR, NGAL in both groups before and after CAG ↑ Cr and NGAL and ↓ eGFR in both groups compared to levels before CAG |
– | Nebivolol did not prevent CIN in patients undergoing CAG | [113] |
| Pilot study | Patients with Cr > 2 mg/dL undergoing CAG treated with iomeprol | MESNA/800 mg/IV/30 min prior to and up to 4 h after iomeprol (n = 12) |
↓ CIN ↓ Cr at 48 h |
– | MESNA prevented CIN in patients with renal impairment | [112] |
α-GST, α-glutathione S-transferase; BUN, blood urea nitrogen; CAD, coronary artery disease; CAG, coronary angiography; CHF, congestive heart failure; CIN, contrast-induced nephropathy; CKD, chronic kidney disease; CM, contrast media; Cr, creatinine; CrCl, creatinine clearance; CT, computed tomography; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; GSH, glutathione; IV, intravenously; LDL, low-density lipoprotein; LOOHs, lipid hydroperoxides; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NAC, N-acetylcysteine; NGAL, neutrophil gelatinase-associated lipocalin; NO, nitric oxide; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction
N-Acetylcysteine (NAC)
For nearly two decades, many randomized-controlled trials have investigated the roles of oral NAC for CIN prevention in patients receiving PCI or diagnostic CAG undergoing computed tomography. They demonstrated the protective effect of NAC to CIN in both low and high risk patients compared with placebo [82–91]. The prospective studies demonstrated that NAC prevented the decline in urinary NO end-products [85, 91]. However, many conflicting reports exist, with no significant evident benefits of oral NAC in CIN prevention [92–106]. Therefore, routine administration of NAC for the prevention of CIN or longer-term adverse events after angiographic procedures is not recommended [93].
Decisive factors in NAC for CIN prevention are dosage and treatment duration. NAC was commonly given only for two days prior to CM administration in those trials. It is possible that the duration of NAC treatment was too short to be effective in counteracting CIN-induced ROS production. Furthermore, since NAC has a very short plasma half-life, dosing twice daily could be insufficient to achieve consistent renal protective effects [7]. Future studies are needed to test this hypothesis. A summary of these reports on the effects of oral administration of NAC to prevent CIN is shown in Table 6.
Effects of intravenous administration of NAC on CIN protection has been investigated in patients requiring emergent CAG. In a prospective randomized-controlled study in low risk patients, short-term intravenous NAC treatment could prevent CIN [107]. However, 7% of the patients developed anaphylactoid reactions in that report. In addition, conflicting reports exist [108, 109]. Intravenous NAC reduced oxidative stress after reperfusion of myocardial infarction, however it did not provide additional clinical benefit to nephropathy [108, 109]. Also, intravenous NAC at higher doses could be associated with significant side effects (anaphylactoid reaction, hypotension, bronchospasm) [12]. Despite equipoise on its efficacy, NAC has been widely used in clinical practice in high risk patients due to its low cost, ready availability, easy administration, and limited toxicity in an oral form.
Ascorbic acid, sodium bicarbonate and sodium-2-mercaptoethane sulphonate (MESNA)
In a randomized, double-blind, placebo-controlled trial of patients with Cr ≥ 1.2 mg/dL undergoing CAG, the use of ascorbic acid was associated with a significant reduction in the rate of CIN [110]. For sodium bicarbonate, a randomized trial in patients undergoing CAG demonstrated that bicarbonate supplementation prevented CIN when it was given 6 h prior to CAG and administered continuously for another 6 h after CAG [111]. MESNA has the potential to act as a ROS scavenger. In a pilot study in CKD patients undergoing CAG, renal function improved after MESNA pre and posttreatment [112]. These findings indicated that these antioxidants could prevent CIN in patients with renal impairment. However, future studies may add weight to these limited reports.
Nebivolol
Although in vivo studies report the benefits of antioxidant effects by β-receptor antagonists leading to prevention of CIN, [56, 57] a report from a clinical study demonstrated otherwise. In a cross-sectional case–control study, the patients with risk factor for CIN that received nebivolol had no change in renal function before and after CAG, and did not prevent CIN in patients undergoing CAG [113]. These inconsistent reports could be due to a small sample size from a single center and different times of follow-up on Cr since a 1 month follow up might allow the development of a tolerance to the vasodilatory effects of the drug.
In summary, this review provides a comprehensive narrative review that summarizes findings of the pathogenesis of CIN from in vitro and in vivo studies and the novel interventions for prevention of CIN. Consistent as well as controversial reports regarding the clinical findings are also summarized for potential interventions to prevent CIN. This review provides fundamental knowledge for future basic and clinical studies to find novel interventions to prevent CIN in a clinical setting. However, since our review does not include the non-English original articles it is possible that other potential interventions to prevent CIN be missing.
Conclusions
CIN is associated with adverse outcomes. These include renal replacement therapy, prolonged hospitalization, increased mortality, and increased financial burden. For this reason, the appropriate prophylactic interventions before CM administration in high-risk patients are important in reducing CIN. Since hypoxic-toxic injury, including altered renal microcirculation, medullary hypoxia and ROS-mediated cellular injury is a fundamental pathogenesis of CIN, understanding these various complex pathways could lead to prevention. Although a variety of experimental studies and clinical trials have demonstrated potential pharmacological interventions to prevent CIN, inconclusive results exist. Future double-blinded randomized-controlled trials with large populations of oral or intravenous antioxidants as well as other novel compounds are needed to warrant their use to prevent CIN in patients exposed to CM.
Acknowledgements
Not applicable.
Abbreviations
- AKI
Acute kidney injury
- ATF2
Activating transcriptional factor 2
- Bax
B-cell lymphoma 2-associated X protein
- Bcl-2
B-cell lymphoma-2
- CAG
Coronary angiography
- CI-AKI
Contrast-induced acute kidney injury
- CIN
Contrast-induced nephropathy
- CKD
Chronic kidney disease
- CM
Contrast media
- Cr
Creatinine
- ERK
Extracellular signal-related kinases
- ESRD
End-stage renal disease
- GSH
Glutathione
- HO-1
Heme oxygenase 1
- HSA-Trx
Human serum albumin-thioredoxin-1 fusion protein
- hsCRP
High-sensitivity C-reactive protein
- ICAM-1
Intracellular cell adhesion molecule 1
- IL
Interleukin
- JNK
C-JUN N-terminal kinase
- KDIGO
Kidney Disease Improving Global Outcomes
- KIM-1
Kidney injury molecule-1
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemotactic protein-1
- MDA
Malondialdehyde
- MESNA
Sodium-2-mercaptoethane sulphonate
- MPO
Myeloperoxidase
- NAC
N-Acetylcysteine
- NAD
Nicotinamide adenine dinucleotide
- NF-kB
Nuclear factor-kB
- NGAL
Neutrophil gelatinase-associated lipocalin
- NO
Nitric oxide
- Nox
Nicotinamide adenine dinucleotide phosphate oxidase
- Nrf-2
Nuclear factor erythroid 2-related factor 2
- PCC
Protein carbonyl content
- PCI
Percutaneous coronary intervention
- PGC-1α-FoxO1
Peroxisome proliferator-activated receptor gamma-assisted activating factor-1α-Forkhead-box transcription factor 1
- rMnSOD
Recombinant manganese superoxide dismutase
- ROCK
Rho-kinase
- ROS
Reactive oxygen species
- siRNA
Short interfering ribonucleic acid
- SIRT1
Silent information regulator 1
- SOD
Superoxide dismutase
- TBARS
Thiobarbiturates
- TNF-α
Tumor necrosis factor-α
Authors’ contributions
PK: Data curation, formal analysis, methodology, writing original draft, review and editing; SCC: formal analysis, funding acquisition, investigation, writing-review and editing; NC: Conceptualization, formal analysis, funding acquisition, methodology, supervision, writing-review and editing. All authors read and approved the final manuscript.
Funding
This work was supported by the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC); the Senior Research Scholar grant from the National Research Council of Thailand (SCC), and the Chiang Mai University Center of Excellence Award (NC).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pattharanitima P, Tasanarong A. Pharmacological strategies to prevent contrast-induced acute kidney injury. Biomed Res Int. 2014;2014:236930. doi: 10.1155/2014/236930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Ruiz A, Chandrashekar K, Juncos LA. Changing paradigms in contrast nephropathy. J Am Soc Nephrol. 2017;28(2):397–399. doi: 10.1681/ASN.2016121369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med. 2018;44(1):104–106. doi: 10.1007/s00134-017-4950-6. [DOI] [PubMed] [Google Scholar]
- 4.Haq MFU, Yip CS, Arora P. The conundrum of contrast-induced acute kidney injury. J Thorac Dis. 2020;12(4):1721–1727. doi: 10.21037/jtd.2019.12.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iordache AM, Docea AO, Buga AM, Mitrut R, Albulescu D, Zlatian O, et al. The incidence of skin lesions in contrast media-induced chemical hypersensitivity. Exp Ther Med. 2019;17(2):1113–1124. doi: 10.3892/etm.2018.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Work Group Membership. Kidney Int Suppl. 2012;2(1):2. [DOI] [PMC free article] [PubMed]
- 7.Pflueger A, Abramowitz D, Calvin AD. Role of oxidative stress in contrast-induced acute kidney injury in diabetes mellitus. Med Sci Monit. 2009;15(6):125–136. [PubMed] [Google Scholar]
- 8.Mohammed NM, Mahfouz A, Achkar K, Rafie IM, Hajar R. Contrast-induced nephropathy. Heart Views. 2013;14(3):106–116. doi: 10.4103/1995-705X.125926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullough PA, Choi JP, Feghali GA, Schussler JM, Stoler RM, Vallabahn RC, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2016;68(13):1465–1473. doi: 10.1016/j.jacc.2016.05.099. [DOI] [PubMed] [Google Scholar]
- 10.Briguori C, Marenzi G. Contrast-induced nephropathy: pharmacological prophylaxis. Kidney Int Suppl. 2006;100:S30–S38. doi: 10.1038/sj.ki.5000372. [DOI] [PubMed] [Google Scholar]
- 11.Kini AS, Mitre CA, Kamran M, Suleman J, Kim M, Duffy ME, et al. Changing trends in incidence and predictors of radiographic contrast nephropathy after percutaneous coronary intervention with use of fenoldopam. Am J Cardiol. 2002;89(8):999–1002. doi: 10.1016/S0002-9149(02)02259-2. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146–2155. doi: 10.1056/NEJMra1805256. [DOI] [PubMed] [Google Scholar]
- 13.Heyman SN, Rosen S, Khamaisi M, Idee JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45(4):188–195. doi: 10.1097/RLI.0b013e3181d2eed8. [DOI] [PubMed] [Google Scholar]
- 14.Pritchard AL, Hayward NK. Molecular pathways: mitogen-activated protein kinase pathway mutations and drug resistance. Clin Cancer Res. 2013;19(9):2301–2309. doi: 10.1158/1078-0432.CCR-12-0383. [DOI] [PubMed] [Google Scholar]
- 15.Pisani A, Riccio E, Andreucci M, Faga T, Ashour M, Di Nuzzi A, et al. Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed Res Int. 2013;2013:868321. doi: 10.1155/2013/868321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu GL, Lei R, Duan SB, Tang MM, Luo M, Xu Q. Atorvastatin alleviates iodinated contrast media-induced cytotoxicity in human proximal renal tubular epithelial cells. Exp Ther Med. 2017;14(4):3309–3313. doi: 10.3892/etm.2017.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong BY, Lee HY, Park CG, Kang J, Yu SL, Choi DR, et al. Oxidative stress caused by activation of NADPH oxidase 4 promotes contrast-induced acute kidney injury. PLoS ONE. 2018;13(1):e0191034. doi: 10.1371/journal.pone.0191034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmon RC, Duffy SP, Terneus MV, Ball JG, Valentovic MA. Characterization of a novel model for investigation of radiocontrast nephrotoxicity. Nephrol Dial Transplant. 2009;24(3):763–768. doi: 10.1093/ndt/gfn540. [DOI] [PubMed] [Google Scholar]
- 19.Lee HC, Sheu SH, Yen HW, Lai WT, Chang JG. JNK/ATF2 pathway is involved in iodinated contrast media-induced apoptosis. Am J Nephrol. 2010;31(2):125–133. doi: 10.1159/000259899. [DOI] [PubMed] [Google Scholar]
- 20.Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett. 2019;24:36. doi: 10.1186/s11658-019-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong YA, Bae SY, Ahn SY, Kim J, Kwon YJ, Jung WY, et al. Resveratrol ameliorates contrast induced nephropathy through the activation of SIRT1-PGC-1alpha-Foxo1 signaling in mice. Kidney Blood Press Res. 2017;42(4):641–653. doi: 10.1159/000481804. [DOI] [PubMed] [Google Scholar]
- 22.Wirth A. Rho kinase and hypertension. Biochim Biophys Acta. 2010;1802(12):1276–1284. doi: 10.1016/j.bbadis.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang H, Yang Z, Miao D, Zhang D. Rho kinase inhibitor, Fasudil, attenuates contrast-induced acute kidney injury. Basic Clin Pharmacol Toxicol. 2018;122(2):278–287. doi: 10.1111/bcpt.12895. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Ding M, Zhu P, Huang H, Zhuang Q, Shen J, et al. New insights into the Nrf-2/HO-1 signaling axis and its application in pediatric respiratory diseases. Oxid Med Cell Longev. 2019;2019:3214196. doi: 10.1155/2019/3214196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, Heretis I, Wilks MF, Spandidos DA, et al. Contrast-induced nephropathy: basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther. 2017;180:99–112. doi: 10.1016/j.pharmthera.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Tongqiang L, Shaopeng L, Xiaofang Y, Nana S, Xialian X, Jiachang H, et al. Salvianolic acid B prevents iodinated contrast media-induced acute renal injury in rats via the PI3K/Akt/Nrf2 pathway. Oxid Med Cell Longev. 2016;2016:7079487. doi: 10.1155/2016/7079487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Z, Liao G, Zhou Q, Lv D, Holthfer H, Zou H. Sulforaphane attenuates contrast-induced nephropathy in rats via Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2016;2016:9825623. doi: 10.1155/2016/9825623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman AI, Olszanecki R, Yang LM, Quan S, Li M, Omura S, et al. Heme oxygenase-1 protects against radiocontrast-induced acute kidney injury by regulating anti-apoptotic proteins. Kidney Int. 2007;72(8):945–953. doi: 10.1038/sj.ki.5002447. [DOI] [PubMed] [Google Scholar]
- 29.Quintavalle C, Fiore D, De Micco F, Visconti G, Focaccio A, Golia B, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation. 2012;126(25):3008–3016. doi: 10.1161/CIRCULATIONAHA.112.103317. [DOI] [PubMed] [Google Scholar]
- 30.Deng J, Wu G, Yang C, Li Y, Jing Q, Han Y. Rosuvastatin attenuates contrast-induced nephropathy through modulation of nitric oxide, inflammatory responses, oxidative stress and apoptosis in diabetic male rats. J Transl Med. 2015;13:53. doi: 10.1186/s12967-015-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X-L, Zhang T, Hu L-H, Sun S-Q, Zhang W-F, Sun Z, et al. Comparison of effects of different statins on contrast-induced acute kidney injury in rats: histopathological and biochemical findings. Oxidative Med Cell Longevity. 2017;2017:1–10. doi: 10.1155/2017/6282486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Otaibi KE, Al Elaiwi AM, Tariq M, Al-Asmari AK. Simvastatin attenuates contrast-induced nephropathy through modulation of oxidative stress, proinflammatory myeloperoxidase, and nitric oxide. Oxid Med Cell Longev. 2012;2012:831748. doi: 10.1155/2012/831748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu S, Hu L, Wang X, Sun S, Zhang T, Sun Z, et al. Xuezhikang ameliorates contrast media-induced nephropathy in rats via suppression of oxidative stress, inflammatory responses and apoptosis. Ren Fail. 2016;38(10):1717–1725. doi: 10.1080/0886022X.2016.1207052. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Jiao Z, Liu Y, Chen J, Li G, Liu T, et al. Probucol Protects against contrast-induced acute kidney injury via the extracellular signal-regulated kinases 1 and 2 (ERK1/2)/JNK-caspase 3 pathway in diabetic Rats. Med Sci Monit. 2019;25:1038–1045. doi: 10.12659/MSM.913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kongkham S, Sriwong S, Tasanarong A. Protective effect of alpha tocopherol on contrast-induced nephropathy in rats. Nefrologia. 2013;33(1):116–123. doi: 10.3265/Nefrologia.pre2012.Nov.11736. [DOI] [PubMed] [Google Scholar]
- 36.Kunak CS, Ugan RA, Cadirci E, Karakus E, Polat B, Un H, et al. Nephroprotective potential of carnitine against glycerol and contrast-induced kidney injury in rats through modulation of oxidative stress, proinflammatory cytokines, and apoptosis. Br J Radiol. 2016;89(1058):20140724. doi: 10.1259/bjr.20140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodama A, Watanabe H, Tanaka R, Tanaka H, Chuang VT, Miyamoto Y, et al. A human serum albumin-thioredoxin fusion protein prevents experimental contrast-induced nephropathy. Kidney Int. 2013;83(3):446–454. doi: 10.1038/ki.2012.429. [DOI] [PubMed] [Google Scholar]
- 38.Ari E, Kedrah AE, Alahdab Y, Bulut G, Eren Z, Baytekin O, et al. Antioxidant and renoprotective effects of paricalcitol on experimental contrast-induced nephropathy model. Br J Radiol. 2012;85(1016):1038–1043. doi: 10.1259/bjr/16327485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozturk O, Eroglu HA, Ustebay S, Kuzucu M, Adali Y. An experimental study on the preventive effects of N-acetyl cysteine and ozone treatment against contrast-induced nephropathy. Acta Cir Bras. 2018;33(6):508–517. doi: 10.1590/s0102-865020180060000005. [DOI] [PubMed] [Google Scholar]
- 40.Xing Y, Wei RB, Tang L, Yang Y, Zheng XY, Wang ZC, et al. Protective effect of salidroside on contrast-induced nephropathy in comparison with N-acetylcysteine and its underlying mechanism. Chin J Integr Med. 2015;21(4):266–273. doi: 10.1007/s11655-015-2137-y. [DOI] [PubMed] [Google Scholar]
- 41.Pisani A, Sabbatini M, Riccio E, Rossano R, Andreucci M, Capasso C, et al. Effect of a recombinant manganese superoxide dismutase on prevention of contrast-induced acute kidney injury. Clin Exp Nephrol. 2014;18(3):424–431. doi: 10.1007/s10157-013-0828-2. [DOI] [PubMed] [Google Scholar]
- 42.Karaman A, Diyarbakir B, Durur-Subasi I, Kose D, Ozbek-Bilgin A, Topcu A, et al. A novel approach to contrast-induced nephrotoxicity: the melatonergic agent agomelatine. Br J Radiol. 2016;89(1061):20150716. doi: 10.1259/bjr.20150716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gazi S, Altun A, Erdogan O. Contrast-induced nephropathy: preventive and protective effects of melatonin. J Pineal Res. 2006;41(1):53–57. doi: 10.1111/j.1600-079X.2006.00336.x. [DOI] [PubMed] [Google Scholar]
- 44.Onk D, Onk OA, Turkmen K, Erol HS, Ayazoglu TA, Keles ON, et al. Melatonin attenuates contrast-induced nephropathy in diabetic rats: the role of interleukin-33 and oxidative stress. Mediators Inflamm. 2016;2016:9050828. doi: 10.1155/2016/9050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gui D, Huang J, Liu W, Guo Y, Xiao W, Wang N. Astragaloside IV prevents acute kidney injury in two rodent models by inhibiting oxidative stress and apoptosis pathways. Apoptosis. 2013;18(4):409–422. doi: 10.1007/s10495-013-0801-2. [DOI] [PubMed] [Google Scholar]
- 46.Buyuklu M, Kandemir FM, Ozkaraca M, Set T, Bakirci EM, Topal E. Protective effect of curcumin against contrast induced nephropathy in rat kidney: what is happening to oxidative stress, inflammation, autophagy and apoptosis? Eur Rev Med Pharmacol Sci. 2014;18(4):461–470. [PubMed] [Google Scholar]
- 47.Ozkan G, Ulusoy S, Orem A, Ersoz S, Alkanat M, Yucesan FB, et al. Protective effect of the grape seed proanthocyanidin extract in a rat model of contrast-induced nephropathy. Kidney Blood Press Res. 2012;35(6):445–453. doi: 10.1159/000337926. [DOI] [PubMed] [Google Scholar]
- 48.Buyuklu M, Kandemir FM, Ozkaraca M, Set T, Bakirci EM, Topal E, et al. Benefical effects of lycopene against contrast medium-induced oxidative stress, inflammation, autophagy, and apoptosis in rat kidney. Hum Exp Toxicol. 2015;34(5):487–496. doi: 10.1177/0960327114542964. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Zhang G, Zhou Y, Gui D, Li J, Xing T, et al. Magnolin protects against contrast-induced nephropathy in rats via antioxidation and antiapoptosis. Oxid Med Cell Longev. 2014;2014:203458. doi: 10.1155/2014/203458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tasanarong A, Kongkham S, Itharat A. Antioxidant effect of Phyllanthus emblica extract prevents contrast-induced acute kidney injury. BMC Complement Altern Med. 2014;14:138. doi: 10.1186/1472-6882-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu DZ, Li YH, Chu PY, Periasamy S, Liu MY. Sesame oil prevents acute kidney injury induced by the synergistic action of aminoglycoside and iodinated contrast in rats. Antimicrob Agents Chemother. 2011;55(6):2532–2536. doi: 10.1128/AAC.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Souza SV, Peters B, Coco LZ, Alves GM, de Assis A, Nogueira BV, et al. Silymarin protects against radiocontrast-induced nephropathy in mice. Life Sci. 2019;228:305–315. doi: 10.1016/j.lfs.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 53.Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao Q, et al. Antithrombin III protects against contrast-induced nephropathy. EBioMedicine. 2017;17:101–107. doi: 10.1016/j.ebiom.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quiros Y, Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Morales AI, Lopez-Novoa JM. Cardiotrophin-1 administration prevents the renal toxicity of iodinated contrast media in rats. Toxicol Sci. 2013;132(2):493–501. doi: 10.1093/toxsci/kft007. [DOI] [PubMed] [Google Scholar]
- 55.Hussien NI, Sorour SM, El-Kerdasy HI, Abdelrahman BA. The glucagon-like peptide-1 receptor agonist Exendin-4, ameliorates contrast-induced nephropathy through suppression of oxidative stress, vascular dysfunction and apoptosis independent of glycaemia. Clin Exp Pharmacol Physiol. 2018;45(8):808–818. doi: 10.1111/1440-1681.12944. [DOI] [PubMed] [Google Scholar]
- 56.Toprak O, Cirit M, Tanrisev M, Yazici C, Canoz O, Sipahioglu M, et al. Preventive effect of nebivolol on contrast-induced nephropathy in rats. Nephrol Dial Transplant. 2008;23(3):853–859. doi: 10.1093/ndt/gfm691. [DOI] [PubMed] [Google Scholar]
- 57.Akgullu C, Hekim T, Eryilmaz U, Boyacioglu M, Gungor H, Meteoglu I, et al. The usefulness of carvedilol and nebivolol in preventing contrast nephropathy in rats. Ren Fail. 2015;37(3):511–517. doi: 10.3109/0886022X.2015.1006087. [DOI] [PubMed] [Google Scholar]
- 58.Almeida LS, Barboza JR, Freitas FPS, Porto ML, Vasquez EC, Meyrelles SS, et al. Sildenafil prevents renal dysfunction in contrast media-induced nephropathy in Wistar rats. Hum Exp Toxicol. 2016;35(11):1194–1202. doi: 10.1177/0960327115626582. [DOI] [PubMed] [Google Scholar]
- 59.Iordache AM, Buga AM, Albulescu D, Vasile RC, Mitrut R, Georgiadis G, et al. Phosphodiesterase-5 inhibitors ameliorate structural kidney damage in a rat model of contrast-induced nephropathy. Food Chem Toxicol. 2020;143:111535. doi: 10.1016/j.fct.2020.111535. [DOI] [PubMed] [Google Scholar]
- 60.Iordache AM, Docea AO, Buga AM, Zlatian O, Ciurea ME, Rogoveanu OC, et al. Sildenafil and tadalafil reduce the risk of contrast-induced nephropathy by modulating the oxidant/antioxidant balance in a murine model. Food Chem Toxicol. 2020;135:111038. doi: 10.1016/j.fct.2019.111038. [DOI] [PubMed] [Google Scholar]
- 61.Georgiadis G, Zisis IE, Docea AO, Tsarouhas K, Fragkiadoulaki I, Mavridis C, et al. Current concepts on the reno-protective effects of phosphodiesterase 5 inhibitors in acute kidney injury: systematic search and review. J Clin Med. 2020;9:5. doi: 10.3390/jcm9051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X, Yan X, Yang D, Zhou J, Song J, Yang D. Rapamycin attenuates mitochondrial injury and renal tubular cell apoptosis in experimental contrast-induced acute kidney injury in rats. Biosci Rep. 2018;38:6. doi: 10.1042/BSR20180876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aksu F, Aksu B, Unlu N, Karaca T, Ayvaz S, Erman H, et al. Antioxidant and renoprotective effects of sphingosylphosphorylcholine on contrast-induced nephropathy in rats. Ren Fail. 2016;38(7):1089–1098. doi: 10.1080/0886022X.2016.1194142. [DOI] [PubMed] [Google Scholar]
- 64.Barlak A, Akar H, Yenicerioglu Y, Yenisey C, Meteoglu I, Yilmaz O. Effect of sodium bicarbonate in an experimental model of radiocontrast nephropathy. Ren Fail. 2010;32(8):992–999. doi: 10.3109/0886022X.2010.502282. [DOI] [PubMed] [Google Scholar]
- 65.Khanal S, Attallah N, Smith DE, Kline-Rogers E, Share D, O'Donnell MJ, et al. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med. 2005;118(8):843–849. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 66.Khosravi A, Dolatkhah M, Hashemi HS, Rostami Z. Preventive effect of atorvastatin (80 mg) on contrast-induced nephropathy after angiography in high-risk patients: double-blind randomized clinical trial. Nephrourol Mon. 2016;8(3):e29574. doi: 10.5812/numonthly.29574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syed MH, Khandelwal PN, Thawani VR, Katare SS. Efficacy of atorvastatin in prevention of contrast-induced nephropathy in high-risk patients undergoing angiography: a double-blind randomized controlled trial. J Pharmacol Pharmacother. 2017;8(2):50–53. doi: 10.4103/jpp.JPP_156_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patti G, Ricottini E, Nusca A, Colonna G, Pasceri V, D'Ambrosio A, et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty–contrast-induced nephropathy] trial. Am J Cardiol. 2011;108(1):1–7. doi: 10.1016/j.amjcard.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Li W, Fu X, Wang Y, Li X, Yang Z, Wang X, et al. Beneficial effects of high-dose atorvastatin pretreatment on renal function in patients with acute ST-segment elevation myocardial infarction undergoing emergency percutaneous coronary intervention. Cardiology. 2012;122(3):195–202. doi: 10.1159/000339472. [DOI] [PubMed] [Google Scholar]
- 70.Ozhan H, Erden I, Ordu S, Aydin M, Caglar O, Basar C, et al. Efficacy of short-term high-dose atorvastatin for prevention of contrast-induced nephropathy in patients undergoing coronary angiography. Angiology. 2010;61(7):711–714. doi: 10.1177/0003319710364216. [DOI] [PubMed] [Google Scholar]
- 71.Sanei H, Hajian-Nejad A, Sajjadieh-Kajouei A, Nazemzadeh N, Alizadeh N, Bidram P, et al. Short term high dose atorvastatin for the prevention of contrast-induced nephropathy in patients undergoing computed tomography angiography. ARYA Atheroscler. 2014;10(5):252–258. [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou X, Jin YZ, Wang Q, Min R, Zhang XY. Efficacy of high dose atorvastatin on preventing contrast induced nephropathy in patients underwent coronary angiography. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37(5):394–396. [PubMed] [Google Scholar]
- 73.Acikel S, Muderrisoglu H, Yildirir A, Aydinalp A, Sade E, Bayraktar N, et al. Prevention of contrast-induced impairment of renal function by short-term or long-term statin therapy in patients undergoing elective coronary angiography. Blood Coagul Fibrinolysis. 2010;21(8):750–757. doi: 10.1097/MBC.0b013e32834014a4. [DOI] [PubMed] [Google Scholar]
- 74.Han Y, Zhu G, Han L, Hou F, Huang W, Liu H, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63(1):62–70. doi: 10.1016/j.jacc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 75.Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome) J Am Coll Cardiol. 2014;63(1):71–79. doi: 10.1016/j.jacc.2013.04.105. [DOI] [PubMed] [Google Scholar]
- 76.Sanadgol H, Abdani S, Tabatabaiee P, Mohammadi M. Protective effect of high dose short term statin therapy with normal saline in prevention of contrast-induced nephropathy among iodixanol-receiving patients. J Renal Inj Prev. 2012;1(1):43–45. doi: 10.12861/jrip.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xinwei J, Xianghua F, Jing Z, Xinshun G, Ling X, Weize F, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2009;104(4):519–524. doi: 10.1016/j.amjcard.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 78.Jo SH, Koo BK, Park JS, Kang HJ, Cho YS, Kim YJ, et al. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial–a randomized controlled study. Am Heart J. 2008;155(3):499. doi: 10.1016/j.ahj.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 79.Toso A, Maioli M, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105(3):288–292. doi: 10.1016/j.amjcard.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 80.Kandula P, Shah R, Singh N, Markwell SJ, Bhensdadia N, Navaneethan SD. Statins for prevention of contrast-induced nephropathy in patients undergoing non-emergent percutaneous coronary intervention. Nephrology (Carlton) 2010;15(2):165–170. doi: 10.1111/j.1440-1797.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 81.Cai L, Bai X, Lei H, Wu H, Liu Y, Zhu Q, et al. High plasma exposure of statins associated with increased risk of contrast-induced acute kidney injury in chinese patients with coronary artery disease. Front Pharmacol. 2018;9:427. doi: 10.3389/fphar.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343(3):180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 83.Briguori C, Manganelli F, Scarpato P, Elia PP, Golia B, Riviezzo G, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002;40(2):298–303. doi: 10.1016/S0735-1097(02)01958-7. [DOI] [PubMed] [Google Scholar]
- 84.Diaz-Sandoval LJ, Kosowsky BD, Losordo DW. Acetylcysteine to prevent angiography-related renal tissue injury (the APART trial) Am J Cardiol. 2002;89(3):356–358. doi: 10.1016/S0002-9149(01)02243-3. [DOI] [PubMed] [Google Scholar]
- 85.Efrati S, Dishy V, Averbukh M, Blatt A, Krakover R, Weisgarten J, et al. The effect of N-acetylcysteine on renal function, nitric oxide, and oxidative stress after angiography. Kidney Int. 2003;64(6):2182–2187. doi: 10.1046/j.1523-1755.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 86.Kay J, Chow WH, Chan TM, Lo SK, Kwok OH, Yip A, et al. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA. 2003;289(5):553–558. doi: 10.1001/jama.289.5.553. [DOI] [PubMed] [Google Scholar]
- 87.MacNeill BD, Harding SA, Bazari H, Patton KK, Colon-Hernadez P, DeJoseph D, et al. Prophylaxis of contrast-induced nephropathy in patients undergoing coronary angiography. Catheter Cardiovasc Interv. 2003;60(4):458–461. doi: 10.1002/ccd.10684. [DOI] [PubMed] [Google Scholar]
- 88.Ochoa A, Pellizzon G, Addala S, Grines C, Isayenko Y, Boura J, et al. Abbreviated dosing of N-acetylcysteine prevents contrast-induced nephropathy after elective and urgent coronary angiography and intervention. J Interv Cardiol. 2004;17(3):159–165. doi: 10.1111/j.1540-8183.2004.09880.x. [DOI] [PubMed] [Google Scholar]
- 89.Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol. 2002;40(8):1383–1388. doi: 10.1016/S0735-1097(02)02308-2. [DOI] [PubMed] [Google Scholar]
- 90.Baskurt M, Okcun B, Abaci O, Dogan GM, Kilickesmez K, Ozkan AA, et al. N-acetylcysteine versus N-acetylcysteine + theophylline for the prevention of contrast nephropathy. Eur J Clin Invest. 2009;39(9):793–799. doi: 10.1111/j.1365-2362.2009.02173.x. [DOI] [PubMed] [Google Scholar]
- 91.Drager LF, Andrade L, Toledo JF, Laurindo FR, MachadoCesar LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19(7):1803–1807. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 92.Kimmel M, Butscheid M, Brenner S, Kuhlmann U, Klotz U, Alscher DM. Improved estimation of glomerular filtration rate by serum cystatin C in preventing contrast induced nephropathy by N-acetylcysteine or zinc–preliminary results. Nephrol Dial Transplant. 2008;23(4):1241–1245. doi: 10.1093/ndt/gfm785. [DOI] [PubMed] [Google Scholar]
- 93.Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378(7):603–614. doi: 10.1056/NEJMoa1710933. [DOI] [PubMed] [Google Scholar]
- 94.Investigators ACT. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT) Circulation. 2011;124(11):1250–1259. doi: 10.1161/CIRCULATIONAHA.111.038943. [DOI] [PubMed] [Google Scholar]
- 95.Allaqaband S, Tumuluri R, Malik AM, Gupta A, Volkert P, Shalev Y, et al. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc Interv. 2002;57(3):279–283. doi: 10.1002/ccd.10323. [DOI] [PubMed] [Google Scholar]
- 96.Amini M, Salarifar M, Amirbaigloo A, Masoudkabir F, Esfahani F. N-acetylcysteine does not prevent contrast-induced nephropathy after cardiac catheterization in patients with diabetes mellitus and chronic kidney disease: a randomized clinical trial. Trials. 2009;10:45. doi: 10.1186/1745-6215-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boccalandro F, Amhad M, Smalling RW, Sdringola S. Oral acetylcysteine does not protect renal function from moderate to high doses of intravenous radiographic contrast. Catheter Cardiovasc Interv. 2003;58(3):336–341. doi: 10.1002/ccd.10389. [DOI] [PubMed] [Google Scholar]
- 98.Durham JD, Caputo C, Dokko J, Zaharakis T, Pahlavan M, Keltz J, et al. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62(6):2202–2207. doi: 10.1046/j.1523-1755.2002.00673.x. [DOI] [PubMed] [Google Scholar]
- 99.Ferrario F, Barone MT, Landoni G, Genderini A, Heidemperger M, Trezzi M, et al. Acetylcysteine and non-ionic isosmolar contrast-induced nephropathy–a randomized controlled study. Nephrol Dial Transplant. 2009;24(10):3103–3107. doi: 10.1093/ndt/gfp306. [DOI] [PubMed] [Google Scholar]
- 100.Fung JW, Szeto CC, Chan WW, Kum LC, Chan AK, Wong JT, et al. Effect of N-acetylcysteine for prevention of contrast nephropathy in patients with moderate to severe renal insufficiency: a randomized trial. Am J Kidney Dis. 2004;43(5):801–808. doi: 10.1053/j.ajkd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 101.Gomes VO, Figueredo CE, Caramori P, Lasevitch R, Bodanese LC, Araujo A, et al. N-acetylcysteine does not prevent contrast induced nephropathy after cardiac catheterisation with an ionic low osmolality contrast medium: a multicentre clinical trial. Heart. 2005;91(6):774–778. doi: 10.1136/hrt.2004.039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldenberg I, Shechter M, Matetzky S, Jonas M, Adam M, Pres H, et al. Oral acetylcysteine as an adjunct to saline hydration for the prevention of contrast-induced nephropathy following coronary angiography. A randomized controlled trial and review of the current literature. Eur Heart J. 2004;25(3):212–218. doi: 10.1016/j.ehj.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Oldemeyer JB, Biddle WP, Wurdeman RL, Mooss AN, Cichowski E, Hilleman DE. Acetylcysteine in the prevention of contrast-induced nephropathy after coronary angiography. Am Heart J. 2003;146(6):E23. doi: 10.1016/S0002-8703(03)00511-8. [DOI] [PubMed] [Google Scholar]
- 104.Yang K, Liu W, Ren W, Lv S. Different interventions in preventing contrast-induced nephropathy after percutaneous coronary intervention. Int Urol Nephrol. 2014;46(9):1801–1807. doi: 10.1007/s11255-014-0765-3. [DOI] [PubMed] [Google Scholar]
- 105.Ozcan EE, Guneri S, Akdeniz B, Akyildiz IZ, Senaslan O, Baris N, et al. Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy. A comparison of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures. A single-center prospective controlled trial. Am Heart J. 2007;154(3):539–544. doi: 10.1016/j.ahj.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 106.Saitoh T, Satoh H, Nobuhara M, Machii M, Tanaka T, Ohtani H, et al. Intravenous glutathione prevents renal oxidative stress after coronary angiography more effectively than oral N-acetylcysteine. Heart Vessels. 2011;26(5):465–472. doi: 10.1007/s00380-010-0078-0. [DOI] [PubMed] [Google Scholar]
- 107.Baker CS, Wragg A, Kumar S, De Palma R, Baker LR, Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol. 2003;41(12):2114–2118. doi: 10.1016/S0735-1097(03)00487-X. [DOI] [PubMed] [Google Scholar]
- 108.Thiele H, Hildebrand L, Schirdewahn C, Eitel I, Adams V, Fuernau G, et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol. 2010;55(20):2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 109.Kefer JM, Hanet CE, Boitte S, Wilmotte L, De Kock M. Acetylcysteine, coronary procedure and prevention of contrast-induced worsening of renal function: which benefit for which patient? Acta Cardiol. 2003;58(6):555–560. doi: 10.2143/AC.58.6.2005321. [DOI] [PubMed] [Google Scholar]
- 110.Spargias K, Alexopoulos E, Kyrzopoulos S, Iokovis P, Greenwood DC, Manginas A, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110(18):2837–2842. doi: 10.1161/01.CIR.0000146396.19081.73. [DOI] [PubMed] [Google Scholar]
- 111.Abouzeid S, Mosbah O. Evaluation of different sodium bicarbonate regimens for the prevention of contrast medium-induced nephropathy. Electron Physician. 2016;8(2):1973–1977. doi: 10.19082/1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haeussler U, Riedel M, Keller F. Free reactive oxygen species and nephrotoxicity of contrast agents. Kidney Blood Press Res. 2004;27(3):167–171. doi: 10.1159/000079805. [DOI] [PubMed] [Google Scholar]
- 113.Altunoren O, Balli M, Eren N, Tasolar H, Arpaci A, Caglayan CE, et al. Is nebivolol really effective in preventing contrast induced nephropathy? Kidney Blood Press Res. 2015;40(5):533–541. doi: 10.1159/000368529. [DOI] [PubMed] [Google Scholar]
- 114.Kaya A, Kurt M, Tanboga IH, Isik T, Ekinci M, Aksakal E, et al. Rosuvastatin versus atorvastatin to prevent contrast induced nephropathy in patients undergoing primary percutaneous coronary intervention (ROSA-cIN trial) Acta Cardiol. 2013;68(5):489–494. doi: 10.1080/AC.68.5.2994472. [DOI] [PubMed] [Google Scholar]
- 115.Ma H, Liu Y, Xie H, Zhang G, Zhan H, Liu Z, et al. The renoprotective effects of simvastatin and atorvastatin in patients with acute coronary syndrome undergoing percutaneous coronary intervention: an observational study. Medicine (Baltimore) 2017;96(32):e7351. doi: 10.1097/MD.0000000000007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.