Abstract
Background
People with criminal justice involvement contribute remarkably to the rising hepatitis C virus (HCV) burden; however, the continuum of care is a major barrier to prison-based programs. We aimed to evaluate a comprehensive HCV care model in an Iranian provincial prison.
Methods
Between 2017–2018, in the Karaj Central Prison, newly admitted male inmates received HCV antibody testing and venipuncture for RNA testing (antibody-positive only). Participants with positive RNA underwent direct-acting antiviral (DAA) therapy (Sofosbuvir/Daclatasvir). Sustained virological response was evaluated at 12 weeks post-treatment (SVR12).
Results
Overall, from 3485 participants, 182 (5.2%) and 117 (3.4%) tested positive for HCV antibody and RNA, respectively. Among 116 patients who were eligible for treatment, 24% (n = 28) were released before treatment and 72% (n = 83) initiated DAA therapy, of whom 81% (n = 67/83) completed treatment in prison, and the rest were released. Of total released patients, 68% (n = 30/44) were linked to care in community, and 70% (n = 21/30) completed treatment, including 60% (n = 12/20) and 90% (n = 9/10) among those who were released before and during treatment, respectively. The overall HCV treatment uptake and completion were 89% (n = 103/116) and 85% (n = 88/103), respectively. From people who completed treatment, 43% (n = 38/88) attended for response assessment and all were cured (SVR12 = 100%).
Conclusions
Integrated HCV care models are highly effective and can be significantly strengthened by post-release interventions. The close collaboration of community and prison healthcare systems is crucial to promote high levels of treatment adherence. Future studies should investigate the predictors of engagement with HCV care following release.
Keywords: Hepatitis C virus (HCV), HCV treatment, Linkage to care, Prison healthcare, Harm reduction, HCV elimination
Background
Following the introduction of highly effective antiviral agents, the hepatitis C virus (HCV) infection has become curable in the recent decade [1]; hence treatment of infected people has been introduced as a key strategy for disease prevention in communities [2]. Imprisonment and the increased risk of transmission after release remarkably contribute to the rising HCV burden worldwide [3, 4]. Accordingly, many countries are scaling up prison-based programs to reach the World Health Organization (WHO) target of viral hepatitis elimination as a public health issue by 2030 [5, 6]. A few studies have reported near- or micro-elimination of HCV in specific prison settings; however, evidence around the post-release engagement with care is scarce [7].
HCV case-finding among the prison population who often are underserved by community healthcare services has appeared to be cost-effective [8]. Despite the encouragement brought by recent successes, many countries have challenges expanding HCV care among people with criminal justice involvement, and access to health services often ends with patient’s release back into community [9, 10]. Retrospective studies from the USA have shown that only 10% of formerly incarcerated patients are linked to HCV care after release [11, 12]. Short prison sentences lead to high rates of treatment discontinuations, which highlights the necessity of ensuring care continuity upon release [13]. However, the transition period is accompanied by many competing priorities that often prevent patients from ongoing engagement with care [9, 11]. Such priorities include inadequate social and financial support, which often result in a return to drug-related activities and may erode all health benefits gained during incarceration [9, 14, 15].
Lack of appropriate discharge planning for HCV treatment, as well as mental disorders and substance use treatment, results in difficulty navigating through community healthcare services after release [16, 17]. Besides, poor integration between prison and community is another obstacle that can hinder immediate linkage to care and contribute to the cycle of suboptimal achievement of HCV elimination programs [18]. Community-based organizations and NGOs can play an invaluable role in the community reintegration of offenders [19]. To date, studies on linkage to care from incarceration have been mainly focused on people with HIV infection, and a variety of strategies, including case management and patient navigation, have been introduced to facilitate the transition period for these patients [15, 20]. Although developing effective care models require knowledge on the gaps in continuity of care and potential solutions [11], evidence lack around feasibility and efficiency of such interventions among HCV patients [21, 22]. Community reintegration and post-release continuity of care are current priority areas for prison healthcare research [23].
To date, no study is published on the effectiveness of HCV interventions among people with criminal justice involvement in low- or middle-income countries [24]. In recent decades, the Iranians Prisons Organization has adopted progressive harm-reduction policies; however, HCV screening and treatment are not yet provided routinely at correctional facilities. We aimed to implement a comprehensive HCV care model in a provincial prison in Iran, as a middle-income country.
Methods
Study population
This interventional study was conducted in the context of a national pilot on “Screening, diagnosis, and treatment of hepatitis C in Iranian prisons.” Between June 2017 and February 2018, all newly admitted male inmates in the Central Prison of Karaj, who aged above 18 years, were recruited given providing written consent. The exclusion criteria were hepatitis B infection, chronic kidney disease, cirrhosis, and HIV co-infection due to the antiretroviral drug interactions. After enrollment, the study was ongoing for about two years and patients were followed by June 2019 to complete treatment and response assessment. The review board of the Digestive Diseases Research Institute of Tehran University of Medical Sciences approved the study protocol.
Study site
The Central Prison of Karaj is a large prison located in Karaj city, Alborz province, which is effectively a suburb of the capital city of Iran. This prison with 14 wards—inmates residing in 10 wards and four wards provide food and other services—is home to 6000 inmates at any given time and has approximately 30 new admissions daily; the majority are involved with drug-related charges (five out of 10 wards). A baseline behavioral survey was conducted in 2007, just before the introduction of methadone maintenance treatment (MMT) in this prison. According to that survey, the prevalence of drug use and injecting drug use was 93% and 42%, respectively; participants also reported having been incarcerated an average of five times before their current prison sentence [25]. The Central Prison of Karaj has a triangular clinic with one general practitioner, one psychologist, and several nurses who provide healthcare services, including HIV testing and methadone dispensing. However, there is no HCV screening or treatment program available.
Sample collection
Before starting the project, several workshops were held by the study coordinators to educate the prison healthcare staff and ensure sampling methods. All inmates received a rapid diagnostic test (RDT) for the HCV antibody using a finger-stick blood specimen. Irrespective of the result, participants underwent venipuncture for another antibody testing by a fourth-generation enzyme-linked immunosorbent assay (ELISA). Blood samples were transferred daily to a reference laboratory outside. In case of discordant results, the plasma sample was re-evaluated by RDT in the laboratory to recognize testing errors in prison. On samples with confirmed positive antibody, quantitative HCV RNA test (The Artus HCV RG RT-PCR Kit, Qiagen) and genotyping were performed by reverse transcription-polymerase chain reaction (RT-PCR) followed by sequencing [26]. Sample collection procedures have been previously detailed elsewhere [27].
Treatment in prison
All required education for treatment and monitoring were delivered by a liver specialist to the physician and nurses. The HCV coordinator in prison was responsible for receiving test results from the laboratory, confirming the accuracy of the patient’s contact information, and leading those with positive HCV RNA to the triangular clinic for pre-treatment counseling and biobehavioral assessment by questionnaire. Further evaluations, including complete blood count, liver enzymes, creatinine, and hepatitis B testing, were conducted in the prison laboratory before treatment initiation. AST to Platelet Ratio Index (APRI) was used for liver disease assessment, calculated as follows: [AST (U/l)/upper limit of normal (considered as 40 U/l)/platelet count (109/l)] × 100. Patients received daily treatment with one tablet of a direct-acting antiviral (DAA) that was a locally-manufactured combination of 400 mg Sofosbuvir and 60 mg Daclatasvir (Sovodak®, Rojan Pharma, Tehran, Iran). The duration of therapy was 12 weeks for participants without cirrhosis (APRI < 2), and those with cirrhosis (APRI ≥ 2) were referred to a specialist health center outside the prison. The prison nurses were responsible for dispensing medication through directly observed therapy (DOT) in the clinic every morning.
Treatment in community
If released during the study, patients were referred to the Alborz district health network (DHN), where several physicians and different healthcare providers are in charge. In Iran, DHN is identified as the setting responsible for providing health services at the township and rural level, under the supervision of state Universities of Medical Sciences. The prison HCV coordinator had to inform the network of patient’s releases and their contact details. Five tablets were provided at the patient’s disposal upon release, considering the time it takes to be linked to the network. DHN personnel were attempting to contact patients for appointment scheduling by reminder calls or reaching their residential address. Treatment was pursued by a general practitioner after receiving medical records from the prison.
Study outcomes
The study outcomes include HCV prevalence and treatment uptake. Linkage to HCV care, defined as a documented visit in the network, was measured among people with positive HCV RNA who released before treatment initiation or completion. The other outcome was response assessment, measured by sustained virological response 12 weeks post-treatment (SVR12). SVR12 was defined as undetectable HCV RNA, performed by PCR on the venipuncture blood samples.
Statistical analysis
Categorical variables were expressed as frequencies and percentages. The prevalence of HCV antibody and HCV RNA was calculated among all participants. Treatment uptake was measured among participants with positive HCV RNA testing, and treatment completion was evaluated among individuals who initiated treatment. Response assessment was based on intention-to-treat (ITT) among all people with positive HCV RNA who were eligible for treatment, and modified intention-to-treat (mITT) that included patients who had completed treatment.
Results
Overall, 3485 newly admitted male inmates participated in the study, from whom 5.2% (n = 182) tested positive for HCV antibody. The prevalence of HCV RNA among all inmates was 3.4% (n = 117), indicating a viremic rate of 64% (n = 117/182) in this prison. The most frequent genotypes were 3a and 1a with 52% (n = 61) and 44% (n = 51) prevalence, respectively; other genotypes included 1b (3%, n = 4) and 3h (1%, n = 1).
Questionnaire data were available for half of the participants with positive HCV RNA (n = 60). The median age was 38 years (interquartile range (IQR) 34–44 years); the majority were heterosexual (91%), and had a drug-related sentence (73%). A history of previous incarceration was reported in 63%, and the mean (SD) incarceration time in the last year was 92 (147) days.
The majority had not finished high school (82%), were not currently employed (63%), had a minimum wage monthly income or below (65%), and all had a history of drug use (100%). During the last six months, one-quarter of patients had unstable housing (25%), and the majority had lived more than half of this time with people who inject drugs (PWID) (53%) and more than half of their friends were current drug users (67%). Compared to all patients, those who attended SVR testing appointments were older, had higher education, monthly income, and employment, and a lower proportion of them had a history of incarceration and drug-related sentences (Table 1).
Table 1.
Characteristics of Karaj prison participants with positive HCV RNA testing
| Total | People attended SVR visit | |
|---|---|---|
| Characteristics, n % | n = 60 | n = 23 |
| Age, median (IQR) | 38 (34, 44) | 39 (34, 45) |
| Male sex | 60 (100%) | 23 (100%) |
| Drug-related sentences | 38 (73.1%) | 13 (61.9%) |
| History of incarceration | 19 (63.3%) | 7 (53.8%) |
| Mean incarceration days† (SD) | 92 (147) | 114 (158) |
| Sexual orientation | ||
| Heterosexual | 53 (91.4%) | 20 (95.2%) |
| Homo/bisexual | 5 (8.6%) | 1 (4.8%) |
| Education | ||
| Did not finish high school | 49 (81.7%) | 16 (69.6%) |
| Finished high school | 10 (16.7%) | 7 (30.4%) |
| Higher education | 1 (1.7%) | 0 (0.0%) |
| Employment | ||
| Unemployed | 38 (63.3%) | 8 (44.4%) |
| Part-time | 13 (21.7%) | 6 (33.3%) |
| Full-time | 9 (15.0%) | 4 (22.2%) |
| Monthly income | ||
| Minimum wage or below | 39 (65.0%) | 14 (60.9%) |
| Living wage | 10 (16.7%) | 5 (21.7%) |
| Above living wage | 11 (18.3%) | 4 (17.4%) |
| Place of residence | ||
| Own house | 4 (8.9%) | 2 (10.5%) |
| Rental/Parents house | 30 (66.7%) | 13 (68.4%) |
| Homeless | 11 (24.4%) | 4 (21.1%) |
| Number of housings within 6 months | ||
| One | 43 (72.9%) | 16 (72.7%) |
| Two or more | 15 (25.4%) | 6 (27.3%) |
| Lived with PWID‡ within 6 months | ||
| Never | 23 (40.4%) | 10 (45.5%) |
| Less than half the time | 4 (7.0%) | 0 (0.0%) |
| Half the time or more | 30 (52.6%) | 12 (54.6%) |
| Number of friends with drug use | ||
| None | 10 (18.2%) | 3 (14.3%) |
| Less than half | 8 (14.6%) | 4 (19.1%) |
| Half or more | 37 (67.3%) | 14 (66.7%) |
| Feeling of anxiety or depression | 46 (79.3%) | 16 (72.7%) |
| Sense of well-being§, mean (SD) | 63 (19) | 68 (21) |
†In the previous year ‡people who inject drugs
§In a scale from zero to one hundred
Drug use patterns
The median age at first drug use was 18 (IQR 15–22 years), and the majority had a history of use in the last six months (67%). From people who reported drug use in the previous month (42%, n = 24/57), 79% had used daily, most commonly Heroine and/or Methamphetamine (75%). Overall, 48% (28/59) had a history of injection; the median age at first injection was 20 (IQR 18–25 years), 25% (n = 7/28) had injected within the last six months and 14% (n = 4/28) within the previous month. From people with recent injection (past month), the majority had daily injection (75%), all Heroine (100%), and had shared needles or syringes (75%). People who attended SVR appointments were less likely to had injected within the last six months (9% vs. 25%, among those with history of injection) and shared needles or syringes (0% vs. 75%, among those with injection in the previous month), compared to all patients (Table 2).
Table 2.
Drug use patterns and HCV care history among Karaj prison participants with positive HCV RNA
| Total | People attended SVR visit | |
|---|---|---|
| Characteristics, n % | n = 60 | n = 23 |
| Drug use, ever | 57 (100%) | 22 (100%) |
| Age at first drug use, median (IQR) | 18 (15, 22) | 18 (16, 20) |
| Drug use within 6 months | 38 (66.7%) | 16 (69.6%) |
| Drug use in the last month | 24 (42.1%) | 9 (39.1%) |
| Daily use | 19 (79.2%) | 7 (77.8%) |
| Most commonly used drugs | ||
| Heroine and/or Methamphetamine | 18 (75.0%) | 8 (88.9%) |
| Methadone | 6 (25.0%) | 1 (11.1%) |
| Injecting drug use, ever | 28 (47.5%) | 11 (50.0%) |
| Age at first injection | 20 (18, 25) | 20 (18, 27) |
| Injection within 6 months | 7 (25.0%) | 1 (9.1%) |
| Injection within the last month | 4 (14.3%) | 1 (9.1%) |
| Daily injection | 3 (75.0%) | 1 (100%) |
| Most commonly injected Heroine | 3 (100%) | 1 (100%) |
| Shared needle or syringe | 3 (75.0%) | 0 (0.0%) |
| Smoking daily, current | 50 (87.7%) | 18 (78.3%) |
| Alcohol use, ever | 10 (18.9%) | 5 (23.8%) |
| Opioid agonist therapy (OAT) | ||
| Current | 29 (55.8%) | 10 (50.0%) |
| History, not current | 17 (32.7%) | 7 (35.0%) |
| Never | 6 (11.5%) | 3 (15.0%) |
| HCV knowledge† | 4 (6.7%) | 2 (8.7%) |
| HCV screening, ever | 10 (16.7%) | 4 (17.4%) |
| HCV treatment uptake, ever | 3 (5.0%) | 1 (5.3%) |
| Willingness to receive HCV treatment | 53 (93.0%) | 19 (90.5%) |
†Answered three out of five questions correctly
History of HCV care and knowledge
History of HCV screening (antibody testing) and treatment uptake was 17% (n = 10/60) and 5% (n = 3/60), respectively. Out of five questions around HCV knowledge, 7% (n = 4/60) answered three or more questions accurately. The majority had a strong willingness to receive HCV treatment (93%, n = 53/57) (Table 2).
HCV treatment and linkage to care
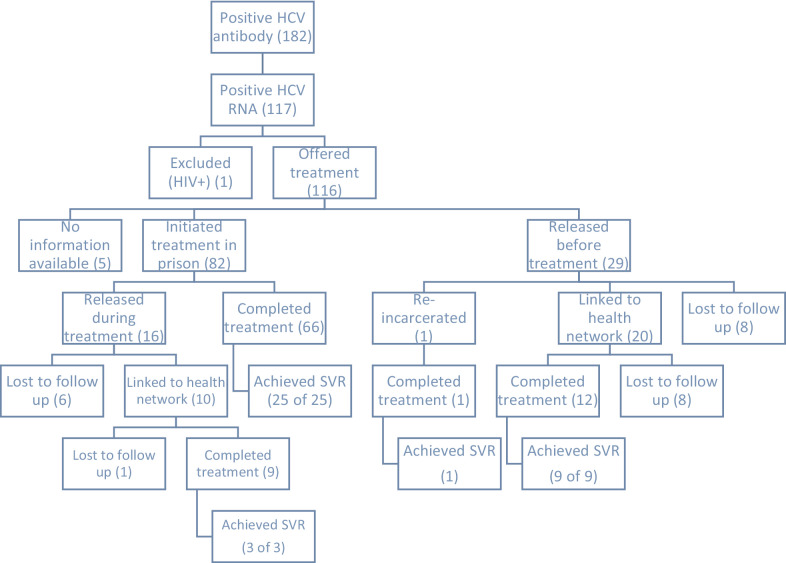
One patient did not meet the criteria for treatment in prison due to concurrent HIV antiretroviral therapy. From 116 patients who were eligible for initiating treatment—all were candidates for a 12-week DAA therapy—24% (n = 28) were released and 72% (n = 83) initiated treatment in prison, including one individual who was released before treatment uptake and reincarcerated. Information on the treatment status of 5 other patients remains unknown.
From patients who received treatment in prison, 81% (n = 67/83) completed their course on-site and the rest were released. From those who were released during treatment, 63% (n = 10/16) were followed by the network, and the majority completed treatment (90%, n = 9/10). Among patients released before treatment initiation, 71% (n = 20/28) were linked to HCV care in the network, and the remaining were lost to follow-up. Among those who initiated treatment in the network, 60% (n = 12/20) completed and the rest discontinued treatment for unspecified reasons. Therefore, among total petients who were released before or during treatment, 68% (n = 30/44) were successfully followed and linked to care in the community and 70% (n = 21/30) completed treatment (Fig. 1).
Fig. 1.
Schematic view of HCV care cascade among Karaj prison participants. SVR: Sustained virological response 12 weeks post-treatment (among those who were tested)
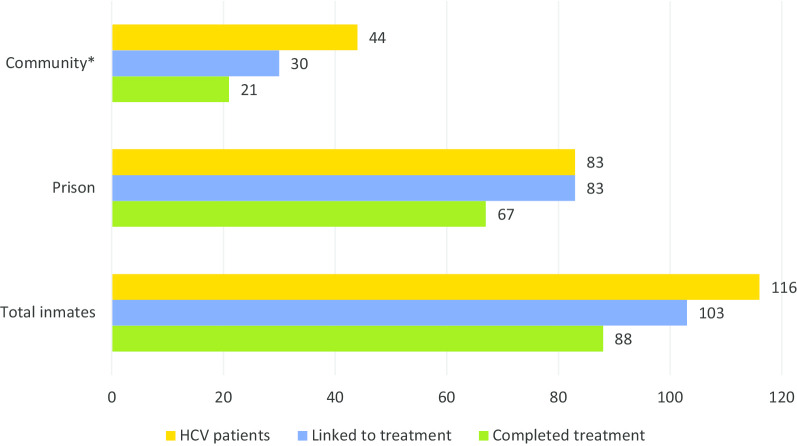
Overall, a total number of 103 patients initiated treatment in prison or network, resulting in a treatment uptake of 89% (n = 103/116). From this proportion, 85% (n = 88/103) completed treatment in prison or network. Forty-three percent (n = 38/88) of patients who had completed treatment were available for SVR assessment, who all had cured. People who initiated treatment in the community had a higher ITT SVR compared to those who initiated in prison [45% (n = 9/20) vs. 35% (n = 29/83)]. Similarly, mITT SVR for patients who completed treatment in the community was higher compared to those who completed in prison [57% (n = 12/21) vs. 39% (n = 26/67)] (Fig. 2).
Fig. 2.
Care cascade among Karaj prison participants with positive HCV RNA, during imprisonment and after release
Discussion
To our knowledge, this is the first study that evaluates the impact of an HCV care program among newly admitted inmates in Iran, and one of the first studies that investigate the post-release engagement with HCV care worldwide. The prevalence of HCV antibody in this study was lower than the national estimations within prisons (5.2% vs. 8% to 28%) [28, 29], which may indicate the lower HCV infection rate among new inmates to the entire prison population. The overall engagement in treatment with 89% uptake and 85% completion rate was high, indicating the feasibility of HCV interventions among people in custody. The majority of patients who were released before or during treatment were linked to care (68%) and completed treatment (70%) in community. In comparison with retrospective studies that showed 10 to 25% linkage to HCV care after release [11, 12, 30], these findings and encouraging cure rates in our study indicate that HCV programs can be strengthened remarkably by accurate post-release patient navigation.
HCV testing and treatment history
One-sixth of patients with available data had a history of testing, and only 5% had received treatment, indicating the missed opportunities for HCV care in correctional settings. These low rates are comparable to previous reports from Iran as well as several high-income countries [12, 31, 32]. According to a 2020 report, among people incarcerated in US prisons, only 3% have access to HCV treatment, which underlines the necessity of escalating prison-based screening and linkage to care programs [33]. Although general knowledge around HCV infection was extremely poor, willingness to initiate treatment was promising; educational initiatives during imprisonment are highly recommended and may persuade people to seek their infection status post-release.
HCV prevalence and risk behaviors
The prevalence of HCV RNA among new inmates in this prison was slightly lower than our previous study (3.4 vs. 4.8%), which had been estimated among both new inmates and residents in Northern Iran [32]. Despite the other Iranian reports, genotype 3a was more frequent than 1a in our study population [34]. Drug-related charges were common among all patients, and the majority had high-risk friendship networks or household members. Indicators of socioeconomic marginalization and risk behaviors in the previous month were less commonly seen among people who attended SVR assessment. Combined harm reduction services, including social support and stable housing, together with expanded opioid agonist therapy (OAT) programs, are crucial to control HCV epidemic in Iran and other countries [35, 36].
HCV treatment uptake and completion
Evidence surrounding prison-based HCV care interventions in the DAA era is scarce [37]. High treatment uptake and completion achieved in our study underlines great willingness towards treatment among people with HCV in prisons; these outcomes are comparable with another DAA-based prison study from Italy [38]. However, due to the heterogeneity of correctional settings and release patterns, effective intervention in a single prison may not be applicable in another. The median length of stay ranges from less than 48 h in jails to long-term housings in prisons, which highlights the necessity of adopting different healthcare strategies [39]. According to a US study, people who were released on parole were more likely to fill an antiretroviral therapy prescription than those with a standard release [40]. Thus, HCV programs should be tailored to the peculiar characteristics of the environment in which they are introduced [32, 39].
HCV treatment outcomes
Previous DAA-based studies have observed high cure rates among current and former prison inmates that are consistent with our results, such as a recent report from New South Wales (NSW) (ITT 57%, mITT 92%). The lower ITT SVR in this study (42%) compared to the NSW can be explained by our two-fold higher release rates [41]. Similarly, although Pontali et al. have reported a higher ITT SVR (91%) in an Italian prison, only 6% of their patients discontinued treatment due to release. In a Scottish research, SVR assessment showed similar results for people who initiated treatment in community and prison (63% vs. 61%) [42], and a higher response was observed among people who were not released or transferred, compared to those who were released during treatment (75% vs. 45%). We observed slightly better ITT outcomes for those who commenced therapy in the community than prison (45% vs. 35%), which can be partly explained by a higher likelihood of adherence to treatment for people who are reached by the health networks after community return, compared to all released inmates. The ITT SVR among former inmates who initiated treatment in community was similar to a study from New York City jails (45% vs. 41%); however, mITT SVR in our study was higher than their observed cure rates (57% vs. 47%) [15]. This difference may suggest a lower risk of reinfection or treatment failure in the Iranian community compared to the USA. These comparisons highlight the significant impact of release patterns on treatment response assessment and its interpretations in different settings, which could incorporate into a better prison- and community-based HCV planning.
Post-release HCV care
There is a growing body of evidence on successful transitional programs to engage patients with healthcare services after release—mainly conducted by community-based providers and NGOs—ranging from reminder calls to intensive case management [43]. Three studies from the USA have reported that only one-quarter of patients who returned to the community were linked to HCV care after incarceration [15, 30, 44]. However, we showed that more than two-thirds of patients could be linked to care following release, highlighting the critical role of active patient navigation in engaging patients with post-release care. The period of leaving incarceration is a particularly vulnerable time and many people may not receive sufficient long-term support during this period, which may lead to poor health outcomes, including treatment failure and reinfection [45]. Retention in treatment is also essential to prevent the risk of developing drug resistance [46]. Due to the similar competing priorities, factors that are considered as facilitators among people with HIV can be applied to the formerly incarcerated population with HCV to obtain synergistic effects. These include treatment for substance use and mental disorders, transportation assistance, offer drug-free transitional housing, and peer support [11, 22, 47]. Unfortunately, we were not able to provide such facilities in our study due to budget limitations.
Limitations
The main limitation of this work was the lack of close observation on the study procedures. To provide real-world information, we aimed to assign the entire work to prison staff and community providers, which resulted in some shortcomings in patient navigation and data collection, including the loss of several medical records. Some staff changes in prison interrupted our data collection process, and tracking down all questionnaires was impossible due to peculiar restrictions of the prison environment. Consistent with the WHO report on Prisons and Health, the penitentiary healthcare system should work in close collaboration with community providers to ensure that treatment is not interrupted when people enter or leave prison and also transferred within the justice system [46]. As we only recruited newly admitted inmates, the interpretation of our results for prison residents should be with caution. Besides, women were underrepresented in this study.
Conclusions
This work supports the feasibility of successful integrated HCV care models in custodial settings, strengthened significantly by post-release interventions. Establishing a multidisciplinary program through the collaboration of community and prison healthcare systems could promote health outcomes. A comprehensive approach should include appropriate discharge planning, increased referral resources, and patient navigation to encourage adherence to treatment among people who cycle through custody. More robust care models incorporating a variety of supportive services and risk reduction measures are needed to guarantee continuity of HCV care, and future studies should investigate the predictors of engagement with treatment and virological cure following release into the community.
Acknowledgements
The authors would like to acknowledge the Karaj Central Prison staff and the Alborz district health network for their valuable contributions, and all prison inmates for their participation in this work.
Abbreviations
- APRI
AST to platelet ratio index
- AST
Aspartate aminotransferase
- DAA
Direct-acting antiviral
- DHN
District health network
- DOT
Directly observed therapy
- ELISA
Enzyme-linked immunosorbent assay
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- IQR
Interquartile range
- ITT
Intention-to-treat
- mITT
Modified intention-to-treat
- MMT
Methadone maintenance treatment
- PWID
People who inject drugs
- RDT
Rapid diagnostic test
- RT-PCR
Reverse transcription-polymerase chain reaction
- SVR
Sustained virological response
- WHO
World Health Organization
Authors’ contributions
SH drafted the manuscript. HS and SMA provided critical feedback and were major contributors to revising the manuscript. HP and SM conceived the original idea and designed the project. BT, MT, RA, and FH conducted the study and were involved in data collection. RM, MMG, and RR supervised the work. BA processed the data and performed the analysis. MS and FA provided insight into the interpretation of the results. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The review board of the Digestive Diseases Research Institute of Tehran University of Medical Sciences approved the research protocol. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
Shahin Merat and Hossein Poustchi own stocks and shares in Rojan Pharma.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sanam Hariri and Heidar Sharafi should be considered joint first authors
References
- 1.Hajarizadeh B, Grebely J, Matthews GV, Martinello M, Dore GJ. The path towards hepatitis C elimination in Australia following universal access to interferon-free treatments. J Hepatol. 2017;66(1):S291–S292. doi: 10.1016/S0168-8278(17)30899-1. [DOI] [Google Scholar]
- 2.Zelenev A, Li J, Mazhnaya A, Basu S, Altice FL. Hepatitis C virus treatment as prevention in an extended network of people who inject drugs in the USA: a modelling study. Lancet Infect Dis. 2018;18(2):215–224. doi: 10.1016/S1473-3099(17)30676-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone J, Martin NK, Hickman M, Hutchinson SJ, Aspinall E, Taylor A, et al. Modelling the impact of incarceration and prison-based hepatitis C virus (HCV) treatment on HCV transmission among people who inject drugs in Scotland. Addiction. 2017;112(7):1302–1314. doi: 10.1111/add.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moazen B, Saeedi Moghaddam S, Silbernagl MA, Lotfizadeh M, Bosworth RJ, Alammehrjerdi Z, et al. Prevalence of drug injection, sexual activity, tattooing, and piercing among prison inmates. Epidemiol Rev. 2018;40(1):58–69. doi: 10.1093/epirev/mxy002. [DOI] [PubMed] [Google Scholar]
- 5.Dolan K, Wirtz AL, Moazen B, Ndeffo-mbah M, Galvani A, Kinner SA, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388(10049):1089–1102. doi: 10.1016/S0140-6736(16)30466-4. [DOI] [PubMed] [Google Scholar]
- 6.Organization WH. Global hepatitis report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 7.Bartlett SR, Fox P, Cabatingan H, Jaros A, Gorton C, Lewis R, et al. Demonstration of near-elimination of hepatitis C virus among a prison population: the lotus glen correctional centre hepatitis C treatment project. Clin Infect Dis. 2018;67(3):460–463. doi: 10.1093/cid/ciy210. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri R, Starnini G, Carbonara S, Pontali E, Leo G, Romano A, et al. Management of HCV infection in the penitentiary setting in the direct-acting antivirals era: practical recommendations from an expert panel. Infection. 2017;45(2):131–138. doi: 10.1007/s15010-016-0973-0. [DOI] [PubMed] [Google Scholar]
- 9.Crowley D, Cullen W, Lambert JS, Van Hout MC. Competing priorities and second chances: a qualitative exploration of prisoners' journeys through the Hepatitis C continuum of care. PLoS ONE. 2019;14(9):e0222186. doi: 10.1371/journal.pone.0222186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein SJ, Wright LN, Birkhead GS, Mojica BA, Klopf LC, Klein LA, et al. Promoting HCV treatment completion for prison inmates: New York State's hepatitis C continuity program. Public Health Rep. 2007;122(Suppl 2):83–88. doi: 10.1177/00333549071220S216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawks L, Norton BL, Cunningham CO, Fox AD. The Hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23(6):473–478. doi: 10.1111/jvh.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochstatter KR, Stockman LJ, Holzmacher R, Greer J, Seal DW, Taylor QA, et al. The continuum of hepatitis C care for criminal justice involved adults in the DAA era: a retrospective cohort study demonstrating limited treatment uptake and inconsistent linkage to community-based care. Health Just. 2017;5(1):10. doi: 10.1186/s40352-017-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocoros N, Nettle E, Church D, Bourassa L, Sherwin V, Cranston K, et al. Screening for Hepatitis C as a Prevention Enhancement (SHAPE) for HIV: an integration pilot initiative in a Massachusetts County correctional facility. Public Health Rep. 2014;129(Suppl 1):5–11. doi: 10.1177/00333549141291S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan E, Ward S, Zeki R, Wayland S, Sherwood J, Wang A, et al. Recidivism, health and social functioning following release to the community of NSW prisoners with problematic drug use: study protocol of the population-based retrospective cohort study on the evaluation of the Connections Program. BMJ Open. 2019;9(7):e030546. doi: 10.1136/bmjopen-2019-030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akiyama MJ, Columbus D, MacDonald R, Jordan AO, Schwartz J, Litwin AH, et al. Linkage to hepatitis C care after incarceration in jail: a prospective, single arm clinical trial. BMC Infect Dis. 2019;19(1):703. doi: 10.1186/s12879-019-4344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begun AL, Early TJ, Hodge A. Mental health and substance abuse service engagement by men and women during community reentry following incarceration. Admin Policy Mental Health Mental Health Serv Res. 2016;43(2):207–218. doi: 10.1007/s10488-015-0632-2. [DOI] [PubMed] [Google Scholar]
- 17.Binswanger IA, Nowels C, Corsi KF, Long J, Booth RE, Kutner J, et al. “From the prison door right to the sidewalk, everything went downhill”, A qualitative study of the health experiences of recently released inmates. Int J Law Psychiatry. 2011;34(4):249–255. doi: 10.1016/j.ijlp.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Van Dorn RA, Desmarais SL, Rade CB, Burris EN, Cuddeback GS, Johnson KL, et al. Jail-to-community treatment continuum for adults with co-occurring substance use and mental disorders: study protocol for a pilot randomized controlled trial. Trials. 2017;18(1):365. doi: 10.1186/s13063-017-2088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nations U. Additional Guidance on Aftercare and Reintegration Programmes for Violent Extremist Offenders. 2014.
- 20.Myers JJ, Kang Dufour M-S, Koester KA, Morewitz M, Packard R, Monico Klein K, et al. The effect of patient navigation on the likelihood of engagement in clinical care for HIV-infected individuals leaving jail. Am J Public Health. 2018;108(3):385–392. doi: 10.2105/AJPH.2017.304250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeliger KB, Altice FL, Desai MM, Ciarleglio MM, Gallagher C, Meyer JP. Predictors of linkage to HIV care and viral suppression after release from jails and prisons: a retrospective cohort study. Lancet HIV. 2018;5(2):e96–e106. doi: 10.1016/S2352-3018(17)30209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanes-Lane M, Dussault C, Linthwaite B, Cox J, Klein MB, Sebastiani G, et al. Using the barriers and facilitators to linkage to HIV care to inform hepatitis C virus (HCV) linkage to care strategies for people released from prison: findings from a systematic review. J Viral Hepat. 2020;27(2):205–220. doi: 10.1111/jvh.13220. [DOI] [PubMed] [Google Scholar]
- 23.Kouyoumdjian FG, Schuler A, McIsaac KE, Pivnick L, Matheson FI, Brown G, et al. Using a Delphi process to define priorities for prison health research in Canada. BMJ Open. 2016;6(1):e010125. doi: 10.1136/bmjopen-2015-010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronfli N, Linthwaite B, Kouyoumdjian F, Klein MB, Lebouché B, Sebastiani G, et al. Interventions to increase testing, linkage to care and treatment of hepatitis C virus (HCV) infection among people in prisons: a systematic review. Int J Drug Policy. 2018;57:95–103. doi: 10.1016/j.drugpo.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Zamani S, Farnia M, Torknejad A, Alaei BA, Gholizadeh M, Kasraee F, et al. Patterns of drug use and HIV-related risk behaviors among incarcerated people in a prison in Iran. J Urban Health. 2010;87(4):603–616. doi: 10.1007/s11524-010-9450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadjbaf D, Keshvari M, Alavian SM, Pouryasin A, Behnava B, Salimi S, et al. The prevalence of hepatitis C virus core amino acid 70 substitution and genotypes of polymorphisms near the IFNL3 gene in Iranian patients with chronic hepatitis C. Hepat Mon. 2016;16(6):e37011. doi: 10.5812/hepatmon.37011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharafi H, Poustchi H, Azimian F, Tamadoni B, Ramezani R, Gouya MM, et al. Performance of a rapid diagnostic test for screening of hepatitis C in a real-life prison setting. J Clin Virol. 2019;113:20–23. doi: 10.1016/j.jcv.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Moradi G, Gouya M-M, Azimizan Zavareh F, Mohamadi Bolbanabad A, Darvishi S, Aghasadeghi MR, et al. Prevalence and risk factors for HBV and HCV in prisoners in Iran: a national bio-behavioural surveillance survey in 2015. Trop Med Int Health. 2018;23(6):641–649. doi: 10.1111/tmi.13065. [DOI] [PubMed] [Google Scholar]
- 29.Behzadifar M, Gorji HA, Rezapour A, Bragazzi NL. Prevalence of hepatitis C virus infection among prisoners in Iran: a systematic review and meta-analysis. Harm Reduct J. 2018;15(1):24. doi: 10.1186/s12954-018-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckwith CG, Kurth AE, Bazerman LB, Patry EJ, Cates A, Tran L, et al. A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections. J Public Health (Oxf) 2016;38(1):130–137. doi: 10.1093/pubmed/fdv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young S, Wood E, Milloy MJ, DeBeck K, Dobrer S, Nosova E, et al. Hepatitis C cascade of care among people who inject drugs in Vancouver. Canada Subst Abus. 2018;39(4):461–468. doi: 10.1080/08897077.2018.1485128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hariri S, Sharafkhah M, Alavi M, Roshandel G, Fazel A, Amiriani T, et al. A simple risk-based strategy for hepatitis C virus screening among incarcerated people in a low- to middle-income setting. Harm Reduct J. 2020;17(1):56. doi: 10.1186/s12954-020-00400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correctional institutions are a critical intervention point for hepatitis C elimination. O’Neill Institute for National and Global Health Law. March 2020. https://oneill.law.georgetown.edu/wp-content/uploads/HepC_Corrections_FINAL_03272020.pdf.
- 34.Mahmud S, Akbarzadeh V, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Iran: Systematic review and meta-analyses. Sci Rep. 2018;8(1):150. doi: 10.1038/s41598-017-18296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane review and meta-analysis. Addiction (Abingdon, England) 2018;113(3):545–563. doi: 10.1111/add.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherz N, Bruggmann P, Brunner N. Direct-acting antiviral therapy for hepatitis C infection among people receiving opioid agonist treatment or heroin assisted treatment. Int J Drug Policy. 2018;62:74–77. doi: 10.1016/j.drugpo.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Vroling H, Oordt-Speets AM, Madeddu G, Babudieri S, Monarca R, O'Moore E, et al. A systematic review on models of care effectiveness and barriers to Hepatitis C treatment in prison settings in the EU/EEA. J Viral Hepat. 2018;25(12):1406–1422. doi: 10.1111/jvh.12998. [DOI] [PubMed] [Google Scholar]
- 38.Pontali E, Fiore V, Ialungo AM, Ranieri R, Mollaretti O, Barbarini G, et al. Treatment with direct-acting antivirals in a multicenter cohort of HCV-infected inmates in Italy. Int J Drug Policy. 2018;59:50–53. doi: 10.1016/j.drugpo.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Spaulding AC, Perez SD, Seals RM, Hallman MA, Kavasery R, Weiss PS. Diversity of release patterns for jail detainees: implications for public health interventions. Am J Public Health. 2011;101(Suppl 1):S347–S352. doi: 10.2105/AJPH.2010.300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, et al. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301(8):848–857. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Overton K, Clegg J, Pekin F, Wood J, McGrath C, Lloyd A, et al. Outcomes of a nurse-led model of care for hepatitis C assessment and treatment with direct-acting antivirals in the custodial setting. Int J Drug Policy. 2019;72:123–128. doi: 10.1016/j.drugpo.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Aspinall EJ, Mitchell W, Schofield J, Cairns A, Lamond S, Bramley P, et al. A matched comparison study of hepatitis C treatment outcomes in the prison and community setting, and an analysis of the impact of prison release or transfer during therapy. J Viral Hepat. 2016;23(12):1009–1016. doi: 10.1111/jvh.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu C, Jurgutis J, Edwards D, O'Shea T, Regenstreif L, Bodkin C, et al. "When you first walk out the gates…where do [you] go?": Barriers and opportunities to achieving continuity of health care at the time of release from a provincial jail in Ontario. PLoS ONE. 2020;15(4):e0231211. doi: 10.1371/journal.pone.0231211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenbachler BT, Smith BD, Seña AC, Hilton A, Bachman S, Lunda M, et al. Hepatitis C Virus testing and linkage to care in North Carolina and South Carolina Jails, 2012–2014. Public Health Rep. 2016;131(Suppl 2):98–104. doi: 10.1177/00333549161310S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kendall S, Redshaw S, Ward S, Wayland S, Sullivan E. Systematic review of qualitative evaluations of reentry programs addressing problematic drug use and mental health disorders amongst people transitioning from prison to communities. Health Justice. 2018;6(1):4. doi: 10.1186/s40352-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.(WHO) WHO. Prisons and Health. 2020.
- 47.Teixeira PA, Jordan AO, Zaller N, Shah D, Venters H. Health outcomes for HIV-infected persons released from the New York City jail system with a transitional care-coordination plan. Am J Public Health. 2015;105(2):351–357. doi: 10.2105/AJPH.2014.302234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.