Abstract
Introduction
There is no standard treatment for locoregional recurrent (LR) esophageal squamous cell carcinoma (ESCC) patients treated with radiotherapy (RT) previously. This retrospective study aimed to examine the efficacy and toxicity of re-irradiation (re-RT) for ESCC patients with LR.
Patients and methods
A total of 252 patients were enrolled. Gross tumor volumes for re-RT were defined using contrast enhanced computed tomography and/or positron emission tomography/computed tomography. Overall survival (OS), after recurrence survival (ARS) and toxicities were assessed.
Results
Through a median follow-up of 38 months, the median OS and ARS were 39.0 and 13.0 months, respectively. The 6-, 12-, and 24-month ARS rates were 81.9%, 50.5%, and 21.8%, respectively. Multivariate analyses showed that chemotherapy, esophageal stenosis and recurrence-free interval (RFI) may be independent prognostic factors for ARS. The incidence of esophageal fistula/perforation (EP), radiation-induced pneumonitis and esophagorrhagia was 21.4%, 12.8% and 9.1%, respectively. RFI ≤ 12 months, esophageal stenosis and fat space between tumor and adjacent tissue disappeared were independent risk factors for the development of EP after re-RT.
Conclusions
Re-RT was feasible for LR ESCC patients after RT initially, the complication occurred in re-RT is acceptable. Patients with RFI ≤ 12 months, esophageal stenosis and fat space between tumor and adjacent tissue disappeared should be closely observed during and after re-RT.
Keywords: Esophageal squamous cell carcinoma, Locoregional recurrence, Re-irradiation, Prognosis
Introduction
Esophageal cancer is the 6th most common cause of cancer deaths worldwide because of its high malignant potential and poor prognosis [1]. Locoregional recurrence (LR) is the major type of failure form in 24–50% of the patients after initial therapy such as surgery and/or chemoradiotherapy (CRT) [2, 3], and in-field relapse after radiotherapy (RT) occurred in more than 20% of patients [4, 5]. The 5-year survival rate drops dramatically down to 0–11% once recurrence occurs [6, 7].
Patients with good physical conditions need to be given active treatment to local recurrent disease to achieve better survival. There is no standard treatment for patients with LR so far. Surgery plays an important role in achieving locoregional control in patients with LR esophageal carcinoma [8] but salvage esophagectomy may cause serious surgery-related complication and hospital mortality. CRT has curative potential for LR esophageal squamous cell carcinoma (ESCC) patients, but the clinical benefit and safety is not demonstrated very well due to the small number of cases [9–17]. In this study, we retrospectively analyzed the efficacy and toxicity of re-irradiation (re-RT) in patients with LR after radical radiotherapy or postoperative adjuvant radiotherapy on a relative large sample.
Materials and methods
Patients
All of the 252 ESCC patients were confirmed by pathology, collected from our hospital from January 2000 to December 2018. Patients were selected meeting the following criteria: (1) primary ESCC was pathological confirmed, (2) histological and/or positron emission tomography/computed tomography confirmation of LR including primary failure (PF), regional lymph node recurrence (LN) or PF combined LN relapsed in-field after initial RT; (3) no evidence of esophageal perforation or ulcer. The exclusion criteria were as follows: (1) history of other malignancies, (2) distant metastases.
Clinical or pathological stage was done according to the 7th edition of the American Joint Committee on Cancer TNM staging system. Toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 3.0. The current study was approved by the Ethics Committee of our hospital.
Treatment
RT was delivered via a 6 MV X-ray linear accelerator, the total doses of primary RT and re-RT are listed in Tables 1 and 2. For the re-RT, the gross tumor volume (GTV) after recurrence was defined as the region of recurrence determined by contrast enhanced computed tomography and/or positron emission tomography/computed tomography. The clinical target volume (CTV) was defined as the GTV plus a margin of 1.0–2.0 cm on each side. The planning target volume (PTV) for re-RT was defined as the CTV or GTV plus a 0.5–1.0 cm margin in all directions, according to previous RT techniques and exposure dose to organs at risk. Partially or all the re-RT target volumes were in the initial radiation fields. The biological effectiveness of radiation schedule was calculated by the biologically effective dose (BED) formula: BED = n × d (1 + d/(α/β)), α/β = 10 [18]. The total dose to the spinal cord was limited not to exceed the maximal dose of 45 Gy except for a few patients, considering the time interval between primary RT and re-RT. V20 were limited within 30% in the first RT and less than 20% during re-RT for the total lungs.
Table 1.
Characteristics of 252 patients with locoregional recurrent ESCC at initial treatment
| Variables | Number | Percent |
|---|---|---|
| Age (years) | ||
| > 60 | 126 | 50.0 |
| ≤ 60 | 126 | 50.0 |
| Gender | ||
| Male | 206 | 81.7 |
| Female | 46 | 18.3 |
| Smoking | ||
| Yes | 122 | 48.4 |
| No | 130 | 51.6 |
| Alcohol consumption | ||
| Yes | 108 | 42.9 |
| No | 144 | 57.1 |
| Family history of malignancy | ||
| Yes | 45 | 17.9 |
| No | 207 | 82.1 |
| Primary tumor location | ||
| Upper thoracic | 72 | 28.6 |
| Middle and lower thoracic | 180 | 71.4 |
| Length (cm) | ||
| ≤ 4 | 152 | 60.3 |
| 4 < to ≤ 6 | 71 | 28.2 |
| > 6 | 29 | 11.5 |
| Macroscopic tumor type | ||
| Medullary | 134 | 53.2 |
| Ulcerative | 83 | 32.9 |
| Constrictive | 15 | 6.0 |
| Mushroom | 20 | 7.9 |
| Tumor differentiation | ||
| Higher | 36 | 14.3 |
| Middle | 143 | 56.7 |
| Lower | 73 | 29.0 |
| T stage | ||
| T1–T2 | 79 | 31.3 |
| T3–T4 | 173 | 68.7 |
| N stage | ||
| N0 | 110 | 43.7 |
| N1 | 111 | 44.0 |
| N2 | 31 | 12.3 |
| Initial clinical/pathological stage | ||
| I–II | 117 | 46.4 |
| III–Iva | 135 | 53.6 |
| Radiation dose (Gy) | ||
| ≤ 50 | 64 | 25.4 |
| > 50 | 188 | 74.6 |
| Daily dose (Gy) | ||
| 1.8 | 61 | 24.2 |
| 2.0 | 180 | 71.4 |
| > 2.0 | 11 | 4.4 |
| Initial treatment | ||
| CRT | 167 | 66.3 |
| Surgery + CRT | 85 | 33.7 |
| Radiotherapy technique | ||
| Conventional treatment | 20 | 7.9 |
| 3D-CRT | 113 | 44.9 |
| IMRT | 119 | 47.2 |
Table 2.
Characteristics of 252 patients with locoregional recurrent ESCC at re-RT
| Variables | Number | Percent |
|---|---|---|
| Age (years) | ||
| > 60 | 167 | 66.3 |
| ≤ 60 | 85 | 33.7 |
| Pattern of recurrence | ||
| Regional lymph node recurrence only | 108 | 42.9 |
| Local failure | 96 | 38.1 |
| Both | 48 | 19.0 |
| Recurrence-free interval (months) | ||
| ≤ 12 | 76 | 30.2 |
| > 12 | 176 | 69.8 |
| Chemotherapy | ||
| Yes | 198 | 78.6 |
| No | 54 | 21.4 |
| Radiation dose (Gy), BED10 | ||
| ≤ 60 | 133 | 52.8 |
| > 60 | 119 | 47.2 |
| Daily dose (Gy) | ||
| 1.15–1.5 bid | 48 | 19.0 |
| 1.8 | 60 | 23.8 |
| 2 | 144 | 57.1 |
| Radiotherapy technique | ||
| 3D-CRT | 40 | 15.9 |
| IMRT | 212 | 84.1 |
All patients received chemotherapy (CT) at the initial treatment, which was treated with 2 to 6 courses CT (median 4). CT regimens were mainly as 5-fluorouracil or paclitaxel plus cisplatin. 198 (78.6%) patients received CT combined with re-RT. The CT regimen was basically the same as before, and the number of cycles of CT was 1–6 (median 2).
Follow-up
Overall survival (OS) time was defined as from the time of the initial treatment to death or the time of last follow-up. The after-recurrence survival (ARS) time was calculated from the date of relapse to the date of death or last follow-up. The recurrence-free interval (RFI) was defined as the time of interval from the end of initial treatment to the recurrence diagnosis [17]. The degree of esophageal stenosis was evaluated with esophagography by the method described before [19] and level ≥ 2 was defined as esophageal stenosis in this study. Patients were considered censored if without end events at the end of the study. Esophageal fistula/perforation (EP), radiation pneumonitis (RP) and esophagorrhagia were recorded.
Statistical analysis
All statistical tests were conducted by using the SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA) and all figures were produced using GraphPad Prism 8.0 (Graphpad Software, USA). p value < 0.05 was considered statistically significant. The rates of survival curves were calculated using the Kaplan–Meier analysis method and log-rank tests. The Cox regression model was employed for the univariate analysis and multivariate analysis. The risk factors associated with the development of EP were analyzed by the forward logistic regression method.
Results
Patient characteristics
At initial treatment, 167 patients received radical RT and 85 patients received RT after surgery. The characteristics of the tumors and cohort are summarized according to the initial treatment (Table 1, 2). The median age was 66 years (range 39–88 years) at the time of diagnosis and 69 years (range 45–90 years) in the re-RT. The median RFI was 20 months (range 3–204 months). The median length of these lesions at initial diagnosis was 5.0 cm (range 1.5–13.5 cm). PF and LN were the most common failure pattern for radical RT (50.3%) and surgery (70.6%) respectively. The majority of patients (95.6%) received conventional fractionation treatment in the initial RT and 19.0% patients received hyperfractionation radiotherapy in re-RT. Among ESCC patients, the median initial radiation dose was 72 Gy (range 42.5–84.0 Gy), median re-RT dose was 60 Gy (range 12.0–86.3 Gy), and median total radiation dose (BED) was 131.5 Gy (range 84.0–158.3 Gy). 150 patients (59.5%) had Level 1, 65 patients (25.8%) had Level 2, 20 patients (7.9%) had Level 3, and 17 patients (6.8%) had Level 4 stenosis.
OS and ARS
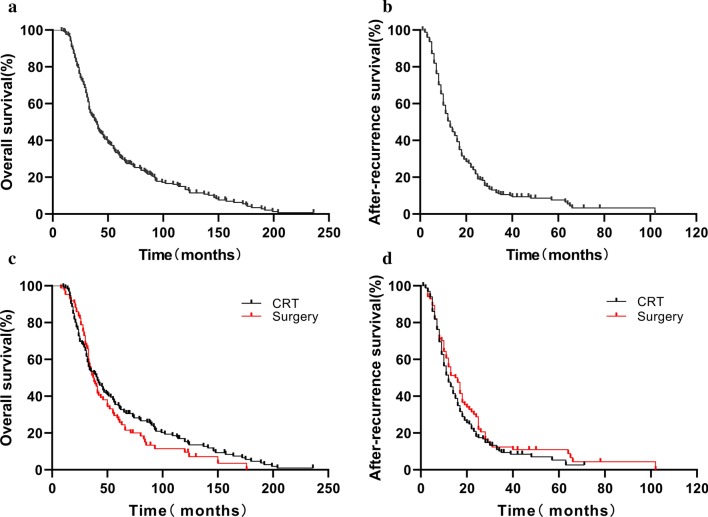
From initially diagnosed with ESCC, median follow-up was 38 months (range 8–236 months). 18 patients (7.1%) were still living, 224 patients (88.9%) had died including 1 patient died of suicide, 10 patients (4.0%) were lost to follow-up. The median OS of the 252 patients was 39.0 months (Fig. 1a) and the 1-, 3-, and 5-year OS rates were 97.6%, 52.8%, and 32.1%, respectively. The median ARS was 13.0 months (Fig. 1b) and the 6-, 12-, and 24-month ARS rates were 81.9%, 50.5%, and 21.8%, respectively. For the radical RT group, the median OS was 41.0 months (Fig. 1c) and the 1-, 3-, and 5-year OS rates were 98.8%, 53.6%, and 34.8%, respectively. The median ARS was 12.0 months (Fig. 1d) and the 6-, 12-, and 24-month ARS rates were 81.8%, 47.7%, and 18.1%, respectively. For the surgery group, the median OS was 38.0 months (Fig. 1c) and the 1-, 3-, and 5-year OS rates were 95.3%, 51.4%, and 27.0%, respectively. The median ARS of the 85 patients were 16.0 months (Fig. 1d) and the 6-, 12-, and 24-month ARS rates were 82.2%, 56.0%, and 28.8%, respectively.
Fig. 1.
a Kaplan–Meier curve of OS for 252 patients with locoregional recurrent ESCC. b Kaplan–Meier curve of ARS for 252 patients with locoregional recurrent ESCC. c Kaplan–Meier curve of OS for 167 patients received RT initially and RT after surgery, respectively. d Kaplan–Meier curve of ARS for 85 patients received RT initially and RT after surgery
Prognostic factor analysis for ARS
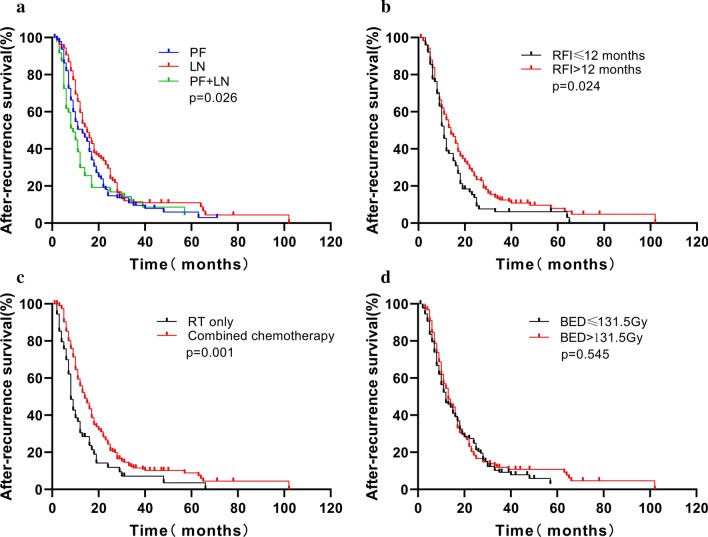
We evaluated the relationship between ARS and clinicopathological features. In the univariate analysis (Table 3), median ARS was significantly longer for patients who occurred LN recurrence compared with those who occurred PF with/without LN recurrence (15.0 vs. 9.0 vs. 13.0 months, p = 0.026) (Fig. 2a). The median ARS of patients with RFI time > 12 months was longer than the patients with RFI time ≤ 12 months (14 vs. 11 months, p = 0.024) (Fig. 2b). Re-RT combined with chemotherapy can improve ARS (14 vs. 8 months, p = 0.001) (Fig. 2c). The ARS was similar for patients received a total radiation BED > 131.5 Gy compared with those for patients received an total radiation dose ≤ 131.5 Gy (p = 0.545) (Fig. 2d). Multivariate factor analysis for ARS revealed that CT, esophageal stenosis and RFI time may be independent prognostic factors for ARS (Table 4).
Table 3.
Prognostic factors for ARS by univariate analysis
| Variable | No. of patients | Survival rate, % | MST, mo (95% CI) | p Value | ||
|---|---|---|---|---|---|---|
| 6-m | 12-m | 24-m | ||||
| Sex | 0.795 | |||||
| Male | 206 | 81.8 | 50.8 | 21.8 | 12 (10.250–13.750) | |
| Female | 46 | 82.6 | 49.1 | 21.8 | 11 (7.202–14.798) | |
| Re-RT age | 0.316 | |||||
| ≤ 60 | 85 | 83.3 | 54.8 | 26.9 | 16 (12.706–19.294) | |
| > 60 | 167 | 81.2 | 48.3 | 19.1 | 12 (9.958–14.042) | |
| Alcohol abuse | 0.687 | |||||
| Yes | 108 | 82.2 | 48.2 | 19.3 | 12 (9.679–14.321) | |
| No | 144 | 81.7 | 52.3 | 23.8 | 13 (10.172–15.828) | |
| Smoking | 0.683 | |||||
| Yes | 122 | 81.0 | 48.2 | 19.9 | 12 (10.182–13.872) | |
| No | 130 | 82.8 | 52.7 | 23.6 | 13 (9.675–16.325) | |
| Primary tumor location | 0.773 | |||||
| Upper | 72 | 83.3 | 51.4 | 17.2 | 13 (8.857–17.143) | |
| Middle/lower | 180 | 81.3 | 50.1 | 23.6 | 13 (11.044–14.956) | |
| Length (cm) | 0.573 | |||||
| ≤ 4 | 152 | 86.0 | 51.5 | 23.2 | 13 (10.043–15.957) | |
| > 4 to ≤ 6 | 71 | 76.1 | 45.7 | 16.4 | 12 (9.349–14.651) | |
| > 6 | 29 | 75.0 | 51.7 | 27.3 | 14 (10.111–17.889) | |
| Tumor differentiation (n, %) | 0.647 | |||||
| Higher | 36 | 77.1 | 51.4 | 31.3 | 17 (9.200–24.800) | |
| Middle | 143 | 84.4 | 49.7 | 18.3 | 12 (10.019–13.981) | |
| Lower | 73 | 79.4 | 51.5 | 23.7 | 13 (9.684–16.316) | |
| Failure patterns | 0.026 | |||||
| Primary failure | 93 | 82.1 | 50.5 | 14.7 | 13 (9.216–16.784) | |
| Regional lymph node recurrence | 106 | 90.6 | 59.5 | 29.5 | 15 (12.118–17.882) | |
| Combined | 53 | 61.8 | 29.8 | 11.5 | 9 (6.127–11.873) | |
| Initial treatment | 0.173 | |||||
| CRT | 167 | 82.2 | 56.0 | 28.8 | 16 (12.311–19.689) | |
| Surgery | 85 | 81.8 | 47.7 | 18.8 | 12 (9.960–14.040) | |
| Initial stage | 0.966 | |||||
| I–II | 117 | 83.6 | 51.7 | 19.4 | 14 (11.386–16.614) | |
| III–Iva | 135 | 80.4 | 49.4 | 23.7 | 12 (9.942–14.058) | |
| RFI time | 0.024 | |||||
| ≤ 12 months | 76 | 77.6 | 38.9 | 13.8 | 11 (9.600–12.400) | |
| > 12 months | 176 | 83.8 | 55.6 | 25.4 | 14 (11.622–16.378) | |
| Chemotherapy | 0.001 | |||||
| Yes | 198 | 85.2 | 55.8 | 24.4 | 14 (11.739–16.261) | |
| No | 54 | 94.4 | 30.5 | 11.9 | 8 (6.602–9.398) | |
| Salvage radiation dose (BED10) | 0.326 | |||||
| ≤ 60 Gy | 133 | 80.9 | 45.0 | 23 | 12 (10.440–13.560) | |
| > 60 Gy | 119 | 83.1 | 56.6 | 20.7 | 14 (11.791–16.209) | |
| Total radiation dose (BED10) | 0.545 | |||||
| ≤ 131.5 Gy | 130 | 79.5 | 46.7 | 24.9 | 12 (9.115–14.885) | |
| > 131.5 Gy | 122 | 84.4 | 54.5 | 18.5 | 13 (10.159–15.841) | |
| Esophageal stenosis | 0.070 | |||||
| Yes | 102 | 82.0 | 50.4 | 14.6 | 13 (10.599–15.401) | |
| No | 150 | 81.9 | 50.6 | 26.5 | 13 (10.200–15.800) | |
| Pain in the chest or/and back | 0.761 | |||||
| Yes | 25 | 80.0 | 42.5 | 18.9 | 11 (6.314–15.686) | |
| No | 227 | 82.1 | 51.4 | 22.1 | 13 (11.117–14.883) | |
| Fat space between tumor and adjacent tissue disappeared | 0.121 | |||||
| Yes | 64 | 82.6 | 46.1 | 12.9 | 12 (9.416–14.584) | |
| No | 188 | 81.7 | 52.1 | 24.7 | 13 (10.394–15.606) | |
| BMI | 0.269 | |||||
| ≤ 20 | 74 | 82.2 | 46.0 | 17 | 12 (9.745–14.255) | |
| > 20 | 178 | 81.8 | 52.4 | 23.8 | 14 (11.483–16.517) | |
| Hemoglobin (g/L) | 0.159 | |||||
| ≤ 12 | 95 | 80.6 | 46.8 | 18.7 | 12 (9.446–15.554) | |
| > 12 | 157 | 82.7 | 52.7 | 23.7 | 13 (10.200–15.800) | |
| Albumin (g/L) | 0.281 | |||||
| ≤ 40 | 87 | 83.7 | 48.0 | 17.9 | 12 (10.095–13.905) | |
| > 40 | 165 | 80.9 | 51.8 | 23.8 | 13 (10.195–15.805) | |
MST median survival time, CI confidence interval
Fig. 2.
Kaplan–Meier survival curves. a Survival of patients who had PF, LN or PF combined with LN relapse. b Survival of patients who had an RFI ≤ 12 months versus RFI > 12 months. c Survival of patients who received RT only versus RT combined chemotherapy. d Survival of patients who received total RT dose > 131.5 Gy versus ≤ 131.5 Gy
Table 4.
Multivariate Cox analysis of the ARS for re-RT
| Variable | p Value | RR (95% CI) |
|---|---|---|
| Failure patterns | 0.997 | – |
| Initial treatment | 0.287 | – |
| RFI time | 0.042 | 0.743 (0.559–0.989) |
| Chemotherapy | 0.001 | 0.580 (0.420–0.802) |
| Salvage radiation dose | 0.379 | – |
| Esophageal stenosis | 0.045 | 1.319 (1.007–1.729) |
| Fat space between tumor and adjacent tissue disappeared | 0.158 | – |
| Hb level | 0.155 | – |
RR relative risk, CI confidence interval
Toxicity
EP was the most common complication in all patients received re-RT (21.4%, 54/252), 40 patients occurred in initial CRT group and 14 patients in surgery group (Table 5). 12 patients were treated with stenting and 14 patients were treated with bouginage before re-RT, and 8 patients developed EP after endoscopic treatment, of whom 6 patients received stenting and 2 patients had bouginage treatment. Radiation-induced pneumonitis was observed in 32 patients (12.8%), 10 patient (4.0%) experienced grade 3 radiation pneumonitis and 3 patients died due to radiation pneumonitis. Esophagorrhagia was noted in 23 patients (9.1%), which was more frequent in patients who received surgery initially (12.9%, 11/85). 17 patients in CRT group and 6 patients in surgery group died of esophagorrhagia, respectively.
Table 5.
Comparison of baseline characteristics between the patients with EP ( +) and EP (−) after re-RT
| Variables | EP (−) | EP ( + ) | p |
|---|---|---|---|
| Re-RT age (years) (n, %) | 0.693 | ||
| ≤ 60 | 68 (34.3) | 17 (31.5) | |
| > 60 | 130 (65.7) | 37 (68.5) | |
| Gender (n, %) | 0.955 | ||
| Male | 162 (81.8) | 44 (81.5) | |
| Female | 36 (18.2) | 10 (18.5) | |
| Smoking (n, %) | 0.965 | ||
| Yes | 96 (48.5) | 26 (48.1) | |
| No | 102 (51.5) | 28 (51.9) | |
| Alcohol consumption (n, %) | 0.206 | ||
| Yes | 80 (40.4) | 27 (50) | |
| No | 118 (59.6) | 27 (50) | |
| Primary tumor location (n, %) | |||
| Upper | 53 (26.8) | 19 (35.2) | 0.225 |
| Middle and lower thoracic | 145 (73.2) | 35 (64.8) | |
| Length, cm (n, %) | 0.872 | ||
| ≤ 4 | 121 (61.1) | 31 (57.4) | |
| 4 < to ≤ 6 | 55 (27.8) | 16 (29.6) | |
| > 6 | 22 (11.1) | 7 (13.0) | |
| Ulcerative type (n, %) | 0.546 | ||
| Yes | 109 (55.1) | 25 (46.3) | |
| No | 89 (44.9) | 29 (53.7) | |
| Tumor differentiation (n, %) | 0.882 | ||
| Higher | 29 (14.6) | 7 (13.0) | |
| Middle | 113 (57.1) | 30 (55.6) | |
| Lower | 56 (28.3) | 17 (31.4) | |
| T stage (n, %) | 0.962 | ||
| T1–2 | 63 (31.8) | 17 (31.5) | |
| T3–4 | 135 (61.2) | 37 (68.5) | |
| Initial clinical/pathological stage (n, %) | 0.775 | ||
| I–II | 91 (46.0) | 26 (48.1) | |
| III–Iva | 107 (54) | 28 (51.9) | |
| Initial treatment (n, %) | 0.171 | ||
| RT | 127 (64.1) | 40 (74.1) | |
| Surgery + RT | 71 (35.9) | 14 (25.9) | |
| Pattern of recurrence (n, %) | 0.010 | ||
| Regional lymph node recurrence only | 90 (45.4) | 15 (27.8) | |
| Local failure | 73 (36.9) | 20 (37.0) | |
| Both | 35 (17.7) | 19 (35.2) | |
| Recurrence-free interval (months) (n, %) | 0.025 | ||
| ≤ 12 | 53 (36.8) | 23 (42.6) | |
| > 12 | 145 (73.2) | 31 (57.4) | |
| Re-RT dose (Gy) (n, %), BED10 | 0.442 | ||
| ≤ 60 | 102 (51.5) | 31 (53.7) | |
| > 60 | 96 (48.5) | 23 (46.3) | |
| Concurrent chemoradiotherapy (n, %) | 0.894 | ||
| Yes | 86 (43.4) | 24 (44.4) | |
| No | 112 (56.6) | 30 (55.6) | |
| Total radiation dose (Gy) (n, %), BED10 | 0.334 | ||
| ≤ 131.5 | 99 (50.0) | 31 (57.4) | |
| > 131.5 | 99 (50.0) | 23 (42.6) | |
| Esophageal stenosis (n, %) | 0.000 | ||
| Yes | 68 (34.3) | 34 (63.0) | |
| No | 130 (65.7) | 20 (37.0) | |
| Fat space between tumor and adjacent tissue disappeared (n, %) | 0.000 | ||
| Yes | 37 (18.7) | 27 (50.0) | |
| No | 161 (81.3) | 27 (50.0) | |
| Pain in the chest or/and back (n, %) | 0.175 | ||
| Yes | 17 (8.6) | 8 (14.8) | |
| No | 181 (91.4) | 46 (85.2) | |
| Median BMI (n, %) | 0.102 | ||
| ≤ 20 | 63 (31.8) | 11 (20.4) | |
| > 20 | 135 (68.2) | 43 (79.6) | |
| Hemoglobin (n, %) | 0.141 | ||
| ≤ 12 | 70 (35.4) | 25 (46.3) | |
| > 12 | 128 (64.6) | 29 (53.7) | |
| Albumin (n, %) | 0.447 | ||
| ≤ 40 | 66 (33.3) | 21 (38.9) | |
| > 40 | 132 (66.7) | 33 (61.1) | |
| White blood cells, × 109/L | 5.776 ± 2.285 | 6.520 ± 3.103 | 0.059 |
| Neutrophil, × 109/L | 4.379 ± 2.172 | 5.013 ± 2.936 | 0.090 |
Risk factors for EP
We evaluated the relationship between EP and clinicopathological features (Table 6). Univariate analysis revealed that pattern of recurrence, RFI, esophageal stenosis and fat space between tumor and adjacent tissue disappeared were potential risk factors for EP after re-RT. We then used the forward logical regression method to perform a multivariant analysis for the post re-RT EP risk factors. We found that the RFI ≤ 12 months, esophageal stenosis and fat space between tumor and adjacent tissue disappeared were independent risk factors for the development of EP after re-RT.
Table 6.
Univariate and multivariate logistic analysis of risk factors of EP after re-RT
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Re-RT age (years) | 1.114 | 0.610–2.036 | 0.725 | |||
| Gender (n, %) | 0.978 | 0.450–2.124 | 0.955 | |||
| Smoking (n, %) | 0.987 | 0.540–1.802 | 0.965 | |||
| Alcohol consumption (n, %) | 1.444 | 0.790–2.642 | 0.233 | |||
| Primary tumor location (n, %) | 0.673 | 0.355–1.278 | 0.227 | |||
| Length, cm (n, %) | 1.120 | 0.732–1.714 | 0.602 | |||
| Macroscopic tumor type | 1.210 | 0.881–1.662 | 0.240 | |||
| Tumor differentiation (n, %) | 1.127 | 0.703–1.805 | 0.620 | |||
| T stage (n, %) | 1.049 | 0.693–1.587 | 0.822 | |||
| N stage (n, %) | 0.908 | 0.597–1.382 | 0.652 | |||
| Initial clinical/pathological stage (n, %) | 1.113 | 0.738–1.679 | 0.610 | |||
| Initial treatment | 1.597 | 0.814–3.135 | 0.173 | |||
| Pattern of recurrence (n, %) | 1.360 | 0.911–2.031 | 0.133 | |||
| Recurrence-free interval (months) | 0.493 | 0.264–0.920 | 0.026 | 0.450 | 0.229–0.884 | 0.021 |
| Re-RT dose (Gy) | 0.788 | 0.430–1.447 | 0.443 | |||
| Concurrent chemoradiotherapy | 1.042 | 0.568–1.910 | 0.894 | |||
| Total radiation dose (Gy) | 0.742 | 0.404–1.361 | 0.335 | |||
| Esophageal stenosis | 3.250 | 1.739–6.074 | 0.000 | 2.665 | 1.359–5.225 | 0.004 |
| Pain in the chest or/and back | 2 | 0.752–4.557 | 0.180 | |||
| Fat space between tumor and adjacent tissue disappeared | 4.351 | 2.290–8.269 | 0.000 | 3.347 | 1.702–6.581 | 0.000 |
| BMI | 1.824 | 0.882–3.773 | 0.105 | |||
| Hemoglobin (g/L) | 0.634 | 0.345–1.166 | 0.143 | |||
| Albumin (g/L) | 0.786 | 0.422–1.463 | 0.447 | |||
| White blood cells, × 109/L | 1.133 | 1.1011–1.270 | 0.032 | |||
| Neutrophil, × 109/L | 1.121 | 0.995–1.263 | 0.059 | |||
HR hazard ratio, CI confidence interval
Discussion
LR of ESCC can be a devastating condition, because of the patients should bear obstruction, dysphagia, pain, infection, bleeding, nausea and vomiting with large impact on health-related quality of life. In the whole population, the recurrence rate of regional LN is slightly higher than PF (42.9% vs. 38.1%). PF recurrence rate was lower than previous study [17, 20], just because of patients who underwent RT after surgery initially and received re-RT were included in the analysis. Regional LN and PF were the most common failure pattern in the surgery group (70.6%) and the radical RT group (50.3%), respectively. These patients are usually in good physical condition, and they are expected to get better survival by taking reasonable salvage treatments.
But the role of salvage treatments in patients with LR in the primary tumor bed after RT is controversial [21]. Re-irradiation has been successfully used in many recurrent tumors of various sites with the development of radiotherapy techniques, such as head and neck cancer [7, 22], high grade glioma [23], lung cancer [24], intracranial germ-cell tumors [25], rectal cancer [26, 27] and paediatric tumor [28] with encouraging results. Several small size retrospective studies reported the outcome of re-RT of LR for ESCC patients received RT [14, 17, 29]. The only prospective study reported that re-RT for oligo-recurrence in lymph nodes from esophageal cancer treated by definitive RT or by surgery with additional RT might be acceptable but unsatisfactory [15]. In our present study, we found that there was a significant increase in OS for patients who received re-RT. The 5-years OS rate was 32.1% in salvage radiotherapy patients, and the median survival time was 39.0 months, which is longer than those reported in other studies [13, 14]. The median ARS was 13.0 months (range 3–168 months), which was similar to the results of previous studies [12–17, 29]. Therefore, further research is needed to improve the survival time after recurrence.
The factors that influence the efficacy of re-RT are also of interest to researchers. In the present study, we also found failure pattern was associated with ARS, and LN recurrence had better survival than PF and/or combined with LN (p = 0.026), which is consistent with Hong et al. [17] reports. Although previous study had not found significant difference in the effect of RFI on prognosis [14], but we found that patients with RFI > 12 months had better outcomes (14 vs. 11 months, p = 0.037) through univariate analysis and Cox regression analysis. This might be attributed to differences in the baseline differences in the population and tumor cells that do not respond to the treatment for early recurrences [30].
We also found that the failure pattern was associated with ARS after re-RT in univariant analysis. Hong et al. [17] found that the median survival time (MST) in the LN group was 23 months, whereas the MST in the PF group was 9 months (p = 0.004). The LN group had better ARS than the PF group with/without LN (p = 0.026) in our study, this may be related to the nutritional status of patients with PF and their poorer tolerance to treatment. Minsky et al. [31] confirmed that higher radiation dose did not increase the survival or improved the local/regional control for esophageal cancer in trial INT 0123 and used 50.4 Gy as a standard irradiation dose. We also found that the ARS of the patients received total RT dose with BED > 131.5 Gy were similar to patients received a total dose with BED ≤ 131.5 Gy (p = 0.545). Meanwhile, salvage radiation dose did not affect ARS in our present study (p = 0.326). This may be related to the fact that the basic conditions of the patients are different during the re-RT and the salvage radiation dose is difficult to be fixed. It also partially suggests that increasing radiation dose alone may not improve survival for LR ESCC patients.
It is well known that CT can improve the sensitivity of radiotherapy and improve the therapeutic effect. But in Hong et al. study, no statistical difference in ARS was observed between the groups treated with re-RT alone and re-RT combined with CT (p = 0.70) [17]. In our present study, we found that patients received re-RT combined with CT got better ARS than patients who were not (p = 0.001). We recommended CT for the patients who received re-RT in good physical condition. Patients with RFI > 12 months had better ARS than RFI ≤ 12 months, this may be related to the different sensitivity of tumor to RT, and patients with longer RFI may be more sensitive to RT. Our findings are in agreement with those reported by previous studies [14, 30]. Esophageal stenosis predicts nutritional status and poorer tolerance to treatment, which affects the patient's prognosis. Previous report found that esophageal stenosis was a predictor of poor median overall and recurrence-free survival in patients with oesophageal cancer [32]. We found that esophageal stenosis also affected the prognosis of patients receiving re-RT. Therefore, further assessment of these risk factors will contribute to a more accurate assessment of the patient's prognosis.
In addition to improve the prognosis, it is also important to predict and prevent adverse effects associated with re-RT. Zhou et al. [14] reported that the EP was observed in 11 cases (20.0%) and in 8 cases (13.6%) in the re-RT and non-salvage re-RT group, respectively (p = 0.357). Chen et al. [13] showed that esophagotracheal fistula in 5 patients and esophageal perforation in 2 patients were identified in the re-RT group (n = 36). In the current study, EP occurred in 21.4%, which is similar to pervious reports.
It is important to predict adverse effects associated with re-irradiation. Patients with RFI ≤ 12 months, esophageal stenosis and fat space between tumor and adjacent tissue disappeared had a higher risk of EP through our present analysis. There is no prediction of risk factors for EP caused by re-RT in the past, but the risk factors were similar to patients who received radiotherapy for the first time [18, 33]. Other risk factors for EP in patients undergoing RT for the first time have also been reported [34–36], further study on the risk of EP in re-RT is expected to be included in analysis. Patients with esophageal stenosis might be treated with stenting/bouginage, which is a risk factor for EP especially in patients who underwent RT [37]. In our present study, 30.8% (8/26) of patients received re-RT after endoscopic treatment developed EP, which was much higher than the overall incidence. Therefore, patients received stenting/bouginage should be closely observed during re-RT and nasal feeding diet or intravenous nutrition during re-RT are also optional treatment strategies. RP is another complication which should be concerned in re-RT. The incidence of grade 3 RP was 12.5% for re-RT group in our study, which is similar to previous study [14, 17]. Bleeding rate was very low in re-RT population [13], which was higher in our study may be because of thoracic stomach increases the risk of esophagorrhagia. Although the incidence of complications in re-RT is acceptable according to previous reports and the findings of our study, we should pay more attention to these patients. Further studies are required to assess the risk factors for toxicities through re-RT.
The present study has several limitations. First, the treatment time span was longer, resulting in poor consistency of treatment. Second, we were unable to analyze how the re-RT dose determined, which is probably related to the patient's nutritional status and tolerance to treatment.
Conclusion
Re-RT was feasible for LR ESCC patients after RT initially, it was also proved effective and safe to receive re-RT for initial surgery patients. Combined with chemotherapy and RFI time > 12 months were better prognostic factors for ARS, and patients with esophageal stenosis may have a poor prognosis. Patients with RFI ≤ 12 months, esophageal stenosis and fat space between tumor and adjacent tissue disappeared should be paid more attention, because of these patients are at significantly increased risk of EP.
Acknowledgements
We would like to acknowledge the work of Yeying Fang, Junchao Qian, Lei Feng, which remarkably improved the quality of this paper.
Abbreviations
- LR
Locoregional recurrence
- CRT
Chemoradiotherapy
- RT
Radiotherapy
- ESCC
Esophageal squamous cell carcinoma
- Re-RT
Re-irradiation
- PF
Primary failure
- LN
Lymph node recurrence
- GTV
Gross tumor volume
- CTV
Clinical target volume
- PTV
Planning target volume
- CT
Chemotherapy
- OS
Overall survival
- ARS
After-recurrence survival
- RFI
Recurrence-free interval
- EP
Esophageal fistula/perforation
- RP
Radiation pneumonitis
- MST
Median survival time
Authors’ contributions
KZ: Data collection, statistics, original draft. YS, LS: Data collection. XM: Conceptualization, review and editing the manuscript. JY: Monitor the clinical trial. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81972796, 81972863) and Natural Science Foundation of Shandong Province (Grant No. ZR2019MH010).
Availability of data and materials
Data are available from the author when needed.
Ethical approval and consent to participate
The requirement of patients’ consent was waived because this was a retrospective study.
Consent for publication
Manuscript is approved by all authors for publication.
Competing interests
The authors declared that they have no conflicts of interest to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kaikai Zhao, Email: abcdkaikai35@126.com.
Youjiao Si, Email: abcdsiyoujiao@163.com.
Liangchao Sun, Email: liangchao0911@126.com.
Xiangjiao Meng, Email: mengxiangjiao@126.com.
Jinming Yu, Email: sdyujinming@163.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA:A Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Stahl M, Stuschke M, Lehmann N, Meyer H-J, Walz MK, Seeber S. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 3.Robb WB, Messager M, Dahan L, Mornex F, Maillard E, D'Journo XB, et al. Patterns of recurrence in early-stage oesophageal cancer after chemoradiotherapy and surgery compared with surgery alone. Br J Surg. 2016;103:117–125. doi: 10.1002/bjs.9959. [DOI] [PubMed] [Google Scholar]
- 4.Haefner MF, Lang K, Krug D, Koerber SA, Uhlmann L, Kieser M, et al. Prognostic factors, patterns of recurrence and toxicity for patients with esophageal cancer undergoing definitive radiotherapy or chemo-radiotherapy. J Radiat Res. 2015;56:742–749. doi: 10.1093/jrr/rrv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordu AD, Nieder C, Geinitz H, Kup PG, Deymann LF, Scherer V, et al. Radio(chemo)therapy for locally advanced squamous cell carcinoma of the esophagus: long-term outcome. Strahlentherapie Onkologie Organ der Deutschen Rontgengesellschaft. 2015;191:153–160. doi: 10.1007/s00066-014-0779-x. [DOI] [PubMed] [Google Scholar]
- 6.Shioyama Y, Nakamura K, Ohga S, Nomoto S, Sasaki T, Yamaguchi T, et al. Radiation therapy for recurrent esophageal cancer after surgery: clinical results and prognostic factors. Jpn J Clin Oncol. 2007;37:918–923. doi: 10.1093/jjco/hym138. [DOI] [PubMed] [Google Scholar]
- 7.Balermpas P, Keller C, Hambek M, Wagenblast J, Seitz O, Rodel C, et al. Reirradiation with cetuximab in locoregional recurrent and inoperable squamous cell carcinoma of the head and neck: feasibility and first efficacy results. Int J Radiat Oncol Biol Phys. 2012;83:e377–e383. doi: 10.1016/j.ijrobp.2011.12.088. [DOI] [PubMed] [Google Scholar]
- 8.Sohda M, Kuwano H. Current status and future prospects for esophageal cancer treatment. Ann Thor Cardiovasc Surg. 2017;23:1–11. doi: 10.5761/atcs.ra.16-00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashraf TM, Nabi MG, Ali ZS, Wajahat A. Comparative evaluation between re-irradiation and demand endoscopic dilatation vs. endoscopic dilatation alone in patients with recurrent/reactivated residual in-field esophageal malignancies. J Cancer Res Ther. 2008;4:121–125. doi: 10.4103/0973-1482.43140. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi S, Ohguri T, Imada H, Yahara K, Moon SD, Higure A, et al. Multimodal approaches including three-dimensional conformal re-irradiation for recurrent or persistent esophageal cancer: preliminary results. J Radiat Res. 2011;52:812–820. doi: 10.1269/jrr.11066. [DOI] [PubMed] [Google Scholar]
- 11.Jingu K, Matsushita H, Takeda K, Umezawa R, Takahashi C, Sugawara T, et al. Long-term results of radiotherapy combined with nedaplatin and 5-fluorouracil for postoperative loco-regional recurrent esophageal cancer: update on a phase II study. BMC Cancer. 2012;12:542. doi: 10.1186/1471-2407-12-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YS, Lee CG, Kim KH, Kim T, Lee J, Cho Y, et al. Re-irradiation of recurrent esophageal cancer after primary definitive radiotherapy. Radiat Oncol J. 2012;30:182–188. doi: 10.3857/roj.2012.30.4.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Lu Y, Wang Y, Yang H, Xia Y, Chen M, et al. Comparison of salvage chemoradiation versus salvage surgery for recurrent esophageal squamous cell carcinoma after definitive radiochemotherapy or radiotherapy alone. Dis Esophagus. 2014;27:134–140. doi: 10.1111/j.1442-2050.2012.01440.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou ZG, Zhen CJ, Bai WW, Zhang P, Qiao XY, Liang JL, et al. Salvage radiotherapy in patients with local recurrent esophageal cancer after radical radiochemotherapy. Radiat Oncol (Lond) 2015;10:54. doi: 10.1186/s13014-015-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jingu K, Niibe Y, Yamashita H, Katsui K, Matsumoto T, Nishina T, et al. Re-irradiation for oligo-recurrence from esophageal cancer with radiotherapy history: a multi-institutional study. Radiat Oncol (Lond) 2017;12:146. doi: 10.1186/s13014-017-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katano A, Yamashita H, Nakagawa K. Re-irradiation of locoregional esophageal cancer recurrence following definitive chemoradiotherapy: a report of 6 cases. Mol Clin Oncol. 2017;7:681–686. doi: 10.3892/mco.2017.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong L, Huang Y-X, Zhuang Q-Y, Zhang X-Q, Tang L-R, Du K-X, et al. Survival benefit of re-irradiation in esophageal cancer patients with locoregional recurrence: a propensity score-matched analysis. Radiat Oncol (Lond). 2018;13:171. doi: 10.1186/s13014-018-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HY, Ma XM, Ye M, Hou YL, Xie HY, Bai YR. Esophageal perforation during or after conformal radiotherapy for esophageal carcinoma. J Radiat Res. 2014;55:940–947. doi: 10.1093/jrr/rru031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atsumi K, Shioyama Y, Arimura H, Terashima K, Matsuki T, Ohga S, et al. Esophageal stenosis associated with tumor regression in radiotherapy for esophageal cancer: frequency and prediction. Int J Radiat Oncol Biol Phys. 2012;82:1973–1980. doi: 10.1016/j.ijrobp.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 20.Versteijne E, van Laarhoven HW, van Hooft JE, van Os RM, Geijsen ED, van Berge Henegouwen MI, et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus. 2015;28:453–459. doi: 10.1111/dote.12215. [DOI] [PubMed] [Google Scholar]
- 21.Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer. 2010;29:843–854. doi: 10.5732/cjc.010.10151. [DOI] [PubMed] [Google Scholar]
- 22.Takiar V, Garden AS, Ma D, Morrison WH, Edson M, Zafereo ME, et al. Reirradiation of head and neck cancers with intensity modulated radiation therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys. 2016;95:1117–1131. doi: 10.1016/j.ijrobp.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Sminia P, Mayer R. External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers (Basel) 2012;4:379–399. doi: 10.3390/cancers4020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshiaki O, Masao M, Eisaku Y, Ryohei S, Yoshishige O, Toshifumi N, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2002;52:390–396. doi: 10.1016/S0360-3016(01)02644-X. [DOI] [PubMed] [Google Scholar]
- 25.de Rezende ACP, Weltman E, Chen MJ, Helito JK, de Carvalho IT, Sakuraba RK, et al. Intensity-modulated ventricular irradiation for intracranial germ-cell tumors: survival analysis and impact of salvage re-irradiation. PLoS ONE. 2019;14:e0226350. doi: 10.1371/journal.pone.0226350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Meij W, Rombouts AJ, Rutten H, Bremers AJ, de Wilt JH. Treatment of locally recurrent rectal carcinoma in previously (chemo)irradiated patients: a review. Dis Colon Rectum. 2016;59:148–156. doi: 10.1097/DCR.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 27.Al-Haidari G, Skovlund E, Undseth C, Rekstad BL, Larsen SG, Asli LM, et al. Re-irradiation for recurrent rectal cancer—a single-center experience. Acta Oncol. 2020;2020:1–7. doi: 10.1080/0284186X.2020.1725111. [DOI] [PubMed] [Google Scholar]
- 28.Tsang DS, Laperriere NJ. Re-irradiation for paediatric tumours. Clin Oncol (R Coll Radiol) 2019;31:191–198. doi: 10.1016/j.clon.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Wang Z, Jiang S, Shang Y, Wu Y. Evaluating the optimal re-irradiation dose for locally recurrent esophageal squamous cell carcinoma after definitive radiotherapy. Radiat Oncol (Lond) 2019;14:191. doi: 10.1186/s13014-019-1402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu E, Tai P, Malthaner R, Stitt L, Rodrigues G, Dar R, et al. What are the factors that predict outcome at relapse after previous esophagectomy and adjuvant therapy in high-risk esophageal cancer? Curr Oncol (Toronto) 2010;17:46–51. doi: 10.3747/co.v17i6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 32.Deng HY, Li G, Luo J. Does oesophageal stenosis have any impact on survival of oesophageal cancer patients? Interacti Cardiovasc Thor Surg. 2018;27:384–386. doi: 10.1093/icvts/ivy095. [DOI] [PubMed] [Google Scholar]
- 33.Tsushima T, Mizusawa J, Sudo K, Honma Y, Kato K, Igaki H, et al. Risk factors for esophageal fistula associated with chemoradiotherapy for locally advanced unresectable esophageal cancer: a supplementary analysis of JCOG0303. Medicine. 2016;95:e3699. doi: 10.1097/MD.0000000000003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami T, Tsushima T, Omae K, Ogawa H, Shirasu H, Kito Y, et al. Risk factors for esophageal fistula in thoracic esophageal squamous cell carcinoma invading adjacent organs treated with definitive chemoradiotherapy: a monocentric case-control study. BMC Cancer. 2018;18:573. doi: 10.1186/s12885-018-4486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe S, Ogino I, Kunisaki C, Hata M. Relationship between nutritional status and esophageal fistula formation after radiotherapy for esophageal cancer. Cancer Radiother. 2019;23:222–227. doi: 10.1016/j.canrad.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Wang L, He B, Li W, Wen Q, Wang S, et al. Development and validation of a risk prediction model for radiotherapy-related esophageal fistula in esophageal cancer. Radiat Oncol (Lond) 2019;14:181. doi: 10.1186/s13014-019-1385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bick BL, Song LM, Buttar NS, Baron TH, Nichols FC, Maldonado F, et al. Stent-associated esophagorespiratory fistulas: incidence and risk factors. Gastrointest Endosc. 2013;77:181–189. doi: 10.1016/j.gie.2012.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the author when needed.