Abstract
Understanding the mechanisms underlying plants’ adaptation to their environment will require knowledge of the genes and alleles underlying elemental composition. Modern genetics is capable of quickly, and cheaply indicating which regions of DNA are associated with particular phenotypes in question, but most genes remain poorly annotated, hindering the identification of candidate genes. To help identify candidate genes underlying elemental accumulations, we have created the known ionome gene (KIG) list: a curated collection of genes experimentally shown to change uptake, accumulation, and distribution of elements. We have also created an automated computational pipeline to generate lists of KIG orthologs in other plant species using the PhytoMine database. The current version of KIG consists of 176 known genes covering 5 species, 23 elements, and their 1588 orthologs in 10 species. Analysis of the known genes demonstrated that most were identified in the model plant Arabidopsis thaliana, and that transporter coding genes and genes altering the accumulation of iron and zinc are overrepresented in the current list.
Keywords: curated, ionomics, mineral nutrition
1. INTRODUCTION
Understanding the complex relationships that determine plant adaptation will require detailed knowledge of the action of individual genes, the environment, and their interactions. One of the fundamental processes that plants must accomplish is to manage the uptake, distribution, and storage of elements from the environment. Many different physiological, chemical, biochemical, and cell biology processes are involved in moving elements, implicating thousands of genes in every plant species. Modern genetic techniques have made it easy and inexpensive to identify hundreds to thousands of loci for traits, such as the elemental composition (or ionome) of plant tissues. However, moving from loci to genes is still difficult as the number of possible candidates is often extremely large and the ability of researchers to identify a candidate gene from its functional annotations is limited by our current knowledge and inherent biases about what is worth studying (Stoeger et al., 2018; Baxter, 2020).
The most obvious candidates for genes affecting the ionome in a species are orthologs of genes that have been shown to affect elemental accumulation in another species. Indeed, there are multiple examples of orthologs affecting elemental accumulation in distantly related species, such as Arabidopsis thaliana and rice (Oryza sativa), including Na+ transporters from the HKT family (Ren et al., 2005; Baxter et al., 2010); the heavy metal transporters AtHMA3 and OsHMA3 (Chao et al., 2012; Yan et al., 2016); E3 ubiquitin ligase BRUTUS and OsHRZs that regulate the degradation of iron uptake factors (Selote et al., 2015; Hindt et al., 2017; Kobayashi et al., 2013) and the K+ channel AKT1 (Ahmad et al., 2016; Lagarde et al., 1996). To our knowledge, no comprehensive list of genes known to affect elemental accumulation in plants exists. To ameliorate this deficiency, we sought to create a curated list of genes based on peer‐reviewed literature along with a pipeline to identify orthologs of the genes in any plant species and a method for continuously updating the list. Here we present version 1.0 of the known ionome gene (KIG) list.
2. MATERIALS AND METHODS
The list includes all functionally characterized genes from the literature that are linked to changes in the ionome. Criteria for inclusion in the primary KIG list were as follows:
The function or levels of the gene are unambiguously altered (i.e., a confirmed knockout, knockdown or overexpressor). For double mutants, both genes are listed.
The levels of at least one element are significantly altered in plant tissue.
Publication in the form of a peer‐reviewed manuscript.
Note that our definition excludes genes that are linked to metal tolerance or sensitivity but do not alter the ionome, or genes where the levels of the transcript are correlated with elemental accumulation. In order to identify the KIG genes, we created a Google survey that was distributed to members of the Ionomicshub research coordination network (NSF DBI‐0953433), as well as advertising on Twitter and in oral presentations by the authors. We asked submitters to provide the species, gene name (or names where alleles of two genes were required for a phenotype), gene ID(s), tissue(s), element(s) altered, and a DOI link for the primary literature support. Subsequently, authors FKR and LW did an extensive literature search.
2.1. Creating the inferred orthologs list
The known ionome gene list contains known genes from the primary list and their orthologous genes inferred by InParanoid (v4.1) pairwise species comparisons (Remm et al., 2001). The InParanoid files were downloaded from Phytozome for each organism‐to‐organism combination of species in the primary list, plus Glycine max, Sorghum bicolor, Setaria italica, Setaria viridis, and Populus trichocarpa. Orthologs of the primary genes were labeled as “inferred” genes. If a primary gene was also found as an ortholog to a primary gene in another species, the status was changed to “Primary/Inferred” in both species. It is important to note that only primary genes can infer genes; inferred genes cannot infer other genes. The pipeline for transforming the primary list into the known ionomics gene list can be found at https://github.com/baxterlab/KIG.
2.2. Gene Enrichment analysis
Overrepresentation analysis (released July 11, 2019) was performed on the primary and inferred genes in A. thaliana using the GO Consortium's web‐based GO Enrichment Analysis tool powered by the PANTHER (v14) classification system tool (Ashburner et al., 2000; Mi et al., 2017; The Gene Ontology Consortium, 2017). We restricted overrepresentation analysis to A. thaliana because of its dominance in the KIG list and our lack of confidence in the functional annotation of the other species on the list. An analysis performed by Wimalanathan et al. (2018) found that maize gene annotations in databases like Gramene and Phytozome lacked GO annotations outside of automatically assigned, electronic annotations (IEA). IEA annotations are not curated and have the least amount of support out of all the evidence codes (Harris et al., 2004). A. thaliana annotations come from a variety of evidence types, showing a higher degree of curation compared to maize (Wimalanathan et al., 2018). The whole‐genome Arabidopsis thaliana gene list from the PANTHER database was used as the reference list.
We tested both the PANTHER GO‐slim and the GO complete datasets for biological processes, molecular function, and cellular component. GO‐Slim datasets contain a selected subset of terms that give a broad summary of the gene list, whereas the complete dataset contains all the terms returned for a more detailed analysis. The enriched terms (fold enrichment >1 and with a false discovery rate <0.05) from the complete dataset were sorted into five specific categories relating to the ionome based annotation terms:
Ion homeostasis ‐ terms include homeostasis, stress, detoxification, regulation of an ion
Ion transport ‐ terms specifically state transport, export, import or localization of ion(s). Does not include hydrogen ion transport
Metal ion chelation ‐ terms relating to phytochelatins, other chemical reactions or pathways of metal chelator synthesis
Response to ions—vaguely states response to ions, but does not have any parent annotation terms that offer any more clarification (ie. stress response). Broadly this is referring to any change in the state or activity of cell secretion, expression, movement, or enzyme production (Carbon et al., 2009)
Other transport—annotation stating the transfer of anything that is not an ion (glucose, peptides, etc.)
Genes may belong to more than one category, but if they belong to a parent and child term in the same category, they are only counted once.
3. RESULTS
The current primary list (v1.0) consists of 176 genes from A. thaliana, O. sativa, Medicago truncatula, Triticum aestivum, and Zea mays with the majority coming from A. thaliana and O. sativa (Table 1, Figure 1).
TABLE 1.
Primary known ionome genes
| Species | GeneID | GeneName | Elements | Tissue | Citation(s) |
|---|---|---|---|---|---|
| A. thaliana | AT1G01340 | CNGC10 | K, Ca, Mg | Roots, shoots | Guo et al. (2010) |
| A. thaliana | AT1G01580 | FRO2 | Fe | Root | Robinson et al. (1999) |
| A. thaliana | AT1G07600 | MT1A | Cd, Zn, As | Shoots | Zimeri et al. (2005) |
| A. thaliana | AT1G08490 | CPNIFS | Se, S | Roots, shoots | Van Hoewyk et al. (2005) |
| A. thaliana | AT1G12640 | LPCAT1 | P | Leaf | Kisko et al. (2018) |
| A. thaliana | AT1G14040 | PHO1;H3 | P | Shoots | Khan et al. (2014) |
| A. thaliana | AT1G14870 | PCR2 | Zn | Shoots | Song et al. (2010) |
| A. thaliana | AT1G18910 | BTSL2 | Fe, Mn, Zn | Leaf | Hindt et al. (2017) |
| A. thaliana | AT1G20110 | FYVE1 | Fe, Zn, Co, Mn | Root | Barberon et al. (2014) |
| A. thaliana | AT1G30270 | CIPK23 | K | Shoots | Xu et al. (2006) |
| A. thaliana | AT1G30400 | ABCC1 | Cd | Shoots | Park et al. (2012) |
| A. thaliana | AT1G30450 | CCC | Ca, K, Na,S | seeds | McDowell et al. (2013) |
| A. thaliana | AT1G31885 | NIP3;1 | As | Shoots | Xu et al. (2015) |
| A. thaliana | AT1G32450 | AtNRT1.5/ AtNPF7.3 | K, NO3‐ | Shoots, Roots | Li et al. (2017) |
| A. thaliana | AT1G36370 | AtMSA1 | S, Se | Shoots | Huang, et al. (2016) |
| A. thaliana | AT1G56160 | myb72 | Fe, Cd, Zn, Co, Mo | Leaf | Palmer et al. (2013) |
| A. thaliana | AT1G56430 | NAS4 | Fe, Cd, Co, Mo | Leaf | Palmer et al. (2013) |
| A. thaliana | AT1G59870 | PEN3 | Cd | Shoots, roots | Kim et al. (2007) |
| A. thaliana | AT1G60960 | AtIRT3 | Fe | Roots | Lin et al. (2009) |
| A. thaliana | AT1G62180 | AtAPR2 | S, Se | Shoots | Loudet et al. (2007); Chao, et al. (2014) |
| A. thaliana | AT1G63440 | AtHMA5 | Cu | Shoots | Andrés‐Colás et al. (2006) |
| A. thaliana | AT1G66240 | AtAX1 | Cu | Shoots | Shin et al. (2012) |
| A. thaliana | AT1G68320 | MYB62 | P | Roots, shoots | Devaiah et al. (2009) |
| A. thaliana | AT1G71200 | AtCITF1 | Cu | Shoots, Anthers | Yan et al. (2017) |
| A. thaliana | AT1G74770 | BTSL1 | Fe, Mn, Zn | Leaf | Hindt et al. (2017) |
| A. thaliana | AT1G76430 | PHT1;9 | P, As | Roots, shoots | Remy et al. (2012) |
| A. thaliana | AT1G80760 | NIP6;1 | B | Leaves,shoots | Tanaka et al. (2008) |
| A. thaliana | AT1G80830 | AtNRAMP1 | Mn | Shoots, roots | Cailliatte et al. (2010) |
| A. thaliana | AT2G01770 | VIT1 | Fe | Seed | Kim et al. (2006) |
| A. thaliana | AT2G01980 | SOS1/NHX7 | Na | Shoots | Shi et al. (2003) |
| A. thaliana | AT2G13540 | ABH1 | S | seeds | McDowell et al. (2013) |
| A. thaliana | AT2G16770 | AtbZIP23 | Zn | Shoots, roots | Assunção et al. (2010) |
| A. thaliana | AT2G19110 | AtHMA4 | Zn | Shoots, seeds | Hussain et al. (2004); Olsen et al. (2016) |
| A. thaliana | AT2G21045 | AtHAC1 | As | Shoots | Chao, et al. (2014) |
| A. thaliana | AT2G23150 | AtNRAMP3 | Fe, Mn, Zn | Shoots | Lanquar et al. (2010) |
| A. thaliana | AT2G23240 | AtMT4b | Cu, Zn | Seeds | Ren et al. (2012) |
| A. thaliana | AT2G25680 | MOT1 | Mo | Leaf | Baxter, Muthukumar, et al., 2008; Baxter, Vitek, et al., 2008 |
| A. thaliana | AT2G28160 | FRU | Fe | Shoots | Yuan et al. (2008) |
| A. thaliana | AT2G28670 | ESB1 | Ca, Mn, Zn, Na, S, K, As, Se, Mo | Leaf | Baxter et al. (2009) |
| A. thaliana | AT2G32830 | PHT1;5 | P | Roots | Nagarajan et al. (2011) |
| A. thaliana | AT2G33770 | PHO2 | P | Roots, shoots | Liu et al. (2012) |
| A. thaliana | AT2G37430 | ZAT11 | Ni | Shoots | Liu et al. (2014) |
| A. thaliana | AT2G38460 | FPN1 | Co | Leaf | Morrissey et al. (2009) |
| A. thaliana | AT2G38940 | PHT1;4 | P | Roots, shoots | Shin et al. (2004) |
| A. thaliana | AT2G39450 | AtMTP11 | Mn | Shoots, roots | Peiter et al. (2007) |
| A. thaliana | AT2G42000 | AtMT4a | Cu, Zn | Seeds | Ren et al. (2012) |
| A. thaliana | AT2G46430 | CNGC3 | K | Leaf | Gobert et al. (2006) |
| A. thaliana | AT2G46800 | AtMTP1 | Zn | Shoots | Desbrosses‐Fonrouge et al. (2005) |
| A. thaliana | AT2G47160 | BOR1 | B | Shoots | Miwa et al. (2006) |
| A. thaliana | AT3G01310 | VIH2 | P | Shoots | Zhu et al. (2019) |
| A. thaliana | AT3G06060 | TSC10a | Na, K, Rb, Mg, Ca, Fe, Mo | Leaf | Chao et al. (2011) |
| A. thaliana | AT3G06100 | NIP7 | As | NA | Lindsay and Maathuis (2016; Isayenkov and Maathuis (2008) |
| A. thaliana | AT3G08040 | FRD3/MAN1 | Mn | Leaf | Delhaize (1996) |
| A. thaliana | AT3G12750 | AtZIP1 | Mn | Roots | Milner et al. (2013) |
| A. thaliana | AT3G12820 | myb10 | Fe, Cd, Zn, Co, Mo | Leaf | Palmer et al. (2013) |
| A. thaliana | AT3G13320 | CAX2 | Mn, Fe, K, P | Seed | Connorton et al. (2012) |
| A. thaliana | AT3G13405 | mir169a | N | Root | Zhao et al. (2011) |
| A. thaliana | AT3G14280 | S | seeds | McDowell et al. (2013) | |
| A. thaliana | AT3G15380 | AtCTL1 | Na, Fe, Zn, Mn, Mo | Shoots, Roots | Gao et al. (2017) |
| A. thaliana | AT3G18290 | BTS | Fe, Zn, Mn | Leaf | Hindt et al. (2017) |
| A. thaliana | AT3G22890 | AtATPS1 | S | Shoos | Koprivova et al. (2013) |
| A. thaliana | AT3G23210 | bHLH34 | Fe | Root, shoot | Li et al. (2016) |
| A. thaliana | AT3G23430 | PHO1 | P | Shoots | Khan et al. (2014) |
| A. thaliana | AT3G43790 | ZIFL2 | Cs | Leaf | Remy et al. (2015) |
| A. thaliana | AT3G47640 | PYE | Fe, Zn, Mn, Co | Root | Long et al. (2010) |
| A. thaliana | AT3G47950 | AHA4 | Na | Root | Vitart et al. (2001) |
| A. thaliana | AT3G51860 | CAX3 | P, K | Seed | Connorton et al. (2012) |
| A. thaliana | AT3G51895 | SULTR3;1 | S | Leaf | Cao et al. (2013) |
| A. thaliana | AT3G56970 | bHLH38 | Fe | Shoots | Yuan et al. (2008) |
| A. thaliana | AT3G56980 | bHLH39 | Fe | Shoots | Yuan et al. (2008) |
| A. thaliana | AT3G58060 | AtMTP8 | Mn | Shoots, seeds | Eroglu et al. (2016, Eroglu et al. (2017) |
| A. thaliana | AT3G58810 | AtMTP3 | Zn | Shoots | Arrivault et al. (2006) |
| A. thaliana | AT3G58970 | MGT6 | Mg | Roots, shoots | Mao et al. (2014) |
| A. thaliana | AT3G62270 | BOR2 | B | Shoots | Miwa et al. (2013) |
| A. thaliana | AT4G02780 | GA1 | Fe | Root | Wild et al. (2016) |
| A. thaliana | AT4G10310 | AtHKT1;1 | Na | Leaf | Baxter et al. (2010) |
| A. thaliana | AT4G10380 | NIP5;1 | B | Roots, shoots | Takano et al. (2006) |
| A. thaliana | AT4G13420 | HAK5 | Rb, Cs | Roots | Rubio et al. (2008; Qi et al. (2008) |
| A. thaliana | AT4G14410 | bHLH104 | Fe | Root, shoot | Li et al. (2016) |
| A. thaliana | AT4G16370 | OPT3 | Fe, Cd | Leaf | Zhai et al. (2014) |
| A. thaliana | AT4G19690 | IRT1 | Fe, Mn, Co, Cd, Zn | Root | Eide et al. (1996) |
| A. thaliana | AT4G23100 | GSH1 | Cd, As | Shoots | Guo et al. (2008) |
| A. thaliana | AT4G24120 | YSL1 | Fe, Zn, Cu | NA | Waters et al. (2006) |
| A. thaliana | AT4G28610 | AtPHR1 | P | Shoots | Nilsson et al. (2007) |
| A. thaliana | AT4G30110 | AtHMA2 | Zn | Shoots, seeds | Hussain et al. (2004; Olsen et al. (2016) |
| A. thaliana | AT4G30120 | AtHMA3 | Cd, Zn | Leaf | Chao et al. (2012; Pita‐Barbosa et al. (2019) |
| A. thaliana | AT4G33000 | CBL10 | K | Shoots | Ren et al. (2013) |
| A. thaliana | AT4G35040 | AtbZIP19 | Zn | Shoots, roots | Assunção et al. (2010) |
| A. thaliana | AT4G37270 | HMA1 | Zn | Shoots | Kim et al. (2009) |
| A. thaliana | AT5G02600 | NaKR1 | Na, K, Rb | Leaf | Tian et al. (2010) |
| A. thaliana | AT5G03455 | ACR2 | As, P | Roots, shoots | Dhankher et al. (2006) |
| A. thaliana | AT5G03570 | FPN2 | Co, Ni | Leaf | Morrissey et al. (2009); Schaaf et al. (2006) |
| A. thaliana | AT5G09690 | MGT7 | Mg | Shoots | Kamiya et al. (2012) |
| A. thaliana | AT5G13740 | ZIF1 | Zn, Fe | Shoots | Haydon et al. (2012) |
| A. thaliana | AT5G15070 | VIH1 | P | Shoots | Zhu et al. (2019) |
| A. thaliana | AT5G15410 | CNGC2/DND1 | Ca, Mg | seeds | McDowell et al. (2013) |
| A. thaliana | AT5G17290 | APG5 | Fe, Mn, Zn | Leaf, shoots, seeds | Pottier et al. (2019) |
| A. thaliana | AT5G18830 | AtSPL7 | Cu | Shoots, roots | Bernal et al. (2012) |
| A. thaliana | AT5G20650 | COPT5 | Cu | Shoots, roots, seeds | Klaumann et al. (2011) |
| A. thaliana | AT5G35410 | SOS2 | Na | Seeds | McDowell et al. (2013) |
| A. thaliana | AT5G42130 | AtMfl1 | Fe | Leaves, shoots | Tarantino et al. (2011) |
| A. thaliana | AT5G43350 | PHT1;1 | P, As | Shoots | Shin et al. (2004; Catarecha et al. (2007) |
| A. thaliana | AT5G44070 | PCS1 | Zn, Cd, As | Leaf | Kühnlenz et al. (2016; Guo et al. (2008) |
| A. thaliana | AT5G53130 | CNGC1 | Pb | Leaf | Sunkar et al. (2000) |
| A. thaliana | AT5G53550 | YSL3 | Fe, Zn, Cu | NA | Waters et al. (2006) |
| A. thaliana | AT5G54680 | ILR3 | Cd, Co, Fe, Mn, Zn | Leaf | Rampey et al. (2006) |
| A. thaliana | AT5G54810 | AtTSB1 | Cd | Roots,shoots | Sanjaya et al. (2008) |
| A. thaliana | AT5G57620 | AtMYB36 | Li, B, Na, Mg, K, Ca, Mn, Fe, Co, Ni, Cu, Zn, Rb, Sr, Mo, Cd | Shoots | Kamiya et al. (2015) |
| A. thaliana | AT5G59030 | COPT1 | Cu | Seed, Leaf | Sancenón et al. (2004) |
| A. thaliana | AT5G64930 | CPR5 | K | Leaf | Borghi et al. (2011) |
| A. thaliana | AT5G67330 | AtNRAMP3 | Fe, Mn, Zn | Shoots | Lanquar et al. (2010) |
| M. truncatula | Medtr1g010270 | MtMOT1.2 | Mo | Nodules | Gil‐Díez et al. (2018) |
| M. truncatula | Medtr3g088460 | MtNramp1 | Fe | Nodules | Tejada‐Jiménez et al. (2015) |
| M. truncatula | Medtr3g464210 | MtMOT1.3 | Mo | Nodules | Tejada‐Jiménez et al. (2017) |
| M. truncatula | Medtr4g019870 | MtCOPT1 | Cu | Nodules | Senovilla et al. (2018) |
| M. truncatula | Medtr4g064893 | MtMTP2 | Zn | Nodules | León‐Mediavilla et al. (2018) |
| M. truncatula | Medtr4g083570 | MtZIP6 | Zn | Nodules | Abreu et al. (2017) |
| O. sativa | LOC_Os01g03914 | OsMTP9 | Mn | Shoots | Ueno et al. (2015) |
| O. sativa | LOC_Os01g20160 | OsHKT1;5 | Na | Leaf, shoots | Kobayashi et al. (2017) |
| O. sativa | LOC_Os01g45990 | AKT1 | K | NA | Ahmad, et al. (2016) |
| O. sativa | LOC_Os01g64250 | OsHORZ1 | Fe | Shoots,seeds | Kobayashi et al. (2013) |
| O. sativa | LOC_Os01g64890 | OsMGT1 | Mg,Na | Roots, shoots | Chen, et al. (2017) |
| O. sativa | LOC_Os02g06290 | OsHAC4 | As | Seed | Xu et al. (2017) |
| O. sativa | LOC_Os02g10290 | OsHMA4 | Cu | Roots, shoots, seeds | Huang, et al. (2016) |
| O. sativa | LOC_Os02g13870 | OsNIP1;1 | As | Shoots | Sun et al. (2018) |
| O. sativa | LOC_Os02g43370 | OsYSL2 | Fe, Mn | Seeds | Ishimaru et al. (2010) |
| O. sativa | LOC_Os02g43410 | OsYSL15 | Fe | Roots, shoots, seeds | Lee et al. (2009) |
| O. sativa | LOC_Os02g51110 | LSI1 | Se | Roots, shoots | Zhao et al. (2010) |
| O. sativa | LOC_Os02g53490 | OsMTP8.2 | Mn | Shoots, roots | Takemoto et al. (2017) |
| O. sativa | LOC_Os02g56510 | OsPHO1;2 | P | Shoots | Secco et al. (2010) |
| O. sativa | LOC_Os03g05640 | OsPT2 | Se | Roots, shoots | Zhang et al. (2014) |
| O. sativa | LOC_Os03g09140 | OsRab6a | Fe, Zn | Seeds, shoot, roots | Yang and Zhang (2016) |
| O. sativa | LOC_Os03g12530 | OsMTP8.1 | Mn | Shoots, roots | Chen et al. (2013) |
| O. sativa | LOC_Os03g18550 | OsMIT | Fe | Shoots | Bashir et al. (2011) |
| O. sativa | LOC_Os03g19420 | OsNAS2 | Fe | Seeds | Lee et al. (2012) |
| O. sativa | LOC_Os03g21240 | OsPHR2 | P | Shoots | Zhou et al. (2008) |
| O. sativa | LOC_Os04g32920 | OsHAK1 | Cs | Shoots, seeds | Rai et al. (2017) |
| O. sativa | LOC_Os04g38940 | OsVIT1 | Fe,Zn | Shoots, seeds | Zhang et al. (2012) |
| O. sativa | LOC_Os04g45860 | OsYSL9 | Fe | Shoots, seeds | Senoura et al. (2017) |
| O. sativa | LOC_Os04g45900 | OsYSL16 | Cu | Roots, shoots, seeds | Zheng et al. (2012) |
| O. sativa | LOC_Os04g46940 | OsHMA5 | Cu | Roots,s hoots | Deng et al. (2013) |
| O. sativa | LOC_Os04g52310 | OsZIP3 | Zn | Shoots | Sasaki et al. (2015) |
| O. sativa | LOC_Os04g52900 | OsABCC1 | As | Seeds | Song et al. (2014) |
| O. sativa | LOC_Os04g56430 | OsRMC | Fe,Mn,Cu | Root, shoot, seeds | Yang et al. (2013) |
| O. sativa | LOC_Os05g34290 | OsPCS1* | As | Seeds | Hayashi et al. (2017) |
| O. sativa | LOC_Os05g39560 | OsZIP5 | Zn | Leaf | Lee et al. (2010) |
| O. sativa | LOC_Os05g47780 | OsHRZ2 | Fe | Shoots, seeds | Kobayashi et al. (2013) |
| O. sativa | LOC_Os05g48390 | OsPHO2 | P | Leaf | Wang et al. (2009) |
| O. sativa | LOC_Os06g01260 | OsPCS2* | As, Cd | Seeds | Uraguchi et al. (2017) |
| O. sativa | LOC_Os06g05160 | SPDT | P | Seed | Yamaji et al. (2017) |
| O. sativa | LOC_Os06g48720 | OsHMA2 | Zn | Shoots, roots | Takahashi et al. (2012) |
| O. sativa | LOC_Os06g48810 | OsHKT2;1 | Na | Roots, shoots | Horie et al. (2007) |
| O. sativa | LOC_Os07g01810 | TPKb | K | Leaf, root | Ahmad et al. (2016) |
| O. sativa | LOC_Os07g09000 | OsPHF1 | P | Leaf, root | Chen et al. (2011) |
| O. sativa | LOC_Os07g12900 | OsHMA3 | Cd | Shoots, seeds | Tanaka et al. (2016) |
| O. sativa | LOC_Os07g15370 | NRAMP5 | Fe,Mn,Cd | Leaf | Sasaki et al. (2012) |
| O. sativa | LOC_Os08g01120 | OsMOT1;1 | Mo | Shoots, Seed | Huang et al. (2019) |
| O. sativa | LOC_Os08g04390 | OsPRI1 | Fe | Shoots, roots | Zhang et al. (2017) |
| O. sativa | LOC_Os08g05590 | OsNIP3;2 | As | Roots | Chen, Sun, et al. (2017a); Chen, Yamaji, et al. (2017b) |
| O. sativa | LOC_Os08g05600 | OsNIP3;3 | As | Shoots | Sun et al. (2018) |
| O. sativa | LOC_Os08g10480 | OsATX1 | Cu | Shoots, roots, seeds | Zhang, Cao, et al. (2018); Zhang, Chen, et al. (2018) |
| O. sativa | LOC_Os09g23300 | OsVIT2 | Fe, Zn | Shoots, seeds | Zhang et al. (2012) |
| O. sativa | LOC_Os12g03899 | ZIFL12 | Fe | Shoots | Che et al. (2019) |
| O. sativa | LOC_Os12g18410 | OsMIR | Fe | Shoots, Roots, seeds | Ishimaru et al. (2009) |
| O. sativa | LOC_Os12g32400 | OsbHLH133 | Fe | Leaf, root, shoot | Wang, Sun, et al. (2013a); Wang, Ying, et al. (2013b) |
| O. sativa | LOC_Os12g37840 | OsBOR1 | B | Shoots | Nakagawa et al. (2007) |
| O. sativa | Os01g0689300 | OsHRZ1 | Fe | Shoots, seeds | Kobayashi et al. (2013) |
| T. aestivum | 2Al‐TRIAE_CS42_ 2AL_TGACv1_095050_AĂ410 | TaIPK1 | Fe, Zn | Seed | Aggarwal et al. (2018) |
| T. aestivum | Traes_4AS_7220D33B3 | Ta‐PHR1 | P | Shoots | Wang, Sun, et al. (2013a); Wang, Ying, et al. (2013b) |
| T. aestivum | Traes_4BL_7091749BF | TaABCC13 | Ca | Seed | Bhati et al. (2016) |
| T. aestivum | Traes_4DL_3F8034BFD | HKT2;1 | Na | Roots | Laurie et al. (2002) |
| Z. mays | GRMZM2G047616 | ZmHKT1 | Na | Leaf | Zhang, Cao, et al. (2018); Zhang, Chen, et al. (2018) |
| Z. mays | GRMZM2G060952 | YS1 | Fe | Root | Von Wiren et al. (1994) |
| Z. mays | GRMZM2G063306 | YS3 | Fe | Leaf | Chan‐Rodriguez and Walker (2018) |
| Z. mays | GRMZM2G084779 | ZmHAK5 | K | Roots, shoots | Qin et al. (2019) |
| Z. mays | GRMZM2G176209 | TLS1 | B | Shoots, roots, anthers | Durbak et al. (2014) |
FIGURE 1.
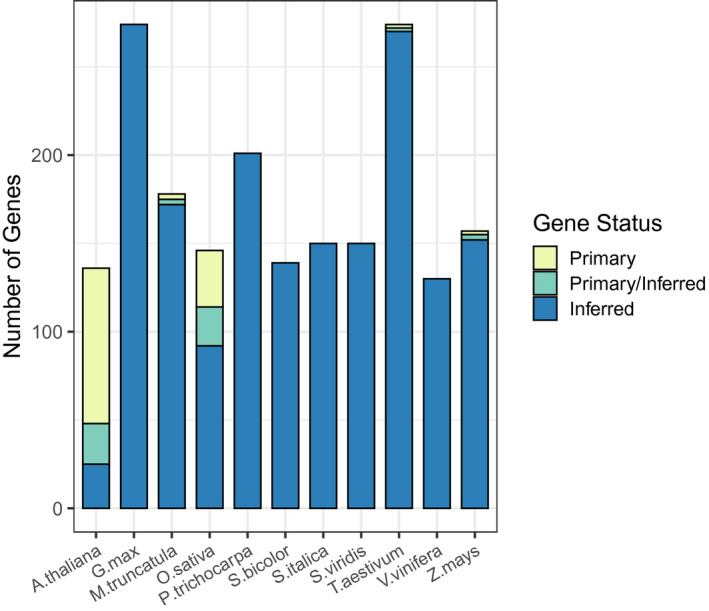
Number of genes for each species that are primary, inferred from other primary genes in other species, or both
Most primary genes have orthologs in other species. Less than 10% of primary genes in A. thaliana, 12% in O. sativa, and one of the four primary genes in wheat (T. aestivum) lack orthologs (Table 2). G. max, P. trichocarpa, S. bicolor, S. italica, and S. viridis currently contain only inferred genes (Table 2, Figure 1).
TABLE 2.
Break down of primary/inferred genes in each species
| Species | Total genes | Primary genes | Primary/inferred genes | Inferred genes | Primary & primary/inferred genes without orthologs |
|---|---|---|---|---|---|
| A. thaliana | 136 | 65.44% | 16.18% | 18.38% | 9.91% |
| O. sativa | 141 | 20.57% | 14.89% | 64.54% | 12.00% |
| M. truncatula | 176 | 1.70% | 1.70% | 96.59% | 0.00% |
| T. aestivum | 267 | 0.75% | 0.75% | 98.50% | 25.00% |
| Z. mays | 152 | 1.32% | 1.97% | 96.71% | 0.00% |
| G. max | 268 | 0.00% | 0.00% | 100.00% | 0.00% |
| P. trichocarpa | 197 | 0.00% | 0.00% | 100.00% | 0.00% |
| S. bicolor | 135 | 0.00% | 0.00% | 100.00% | 0.00% |
| S. italica | 146 | 0.00% | 0.00% | 100.00% | 0.00% |
| S. viridis | 146 | 0.00% | 0.00% | 100.00% | 0.00% |
The YSL genes in A. thaliana and O. sativa are an example that provides evidence for the validity of the KIG list pipeline: AtYSL3, OsYSL9, and OsYSL16 were listed in their respective species as primary genes (Table 1) and after the ortholog search was annotated as primary/inferred genes, referencing each other (Table S1). AtYSL2 in A. thaliana, was not listed as primary gene, but was inferred through its rice orthologs OsYSL9 and OsYSL16. Additionally, AtYSL1 in A. thaliana is not a paralog of AtYSL3 or an ortholog of OsYSL9 and OsYSL16 according to PhytoMine's InParanoid results and is not listed as an ortholog to either of the O. sativa YSL genes in the KIG list. Other examples include AtVIT1 and OsVIT1/OsVIT2 (Kim et al., 2006; Zhang et al., 2012), and the vacuolar Mn transporters AtMTP8 and OsMTP8 (Eroglu et al., 2016; Chen et al., 2013). Thus, we can reliably generate inferred genes and create a species‐specific KIG list for any species in PhytoMine.
The primary list covers 23 elements (Figure 2) according to the reported elements from authors in the primary list, which is more elements than predicted by the GO term annotations for those genes. Some GO annotations for these genes mention only a portion of elements listed by the literature on the primary list. This may be due to GO annotation evidence codes lacking curation or biological data (IEA, ND, NAS) (Wimalanathan et al., 2018), or it may be due to alterations in one element leading to alterations in other elements (Baxter, Muthukumar, et al., 2008; Baxter, Vitek, et al., 2008).
FIGURE 2.
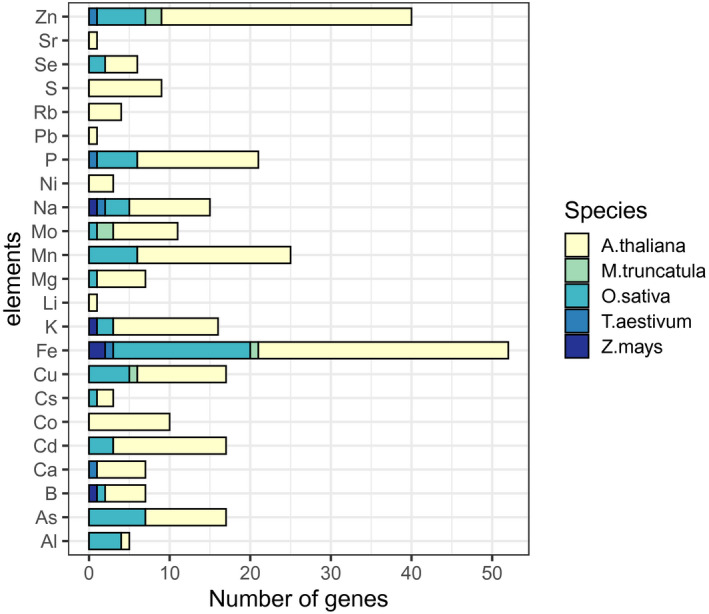
Number of primary genes from each species listing each element
A. thaliana is the only species to have a primary gene listing for each element. There is a bias toward manganese, zinc, and iron which have two, three, and four times more associated genes than the average 13 ± 12 genes of other elements. Iron is the only element to contain genes from all five species in the primary list. In addition to biases toward certain elements, our primary list is also skewed toward an overrepresentation of ionome genes in above‐ground tissue studies (Figure 3). This is likely due to the difficulties in studying the elemental content of below‐ground tissues. All M. truncatula genes come from studies of the nodule in this model legume species.
FIGURE 3.
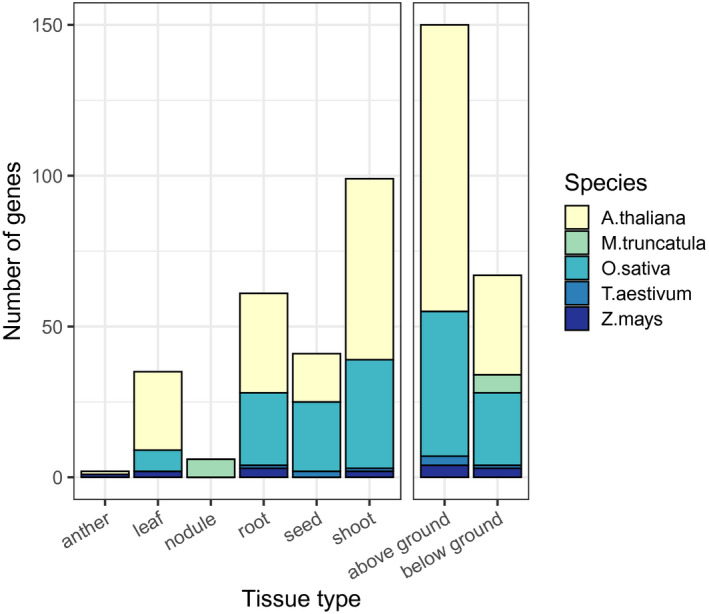
Number of primary genes each type of tissue contributes to the known ionome gene list. Above ground is a summary of anther, leaf, seed, and shoot, while below ground is a summary of root and nodule
Querying the manually curated PANTHER GO‐slim biological process database (PANTHER v14.1, released March 12, 2019) and the GO complete biological process database (GO Ontology database, released October 08, 2019) with the A. thaliana KIG genes returned significantly (FDR < 0.05) overrepresented annotation terms related to the transport, response, and homeostasis of iron, zinc, copper and manganese ions. Additionally, the GO complete results had terms for cadmium, nickel, cobalt, sulfur, arsenic, lead, selenium, boron, magnesium, phosphorus, sodium, potassium, and calcium; covering most of the elements in the KIG list (Figure 4). Even though some genes were annotated as associated in the “other transport” of glycoside, glucose, oligopeptides, or phloem transport, the citations that have added them into our primary list show that their mutant alleles altered elemental accumulation. AtABCC1 is annotated as encoding a glycoside transporter protein, but Park et al. (2012) found overexpression of AtABCC1 increased cadmium concentrations in shoot tissue. The YSL genes and OPT3 are annotated as genes encoding oligopeptide transporters, but more specifically they are encoding predicted phloem‐localized metal‐nicotianamine complex and iron/cadmium transporters, respectively (Waters et al., 2006; Zhai et al., 2014). Last, NRT1.5/NPF7.3 is also annotated as encoding an oligopeptide transporter, but Li et al., (2017) identified it as a xylem loading potassium ion antiporter.
FIGURE 4.
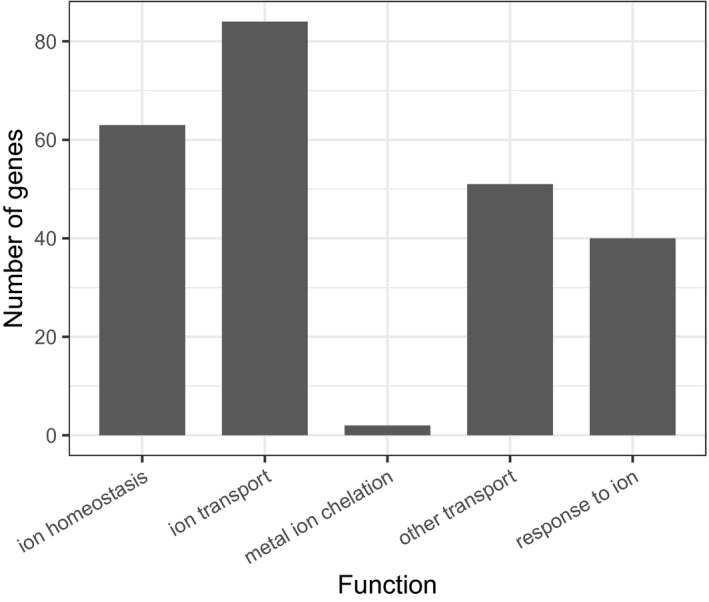
Known ionome genes relating to different terms from the GO complete biological process dataset. Ontology groups of GO Enrichment Analysis from PANTHER
The PANTHER GO‐slim molecular function annotation database found a significant overrepresentation for iron and potassium cation transmembrane transporter activity in the A. thaliana genes. The results using the GO complete molecular function database supported this and additionally included terms for arsenic, cadmium, zinc, boron, manganese, phosphate, sulfur, and magnesium ion transmembrane transporter activity. The GO complete molecular database also returned overrepresented terms for metal ion‐binding and cyclic nucleotide‐binding annotations. The cyclic nucleotide‐binding annotation genes were more specifically cyclic nucleotide ion gated channel genes (Gobert et al., 2006). The PANTHER GO‐slim cell component and GO complete cell component annotation database both returned significant overrepresentation for vacuoles and the plasma membrane, both known to be critical for elemental movement and storage (Barkla & Pantoja, 1996). The molecular function and cell component results are further evidence that our list is dominated by ion transporters.
To test the completeness of the KIG list, we searched PANTHER’s biological processes annotations for the number of A. thaliana genes encoding predicted elemental transporters. We found 618 A. thaliana genes predicted to encode elemental transport, and only 40 of these PANTHER genes are listed in the KIG list. We checked these results against ThaleMine (v1.10.4, updated on June 13, 2017) genes with the term “ion transport” in the gene name, description, or GO annotation and found only 358 genes, with 52 of these genes listed in the A. thaliana known ionome gene list. Interestingly, 219 of the genes from ThaleMine were not found in the 634 from PANTHER.
4. DISCUSSION
Here we have produced a curated list of genes known to alter the elemental composition of plant tissues. We envision several possible uses for this list:
Researchers can use the list to identify candidate genes in loci from QTL and GWAS experiments.
This list can serve as a gold standard for computational approaches.
The list can serve as a reading list for those interested in learning about elemental accumulation.
It is important to highlight that the inferred genes lists are not likely to be perfect predictors of the causal genes. Our use of InParanoid orthologs may exclude homologs that are likely candidates. Additionally, the reasons that some genes have been studied could be the result of human bias toward research topics (Baxter, 2020). The list is highly enriched for (a) transporters, (b) genes that affect elemental accumulation in above‐ground tissues, and (c) genes that affect the accumulation of Fe and Zn. Transporter genes became obvious candidates for studying plant nutrition when disruption allele collections were produced (McDowell et al., 2013). Above‐ground tissues are easier to study without contamination from the soil, and such studies are, therefore, more prevalent. Finally, while Fe and Zn are important biochemical cofactors, these elements are likely enriched in the KIG list due to their considerable interest in the community where the ionomics approach was developed. This is further illustrated in the PANTHER GO‐slim databases, where Fe was the only element to have its overrepresented response, homeostasis, and transport‐related GO terms show up in the PANTHER GO‐slim biological process and molecular function databases, which are selected subsets of terms meant to broadly summarize data. Overrepresented terms related to other KIG list elements are only found in the GO complete databases. Taken together, these factors warn against forming conclusions about the nature of all elemental accumulation genes based on this limited dataset.
Most entries on this list are derived from model organisms, suggesting that most of our knowledge about genes that affect elemental accumulation comes from these species. A. thaliana and M. truncatula account for 64% of the primary genes list, which is probably a lower bound for the influence of knowledge generated in model organisms. Several of the genes in crop plants were found due to being orthologs of genes in the model organisms (Ahmad, et al., 2016; Xu et al., 2017), and on closer inspection of the 50 papers identifying primary genes in rice, 38 cited a gene in Arabidopsis (not necessarily the direct ortholog) as a source for why the gene was investigated. The higher quality of the GO terms in Arabidopsis, when compared to other species, is another reflection of this disparity of knowledge and a significant hindrance when trying to clone genes in other organisms.
4.1. Call for more submissions
While we have done our best to ensure that the current list is useful and thorough, it is possible we are still missing genes. We ask readers who know of genes that we are missing to contribute by submitting them here: https://docs.google.com/forms/d/e/1FAIpQLSdmS_zeOlxTOLmq2wB45BuSQml1LMKtKnWSatmFRGR2Q1o0Ew/viewform?c=0&w=1 or email corresponding author. KIG lists v1.0 for each of the species can be viewed in Table S1, and future updates to the list can be found at https://docs.google.com/spreadsheets/d/1XI2l1vtVJiHrlXLeOS5yTQQnLYq7BOHpmjuC‐kUejUU/edit?usp=sharing.
AUTHORS CONTRIBUTIONS
Contributed genes: IB, FKR, FM, SC, EW, PK. Analyzed data: LW, GZ. Wrote paper: LW, FKR, IB. Edited paper: FKR, FM, SC, EW, PK, GZ, LW, IB.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors thank the editors and reviewers for their consideration and comments.
Whitt L, Ricachenevsky FK, Ziegler G, et al. A curated list of genes that control elemental accumulation in plants. Plant Direct. 2020;4:1–15. 10.1002/pld3.272
REFERENCES
- Abreu, I. , Saéz, Á. , Castro‐Rodríguez, R. , Escudero, V. , Rodríguez‐Haas, B. , Senovilla, M. , Larue, C. , Grolimund, D. , Tejada‐Jiménez, M. , Imperial, J. , & González‐Guerrero, M. (2017). Medicago truncatula zinc‐iron permease6 provides zinc to rhizobia‐infected nodule cells. Plant, Cell & Environment, 40(11), 2706–2719. [DOI] [PubMed] [Google Scholar]
- Aggarwal, S. , Kumar, A. , Bhati, K. K. , Kaur, G. , Shukla, V. , Tiwari, S. , & Pandey, A. K. (2018). RNAi‐mediated downregulation of inositol pentakisphosphate kinase (IPK1) in wheat grains decreases phytic acid levels and increases Fe and Zn Accumulation. Frontiers in Plant Science, 9(March), 259 10.3389/fpls.2018.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, I. , Devonshire, J. , Mohamed, R. , Schultze, M. , & Maathuis, F. J. M. (2016). Overexpression of the potassium channel TPKb in small vacuoles confers osmotic and drought tolerance to rice. The New Phytologist, 209(3), 1040–1048. [DOI] [PubMed] [Google Scholar]
- Ahmad, I. , Mian, A. , & Maathuis, F. J. M. (2016). Overexpression of the rice AKT1 potassium channel affects potassium nutrition and rice drought tolerance. Journal of Experimental Botany, 67(9), 2689–2698. 10.1093/jxb/erw103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés‐Colás, N. , Sancenón, V. , Rodríguez‐Navarro, S. , Mayo, S. , Thiele, D. J. , Ecker, J. R. , Puig, S. , & Peñarrubia, L. (2006). The arabidopsis heavy metal P‐type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. The Plant Journal, 45(2), 225–236. 10.1111/j.1365-313X.2005.02601.x [DOI] [PubMed] [Google Scholar]
- Arrivault, S. , Senger, T. , & Krämer, U. (2006). The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe Deficiency and Zn oversupply. The Plant Journal, 46(5), 861–879. 10.1111/j.1365-313X.2006.02746.x [DOI] [PubMed] [Google Scholar]
- Ashburner, M. , Ball, C. A. , Blake, J. A. , Botstein, D. , Butler, H. , Cherry, J. M. , Davis, A. P. , Dolinski, K. , Dwight, S. S. , Eppig, J. T. , Harris, M. A. , Hill, D. P. , Issel‐Tarver, L. , Kasarskis, A. , Lewis, S. , Matese, J. C. , Richardson, J. E. , Ringwald, M. , Rubin, G. M. , & Sherlock, G. (2000). Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nature Genetics, 25(1), 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção, A. G. L. , Herrero, E. , Lin, Y.‐F. , Huettel, B. , Talukdar, S. , Smaczniak, C. , Immink, R. G. H. , van Eldik, M. , Fiers, M. , Schat, H. , & Aarts, M. G. M. (2010). Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10296–10301. 10.1073/pnas.1004788107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon, M. , Dubeaux, G. , Kolb, C. , Isono, E. , Zelazny, E. , & Vert, G. (2014). Polarization of IRON‐REGULATED TRANSPORTER 1 (IRT1) to the plant‐soil interface plays crucial role in metal homeostasis. Proceedings of the National Academy of Sciences of the Unnited States of America, 111(22), 8293–8298. 10.1073/pnas.1402262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla, B. J. , & Pantoja, O. (1996). Physiology of ion transport across the tonoplast of higher plants. Annual Review of Plant Physiology and Plant Molecular Biology, 47(June), 159–184. 10.1146/annurev.arplant.47.1.159 [DOI] [PubMed] [Google Scholar]
- Bashir, K. , Ishimaru, Y. , Shimo, H. , Nagasaka, S. , Fujimoto, M. , Takanashi, H. , Tsutsumi, N. , An, G. , Nakanishi, H. , & Nishizawa, N. K. (2011). The Rice Mitochondrial Iron Transporter Is Essential for Plant Growth. Nature Communications, 2, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, I. (2020). We aren’t good at picking candidate genes, and it’s slowing us down. Current Opinion in Plant Biology, 54(April), 57–60. [DOI] [PubMed] [Google Scholar]
- Baxter, I. , Brazelton, J. N. , Danni, Y. U. , Huang, Y. S. , Lahner, B. , Yakubova, E. , Li, Y. , Bergelson, J. , Borevitz, J. O. , Nordborg, M. , & Vitek, O. (2010). A coastal cline in sodium accumulation in arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genetics, 6(11), e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, I. , Hosmani, P. S. , Rus, A. , Lahner, B. , Borevitz, J. O. , Muthukumar, B. , Mickelbart, M. V. , Schreiber, L. , Franke, R. B. , & Salt, D. E. (2009). Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in arabidopsis. PLoS Genetics, 5(5), e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, I. , Muthukumar, B. , Park, H. C. , Buchner, P. , Lahner, B. , Danku, J. , Zhao, K. , Lee, J. , Hawkesford, M. J. , Guerinot, M. L. , & Salt, D. E. (2008). Variation in molybdenum content across broadly distributed populations of arabidopsis thaliana is controlled by a Mitochondrial Molybdenum Transporter (MOT1). PLoS Genetics, 4(2), e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, I. R. , Vitek, O. , Lahner, B. , Muthukumar, B. , Borghi, M. , Morrissey, J. , Guerinot, M. L. , & Salt, D. E. (2008). The leaf ionome as a multivariable system to detect a plant’s physiological status. Proceedings of the National Academy of Sciences of the United States of America, 105(33), 12081–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal, M. , Casero, D. , Singh, V. , Wilson, G. T. , Grande, A. , Yang, H. , Dodani, S. C. , Pellegrini, M. , Huijser, P. , Connolly, E. L. , & Merchant, S. S. (2012). Transcriptome sequencing identifies SPL7‐regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in arabidopsis. The Plant Cell, 24(2), 738–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhati, K. K. , Alok, A. , Kumar, A. , Kaur, J. , Tiwari, S. , & Pandey, A. K. (2016). Silencing of ABCC13 transporter in wheat reveals its involvement in grain development, phytic acid accumulation and lateral root formation. Journal of Experimental Botany, 67(14), 4379–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi, M. , Rus, A. , & Salt, D. E. (2011). Loss‐of‐Function of constitutive expresser of pathogenesis related genes5 affects potassium homeostasis in arabidopsis thaliana. PLoS One, 6(10), e26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailliatte, R. , Schikora, A. , Briat, J.‐F. , Mari, S. , & Curie, C. (2010). High‐affinity manganese uptake by the metal transporter NRAMP1 is essential for arabidopsis growth in low manganese conditions. The Plant Cell, 22(3), 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, M.‐J. , Wang, Z. , Wirtz, M. , Hell, R. , Oliver, D. J. , & Xiang, C.‐B. (2013). SULTR3;1 is a chloroplast‐localized sulfate transporter in arabidopsis thaliana. The Plant Journal, 73(4), 607–616. [DOI] [PubMed] [Google Scholar]
- Carbon, S. , Ireland, A. , Mungall, C. J. , Shu, S. , Marshall, B. , Lewis, S. , AmiGO Hub and Web Presence Working Group . (2009). AmiGO: online access to ontology and annotation data. Bioinformatics, 25(2), 288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarecha, P. , Segura, M. D. , Franco‐Zorrilla, J. M. , García‐Ponce, B. , Lanza, M. , Solano, R. , Paz‐Ares, J. , & Leyva, A. (2007). A mutant of the arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. The Plant Cell, 19(3), 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan‐Rodriguez, D. , & Walker, E. L. (2018). Analysis of yellow striped mutants of zea mays reveals novel loci contributing to iron deficiency chlorosis. Frontiers in Plant Science, 9(February), 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, D.‐Y. , Baraniecka, P. , Danku, J. , Koprivova, A. , Lahner, B. , Luo, H. , Yakubova, E. , Dilkes, B. , Kopriva, S. , & Salt, D. E. (2014). Variation in sulfur and selenium accumulation is controlled by naturally occurring isoforms of the key sulfur assimilation enzyme ADENOSINE 5’‐PHOSPHOSULFATE REDUCTASE2 across the arabidopsis species range. Plant Physiology, 166(3), 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, D.‐Y. , Chen, Y. I. , Chen, J. , Shi, S. , Chen, Z. , Wang, C. , Danku, J. M. , Zhao, F.‐J. , & Salt, D. E. (2014). Genome‐wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biology, 12(12), e1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, D.‐Y. , Gable, K. , Chen, M. , Baxter, I. , Dietrich, C. R. , Cahoon, E. B. , Guerinot, M. L. , Lahner, B. , Lü, S. , Markham, J. E. , & Morrissey, J. , (2011). Sphingolipids in the root play an important role in regulating the leaf ionome in arabidopsis thaliana. The Plant Cell, 23(3), 1061–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, D.‐Y. , Silva, A. , Ivan Baxter, Y. S. , Huang, M. N. , Danku, J. , Lahner, B. , Yakubova, E. , & Salt, D. E. (2012). Genome‐wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in arabidopsis thaliana. PLoS Genetics, 8(9), e1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, J. , Yokosho, K. , Yamaji, N. , & Ma, J. F. (2019). A Vacuolar phytosiderophore transporter alters iron and zinc accumulation in polished rice grains. Plant Physiology, 181(1), 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Liu, Y. U. , Ni, J. , Wang, Y. , Bai, Y. , Shi, J. , Gan, J. , Zhongchang, W. U. , & Ping, W. U. (2011). OsPHF1 regulates the plasma membrane localization of low‐ and high‐affinity inorganic phosphate transporters and determines inorganic phosphate uptake and translocation in rice. Plant Physiology, 157(1), 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. I. , Sun, S.‐K. , Tang, Z. , Liu, G. , Moore, K. L. , Maathuis, F. J. M. , Miller, A. J. , McGrath, S. P. , & Zhao, F.‐J. (2017). The nodulin 26‐like intrinsic membrane protein OsNIP3;2 is involved in arsenite uptake by lateral roots in rice. Journal of Experimental Botany, 68(11), 3007–3016. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Fujii, Y. , Yamaji, N. , Masuda, S. , Takemoto, Y. , Kamiya, T. , Yusuyin, Y. , Iwasaki, K. , Kato, S. I. , Maeshima, M. , & Ma, J. F. (2013). Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. Journal of Experimental Botany, 64(14), 4375–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. C. , Yamaji, N. , Horie, T. , Che, J. , Li, J. , An, G. , & Ma, J. F. (2017). A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physiology, 174(3), 1837–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connorton, J. M. , Webster, R. E. , Cheng, N. , & Pittman, J. K. (2012). Knockout of multiple arabidopsis cation/H(+) exchangers suggests isoform‐specific roles in metal stress response, germination and seed mineral nutrition. PLoS One, 7(10), e47455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize, E. (1996). A metal‐accumulator mutant of arabidopsis thaliana. Plant Physiology, 111(3), 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, F. , Yamaji, N. , Xia, J. , & Ma, J. F. (2013). A Member of the heavy metal P‐Type ATPase OsHMA5 Is involved in xylem loading of copper in rice. Plant Physiology, 163(3), 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbrosses‐Fonrouge, A.‐G. , Voigt, K. , Schröder, A. , Arrivault, S. , Thomine, S. , & Krämer, U. (2005). Arabidopsis thaliana MTP1 Is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Letters, 579(19), 4165–4174. [DOI] [PubMed] [Google Scholar]
- Devaiah, B. N. , Madhuvanthi, R. , Karthikeyan, A. S. , & Raghothama, K. G. (2009). Phosphate Starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in arabidopsis. Molecular Plant, 2(1), 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhankher, O. P. , Rosen, B. P. , McKinney, E. C. , & Meagher, R. B. (2006). Hyperaccumulation of Arsenic in the Shoots of Arabidopsis Silenced for Arsenate Reductase (ACR2). Proceedings of the National Academy of Sciences of the United States of America, 103(14), 5413–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbak, A. R. , Phillips, K. A. , Pike, S. , O’Neill, M. A. , Mares, J. , Gallavotti, A. , Malcomber, S. T. , Gassmann, W. , & McSteen, P. (2014). Transport of boron by the tassel‐less1 aquaporin is critical for vegetative and reproductive development in maize. The Plant Cell, 26(7), 2978–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide, D. , Broderius, M. , Fett, J. , & Guerinot, M. L. (1996). A novel iron‐regulated metal transporter from plants identified by functional expression in yeast. Proceedings of the National Academy of Sciences of the United States of America, 93(11):5624–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu, S. , Giehl, R. F. H. , Meier, B. , Takahashi, M. , Terada, Y. , Ignatyev, K. , Andresen, E. , Küpper, H. , Peiter, E. , & von Wirén, N. (2017). Metal tolerance protein 8 mediates manganese homeostasis and iron reallocation during seed development and germination. Plant Physiology, 174(3), 1633–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu, S. , Meier, B. , von Wirén, N. , & Peiter, E. (2016). The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency‐induced chlorosis in arabidopsis. Plant Physiology, 170(2), 1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y.‐Q. , Chen, J.‐G. , Chen, Z.‐R. , An, D. , Lv, Q.‐Y. , Han, M.‐L. , Wang, Y.‐L. , Salt, D. E. , & Chao, D.‐Y. (2017). A new vesicle trafficking regulator CTL1 plays a crucial role in ion homeostasis. PLoS Biology, 15(12), e2002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐Díez, P. , Tejada‐Jiménez, M. , León‐Mediavilla, J. , Wen, J. , Mysore, K. S. , Imperial, J. , & González‐Guerrero, M. (2018). MtMOT1.2 is responsible for molybdate supply to medicago truncatula nodules. Plant, Cell & Environment, 42(1), 310–320. 10.1111/pce.13388 [DOI] [PubMed] [Google Scholar]
- Gobert, A. , Park, G. , Amtmann, A. , Sanders, D. , & Maathuis, F. J. M. (2006). Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non‐selective ion transporter involved in germination and cation transport. Journal of Experimental Botany, 57(4), 791–800. [DOI] [PubMed] [Google Scholar]
- Guo, J. , Dai, X. , Wenzhong, X. U. , & Ma, M. I. (2008). Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in arabidopsis thaliana. Chemosphere, 72(7), 1020–1026. [DOI] [PubMed] [Google Scholar]
- Guo, K. M. , Babourina, O. , Christopher, D. A. , Borsic, T. , & Rengel, Z. (2010). The cyclic nucleotide‐gated channel AtCNGC10 transports Ca2+ and Mg2+ in arabidopsis. Physiologia Plantarum, 139(3), 303–312. [DOI] [PubMed] [Google Scholar]
- Harris, M. A. , Clark, J. , Ireland, A. , Lomax, J. , Ashburner, M. , Foulger, R. , Eilbeck, K. et al (2004). The gene ontology (GO) database and informatics resource. Nucleic Acids Research, 32(Database issue): D258–D261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, S. , Kuramata, M. , Abe, T. , Takagi, H. , Ozawa, K. , & Ishikawa, S. (2017). Phytochelatin synthase OsPCS1 plays a crucial role in reducing arsenic levels in rice grains. The Plant Journal, 91(5), 840–848. [DOI] [PubMed] [Google Scholar]
- Haydon, M. J. , Kawachi, M. , Wirtz, M. , Hillmer, S. , Hell, R. , & Krämer, U. (2012). Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in arabidopsis. The Plant Cell, 24(2), 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindt, M. N. , Akmakjian, G. Z. , Pivarski, K. L. , Punshon, T. , Baxter, I. , Salt, D. E. , & Guerinot, M. L. (2017). BRUTUS and Its paralogs, BTS LIKE1 and BTS LIKE2, encode important negative regulators of the iron deficiency response in arabidopsis thaliana. Metallomics, 9(7), 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoewyk, V. , Douglas, G. F. , Garifullina, A. R. , Ackley, S. E. , Abdel‐Ghany, M. A. , Marcus, S. F. , Ishiyama, K. , Inoue, E. , Pilon, M. , Takahashi, H. , & Pilon‐Smits, E. A. (2005). Overexpression of AtCpNifS enhances selenium tolerance and accumulation in arabidopsis. Plant Physiology, 139(3), 1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie, T. , Costa, A. , Kim, T. H. , Han, M. J. , Horie, R. , Leung, H.‐Y. , Miyao, A. , Hirochika, H. , An, G. , & Schroeder, J. I. (2007). Rice OsHKT2;1 transporter mediates large Na+ influx component into K+‐starved roots for growth. The EMBO Journal, 26(12), 3003–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X.‐Y. , Chao, D.‐Y. , Koprivova, A. , Danku, J. , Wirtz, M. , Müller, S. , Sandoval, F. J. , Bauwe, H. , Roje, S. , Dilkes, B. , & Hell, R. (2016). Nuclear localised MORE SULPHUR ACCUMULATION1 epigenetically regulates sulphur homeostasis in arabidopsis thaliana. PLoS Genetics, 12(9), e1006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X.‐Y. , Deng, F. , Yamaji, N. , Pinson, S. R. M. , Fujii‐Kashino, M. , Danku, J. , Douglas, A. , Guerinot, M. L. , Salt, D. E. , & Ma, J. F. (2016). A heavy metal P‐Type ATPase OsHMA4 prevents copper accumulation in rice grain. Nature Communications, 7(July), 12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X.‐Y. , Liu, H. , Zhu, Y.‐F. , Pinson, S. R. M. , Lin, H.‐X. , Guerinot, M. L. , Zhao, F.‐J. , & Salt, D. E. (2019). Natural variation in a molybdate transporter controls grain molybdenum concentration in rice. The New Phytologist, 221(4), 1983–1997. [DOI] [PubMed] [Google Scholar]
- Hussain, D. , Haydon, M. J. , Wang, Y. , Wong, E. , Sherson, S. M. , Young, J. , Camakaris, J. , Harper, J. F. , & Cobbett, C. S. (2004). P‐type ATPase heavy metal transporters with roles in essential zinc homeostasis in arabidopsis. The Plant Cell, 16(5), 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayenkov, S. V. , & Maathuis, F. J. M. (2008). The arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Letters, 582(11), 1625–1628. [DOI] [PubMed] [Google Scholar]
- Ishimaru, Y. , Bashir, K. , Fujimoto, M. , An, G. , Itai, R. N. , Tsutsumi, N. , Nakanishi, H. , & Nishizawa, N. K. (2009). Rice‐specific mitochondrial iron‐regulated gene (MIR) plays an important role in iron homeostasis. Molecular Plant, 2(5), 1059–1066. [DOI] [PubMed] [Google Scholar]
- Ishimaru, Y. , Masuda, H. , Bashir, K. , Inoue, H. , Tsukamoto, T. , Takahashi, M. , Nakanishi, H. , Aoki, N. , Hirose, T. , Ohsugi, R. , & Nishizawa, N. K. (2010). Rice metal‐nicotianamine transporter, OsYSL2, is required for the long‐distance transport of iron and manganese. The Plant Journal, 62(3), 379–390. [DOI] [PubMed] [Google Scholar]
- Kamiya, T. , Borghi, M. , Wang, P. , Danku, J. M. C. , Kalmbach, L. , Hosmani, P. S. , Naseer, S. , Fujiwara, T. , Geldner, N. , & Salt, D. E. (2015). The MYB36 transcription factor orchestrates Casparian strip formation. Proceedings of the National Academy of Sciences of the United States of America, 112(33), 10533–10538. 10.1073/pnas.1507691112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, T. , Yamagami, M. , Hirai, M. Y. , & Fujiwara, T. (2012). Establishment of an in planta magnesium monitoring system using CAX3 promoter‐luciferase in arabidopsis. Journal of Experimental Botany, 63(1), 355–363. 10.1093/jxb/err283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, G. A. , Bouraine, S. , Wege, S. , Li, Y. , de Carbonnel, M. , Berthomieu, P. , Poirier, Y. , & Rouached, H. (2014). Coordination between zinc and phosphate homeostasis involves the transcription factor PHR1, the phosphate exporter PHO1, and its homologue PHO1;H3 in arabidopsis. Journal of Experimental Botany, 65(3), 871–884. 10.1093/jxb/ert444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.‐Y. , Bovet, L. , Maeshima, M. , Martinoia, E. , & Lee, Y. (2007). The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance: Role of AtPDR8 in cadmium resistance. The Plant Journal, 50(2), 207–218. 10.1111/j.1365-313X.2007.03044.x [DOI] [PubMed] [Google Scholar]
- Kim, S. A. , Punshon, T. , Lanzirotti, A. , Li, L. , Alonso, J. M. , Ecker, J. R. , Kaplan, J. , & Guerinot, M. L. (2006). Localization of iron in arabidopsis seed requires the vacuolar membrane transporter VIT1. Science, 314(5803), 1295–1298. 10.1126/science.1132563 [DOI] [PubMed] [Google Scholar]
- Kim, Y.‐Y. , Choi, H. , Segami, S. , Cho, H.‐T. , Martinoia, E. , Maeshima, M. , & Lee, Y. (2009). AtHMA1 contributes to the detoxification of excess Zn(II) in arabidopsis. The Plant Journal, 58(5), 737–753. 10.1111/j.1365-313X.2009.03818.x [DOI] [PubMed] [Google Scholar]
- Kisko, M. , Bouain, N. , Safi, A. , Medici, A. , Akkers, R. C. , Secco, D. , Fouret, G. , Krouk, G. , Aarts, M. G. M. , Busch, W. , & Rouached, H. (2018). LPCAT1 controls phosphate homeostasis in a zinc‐dependent manner. eLife, 7, e32077. 10.7554/eLife.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaumann, S. , Nickolaus, S. D. , Fürst, S. H. , Starck, S. , Sabine Schneider, H. , Neuhaus, E. , & Trentmann, O. (2011). The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in arabidopsis thaliana. The New Phytologist, 192(2), 393–404. 10.1111/j.1469-8137.2011.03798.x [DOI] [PubMed] [Google Scholar]
- Kobayashi, N. I. , Yamaji, N. , Yamamoto, H. , Okubo, K. , Ueno, H. , Costa, A. , Tanoi, K. , Matsumura, H. , Fujii‐Kashino, M. , Horiuchi, T. , & Nayef, M. A. (2017). OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. The Plant Journal, 91(4), 657–670. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T. , Nagasaka, S. , Senoura, T. , Itai, R. N. , Nakanishi, H. , & Nishizawa, N. K. (2013). Iron‐binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nature Communications, 4, 2792 10.1038/ncomms3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova, A. , Giovannetti, M. , Baraniecka, P. , Lee, B.‐R. , Grondin, C. , Loudet, O. , & Kopriva, S. (2013). Natural variation in the ATPS1 isoform of ATP sulfurylase contributes to the control of sulfate levels in arabidopsis. Plant Physiology, 163(3), 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnlenz, T. , Hofmann, C. , Uraguchi, S. , Schmidt, H. , Schempp, S. , Weber, M. , Lahner, B. , Salt, D. E. , & Clemens, S. (2016). Phytochelatin synthesis promotes leaf Zn accumulation of arabidopsis thaliana plants grown in soil with adequate Zn supply and is essential for survival on Zn‐contaminated soil. Plant & Cell Physiology, 57(11), 2342–2352. [DOI] [PubMed] [Google Scholar]
- Lagarde, D. , Basset, M. , Lepetit, M. , Conejero, G. , Gaymard, F. , Astruc, S. , & Grignon, C. (1996). Tissue‐specific expression of arabidopsis AKT1 gene is consistent with a role in K+ nutrition. The Plant Journal, 9(2), 195–203. [DOI] [PubMed] [Google Scholar]
- Lanquar, V. , Ramos, M. S. , Lelièvre, F. , Barbier‐Brygoo, H. , Krieger‐Liszkay, A. , Krämer, U. , & Thomine, S. (2010). Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 Is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiology, 152(4), 1986–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie, S. , Feeney, K. A. , Maathuis, F. J. M. , Heard, P. J. , Brown, S. J. , & Leigh, R. A. (2002). A role for HKT1 in sodium uptake by wheat roots. The Plant Journal, 32(2), 139–149. [DOI] [PubMed] [Google Scholar]
- Lee, S. , Chiecko, J. C. , Kim, S. A. , Walker, E. L. , Lee, Y. , Guerinot, M. L. , & An, G. (2009). Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiology, 150(2), 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Jeong, H. J. , Kim, S. A. , Lee, J. , Guerinot, M. L. , & An, G. (2010). OsZIP5 is a plasma membrane zinc transporter in rice. Plant Molecular Biology, 73(4–5), 507–517. [DOI] [PubMed] [Google Scholar]
- Lee, S. , Kim, Y.‐S. , Jeon, U. S. , Kim, Y.‐K. , Schjoerring, J. K. , & An, G. (2012). Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Molecules and Cells, 33(3), 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León‐Mediavilla, J. , Senovilla, M. , Montiel, J. , Gil‐Díez, P. , Saez, Á. , Kryvoruchko, I. S. , Reguera, M. , Udvardi, M. K. , Imperial, J. , & González‐Guerrero, M. (2018). MtMTP2‐facilitated zinc transport into intracellular compartments is essential for nodule development in medicago truncatula. Frontiers in Plant Science, 9(July), 990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Miao, Y. U. , Xin‐Qiao, D. U. , Wang, Z.‐F. , Wei‐Hua, W. U. , Quintero, F. J. , Jin, X.‐H. , Li, H.‐D. , & Wang, Y. I. (2017). NRT1.5/NPF7.3 functions as a proton‐coupled H+/K+ antiporter for K+ loading into the xylem in arabidopsis. The Plant Cell, 29(8), 2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Zhang, H. , Ai, Q. , Liang, G. , & Diqiu, Y. U. (2016). Two bHLH transcription factors, bHLH34 and bHLH104, regulate iron homeostasis in arabidopsis thaliana. Plant Physiology, 170(4), 2478–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y.‐F. , Liang, H.‐M. , Yang, S.‐Y. , Boch, A. , Clemens, S. , Chen, C.‐C. , Jing‐Fen, W. U. , Huang, J.‐L. , & Yeh, K.‐C. (2009). Arabidopsis IRT3 Is a zinc‐regulated and plasma membrane localized zinc/iron transporter. The New Phytologist, 182(2), 392–404. [DOI] [PubMed] [Google Scholar]
- Lindsay, E. R. , & Maathuis, F. J. M. (2016). Arabidopsis thaliana NIP7;1 is involved in tissue arsenic distribution and tolerance in response to arsenate. FEBS Letters, 590(6), 779–786. [DOI] [PubMed] [Google Scholar]
- Liu, T.‐Y. , Huang, T.‐K. , Tseng, C.‐Y. , Lai, Y.‐S. , Lin, S.‐I. , Lin, W.‐Y. , Chen, J.‐W. , & Chiou, T.‐J. (2012). PHO2‐dependent degradation of PHO1 modulates phosphate homeostasis in arabidopsis. The Plant Cell, 24(5), 2168–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X.‐M. , An, J. , Han, H. J. , Kim, S. H. , Lim, C. O. , Yun, D.‐J. , & Chung, W. S. (2014). ZAT11, a zinc finger transcription factor, is a negative regulator of nickel ion tolerance in arabidopsis. Plant Cell Reports, 33(12), 2015–2021. [DOI] [PubMed] [Google Scholar]
- Long, T. A. , Tsukagoshi, H. , Busch, W. , Lahner, B. , Salt, D. E. , & Benfey, P. N. (2010). The bHLH transcription factor POPEYE regulates response to iron deficiency in arabidopsis roots. The Plant Cell, 22(7), 2219–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet, O. , Saliba‐Colombani, V. , Camilleri, C. , Calenge, F. , Gaudon, V. , Koprivova, A. , North, K. A. , Kopriva, S. , & Daniel‐Vedele, F. (2007). Natural variation for sulfate content in arabidopsis thaliana is highly controlled by APR2. Nature Genetics, 39(7), 896–900. [DOI] [PubMed] [Google Scholar]
- Mao, D. , Chen, J. , Tian, L. , Liu, Z. , Yang, L. , Tang, R. , Li, J. , Lu, C. , Yang, Y. , Shi, J. , & Chen, L. (2014). Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. The Plant Cell, 26(5), 2234–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, S. C. , Akmakjian, G. , Sladek, C. , Mendoza‐Cozatl, D. , Morrissey, J. B. , Saini, N. , Mittler, R. , Baxter, I. , Salt, D. E. , Ward, J. M. , & Schroeder, J. I. (2013). Elemental concentrations in the seed of mutants and natural variants of arabidopsis thaliana grown under varying soil conditions. PLoS One, 8(5), e63014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi, H. , Huang, X. , Muruganujan, A. , Tang, H. , Mills, C. , Kang, D. , & Thomas, P. D. (2017). PANTHER version 11: Expanded annotation data from gene ontology and reactome pathways, and data analysis tool enhancements. Nucleic Acids Research, 45(D1), D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, M. J. , Seamon, J. , Craft, E. , & Kochian, L. V. (2013). Transport properties of members of the ZIP family in plants and their role in Zn and mn homeostasis. Journal of Experimental Botany, 64(1), 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa, K. , Takano, J. , & Fujiwara, T. (2006). Improvement of seed yields under boron‐limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in arabidopsis thaliana. The Plant Journal, 46(6), 1084–1091. [DOI] [PubMed] [Google Scholar]
- Miwa, K. , Wakuta, S. , Takada, S. , Ide, K. , Takano, J. , Naito, S. , Omori, H. , Matsunaga, T. , & Fujiwara, T. (2013). Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in arabidopsis. Plant Physiology, 163(4), 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey, J. , Baxter, I. R. , Lee, J. , Li, L. , Lahner, B. , Grotz, N. , Kaplan, J. , Salt, D. E. , & Guerinot, M. L. (2009). The ferroportin metal efflux proteins function in iron and cobalt homeostasis in arabidopsis. The Plant Cell, 21(10), 3326–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan, V. K. , Jain, A. , Poling, M. D. , Lewis, A. J. , Raghothama, K. G. , & Smith, A. P. (2011). Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiology, 156(3), 1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, Y. , Hanaoka, H. , Kobayashi, M. , Miyoshi, K. , Miwa, K. , & Fujiwara, T. (2007). Cell‐type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. The Plant Cell, 19(8), 2624–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, L. , Müller, R. , & Nielsen, T. H. (2007). Increased expression of the MYB‐related transcription factor, PHR1, Leads to enhanced phosphate uptake in arabidopsis thaliana. Plant, Cell & Environment, 30(12), 1499–1512. [DOI] [PubMed] [Google Scholar]
- Olsen, L. I. , Hansen, T. H. , Larue, C. , Østerberg, J. T. , Hoffmann, R. D. , Liesche, J. , Krämer, U. , Surblé, S. , Cadarsi, S. , Samson, V. A. , & Grolimund, D. (2016). Mother‐plant‐mediated pumping of zinc into the developing seed. Nature Plants, 2(5), 16036. [DOI] [PubMed] [Google Scholar]
- Palmer, C. M. , Hindt, M. N. , Schmidt, H. , Clemens, S. , & Guerinot, M. L. (2013). MYB10 and MYB72 are required for growth under iron‐limiting conditions. PLoS Genetics, 9(11), e1003953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Song, W.‐Y. , Ko, D. , Eom, Y. , Hansen, T. H. , Schiller, M. , Lee, T. G. , Martinoia, E. , & Lee, Y. (2012). The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury: ABC transporters for PC‐dependent Cd and Hg tolerance. The Plant Journal, 69(2), 278–288. [DOI] [PubMed] [Google Scholar]
- Peiter, E. , Montanini, B. , Gobert, A. , Pedas, P. , Husted, S. , Maathuis, F. J. M. , Blaudez, D. , Chalot, M. , & Sanders, D. (2007). A secretory pathway‐localized cation diffusion facilitator confers plant manganese tolerance. Proceedings of the National Academy of Sciences of the United States of America, 104(20), 8532–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita‐Barbosa, A. , Ricachenevsky, F. K. , Wilson, M. , Dottorini, T. , & Salt, D. E. (2019). Transcriptional plasticity buffers genetic variation in zinc homeostasis. Scientific Reports, 9(1), 19482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier, M. , Dumont, J. , Masclaux‐Daubresse, C. , & Thomine, S. (2019). Autophagy is essential for optimal translocation of iron to seeds in arabidopsis. Journal of Experimental Botany, 70(3), 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Z. , Hampton, C. R. , Shin, R. , Barkla, B. J. , White, P. J. , & Schachtman, D. P. (2008). The HIGH Affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in arabidopsis. Journal of Experimental Botany, 59(3), 595–607. [DOI] [PubMed] [Google Scholar]
- Qin, Y.‐J. , Wei‐Hua, W. U. , & Wang, Y. I. (2019). ZmHAK5 and ZmHAK1 function in K+ uptake and distribution in maize under low K+ conditions. Journal of Integrative Plant Biology, 61(6), 691–705. [DOI] [PubMed] [Google Scholar]
- Rai, H. , Yokoyama, S. , Satoh‐Nagasawa, N. , Furukawa, J. , Nomi, T. , Ito, Y. , Fujimura, S. , Takahashi, H. , Suzuki, R. , Yousra, E. , & Goto, A. (2017). Cesium uptake by rice roots largely depends upon a single gene, HAK1, which encodes a potassium transporter. Plant & Cell Physiology, 58(9), 1486–1493. [DOI] [PubMed] [Google Scholar]
- Rampey, R. A. , Woodward, A. W. , Hobbs, B. N. , Tierney, M. P. , Lahner, B. , Salt, D. E. , & Bartel, B. (2006). An arabidopsis basic helix‐loop‐helix leucine zipper protein modulates metal homeostasis and auxin conjugate responsiveness. Genetics, 174(4), 1841–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remm, M. , Storm, C. E. , & Sonnhammer, E. L. (2001). Automatic clustering of orthologs and in‐paralogs from pairwise species comparisons. Journal of Molecular Biology, 314(5), 1041–1052. [DOI] [PubMed] [Google Scholar]
- Remy, E. , Cabrito, T. R. , Batista, R. A. , Teixeira, M. C. , Sá‐Correia, I. , & Duque, P. (2012). The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the arabidopsis thaliana root during phosphorus starvation. The New Phytologist, 195(2), 356–371. [DOI] [PubMed] [Google Scholar]
- Remy, E. , Cabrito, T. R. , Batista, R. A. , Teixeira, M. C. , Sá‐Correia, I. , & Duque, P. (2015). The major facilitator superfamily transporter ZIFL2 modulates cesium and potassium homeostasis in arabidopsis. Plant & Cell Physiology, 56(1), 148–162. [DOI] [PubMed] [Google Scholar]
- Ren, X.‐L. , Qi, G.‐N. , Feng, H.‐Q. , Zhao, S. , Zhao, S.‐S. , Wang, Y. I. , & Wei‐Hua, W. U. (2013). Calcineurin B‐like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in arabidopsis. The Plant Journal, 74(2), 258–266. [DOI] [PubMed] [Google Scholar]
- Ren, Y. , Liu, Y. , Chen, H. , Li, G. , Zhang, X. , & Zhao, J. (2012). Type 4 metallothionein genes are involved in regulating Zn ion accumulation in late embryo and in controlling early seedling growth in arabidopsis. Plant, Cell & Environment, 35(4), 770–789. 10.1111/j.1365-3040.2011.02450.x [DOI] [PubMed] [Google Scholar]
- Ren, Z.‐H. , Gao, J.‐P. , Li, L.‐G. , Cai, X.‐L. , Huang, W. , Chao, D.‐Y. , Zhu, M.‐Z. , Wang, Z.‐Y. , Luan, S. , & Lin, H.‐X. (2005). A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nature Genetics, 37(10), 1141–1146. 10.1038/ng1643 [DOI] [PubMed] [Google Scholar]
- Robinson, N. J. , Procter, C. M. , Connolly, E. L. , & Guerinot, M. L. (1999). A ferric‐chelate reductase for iron uptake from soils. Nature, 397(February), 694 10.1038/17800 [DOI] [PubMed] [Google Scholar]
- Rubio, F. , Nieves‐Cordones, M. , Alemán, F. , & Martínez, V. (2008). Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high‐affinity range of concentrations. Physiologia Plantarum, 134(4), 598–608. [DOI] [PubMed] [Google Scholar]
- Sancenón, V. , Puig, S. , Mateu‐Andrés, I. , Dorcey, E. , Thiele, D. J. , & Peñarrubia, L. (2004). The arabidopsis copper transporter COPT1 functions in root elongation and pollen development. The Journal of Biological Chemistry, 279(15), 15348–15355. [DOI] [PubMed] [Google Scholar]
- Sanjaya, P.‐Y. , Ruey‐Chih, S. U. , Ko, S.‐S. , Tong, C.‐G. , Yang, R.‐Y. , & Chan, M.‐T. (2008). Overexpression of arabidopsis thaliana tryptophan synthase beta 1 (AtTSB1) in arabidopsis and tomato confers tolerance to cadmium stress. Plant, Cell & Environment, 31(8), 1074–1085. [DOI] [PubMed] [Google Scholar]
- Sasaki, A. , Yamaji, N. , Mitani‐Ueno, N. , Kashino, M. , & Ma, J. F. (2015). A node‐localized transporter OsZIP3 Is responsible for the preferential distribution of Zn to developing tissues in rice. The Plant Journal, 84(2), 374–384. 10.1111/tpj.13005 [DOI] [PubMed] [Google Scholar]
- Sasaki, A. , Yamaji, N. , Yokosho, K. , & Ma, J. F. (2012). Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. The Plant Cell, 24(5), 2155–2167. 10.1105/tpc.112.096925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf, G. , Honsbein, A. , Meda, A. R. , Kirchner, S. , Wipf, D. , & von Wirén, N. (2006). AtIREG2 encodes a tonoplast transport protein involved in iron‐dependent nickel detoxification in arabidopsis thaliana roots. The Journal of Biological Chemistry, 281(35), 25532–25540. [DOI] [PubMed] [Google Scholar]
- Secco, D. , Baumann, A. , & Poirier, Y. (2010). Characterization of the rice PHO1 gene family reveals a key role for ospho1;2 in phosphate homeostasis and the evolution of a distinct clade in dicotyledons. Plant Physiology, 152(3), 1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selote, D. , Samira, R. , Matthiadis, A. , Gillikin, J. W. , & Long, T. A. (2015). Iron‐binding E3 ligase mediates iron response in plants by targeting basic helix‐loop‐helix transcription factors. Plant Physiology, 167(1), 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoura, T. , Sakashita, E. , Kobayashi, T. , Takahashi, M. , Aung, M. S. , Masuda, H. , Nakanishi, H. , & Nishizawa, N. K. (2017). The iron‐chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Molecular Biology, 95(4–5), 375–387. [DOI] [PubMed] [Google Scholar]
- Senovilla, M. , Castro‐Rodríguez, R. , Abreu, I. , Escudero, V. , Kryvoruchko, I. , Udvardi, M. K. , Imperial, J. , & González‐Guerrero, M. (2018). Medicago truncatula copper transporter 1 (MtCOPT1) delivers copper for symbiotic nitrogen fixation. The New Phytologist, 218(2), 696–709. [DOI] [PubMed] [Google Scholar]
- Shi, H. , Lee, B.‐H. , Shaw‐Jye, W. U. , & Zhu, J.‐K. (2003). Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in arabidopsis thaliana. Nature Biotechnology, 21(1), 81–85. [DOI] [PubMed] [Google Scholar]
- Shin, H. , Shin, H.‐S. , Dewbre, G. R. , & Harrison, M. J. (2004). Phosphate transport in arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low‐ and high‐phosphate environments. The Plant Journal, 39(4), 629–642. [DOI] [PubMed] [Google Scholar]
- Shin, L.‐J. , Lo, J.‐C. , & Yeh, K.‐C. (2012). Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiology, 159(3), 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.‐Y. , Choi, K. S. , Kim, D. Y. , Geisler, M. , Park, J. , Vincenzetti, V. , Schellenberg, M. , Kim, S. H. , Lim, Y. P. , Noh, E. W. , Lee, Y. , & Martinoia, E. (2010). Arabidopsis PCR2 Is a zinc exporter involved in both zinc extrusion and long‐distance zinc transport. The Plant Cell, 22(7), 2237–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.‐Y. , Yamaki, T. , Yamaji, N. , Ko, D. , Jung, K.‐H. , Fujii‐Kashino, M. , An, G. , Martinoia, E. , Lee, Y. , & Ma, J. F. (2014). A Rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proceedings of the National Academy of Sciences of the United States of America, 111(44), 15699–15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeger, T. , Gerlach, M. , Morimoto, R. I. , & Nunes, L. A. (2018). Large‐scale investigation of the reasons why potentially important genes are ignored. PLoS Biology, 16(9), e2006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S.‐K. , Chen, Y. I. , Che, J. , Konishi, N. , Tang, Z. , Miller, A. J. , Ma, J. F. , & Zhao, F.‐J. (2018). Decreasing arsenic accumulation in rice by overexpressing OsNIP1;1 and OsNIP3;3 through disrupting arsenite radial transport in roots. The New Phytologist, 219(2), 641–653. [DOI] [PubMed] [Google Scholar]
- Sunkar, R. , Kaplan, B. , Bouché, N. , Arazi, T. , Dolev, D. , Talke, I. N. , Maathuis, F. J. , Sanders, D. , Bouchez, D. , & Fromm, H. (2000). Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous arabidopsis CNGC1 gene confer Pb2+ tolerance. The Plant Journal, 24(4), 533–542. [DOI] [PubMed] [Google Scholar]
- Takahashi, R. , Ishimaru, Y. , Shimo, H. , Ogo, Y. , Senoura, T. , Nishizawa, N. K. , & Nakanishi, H. (2012). The OsHMA2 transporter is involved in root‐to‐shoot translocation of Zn and Cd in rice. Plant, Cell & Environment, 35(11), 1948–1957. [DOI] [PubMed] [Google Scholar]
- Takano, J. , Wada, M. , Ludewig, U. , Schaaf, G. , von Wirén, N. , & Fujiwara, T. (2006). The arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. The Plant Cell, 18(6), 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto, Y. , Tsunemitsu, Y. , Fujii‐Kashino, M. , Mitani‐Ueno, N. , Yamaji, N. , Ma, J. F. , Kato, S.‐I. , Iwasaki, K. , & Ueno, D. (2017). The tonoplast‐localized transporter MTP8.2 contributes to manganese detoxification in the shoots and roots of Oryza Sativa L. Plant & Cell Physiology, 58(9), 1573–1582. [DOI] [PubMed] [Google Scholar]
- Tanaka, M. , Wallace, I. S. , Takano, J. , Roberts, D. M. , & Fujiwara, T. (2008). NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in arabidopsis. The Plant Cell, 20(10), 2860–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, N. , Nishida, S. , Kamiya, T. , & Fujiwara, T. (2016). Large‐scale profiling of brown rice ionome in an ethyl methanesulphonate‐mutagenized hitomebore population and identification of high‐ and low‐cadmium lines. Plant and Soil, 407(1–2), 109–117. [Google Scholar]
- Tarantino, D. , Morandini, P. , Ramirez, L. , Soave, C. , & Murgia, I. (2011). Identification of an arabidopsis mitoferrinlike carrier protein involved in fe metabolism. Plant Physiology and Biochemistry, 49(5), 520–529. [DOI] [PubMed] [Google Scholar]
- Tejada‐Jiménez, M. , Castro‐Rodríguez, R. , Igor Kryvoruchko, M. , Lucas, M. , Udvardi, M. , Imperial, J. , & González‐Guerrero, M. (2015). Medicago truncatula natural resistance‐associated macrophage protein1 is required for iron uptake by rhizobia‐infected nodule cells. Plant Physiology, 168(1), 258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada‐Jiménez, M. , Gil‐Díez, P. , León‐Mediavilla, J. , Wen, J. , Mysore, K. S. , Imperial, J. , & González‐Guerrero, M. (2017). Medicago truncatula Molybdate Transporter Type 1 (MtMOT1.3) is a plasma membrane molybdenum transporter required for nitrogenase activity in root nodules under molybdenum deficiency. The New Phytologist, 216(4), 1223–1235. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium (2017). Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Research, 45(D1), D331–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, H. , Baxter, I. R. , Lahner, B. , Reinders, A. , Salt, D. E. , & Ward, J. M. (2010). Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. The Plant Cell, 22(12), 3963–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, D. , Sasaki, A. , Yamaji, N. , Miyaji, T. , Fujii, Y. , Takemoto, Y. , Moriyama, S. , Che, J. , Moriyama, Y. , Iwasaki, K. & Ma, J. F. (2015). A polarly localized transporter for efficient manganese uptake in rice. Nature Plants, 1(November), 15170. [DOI] [PubMed] [Google Scholar]
- Uraguchi, S. , Tanaka, N. , Hofmann, C. , Abiko, K. , Ohkama‐Ohtsu, N. , Weber, M. , Kamiya, T. , Sone, Y. , Nakamura, R. , Takanezawa, Y. , & Kiyono, M. , (2017). Phytochelatin synthase has contrasting effects on cadmium and arsenic accumulation in rice grains. Plant & Cell Physiology, 58(10), 1730–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitart, V. , Baxter, I. , Doerner, P. , & Harper, J. F. (2001). Evidence for a role in growth and salt resistance of a plasma membrane H+‐ATPase in the root endodermis: salt sensitive H+‐ATPase mutant. The Plant Journal, 27(3), 191–201. [DOI] [PubMed] [Google Scholar]
- Von Wiren, N. , Mori, S. , Marschner, H. , & Romheld, V. (1994). Iron inefficiency in maize mutant ys1 (Zea Mays L. Cv Yellow‐Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiology, 106(1), 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Ying, S. , Huang, H. , Li, K. , Ping, W. U. , & Shou, H. (2009). Involvement of OsSPX1 in phosphate homeostasis in rice. The Plant Journal, 57(5), 895–904. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Sun, J. , Miao, J. , Guo, J. , Shi, Z. , He, M. , Chen, Y. , Zhao, X. , Li, B. , Han, F. , & Tong, Y. (2013). A phosphate starvation response regulator Ta‐PHR1 is involved in phosphate signalling and increases grain yield in wheat. Annals of Botany, 111(6), 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. U. , Ying, Y. , Narsai, R. , Ye, L. , Zheng, L. , Tian, J. , Whelan, J. , & Shou, H. (2013). Identification of OsbHLH133 as a regulator of iron distribution between roots and shoots in Oryza Sativa . Plant, Cell & Environment, 36(1), 224–236. [DOI] [PubMed] [Google Scholar]
- Waters, B. M. , Chu, H.‐H. , Didonato, R. J. , Roberts, L. A. , Eisley, R. B. , Lahner, B. , Salt, D. E. , & Walker, E. L. (2006). Mutations in arabidopsis yellow stripe‐like1 and yellow stripe‐like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiology, 141(4), 1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild, M. , Davière, J.‐M. , Regnault, T. , Sakvarelidze‐Achard, L. , Carrera, E. , Diaz, I. L. , Cayrel, A. , Dubeaux, G. , Vert, G. , & Achard, P. (2016). Tissue‐specific regulation of gibberellin signaling fine‐tunes arabidopsis iron‐deficiency responses. Developmental Cell, 37(2), 190–200. [DOI] [PubMed] [Google Scholar]
- Wimalanathan, K. , Friedberg, I. , Andorf, C. M. , & Lawrence‐Dill, C. J. (2018). Maize GO annotation—methods, evaluation, and review (maize‐GAMER). Plant Direct, 2(4), e00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Li, H.‐D. , Chen, L.‐Q. , Wang, Y. I. , Liu, L.‐L. , He, L. , & Wei‐Hua, W. U. (2006). A protein kinase, interacting with two calcineurin B‐like proteins, regulates K+ transporter AKT1 in arabidopsis. Cell, 125(7), 1347–1360. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Shi, S. , Wang, L. , Tang, Z. , Lv, T. , Zhu, X. , Ding, X. , Wang, Y. , Zhao, F.‐J. , & Zhongchang, W. U. (2017). OsHAC4 Is critical for arsenate tolerance and regulates arsenic accumulation in rice. The New Phytologist, 215(3), 1090–1101. [DOI] [PubMed] [Google Scholar]
- Xu, W. , Dai, W. , Yan, H. , Li, S. , Shen, H. , Chen, Y. , Hua, X. U. , Sun, Y. , He, Z. , & Ma, M. I. (2015). Arabidopsis NIP3;1 plays an important role in arsenic uptake and root‐to‐shoot translocation under arsenite stress conditions. Molecular Plant, 8(5), 722–733. [DOI] [PubMed] [Google Scholar]
- Yamaji, N. , Takemoto, Y. , Miyaji, T. , Mitani‐Ueno, N. , Yoshida, K. T. , & Ma, J. F. (2017). Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature, 541(7635), 92–95. [DOI] [PubMed] [Google Scholar]
- Yan, J. , Chia, J.‐C. , Sheng, H. , Jung, H.‐I. , Tetiana‐Olena Zavodna, L. U. , Zhang, R. H. , Zavodna, T. O. , Zhang, L. , Huang, R. , Jiao, C. , Craft, E. J. , Fei, Z. and Kochian, L. V. (2017). Arabidopsis pollen fertility requires the transcription factors CITF1 and SPL7 that regulate copper delivery to anthers and jasmonic acid synthesis. The Plant Cell, 29(12), 3012–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J. , Wang, P. , Wang, P. , Yang, M. , Lian, X. , Tang, Z. , Huang, C.‐F. , Salt, D. E. , & Zhao, F. J. (2016). A loss‐of‐function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of japonica rice cultivars. Plant, Cell & Environment, 39(9), 1941–1954. [DOI] [PubMed] [Google Scholar]
- Yang, A. N. , Li, Y. , Yunyuan, X. U. , & Zhang, W.‐H. (2013). A receptor‐like protein RMC is involved in regulation of iron acquisition in rice. Journal of Experimental Botany, 64(16), 5009–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, A. N. , & Zhang, W.‐H. (2016). A small GTPase, OsRab6a, is involved in the regulation of iron homeostasis in rice. Plant & Cell Physiology, 57(6), 1271–1280. [DOI] [PubMed] [Google Scholar]
- Yuan, Y. , Huilan, W. U. , Wang, N. , Li, J. , Zhao, W. , Juan, D. U. , Wang, D. , & Ling, H.‐Q. (2008). FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in arabidopsis. Cell Research, 18(3), 385–397. [DOI] [PubMed] [Google Scholar]
- Zhai, Z. , Gayomba, S. R. , Jung, H.‐I. , Vimalakumari, N. K. , Piñeros, M. , Craft, E. , Rutzke, M. A. , Danku, J. , Lahner, B. , Punshon, T. , & Guerinot, M. L. (2014). OPT3 is a phloem‐specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in arabidopsis. The Plant Cell, 26(5), 2249–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Li, Y. , Yao, X. , Liang, G. , & Diqiu, Y. U. (2017). POSITIVE REGULATOR OF IRON HOMEOSTASIS1, OsPRI1, facilitates iron homeostasis. Plant Physiology, 175(1), 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Bin, H. U. , Li, W. , Che, R. , Deng, K. , Li, H. , Feiyan, Y. U. , Ling, H. , Li, Y. , & Chu, C. (2014). OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. The New Phytologist, 201(4), 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Cao, Y. , Wang, Z. , Wang, Z.‐Q. , Shi, J. , Liang, X. , Song, W. , Chen, Q. , Lai, J. , & Jiang, C. (2018). A retrotransposon in an HKT1 family sodium transporter causes variation of leaf na+ exclusion and salt tolerance in maize. The New Phytologist, 217(3), 1161–1176. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Chen, K. , Zhao, F.‐J. , Sun, C. , Jin, C. , Shi, Y. , Sun, Y. , Li, Y. , Yang, M. , Jing, X. , & Luo, J. (2018). OsATX1 interacts with heavy metal P1B‐Type ATPases and affects copper transport and distribution. Plant Physiology, 178(1), 329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. U. , Yong‐Han, X. U. , Yi, H.‐Y. , & Gong, J.‐M. (2012). Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. The Plant Journal: For Cell and Molecular Biology, 72(3), 400–410. [DOI] [PubMed] [Google Scholar]
- Zhao, M. , Ding, H. , Zhu, J.‐K. , Zhang, F. , & Li, W.‐X. (2011). Involvement of miR169 in the nitrogen‐starvation responses in arabidopsis. The New Phytologist, 190(4), 906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. Q. , Mitani, N. , Yamaji, N. , Shen, R. F. , & Ma, J. F. (2010). Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiology, 153(4), 1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L. , Yamaji, N. , Yokosho, K. , & Ma, J. F. (2012). YSL16 is a phloem‐localized transporter of the copper‐nicotianamine complex that is responsible for copper distribution in rice. The Plant Cell, 24(9), 3767–3782. 10.1105/tpc.112.103820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Jiao, F. , Zhongchang, W. U. , Li, Y. , Wang, X. , He, X. , Zhong, W. , & Ping, W. U. (2008). OsPHR2 is involved in phosphate‐starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiology, 146(4), 1673–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Lau, K. , Puschmann, R. , Harmel, R. K. , Zhang, Y. , Pries, V. , Gaugler, P. , Broger, L. , Dutta, A. K. , Jessen, H. J. , Schaaf, G. , Fernie, A. R. , Hothorn, L. A. , Fiedler, D. , & Hothorn, M. (2019). Two bifunctional inositol pyrophosphate kinases/phosphatases control plant phosphate homeostasis. eLife, 8, e43582 10.7554/eLife.43582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimeri, A. M. , Dhankher, O. P. , McCaig, B. , & Meagher, R. B. (2005). The plant MT1 metallothioneins are stabilized by binding cadmiums and are required for cadmium tolerance and accumulation. Plant Molecular Biology, 58(6), 839–855. 10.1007/s11103-005-8268-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


