Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity reaction (HR) mediated by antigens to Aspergillus fumigatus. It is estimated that 2–15% of patients with cystic fibrosis (CF) and between 1% and 5% of asthmatics develop ABPA, affecting approximately 4.8 million people worldwide. The goals of treatment are controlling inflammation, reducing the number of exacerbations and limiting the progression of lung damage. Systemic steroids are therefore used as the mainstay therapy, along with antifungal medications. However, many patients do not respond or develop side effects to treatment. In this scenario, biological drugs such as Omalizumab, Mepolizumab, Benralizumab and Dupilumab have been implemented in clinical practice, even though there is a lack of scientific evidence to support their use. We performed a literature review of the studies carried out which analyzed biologics for the management of ABPA in adult populations with asthma and CF. To our knowledge this is the first literature review that included all biologics. We included a total of 32 studies, all but one were descriptive studies, and the vast majority evaluated the use of Omalizumab. Biologics appeared to have more benefit for patients with ABPA and asthma than CF, specifically at decreasing the frequency of acute exacerbations and by having a steroid-sparing effect. Although a decrease in serum IgE level is considered a measure of therapy success, values may not decline as expected in the context of a significant clinical improvement, highlighting the importance of measuring patient-oriented outcomes. As evidence comes mainly from case series and case reports, randomized controlled trials are needed to evaluate further the safety and efficacy of biologics in ABPA.
The reviews of this paper are available via the supplemental material section.
Keywords: allergic bronchopulmonary aspergillosis, asthma, biologics, cystic fibrosis, monoclonal antibodies
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity reaction (HR) mediated by antigens to Aspergillus fumigatus, which colonize the airways of susceptible subjects, such as asthmatics and patients with cystic fibrosis (CF).1 It is estimated that 2–15% of patients with CF and between 1% and 5% of asthmatics develop ABPA, affecting approximately 4.8 million people worldwide.2,3 In 1952, Dr. Hinson described the first case of ABPA in England. Later on, in 1968, the first formal registry was carried out in the United States and it was not until 1977 that Rosenberg and Patterson established the first diagnostic criteria for this disease.2,4
A high level of suspicion of ABPA is crucial among patients with asthma and CF, especially in patients who present with frequent acute exacerbations or who fail to respond adequately to standard management.5 In 1977, Rosenberg and Patterson6 proposed diagnostic criteria for ABPA, including both primary and secondary criteria, in which any combination of five to eight criteria would make the diagnosis (Table 1).2,6 In 2013, the International Society for Human and Animal Mycology (ISHAM) proposed different diagnostic criteria, applicable to both asthmatic and CF patients, in which some criteria are mandatory such as total serum IgE levels >1000 UI/mL (Table 2),7 which exclude some patients, but both criteria are validated and can be used.2,5 Based on these criteria, patients can be classified into three subgroups: seropositive ABPA, central bronchiectasis ABPA, and severe asthma with fungal sensitization.5
Table 1.
Diagnostic criteria for ABPA by Rosenberg and Patterson (1977).6
| Primary |
| Episodic bronchial obstruction (asthma) |
| Peripheral blood eosinophilia |
| Immediate skin reactivity to Aspergillus antigen |
| Precipitating antibodies against Aspergillus antigen |
| Elevated serum immunoglobulin E concentrations |
| History of pulmonary infiltrates (transient or fixed) |
| Central bronchiectasis |
| Secondary |
| Aspergillus fumigatus in sputum (by repeated culture or microscopic examination) |
| History of expectoration of brown plugs or flecks |
| Arthus reactivity (late skin reactivity) to Aspergillus antigen |
ABPA, allergic bronchopulmonary aspergillosis.
Table 2.
Diagnostic criteria for ABPA by the international society for human and animal mycology (ISHAM) (2013).7
| Baseline conditions: asthma and/or cystic fibrosis. | |
|---|---|
| Mandatory criteria: | |
| 1. IgE specific to A. fumigatus (OR) | >0.35 kU/L |
| 2. A positive skin test against A. fumigatus (AND) | |
| 3. Total serum IgE | >1000 UI/mL |
| Other criteria (at least 2 must be present) | |
| 1. IgG against A. fumigatus (OR) | >27 mg/L |
| 2. Radiological changes typical of ABPA (OR) • Central and proximal cylindrical bronchiectasis • Alterations predominantly in the upper lobe • Nodules • Atelectasis • Air trapping |
|
| 3. Total eosinophil count | >500 cells/UL |
ABPA, allergic bronchopulmonary aspergillosis.
The objectives of ABPA management are controlling inflammation, reducing the number of exacerbations and limiting the progression of lung damage. Systemic steroids are therefore employed as the mainstay therapy, leading to numerous side effects when used for prolonged periods of time, limiting their use.4,8 Antifungals are also useful for patients who require high doses of steroids or present an acute exacerbation of ABPA; they aim to control and decrease the antigenic stimulus generated by the fungus, thus reducing the inflammatory response.4,5
As not all patients respond successfully to standard treatment, biological drugs such as Omalizumab, Mepolizumab, Dupilumab and Benralizumab have been used in the last decade, owing to the inhibition of pathways that have a fundamental role in the activation of inflammatory mediators and cells such as eosinophils, responsible for the immune response observed in this condition.4–8 Biological drugs are Food and Drug Administration approved for the management of severe asthma and are considered off-label for the therapy of ABPA. In addition, it should be mentioned that biologics are far more expensive than the standard of care. Furthermore, data on this subject are scarce and come mainly from observational studies, so the question of its efficacy in the treatment of ABPA persists.
Objective
The objective of this literature review is to collect and analyze all published data to date on asthmatics and CF patients with ABPA, on whom biological medications have been used. We focused mainly on the efficacy of the treatment, understood as its ability to reduce the frequency of pulmonary exacerbations/asthma control test (ACT), total IgE level, dosing of concurrent medications and the absence of pulmonary opacities in chest imaging.
Methods
Types of studies
We included in this review randomized controlled trials (RCTs), observational studies, case series, case reports, letters to the editors and conference abstracts. The latter were only included when they provided sufficient information in the results section.
Types of participants
Participants were both men and women, 19 years old or older, with a history of asthma and/or cystic fibrosis and a confirmed diagnosis of ABPA using the diagnostic criteria described either by Rosenberg and Patterson or the ISHAM (see Tables 1 and 2). All patients had failed to respond adequately to other treatments, such as steroids and antifungal therapies, persisting with symptoms and frequent exacerbations. Also, baseline chest X-rays and computerized tomography (CT) scans reported consistently the presence of multiple and/or bilateral bronchiectasis, atelectasis, bronchial wall thickening, ill-defined opacities and mucoid impaction.
Types of interventions
In all studies patients received one monoclonal antibody (Omalizumab, Mepolizumab, Dupilumab, Benralizumab) alone or in conjunction with systemic/inhaled steroids and antifungal treatment. We found only one randomized placebo-controlled trial, the rest being descriptive studies.
Search methods for identification of studies
We performed an electronic search using PUBMED and EMBASE in May 2020 using both ‘Mesh’ and ‘Emtree’ terms, respectively, as well as free text. The following terms were used: allergic bronchopulmonary aspergillosis, monoclonal antibodies, Omalizumab, Dupilumab, Mepolizumab, Benralizumab, asthma, cystic fibrosis. The results were limited by age (using the ⩾ 19 years old filter). No other filters were used. We selected studies in English and Spanish. References were manually searched for additional relevant studies.
Data collection and analysis
Selection of studies
Studies were screened by two independent investigators (ICE and SS), who analyzed the titles and abstracts for potentially relevant studies. Then each study was assessed against the preset inclusion and exclusion criteria.
Data extraction and management
Two investigators (ICE and SS) extracted the data from all studies using a standardized data collection form. When disagreements were present, they consulted with each other and came to a consensus. For each study, the trial design, characteristics of participants, types of interventions and outcomes were assessed. Specifically, for the two latter, we divided the data into pre-treatment and post-treatment clinical variables. For pre-treatment variables we extracted data for blood eosinophil count, total IgE, forced expiratory volume in 1 s (FEV1), frequency of acute exacerbations, antifungal treatment and chest imaging findings. Post-treatment clinical variables were the same as the pre-clinical variables, plus the status of systemic steroids used. Due to the descriptive nature of most of the included studies and their inherent high risk for bias, we did not aim to perform any statistical analysis on the results. In addition, we did not contact authors to clarify study results further or acquire the original databases. However, we did use the supplemental appendices, when provided, to complement the results of our review.
Results
Description of studies
Results of the search
A PubMed and EMBASE search was performed in May 2020, which identified a total of 365 records. After filtering by age, a total of 141 records was retrieved. After reviewing the selected studies, 81 records were removed for being duplicates. 60 articles were screened in total, of which 57 were fully assessed. Finally, 32 studies were included in the literature review. Figure 1 depicts the flow diagram.
Figure 1.
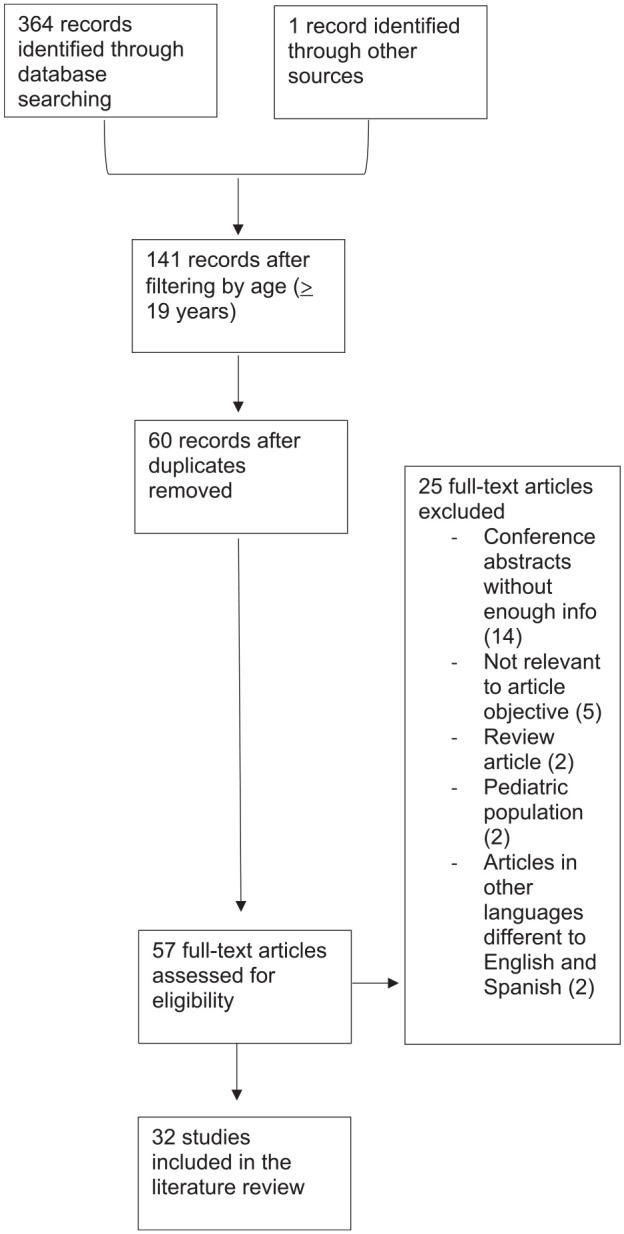
Electronic search flow diagram.
Included studies
Studies in asthmatic population
Demographic information
The studies included a total of 152 patients, with a median age of 50.25 years (range 19–79). Gender distribution was 1:1 (F:M). Only 7 of the 30 studies reported time from when ABPA was diagnosed until the biologic was started, including information on 29 patients, which ranged from 0 to 18 years.9–15 Regarding systemic steroids, 80% of patients throughout the studies were on long-term therapy at the initiation of the biologic. In addition, 48% were receiving or had received antifungal therapy; 106 patients (69.7%) had baseline eosinophil counts >500 cells/µL.10,12,13,16–33 55 patients (36%) had baseline IgE serum values >1000 IU/mL.10–12,14,15,17–19,21,23–28,30–32,34,35
Omalizumab
We found a total of 17 studies which included 104 patients.9–11,14–22,34–38 Seven were case series, defined as ⩾three patients, nine were case reports and one was a randomized double-blind cross-over study; 89 patients (85.5%) were on long-term systemic steroids at the time of initiation of Omalizumab, 14 patients were not receiving steroids (9.2%) and in one case it was not reported. Follow-up ranged from 3 months to 3 years. No studies reported post-treatment imaging findings (Table 3).
Table 3.
Treatment with Omalizumab in patients with ABPA and asthma.
| Pre-treatment clinical variables | Post-treatment clinical variables | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Type of study | Sample | Gender | Age | Blood Eosinophils (Absolute count (cells/µL) or percentage) | Total IgE (IU/mL) | FEV1 (%predicted or L) | Frequency of acute exacerbations | Antifungal treatment | Follow-up time | Total eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (% predicted or L) | Frequency of acute exacerbations | Systemic steroids | Antifungal treatment |
| Koutsokera et al.36 | Case series | 11 | 5M6F | 27.8 (21.8–37.7) | 430 cells/µL (180–560) | 889 (715.5–2991.5) | 39.5% (33–59) | NI | NI | 1 yr | NI | NI | NI | NI | No change | NI |
| Unal16 | Case series | 15 | 9M6F | 48.26 (SD + –9.92a | 692 cells/µL (SD + –346)b | 1380 (647–3339)b | 42.51% (SD + –12.94)a | NI | Itraconazole | 3 yr | NI | NI | 54.11% (SD + –17.18)a | Reduced | Suspended | NI |
| Cunha et al.9 | Case report | 1 | F | 53 | NI | NI | 24% | NI | NI | 1 yr | 108 | 477 | 35.6% | Reduced | Suspended | NI |
| Aguiar et al.10 | Case report | 1 | M | 45 | 570 cells/µL | 2674 | 58% | NI | No | 6 mo 12 mo 18 mo | 200 160 90 | 1683 1600 1950 | 68% 78% | Reduced | Reduced Suspended | N.A. |
| Homma et al.17 | Case report | 1 | M | 51 | 1358 cells/µL | 1500 | 58% | NI | Itraconazole | 4 mo 12 mo | 988 964 | 972 609 | 55.9% 54.7% | Reduced | Reduced by 50% | Discontinued (toxicity) |
| Aydin et al.34 | Case series | 14 | 7M7F | 44.2 (SD + –13.01) | 7.35% (SD + –2.15) | 1056.93 (SD + –555.62) | 59% (SD + –19.40) | 2.7 (SD+ – 1.5) | Itraconazole | 1 yr | NI | 769 (456–2030)bc to 1616 (1348–1808)bd | 71% | Reduced | Reduced | NI |
| Evans et al.15 | Case report | 1 | F | 32 | NI | 22,893 | NI | Itraconazole | 1 yr | NI | NI | 60% | Reduced | Suspended | NI | |
| Voskamp et al.18 | Randomized, double blinded, cross over trial | 13 | 4M9F | 59 | 500 cells/µL | 2314 (376–7860) | 73.2% (47–136) | NI | Itraconazole | 4 mo | NI | NI | 72% (SD + –22.5) | Reduced | NI | NI |
| Collins et al.11 | Case series | 4 | 2M2F | 50.5 | 480 cells/µL | 1730 (565–4362) | 2.28 L (1.3–3.28) | NI | NI | 12 mo | 220 | 914 (466–1638) | 2.24 L | Reduced | Reduced by 50% | NI |
| Sastre et al.18 | Case report | 1 | F | 32 | 1100 cells/µL | 3090 | 60% | NI | Itraconazole | 3 mo | NI | NI | 72.6% | Reduced | Reduced by 66% | NI |
| Tillie-Leblond19 | Case series | 16 | 8M8F | 56 (29–76) | NI | 582 (131–3766) | 5.3 L (3.3–1.1) | 10 subjects: > 3/yr | 75% Itraconazole | 1 yr | NI | NI | 5.5 L (3.6–1.2) | 2 subjects: > 3/yr | Reduced (0 mg/day (0–10 mg)b | NI |
| Pérez-de- Llano et al.20 | Case series | 18 | 5M13F | 49 (SD + –17)a | 610 cells/µL (317–1015)b | 698 (478–977)b | 1.36 L (0.95–2.2)b | 1/year (1–4)b | 55% Itraconazole | 9 mo | NI | NI | 1.85 L (1.27–2.29)b | None | 66% suspended | 16% Itraconazole |
| Lin et al.21 | Case report | 2 | M | 39 | NI | 2462 | 56% | NI | Itraconazole | 3 yr | NI | 789 | 56% | 6 exacerbations (4 yr) | Suspended | Suspended |
| M | 51 | 363 cells/µL | 586 | 40% | NI | Voriconazole | 1 yr | NI | 877 | NI | None | Suspended | Suspended | |||
| Kelso22 | Case report | 1 | F | 79 | NI | 2119 | 44% | NI | Voriconazole | 5 mo | NI | 590 | 44% | Reduced | Reduced by 75% | No |
| Bobolea et al.21 | Case series | 3 | M | 44 | 640 cells/µL | 1000 | 43–62% | 3/yra | Itraconazole | 3 yr | 400 | 400 | 64% | Reduced | Reduced | NI |
| F | 50 | 200 cells/µL | 500 | 60–70% | NI | Itraconazole | 3 yr | 100 | 582 | 80% | Reduced | Suspended | NI | |||
| M | 34 | 100 cells/µL | 5000 | 42–68% | NI | NI | 3 yr | 200 | 1132 | 90% | Reduced | Suspended | NI | |||
| Wolf and Johnson24 | Case report | 1 | M | 77 | NI | 4163 | 61% | NI | Voriconazole | 10 mo | NI | 1089 | NI | No change | Reduced by 87% | NI |
| Quintás Vázquez et al.22 | Case report | 1 | M | 68 | 2200 cells/µL | 327 | 36% | > 3/yra | NI | 6 mo | NI | NI | 53% | Reduced | Suspended | NI |
Mean (SD/range).
Median (range).
Complete response.
Partial response.
ABPA, allergic bronchopulmonary aspergillosis; IU, international units; L, liters; mL, milliliters; mo, months; NI, no information; uL, microliters; yr, years.
All but one study reported baseline FEV1 values (103 patients) and all except two studies reported FEV1 values after treatment (102 patients). At baseline the median for FEV1 (% predicted) was 58% (range 24.36–76.2),9–11,14–22,34,36–38 and after 3 months to 3 years of follow-up FEV1 had a median of 62% of the predicted value (range 35.6–90).9,10,14,16–19,21,22,34,36,38 Three studies reported the value in liters at baseline and after treatment with a median of 2.28 L (range 1.36–5.3) pre-treatment and a median of 2.4 L (range 1.5–5.5) post-treatment.11,20,37
Baseline IgE was reported in all but one study (103 patients), with a median of 1500 IU/mL (range 327–22,893).9–11,14,16,17–20,31,34,35,37,38 After treatment, 12 studies reported IgE, including values for 28 patients, with a median of 789 IU/mL (range 400–1950) after 5 months to 3 years of treatment.9–11,14,15,17,21,34,38 Furthermore, baseline and post-treatment values were reported in 27 of 104 patients of whom 11 patients achieved a reduction greater than 35% in total IgE values (range 40–77.4% reduction) from 5 months to 3 years of treatment.10,11,14,15,17,21,34,38
We found 12 studies with baseline eosinophil count, including values for 83 patients (79.8%), with a median of 570 cells/µL (range 100–2200).10,11,16–22,34,36,38 After treatment, the eosinophil count was reported for 10 patients (9.6%), with a median of 200 cells/µL (range 100–964); 85.7% of these patients had eosinophil counts below 500 cells/µL after treatment.9–11,17,21
Of the 89 patients who were taking steroids before treatment with Omalizumab, 12 patients had no change in systemic steroid use after 1 year (13.4%),34,35 14 patients (15.7%) with follow-up periods ranging from 3 months to 1 year had a reduction in the dosage,11,14,15,17,19,21 and systemic steroids were reported as suspended in 45 patients (50.5%)9,10,16,20–22,34,35,37,38 after 6 months to 3 years of follow-up. One study did not report systemic steroid use16 and there was no precise information on the remaining 19 patients.
At baseline 12 studies reported patients were taking either Itraconazole or Voriconazole,14–21,34,35,37,38 for a total of 73 patients (70%). One study reported the patient was not on antifungal treatment due to hepatotoxicity.10 The four remaining studies, corresponding to 27 patients (26%) did not report on antifungal treatment at baseline. After treatment, it was reported to be suspended in three patients due to toxicity (2.9%).17,38 Furthermore, in the study by Pérez-de-Llano, antifungal treatment with Itraconazole was given to 55% of the subjects at baseline in contrast to 16% of the subjects who were receiving it at 9 months of follow-up.20 There was no additional information on the rest of the patients post-treatment.
Most studies did not report the exact frequency of acute exacerbations prior to or after treatment. Five studies reported the annual rate of exacerbations at baseline, including a total of 50 patients (48%) with a median of 2.7 exacerbations/year (range 1 to >3/year).20–22,34,37 After treatment with Omalizumab, 19 patients (18.2%) had no exacerbations at 9 months to 1 year of treatment,20,38 70 patients (67.3%) had a reduced frequency of exacerbations after 3 months to 3 years of treatment,9–11,14,16–19,21,22,34,35,37 and no change was reported in four patients (3.8%).15,37,38 The remaining 11 patients had no information on this topic.
Mepolizumab
A total of nine studies were included,12,13,23–29 of which seven were case reports and two were case series, including a total of 32 patients; 84.3% of the subjects had been on prior treatment with Omalizumab. Follow-up time was between 2 and 14 months, being superior to 6 months in 71.8% of cases (Table 4).
Table 4.
Treatment with mepolizumab in patients with ABPA and asthma.
| Pre-treatment clinical variables | Post-treatment clinical variables | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Type of study | Sample | Gender | Age | Previous biologic treatment | Absolute eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted or L) | Frequency of acute exacerbations or asthma control test | Antifungal treatment | Follow-up time | Total eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted or L) | Frequency of acute exacerbations or Asthma control test | Systemic steroids | Antifungal treatment |
| Soeda et al.23 | Case report | 2 | F | 54 | No | 1365 | 2145 | 2.39 L | 21 | No | 6 mo | 73 | No change | 2.48 L | 25 | N.A. | No |
| F | 61 | No | 1856 | 162 | NI | 24 | No | 2 mo | 32 | No change | NI | 24 | N.A. | No | |||
| Hirota et al.24 | Case report | 1 | F | 56 | Omalizumab (94 wk) | 1500 | 2421 | 1.42 L (62%) | NI | Itraconazole | 14 mo | 0 | 362 | No change | Improved | Discontinued | NI |
| Tsubouch et al.28 | Case report | 1 | F | 60 | No | 560 | 8327 | 1.59 l (79.3%) | NI | Itraconazole | 5 mo | 20 | 2025 | 1.49 L (80%) | Improved | Dose decrease - 5 mg/day | Itraconazole 200 mg/day |
| Oda, et al.12 | Case report | 1 | M | 33 | No | 6370 | 1505 | 2.36 L | 5 | Itraconazole - 8 mo | 9 mo | 64 (2 mo) | NI | 3.16 L (71%) (6 mo) | 25 (2 mo) | Dose decrease - 2.5 mg/day | NI |
| Terashima et al.26 | Case report | 1 | F | 64 | No | 3017 | 3400 | 1.01 L | 18 | Itraconazole - 3 yr | 2 mo | 174 | No change | 1.28 L | 24 | Discontinued | NI |
| Patel et al.13 | Conference abstract - case series | 4 | NI | NI | Omalizumab | 725 (360–1080)b | 997 (134–1730)b | 52% (26–72%)b | NI | NI | NI | NI | NI | NI | Improved | Discontinued | NI |
| Kubena et al.27 | Case report | 1 | F | 58 | Omalizumab | 1310 | 2242 | NI | 3–4/Yearc | Itraconazole | NI | 100 | 720 | Improved | Improved | Dose decrease - 2.5 mg/day | NI |
| Altman et al.28 | Case report | 1 | F | 58 | Omalizumab | 1100 | 1730 | 0.74 L | NI | Itraconazole - 3 mo Voriconazole - 4 mo | 5 mo | 0 | 298 | 0.66 L | Improved | Discontinued | No |
| Florence et al.29 | Case series | 20 | M9:F11 | 63a | Omalizumab | 660 (438–980)b | NI | 1.50 L (1.08–2.11)b | 3/year (2–4.5)cb/10 (8–13)bd | Itraconazole - 1 Patient | 6 mo | 54 (25–115)b | NI | 1.84 L (1.14–2.37)b | 0/year (0–1)cb/19 (10–21)bd | Dose decrease and discotinued | NI |
Mean (SD/range).
Median (range).
Frequency of acute exacerbations.
Asthma control test.
ABPA, allergic bronchopulmonary aspergillosis.
Baseline FEV1 values were reported for eight studies (30 patients).12,13,23–26,28,29 In seven studies12,23–25,28,29 including 26 patients FEV 1 was reported in liters, with a median of 1.50 L (range 0.74–2.39). In the remaining study FEV1 was reported as the percentage predicted with a median of 52% (range 26–72).13 Following treatment, FEV1 values were reported for 25 patients (83.3%), with a median of 1.66 L (range 0.66–3.16).12,23,25,26,28,29
Baseline IgE levels were reported for 12 patients,12,13,23–28 with a median of 2145 IU/mL (range 134–8327 IU/ml). Four studies including four patients described post-treatment levels, with a median of 720 IU/mL (range 298–3400), reaching a reduction of 66.5% compared to baseline with a follow-up period between 5 and 14 months.24,25,27,28 None of the cases presented a new rise in IgE levels.
Baseline eosinophil count was reported for the entire population with a median of 1337 cells/µL (range 360–6370).12,13,23–29 After treatment, eosinophil count was reported for 28 patients (87.5%),12,23–29 with a median of 54 cells/µL (range 0–174 cells/µL). All of these patients had eosinophil counts below 500 cells/µL after treatment.12,23–29
The use of systemic steroids was reported in eight studies,12,13,24–29 including a total of 30 patients (93.7% of the population); three patients (10%) had a reduction to a dose between 2.5 and 5 mg/daily with follow-up periods ranging from 5 to 9 months12,13,25–27 and the remaining 27 subjects (90%) had them suspended after 2–14 months of treatment.13,24,26,28,29
Only the studies by Kubena et al. and Florence et al., including a total of 21 patients (65.6% of the population) reported frequency of baseline annual acute exacerbations, being three exacerbations/year (range 2–4.5).27,29 Post-treatment, the study by Florence et al.29 reported that the median exacerbation rate for the 20 patients included in the study was 0 exacerbations/year (range 0–1). The remaining studies including a total eight patients (25% of the population)13,24,25,27,28 reported an improvement in the frequency of exacerbations. Regarding the ACT score, it was reported in three studies including a total of four patients.12,23,26 In particular, the study by Soeda et al. reported two patients with baseline values of 21 and 24, who achieved a score above 20 points after treatment (25 and 24, respectively).23 Similarly, the case reports by Oda et al. and Terashima et al. described two patients with a pre-treatment score of 5 and 18, respectively, with post-treatment scores of 25 and 24.12,26
Finally, seven patients (21.8%) had antifungal treatment prior to the initiation of Mepolizumab.12,24–29 The case report by Altman et al. reported that the patient used both Itraconazole and Voriconazole, with withdrawal of the treatment after initiation of Mepolizumab.28 On the other hand, for the patient included in the study by Tsubouchi et al., it was not possible to suspend antifungal treatment.25 The remaining studies including a total of six patients (18.75% of the population) did not describe antifungal use post-treatment.12,13,24,26,27,29
Dupilumab
Two studies were included,30,31 of which the study by Ramonell et al. was a case series and the other one, by Corren et al., was a post hoc analysis made to an RCT (Liberty Asthma Quest),39 including a total of 21 patients. The case series included three patients,30 with a gender distribution of 2:1 (female: male), median age of 51 years (range 33–60). One had been on prior treatment with Mepolizumab and other subject had been on both Omalizumab and Mepolizumab, without a significant improvement30 (Table 5).
Table 5.
Treatment with dupilumab in patients with ABPA and asthma.
| Pre-treatment clinical variables | Post-treatment clinical variables | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Type of study | Sample | Gender | Age | Previous biologic treatment | Absolute eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted or L) | Frequency of acute exacerbations or asthma control test | Antifungal treatment | Follow-up time | Total eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted) | Frequency of acute exacerbations or asthma control test | Systemic steroids | Antifungal treatment | Adverse effects |
| Ramonell et al.30 | Case series | 3 | F | 60 | Omalizumab and Mepolizumab | 1620 | 561 | 1.51 L (58%) | NI | NI | 6 months | 1090 (4 mo) | 380 (3 mo) | 2.18 L (99%) | Improved | Discontinued | NI | hypereosinophilia |
| F | 51 | Mepolizumab | 1040 | > 2000 | 2.75 L (95%) | NI | Itraconazole | 3 months | 160 | 384 | 2.82 L (97%) | Improved | Discontinued | NI | No | |||
| M | 33 | No | 1750 | 11.290 | 1.97 L (37%) | NI | Voriconazole | 3 months | 690 | 1637 | 2.33 L (56%) | Asthma exacerbation after onset dupilumab | NI | NI | Hypereosinophilia | |||
| Corren et al.31 | Post Hoc analysis of an RCTf (35) | 18 | NI | NI | NI | NI | 3383 (1480–5000b | 2.00 L (68%)a | 2.28 (1.53)a,d | NI | 13 months | NI | 691,5 (323–2617)b | 24 w 2.26 52 w 2.33c | Improved | NI | NI | Injection-site reaction |
Mean (SD/range).
Median (range).
Mean (95% CI).
Frequency of acute exacerbations.
Asthma control test.
Liberty Asthma Quest study.
ABPA, allergic bronchopulmonary aspergillosis.
The study by Ramonell et al.30 reported baseline FEV1 values with a median of 1.98 L (range 1.51–2.75), and after 3–6 months of treatment median FEV1 was 2.33 L (range 2.18–2.82). The analysis by Corren et al. reported baseline FEV1 values with a mean of 2.00 L. After treatment, FEV1 was 2.37 L and 2.51 L at weeks 24 and 52, respectively.31
Baseline IgE levels were reported for the 21 patients, with a median of 2691 UI/mL (range 561–11290).30,31 Post-treatment, Ramonell et al. described a decrease in IgE values, reaching a median of 384 IU/mL (range 380–1637) after follow-up periods from 3 to 4 months.30 The study by Corren et al. reported that IgE levels decreased, obtaining values with a median of 691.5 UI/mL (range 323–2617), reaching a reduction of >35% compared to baseline, in patients followed for 13 months.31 None of the patients who were followed for more than 6 months suffered a new rise in IgE levels.30,31
Baseline eosinophil count was reported in both studies with a median of 1330 cells/µL (range 500–1750).30,31 Only the study by Ramonell et al. described the eosinophil count post-treatment, showing a reduction after 3 months of treatment, with a median of 690 cells/µL (range 160–1090).30
The use of systemic steroids was reported in the study by Ramonell et al., in which three patients had a discontinuation of systemic steroids after 3 and 6 months of treatment with Dupilumab.30 The status of systemic steroid use was not reported for the remaining 19 patients (90.4% of the population).30,31
Regarding the frequency of acute exacerbations, the study by Corren et al. described a mean annual exacerbation rate pre-treatment of 2.28 exacerbations/year (SD 1.53).31 The post-treatment exacerbation rate was not reported but both authors describe a reduction in the frequency of acute pulmonary exacerbations, corresponding to 95% of the population,30,31 as only one patient in the study by Ramonel et al. was said to have an acute asthma exacerbation secondary to early hypereosinophilia after to the initiation of Dupilumab.30
In relation to adverse effects, three patients were described to suffer adverse effects to Dupilumab, of which two had an early hypereosinophilic reaction, for which concomitant steroids were administered, without the need to stop treatment.30,31
Benralizumab
For Benralizumab, only two case reports were found,32,33 including two women of 40 and 60 years of age, who had not been on previous treatment with another biologic. Both eosinophils and IgE decreased in both patients after treatment. Follow-up time was only described in the case report by Wong et al., which was of 3 months (Table 6).32
Table 6.
Treatment with Benralizumab in patients with ABPA and asthma.
| Pre-treatment clinical variables | Post-treatment clinical variables | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Type of study | Sample | Gender | Age | Previous biologic treatment | Absolute eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted or L) | Frequency of acute exacerbations | Antifungal treatment | Follow-up time | Total eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted) | Frequency of acute exacerbations | Systemic steroids | Antifungal treatment |
| Wong and Ahluwalia38 | Case report | 1 | F | 40 | No | 2500 | 3950 | NI | Decreased | Itraconazole (4 months) | NI | Decreased | Decreased | NI | Improved | Discontinued | NI |
| Soeda et al.33 | Case report | 1 | F | 60 | NI | 972 | 537 | Decreased | Decreased | No | 3 months | 0 | 415 | NI | Improved | NI | NI |
Mean (SD/range).
Median (range).
Frequency of acute exacerbations.
Asthma control test.
ABPA, allergic bronchopulmonary aspergillosis.
After treatment with Benralizumab, a clinical improvement was described as well as a decrease in the frequency of exacerbations, although the exact rate was not reported.32,33 The patient in the study by Wong et al. was able to discontinue systemic steroids after 3 months.32
Studies in cystic fibrosis population
Omalizumab
We could only find two studies including adults with cystic fibrosis,40,41 in whom omalizumab was initiated for treatment of ABPA. Both studies were case series, including a total of 15 patients between adults and children.40,41 We only extracted data for patients 19 years or older, for a total of nine patients. Gender distribution was 6:3 (male: female) and median age was 24 years old (range 19–41) (Table 7).
Table 7.
Treatment with Omalizumab in patients with ABPA and cystic fibrosis.
| Pre-treatment clinical variables | Post-treatment clinical variables | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Type of study | Sample | Gender | Age | Absolute eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted) | Frequency of acute exacerbations | Systemic steroids | Antifungal treatment | Follow-up time | Total eosinophil count (cell/µL) | Total IgE (IU/mL) | FEV1 (%predicted) | Frequency of acute exacerbations | Systemic steroids | Antifungal treatment |
| Ashkenazi et al.40 | Case series | 6 | M | 32 | NI | 1570 | 69% | NI | Pulse | Yes | 26 m | NI | 387 | 54% | 2 | No change | NI |
| M | 19 | NI | 7560 | 83% | NI | Contraindication | Yes | 8 m | NI | 5240 | 71% | 4 | NI | NI | |||
| F | 27 | NI | 3280 | 61% | NI | Contraindication | Yes | 15 m | NI | 3900 | 62% | 0 | NI | NI | |||
| M | 24 | NI | 472 | 72% | NI | 0.1 mg/kg | Yes | 12 m | NI | 274 | 74% | 3 | No change | NI | |||
| M | 21 | NI | 667 | 39% | NI | 0.25 mg/kg | Yes | 18 m | NI | 172 | 29% | 17 | No change | NI | |||
| F | 41 | NI | 715 | 36% | NI | Contraindication | Yes | 27 m | NI | 488 | 33% | 6 | NI | NI | |||
| Lehmann et al.41 | Case series | 3 | M | 23 | NI | NI | 21% | >1/year | Prednisone | Itraconazole | 13 m | NI | NI | 31% | No change | Reduced | NI |
| F | 33 | NI | NI | 72% | >1/year | Prednisone | Itraconazole | 7 m | NI | NI | 88% | None | Reduced | NI | |||
| M | 23 | NI | NI | 38% | >1/year | Prednisone | Itraconazole | 9 M | NI | NI | 63% | None | Reduced by 50% | NI | |||
ABPA, allergic bronchopulmonary aspergillosis.
Only the study by Ashkenazi et al. reported total IgE levels.40 Baseline IgE had a median of 1142.5 IU/mL (range 472–7560). After 8–27 months of treatment, IgE had a median of 437.5 IU/mL (range 172–5240).
Both studies reported baseline FEV1 values (% predicted). Median FEV1 was 61% (range 21–83). After 7–26 months of treatment, median FEV1 was 62% of the predicted value (range 29–88).
In addition, the study by Lehmann et al. reported that patients had more than one exacerbation/year prior to treatment.41 After 9–13 months of treatment, two patients had not presented with exacerbations and one had no change from baseline. The study by Ashkenazi et al. did not report exacerbation frequency at baseline but reported that after 8–27 months of treatment, all but one patient had acute pulmonary exacerbations, with a median of 3.5 exacerbations (range 0–17).40
In both studies systemic steroid use was reported, in which all but three patients were receiving steroids. After treatment for 9 to 27 months, of the six patients, three had no change in dosing and three had been able to taper the dose down to 50% in one patient.
Discussion
ABPA develops due to repeated inhalation of A. fumigatus spores from susceptible hosts. Human leukocyte antigen (HLA) DR2 and HLA DR5 have been identified as high risk for the development of ABPA, as opposed to HLA DQ2.4 Colonization of the airway is mediated by allergens from the fungus generating epithelial damage and allowing greater adherence and bronchial penetration of A. fumigatus, with a subsequent increase of Interleukin (IL)-6, IL-8, metalloproteinases (MMP) 9, followed by activation of neutrophils triggering much of the bronchial damage.42 There is also a type I hypersensitivity reaction that mediates an increase in total and specific IgE against A. fumigatus, through activation of IL-4 and IL-13 and a greater recruitment, activation and survival of eosinophils by stimulation of IL-5.5 To a lesser extent, a type III and type IV hypersensitivity reaction also develops, leading to an increase in IgG against the fungus and a cellular response mediated by lymphocytic infiltration and neutrophil activation.4,5
Furthermore, after diagnosing the disease (Tables 1 and 2), ABPA can be clinically classified using five stages: stage I: patient in the acute phase who meets the criteria for the disease; stage II: remission phase in which the patient is asymptomatic, without radiological changes nor using systemic steroids and with normal total IgE values for at least 6 months; stage III: exacerbation with evidence of imaging and paraclinical changes, plus doubled IgE values in comparison to remission phase; stage IV: corticosteroid-dependent asthma, in which steroids cannot be tapered nor suspended successfully; stage V: pulmonary fibrotic disease with irreversible fibrosis and chronic cavitation by imaging, usually with negative serology.2 Although this classification is not widely employed, it highlights the importance of initiating an effective management, in a timely fashion, to avoid the progression of the disease to advanced and irreversible stages.
As mentioned earlier, systemic steroids and antifungal medications are considered the standard treatment, with the objective of suppressing anti-inflammatory activity and fungal load, respectively. Prolonged use of steroids is related to systemic adverse effects and an increased risk of opportunistic infections, particularly fungal infections. Furthermore, almost 50% of patients suffer a relapse when the steroid dose is tapered and 20–45% become steroid dependent.43,44 On the other hand, the efficacy of Itraconazole has been reported in less than 50% of patients.29,45 Also, toxicity and pharmacological interactions between these medications must be considered, carefully monitoring blood levels to balance toxicity and azole resistance, if the dose is reduced too much.43,44
The clinical efficacy of therapy is based on the decrease of total IgE levels at least 35%, symptomatic and radiological improvement. Patients should be initially followed every 6–8 weeks, and then every 3–6 months, to evaluate the stability in response to treatment and make the necessary adjustments.4,44 Due to lack of response of many patients to standard of care, the use of biological drugs has been increasing in the past decade to treat ABPA, even though they are not approved by authorities worldwide for this purpose. The proposed mechanism of action for their use in ABPA relies on their inhibition of fundamental pathways for the development of the disease, such as the production of IgE and action against interleukin (IL) 4, IL-13 and IL-5.4,5
In this literature review we included 32 studies that analyzed the use of Omalizumab, Mepolizumab, Dupilumab and Benralizumab in both asthmatic (30 studies) and CF populations (two patients), for the treatment of ABPA. We found a total of 161 adult patients, but evidence came mainly from the asthmatic population (94.4%). Furthermore, around 60% of the studies analyzed Omalizumab use and the remaining studies were distributed among the rest of the biologics. Throughout the studies gender distribution remained equal, with women accounting for 50% of subjects. Likewise, age was consistent across the studies ranging from 28 to 79 years of age for the asthmatic population, consistent with what is described in the literature regarding the initiation of ABPA, which is usually between the third and fifth decades of life.16 The population analyzed in the CF studies was younger ranging from 19 to 41 years of age (see Tables 3–7).
Patients with ABPA have elevated IgE seric values (> 417 IU/mL), which is key in the pathophysiology of the disease.2 Overall, all but one patient included in this review had elevated IgE values, ranging from 500 to 22,893 IU/mL. Furthermore, 36% of patients had values over 1000 IU/mL,10–12,14,15,17–19,21,23–28,30–32,34,35 mandatory for ABPA diagnosis according to the ISHAM criteria (Table 2). Specifically, for patients treated with Omalizumab (in the asthmatic population), baseline values had a median of 1500 IU/mL.10,11,14–22,34–38 It is important to note that an IgE value of ⩽700 IU/mL and a maximum dose of 750 mg/month have been determined as the upper limit for the use of Omalizumab in severe asthma. Furthermore, dosage is calculated according to the patient’s weight and IgE levels. Considering the levels of IgE that patients with ABPA usually have, this calculation is not feasible because the maximum dose would be exceeded; most patients initiate treatment with the maximum dose recommended (375 mg every 2 weeks).44 That being said, from the 27 patients treated with Omalizumab who had pre and post-treatment IgE values, 40.7% achieved a reduction greater than 35% up to 3 years from initiation of treatment, the goal being to see such response after 2 months. The case report by Aguiar et al. showed several of the patients’ IgE values at different time points of treatment; the first one showed a reduction of 37% at 6 months, 40% at 12 months and 27% at 18 months.10 Despite the new rise in IgE the patients remained exacerbation free, questioning the extent to which IgE levels should be interpreted as a marker of disease activity. It is of utmost importance to analyze the success of the therapy in a comprehensive way, relying above all on clinical improvement. However, evidence from the literature suggests that a further increase in IgE greater than 50% of the previous value should be considered as indicative of an exacerbation.15 Moreover, in the case series by Aydin et al., the authors stated that patients with baseline IgE values <1000 UI/mL appeared to have a better response to treatment.34
Regarding Dupilumab, all patients had a decrease in IgE values, ranging from 32.3% to 85.6%, after 3–13 months of treatment.30,31 In contrast, the initiation of Mepolizumab is not determined by baseline IgE levels. Even though all patients began treatment with high levels of IgE, the maximum being 8327 UI/mL, only four out of seven patients who had post-treatment levels reported, had a decrease in IgE values ranging from 68% to 98%.24,25,27,28 The remaining patients had no change in IgE values, had never been treated with systemic steroids or antifungal treatment, but had favorable post treatment ACT levels, highlighting the importance of giving more relevance to patient-centered clinical outcomes. In relation to Benralizumab, follow-up time was very short and not all variables were described. In both cases there was a clinical improvement, IgE levels decreased 22% after 1 month of treatment, which is too soon to draw any conclusions.32,33
As already mentioned, systemic steroids are a fundamental part of the standard of care for these patients, despite their large profile of adverse effects. If adding a biological drug is effective, it should have a steroid-sparing effect directly through the inhibition of inflammatory pathways or indirectly through the decrease of acute exacerbations. Of the patients treated with Omalizumab, 89 patients were taking steroids before treatment, of which 66.2% had either a reduction in the dose or were able to suspend steroids after 3 months to 3 years of treatment,9–11,14–17,19,21,22,34,35,37,38 while 13.4% of patients remained with the same dosage.36,37 The remaining 21% of patients had no information on this aspect. A steroid-sparing effect was also seen in patients who received Mepolizumab, in whom dosing was either reduced or discontinued in 93.7% of patients;12,13,24–29 the two remaining patients never received steroids. Even though the steroid-sparing effect is seen with both Omalizumab and Mepolizumab, it appears to be quicker with the latter. Regarding Dupilumab, the status of steroid use was reported in only two patients out of 21, in whom the drug was suspended. However, two reports described early hypereosinophilia following initiation of treatment with Dupilumab, so the authors of the study by Ramonell et al. underlined the utility of using Dupilumab and steroids together in the early stages of treatment to avoid the increase in eosinophils and possible exacerbations of asthma.30
Radiological findings post-treatment were only described in six patients taking Mepolizumab12,13,23–29 and one patient taking Benralizumab,30 in whom an improvement in imaging findings was reported, mainly referring to the appearance of central bronchiectasis and mucoid impaction. As radiological improvement and absence of disease progression are important variables to determine remission of disease and one of the goals for treating ABPA, respectively, we encourage authors to include such findings in their studies.
Clinical response is the most important parameter to consider efficacy of treatment. In order for patients to be considered candidates for treatment with biologics, they must have had frequent acute exacerbations and a lack of response to standard care. Regarding Omalizumab, 89 patients (95.6%) out of the 93 patients on whom frequency of exacerbations was reported presented either a reduction or no exacerbations at all from 3 months up to 3 years of follow-up,14,20–22,37,38 while only 4.3% of patients had no change.15,37,38 Furthermore, pulmonary function deteriorated in all patients at baseline reporting a significant change after treatment in 54 of the 90 patients in whom pulmonary function was reported pre and post-treatment.9–11,14,16–22,34,36–38 Clinically, an improvement of more than 10% in FEV1 has been considered as relevant based on patient perception.46 Regarding Mepolizumab all patients were reported to have improved in the frequency of exacerbations and ACT scores from 2 to 14 months of treatment, without reporting an exact annual rate, impeding further interpretation.12,23,26 Furthermore, 88.4% of patients had an improvement in pulmonary function, taking 10% as the cut-off point.12,23,25,26,28,29 Similarly, for the patients treated with Dupilumab, a general improvement in frequency of exacerbations, as well as a progress in pulmonary function was reported in 20 of 21 patients, without giving further specific information.30,31
Concerning ABPA and CF patients, we could only find two case series with a total of nine patients who received Omalizumab.40,41 Exacerbations were only absent in three patients, without improvement in the remaining six patients. Furthermore, three patients had an improvement in pulmonary function, while the remaining patients remained stable as compared to baseline or had a further decline, which could be explained by the natural course of CF. The steroid-sparing effect of Omalizumab was reported in six patients, of whom three had no change and three had a reduction in the dosing.
Finally, regarding the safety and tolerability of biologics, there were no serious adverse effects reported across the studies. It is likely that minor adverse effects did occur and remained unreported, making it difficult to appreciate the safety profile of these drugs.
The strength of this review process was that studies were identified and analyzed by two investigators independently, which reduces bias of the results. However, the biggest limitation is that there is substantial heterogeneity among studies, a high risk of bias inherent in these types of descriptive studies and significant differences in sample size, making it inappropriate to attempt to do other statistical analysis on the results. Therefore, this article has a narrative purpose, in which no robust conclusions can be drawn.
Conclusion
ABPA should be considered in patients with asthma and CF who do not respond adequately to standard management and who have frequent acute exacerbations. An opportune diagnosis is key to prevent disease progression and pulmonary fibrosis. Biologics have been used in recent years to treat ABPA in patients with frequent acute exacerbations, use of antifungal medication with no response and stage IV ABPA. However, robust clinical evidence of its efficacy is lacking. They appear to be more effective in adult patients with asthma rather than CF, which could be related to the natural course of each disease, although there are only a few cases describing its effect in the CF population. Biologics seem to decrease the frequency of acute exacerbations and improve ACT asthma scores, as well as improve pulmonary function in at least 60% of the population. Although most patients responded effectively with a decrease in total serum IgE levels, there were cases in which IgE did not reach the 35% decrease but there was a significant clinical improvement, which reflects the importance of giving more value to patient-centered outcomes rather than reaching a specific cut-off value. Furthermore, because biological drugs have different mechanisms of action, IgE level and eosinophil count response is usually variable between them in time and in magnitude. The steroid-sparing effect of biologics was evident across all studies. Patients should be followed with chest imaging to evaluate the compromise of pulmonary parenchyma, as the objective of treatment is not only to reduce exacerbations but prevent progression of lung disease; this was reported in a minority of studies. All biologics appeared to impact all included variables positively, although Omalizumab seems to require more time to reach the objective. More studies are required to evaluate the effectiveness and safety of biological medications in this group of patients, with longer follow-up periods and objective measurements of the impact treatment has in reducing exacerbations, improving quality of life and preventing disease progression.
Supplemental Material
Supplemental material, Author_Response for Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review by Isabel C. Eraso, Saveria Sangiovanni, Eliana I. Morales and Liliana Fernández-Trujillo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review by Isabel C. Eraso, Saveria Sangiovanni, Eliana I. Morales and Liliana Fernández-Trujillo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review by Isabel C. Eraso, Saveria Sangiovanni, Eliana I. Morales and Liliana Fernández-Trujillo in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Isabel C. Eraso: Conceptualization; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Saveria Sangiovanni: Conceptualization; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Eliana I. Morales: Conceptualization; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Liliana Fernández-Trujillo: Conceptualization; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Liliana Fernández-Trujillo  https://orcid.org/0000-0003-0789-9154
https://orcid.org/0000-0003-0789-9154
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Isabel C. Eraso, Faculty of Health Sciences, Universidad Icesi, Cali, Colombia Department of Internal Medicine, Allergology Service, Fundación Valle del Lili, Cali, Colombia
Saveria Sangiovanni, Clinical Research Center, Fundación Valle del Lili, Cali, Colombia.
Eliana I. Morales, Faculty of Health Sciences, Universidad Icesi, Cali, Colombia Department of Internal Medicine, Pulmonology Service, Fundación Valle del Lili, Cali, Colombia
Liliana Fernández-Trujillo, Department of Internal Medicine, Pulmonology Service, Interventional Pulmonology, Avenida Simón Bolívar, Cra. 98 No. 18–49, Fundación Valle del Lili, Tower 6, 4th Floor, Cali 760032, Colombia Faculty of Health Sciences, Universidad Icesi, Cali, Colombia.
References
- 1. Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thorac Soc 2010; 7: 237–244. [DOI] [PubMed] [Google Scholar]
- 2. Patel AR, Patel AR, Singh S, et al. Diagnosing allergic bronchopulmonary aspergillosis: a review. Cureus 2019; 11: e4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maturu V, Agarwal R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: systematic review and meta-analysis. Clin Exp Allergy 2015; 45: 1765–1778. [DOI] [PubMed] [Google Scholar]
- 4. Patel AR, Patel AR, Singh S, et al. Treating allergic bronchopulmonary aspergillosis: a review. Cureus 2019; 11: e4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tracy MC, Okorie CUA, Foley EA, et al. Allergic bronchopulmonary aspergillosis. J Fungi 2016; 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenberg M, Patterson R, Mintzer R, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 1977; 86: 405–414. DOI: 10.7326/0003-4819-86-4-405 [DOI] [PubMed] [Google Scholar]
- 7. Banka R, Kamath A. Application of the ISHAM criteria for diagnosis of ABPA in a clinical cohort at a university teaching hospital. Eur Respir J 2018; 52 (Suppl. 62): PA1136. DOI: 10.1183/13993003.congress-2018.PA1136 [DOI] [Google Scholar]
- 8. Patterson TF, Thompson GR, III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis 2016; 63: e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Cunha FS, Valle SOR, Filho JE, et al. Omalizumab as add-on therapy in patients with asthma and allergic bronchopulmonary aspergillosis. J Bras Pneumol 2018; 44: 439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aguiar R, Fernandes NP, Lopes A, et al. Allergic bronchopulmonary aspergillosis treated successfully with omalizumab. Rev Port Pneumol 2017; 23: 304–306. [DOI] [PubMed] [Google Scholar]
- 11. Collins J, Devos G, Hudes G, et al. Allergic bronchopulmonary aspergillosis treated successfully for one year with omalizumab. J Asthma Allergy 2012; 5: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oda N, Miyahara N, Senoo S, et al. Severe asthma concomitant with allergic bronchopulmonary aspergillosis successfully treated with mepolizumab. Allergol Int 2018; 67: 521–523. [DOI] [PubMed] [Google Scholar]
- 13. Patel J, Ayars AG, Rampur L, et al. Combination anti-IgE and anti-IL5 therapies in patients with severe persistent asthma and allergic bronchopulmonary aspergillosis (ABPA). J Allergy Clin Immunol 2018; 141: AB234. [Google Scholar]
- 14. Kelso JM. Following total IgE concentration in patients with allergic bronchopulmonary aspergillosis on omalizumab. J Allergy Clin Immunol Pract 2015; 4: 364–365. [DOI] [PubMed] [Google Scholar]
- 15. Wolf BL, Johnson A. Unexpected decrease in total IgE in a patient with allergic bronchopulmonary aspergillosis treated with omalizumab. J Allergy Clin Immunol Pract 2014; 2: 111–113. [DOI] [PubMed] [Google Scholar]
- 16. Unal D. Allergic bronchopulmonary aspergillosis: a clinical evaluation of 15 patients and successful omalizumab treatment of five patients. Asthma Allergy Immunol 2019; 17: 103–110. [Google Scholar]
- 17. Homma T, Kurokawa M, Matsukura S, et al. Anti-IgE therapy for allergic bronchopulmonary aspergillosis. J Microbiol Immunol Infect 2013; 49: 459–463. [DOI] [PubMed] [Google Scholar]
- 18. Voskamp AL, Gillman A, Symons K, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2015; 3: 192–199. [DOI] [PubMed] [Google Scholar]
- 19. Sastre I, Blanco J, Mata H, et al. A case of allergic bronchopulmonary aspergillosis treated with omalizumab. J Investig Allergol Clin Immunol 2012; 22: 145–147. [PubMed] [Google Scholar]
- 20. Pérez-de-Llano LA, Vennera MC, Parra A, et al. Effects of omalizumab in Aspergillus-associated airway disease. Thorax 2011; 66: 539–540. [DOI] [PubMed] [Google Scholar]
- 21. Bobolea I, Rodriguez CF, Diaz-Campos R, et al. Measuring total IgE is useful in detecting exacerbations in patients with allergic bronchopulmonary aspergillosis receiving omalizumab. J Allergy Clin Immunol Pract 2016; 4: 361–363. [DOI] [PubMed] [Google Scholar]
- 22. Vázquez LMQ, Piquer MO, de Llano LAP. Tratamiento efectivo con anticuerpo antiinmunoglobulina E en un paciente con aspergilosis broncopulmonar alérgica. Arch Bronconeumol 2009; 45: 207. [DOI] [PubMed] [Google Scholar]
- 23. Soeda S, To M, Kono Y, et al. Case series of allergic bronchopulmonary aspergillosis treated successfully and safely with long-term mepolizumab. Allergol Int 2019; 68: 377–379. [DOI] [PubMed] [Google Scholar]
- 24. Hirota S, Kobayashi Y, Ishiguro T, et al. Allergic bronchopulmonary aspergillosis successfully treated with mepolizumab: case report and review of the literature. Respir Med Case Rep 2019; 26: 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsubouchi H, Tsuchida S, Yanagi S, et al. Successful treatment with mepolizumab in a case of allergic bronchopulmonary aspergillosis complicated with nontuberculous mycobacterial infection. Respir Med Case Reports 2019; 28: 100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terashima T, Shinozaki T, Iwami E, et al. A case of allergic bronchopulmonary aspergillosis successfully treated with mepolizumab. BMC Pulm Med 2018; 18: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kubena P, Duchna H, Capova G, et al. Allergic bronchopulmonary aspergillosis (ABPA) description of typical exacerbation. Classical remission induction and implementation of mepolizumab. Respiration 2018; 95: 488. [Google Scholar]
- 28. Altman M, Lenington J, Bronson S, et al. Combination omalizumab and mepolizumab therapy for refractory allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2017; 5: 1137–1139. [DOI] [PubMed] [Google Scholar]
- 29. Florence S, Elleni-Sofia V, Charles P, et al. Mepolizumab for allergic bronchopulmonary aspergillosis: report of 20 cases from the Belgian Severe Asthma Registry and review of the literature. J Allergy Clin Immunol Pract 2020; 8: 2412–2413e2. [DOI] [PubMed] [Google Scholar]
- 30. Ramonell R, Lee F, Swenson C, et al. Dupilumab treatment for allergic bronchopulmonary aspergillosis: a case series. J Allergy Clin Immunol Pract 2020; 8: 742–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corren J, Sher L, Zhu X, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe asthma and serologic evidence of allergic bronchopulmonary aspergillosis. Ann Allergy, Asthma Immunol 2019; 123: S15. [Google Scholar]
- 32. Wong S, Ahluwalia S. Benralizumab for the treatment of refractory allergic bronchopulmonary aspergillosis. Ann Allergy, Asthma Immunol 2019; 123: S100–S101. [Google Scholar]
- 33. Soeda S, Kono Y, Tsuzuki R, et al. Allergic bronchopulmonary aspergillosis successfully treated with benralizumab. J Allergy Clin Immunol Pract 2019; 7: 1633–1635. [DOI] [PubMed] [Google Scholar]
- 34. Aydin O, Sozener ZC, Soyyigit S, et al. Omalizumab in the treatment of allergic bronchopulmonary aspergillosis: one center’s experience with 14 cases. Allergy Asthma Proc 2015; 36: 493–500. [DOI] [PubMed] [Google Scholar]
- 35. Evans MO, Morris MJ, Coop CA, et al. Omalizumab, an additional therapy for allergic bronchopulmonary aspergillosis. Ann Allergy, Asthma Immunol 2015; 115: 250–251. [DOI] [PubMed] [Google Scholar]
- 36. Koutsokera A, Corriveau S, Sykes J, et al. Omalizumab for asthma and allergic bronchopulmonary aspergillosis in adults with cystic fibrosis. J Cyst Fibros 2020; 19: 119–124. [DOI] [PubMed] [Google Scholar]
- 37. Tillie-Leblond I, Leroyer C, Germaud P, et al. Allergic bronchopulmonary aspergillosis and omalizumab. Allergy 2011; 66: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 38. Lin RY, Sethi S, Bhargave GA. Measured immunoglobulin E in allergic bronchopulmonary aspergillosis treated with omalizumab. J Asthma 2010; 47: 942–945. [DOI] [PubMed] [Google Scholar]
- 39. Busse W, Maspero J, Rabe K, et al. Liberty asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther 2018; 35: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashkenazi M, Sity S, Sarouk I, et al. Omalizumab in allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J Asthma Allergy 2018; 11: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehmann S, Pfannenstiel C, Friedrichs F, et al. Omalizumab: a new treatment option for allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Ther Adv Respir Dis 2014; 8: 141–149. [DOI] [PubMed] [Google Scholar]
- 42. De Córdova-Aguirre JCF, Velasco-Medina AA, Cariño-Cartagena DA, et al. Aspergilosis broncopulmonar alérgica. Rev Alerg Mex 2014; 61: 121–126. [PubMed] [Google Scholar]
- 43. Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013; 43: 850–873. [DOI] [PubMed] [Google Scholar]
- 44. Agarwal R, Sehgal IS, Dhooria S, et al. Developments in the diagnosis and treatment of allergic bronchopulmonary aspergillosis. Expert Rev Respir Med 2016; 10: 1317–1334. [DOI] [PubMed] [Google Scholar]
- 45. Burgel PR, Paugam A, Hubert D, et al. Aspergillus fumigatus in the cystic fibrosis lung: pros and cons of azole therapy. Infect Drug Resist 2016; 9: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Global Strategy for Asthma Management and Prevention. GINA Report, Global Strategy for Asthma Management and Prevention. (2020). https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf (2020; accessed 13 September 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review by Isabel C. Eraso, Saveria Sangiovanni, Eliana I. Morales and Liliana Fernández-Trujillo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review by Isabel C. Eraso, Saveria Sangiovanni, Eliana I. Morales and Liliana Fernández-Trujillo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review by Isabel C. Eraso, Saveria Sangiovanni, Eliana I. Morales and Liliana Fernández-Trujillo in Therapeutic Advances in Respiratory Disease


