Abstract
Chimeric antigen receptor (CAR) T-cell therapy is a rapidly developing method for adoptive immunotherapy of tumours in recent years. CAR T-cell therapies have demonstrated unprecedented efficacy in the treatment of patients with haematological malignancies. A 90% complete response (CR) rate has been reported in patients with advanced relapse or refractory acute lymphoblastic leukaemia, while >50% CR rates have been reported in cases of chronic lymphocytic leukaemia and partial B-cell lymphoma. Despite the high CR rates, a subset of the patients with complete remission still relapse. The mechanism of development of resistance is not clearly understood. Some patients have been reported to demonstrate antigen-positive relapse, whereas others show antigen-negative relapses. Patients who relapse following CAR T-cell therapy, have very poor prognosis and novel approaches to overcome resistance are required urgently. Herein, we have reviewed current literature and research that have investigated the strategies to overcome resistance to CAR T-cell therapy.
Keywords: CAR T-cell therapy, drug resistance, haematological malignancies
Introduction
Chimeric antigen receptors (CARs) are synthetic tumour-specific receptors that are genetically reprogrammed in vitro using a patient’s own T lymphocytes, which bind a tumour antigen in a major histocompatibility complex-independent manner, allowing T cells to recognise and kill antigen-expressing cancer cells. In the past few years, clinical trials using CAR T cells have demonstrated high rates of response in the treatment of patients with haematological malignancies, as well as increased duration of remission in patients with acute lymphoblastic leukaemia (ALL),1,2 chronic lymphocytic leukaemia (CLL),3 and partial B cell lymphomas.4,5 CAR T-cell therapy has provided a new therapeutic option to patients with relapse/refractory haematological malignancies. Based on the results, the United States Food and Drug Administration (FDA) approved tisagenlecleucel in August 2017 for paediatric patients and young adults with B-cell ALL (B-ALL). Furthermore, in October 2017, the FDA approved CAR T-cell therapy for the treatment of B-cell lymphoma.6 A current challenge in CAR T-cell therapy is that a portion of the patients achieving remission following CAR T-cell therapy subsequently undergo relapse. The mechanism of development of resistance to CAR T-cell therapy is not completely understood. Some patients have been reported to demonstrate antigen-positive relapse due primarily to shorter duration of persistence of CAR T cells, whereas others show antigen-negative relapses associated with lineage switching, acquired mutation and alternative splicing, epitope-masking and antigen downregulation.7–15 The current review outlines the diverse strategies to overcome or reduce resistance to CAR T-cell therapy.
Basic structure and development of CAR T-cells
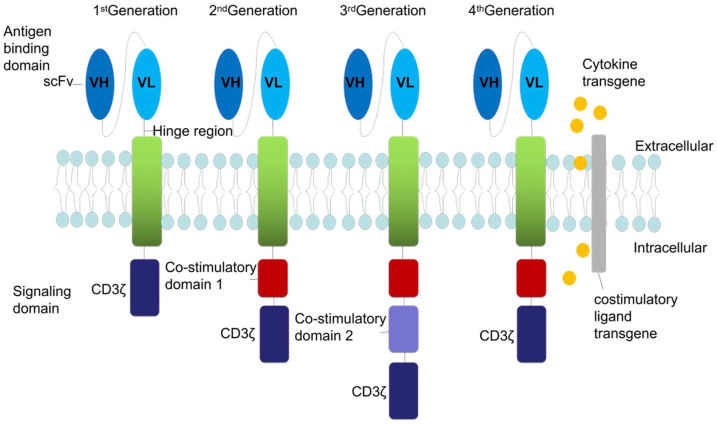
CAR T-cell therapy is a cellular therapy that redirects a patient’s T cells to specifically target and destroy tumour cells. CARs are proteins expressed on the surface of T and natural killer (NK) cells, which contain extracellular binding domains, a hinge region that mediates the linkage of extracellular to transmembrane domains, a transmembrane domain and an intracellular signaling domain (Figure 1).16–20 In 1987, Kuwana et al. first proposed the concept of CAR and constructed a prototype of CAR-T cells that specifically recognised tumour-associated antigens.21 In the first-generation CARs, the intracellular signaling domain comprised solely a CD3ζ chain, a component of the endogenous T-cell receptor (TCR).22 These first-generation CARs showed minimal killing and persistence in vivo along with limited clinical benefits.23–28 Second-generation CARs incorporated co-stimulation into the CD3ζ construct. Most investigators work with second-generation CARs, involving those that express the classical co-stimulatory molecules, namely the tumour necrosis factor (TNF) superfamily members 9 (4-1BB) and 4 (OX40).29,30 However, some investigators have expanded their toolkit to include other types of co-stimulatory molecules into the CAR constructs, such as OX40, 4-1BBL, or inducible co-stimulator (ICOS).31–33 Studies have reported that second-generation CAR T cells demonstrated potent expansion and cytokine secretion abilities, and persistence of anti-tumour T cells both in vitro and in vivo.7,17,34–36 Third-generation CARs containing three or more co-stimulatory domains to boost T-cell activation signals, including CD28, 4-1BB, and CD3ζ, were developed to improve the design and enhance the activation of the second-generation CARs.37–45 The fourth-generation CARs (T cells redirected for universal cytokine killing, TRUCKs) can secrete pro-inflammatory cytokines such as IL-12 into the tumour microenvironment,46,47 which consequently improve the tumour eradication ability of these cells.48–52
Figure 1.
Schematic representation of CAR structure. CAR T cells are composed of three parts: (1) an scFv, (2) a transmembrane domain, and (3) a signal transduction domain of the TCR. First-generation CARs used a CD3ζ as the signal transduction domain of the TCR, whereas second-generation CARs include additional co-stimulatory signaling domains (CD28 or 4-1BB). Third-generation CARs consist of two distinct co-stimulatory domains, such as both CD28 and 4-1BB. Fourth-generation CARs are additionally armored with genes that enable, for example, the expression of cytokines.
CAR, chimeric antigen receptor; scFv, single-chain variable domain of an antibody; TCR, T-cell receptor.
Efficacy of CAR T cells in the treatment of haematological malignancies
Haematological malignancies are one of the most common cancers among patients in China. Presently, haematological malignancies remain incurable and have a high recurrence rate and mortality. In recent years, novel gene and targeted therapies have emerged for the treatment of patients with haematological malignancies; however, clinical remission rates are limited. In 2013, the journal Science summarised the top 10 breakthrough technologies in the scientific community, with tumour immunotherapy topping the list. CAR T-cell therapy, as a special tumour immunotherapy, has demonstrated remarkable results in the treatment of patients with malignant tumours, especially lymphatic haematopoietic malignancies.
B-ALL
CAR T-cell therapy has emerged as a highly effective therapy for patients with relapsed or refractory B-ALL with previously limited treatment options. The therapy was reported to demonstrate complete responses (CRs) ranging from 60% to 90% (Table 1).2,7,48–53 Relapse rates of approximately 30–50% were reported in patients with B-ALL, with the majority being CD19-negative relapses.7 In a phase II, single-cohort, 25-centre global study, 75 patients received an infusion of tisagenlecleucel and were followed up for at least 3 months; the overall remission rate was 81%.54 A total of 45 patients (60%) had complete remission and 16 (21%) had complete remission with incomplete haematological recovery. Among the patients with complete remission, 17 experienced relapse before receiving additional anticancer therapy. Characterisation of CD19 status at the time of relapse showed that 1 patient had CD19-positive and 15 had CD19-negative recurrence, whereas six patients had unknown status. Turtle et al. conducted a clinical trial on 29 patients with B-ALL who received CAR T cells, and demonstrated a complete response (CR) rate of 93%. Among the patients with complete remission, nine had a relapse. Characterisation of CD19 status at the time of relapse showed that two patients had a CD19-negative relapse.55
Table 1.
Summary of CAR T cells in the treatment of B-ALL, B-NHL and CLL.
| Disease | Patient populations | Response and relapse | References |
|---|---|---|---|
| B-ALL | 53 adults | 44/53 (83%) achieved a CR, the median overall survival was 12.9 months. | Park et al.2 |
| 30 paediatric and adults | 27/30 (90%) achieved a CR, seven patients who had a complete remission subsequently had a relapse between 6 weeks and 8.5 months after infusion of CAR T cells. | Maude et al.7 | |
| 75 paediatric and adults | 45/75 (60%) had a CR, the rate of overall survival was 90% at 6 months after infusion and 76% at 12 months after infusion. | Kochenderfer et al.54 | |
| 21 paediatric and adults | 14/21 (66.7%) achieved a CR. | Lee et al.53 | |
| B-NHL | 28 adults | 6/14 DLBCL patients achieved a CR and 10/14 FL patients achieved a CR. | Schuster et al.60 |
| 7 adults | 4/7 (57%) achieved a CR. Three patients are in ongoing CR at 12 months post CAR T cells infusion. | Locke et al.63 | |
| 101 adults | CR rate was 54%. With a median follow-up of 15.4 months, with 40% continuing to have a complete response. | Ye et al.66 | |
| 15 adults | 8/15 (53%) had a CR. Seven patients are in ongoing CR, ranging from 9 to 22 months post CAR T cells infusion. | Schuster et al.60; June and Sadelain61 | |
| 7 adults | 2/7 achieved a CR, one patient attained a PR, another four patients exhibited SD. Two patients are in ongoing CR at 3 months and 13 months post CAR T cells infusion. | June and Sadelain61 | |
| CLL | 14 adults | 8/14 (58%) achieved an objective response, with 4/14 (29%) achieving a CR. CAR T cells persisted for >5 years in two patients with durable CRs. | Porter et al.59 |
| 3 adults | 2CR, 1PR; two of whom experienced long-lasting CR. | Porter et al.59 |
B-ALL, B-cell acute lymphoblastic leukaemia; B-NHL, B-cell non-Hodgkin lymphoma; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukaemia; CR, complete remission; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; PR, partial remission; SD, stable disease.
B-cell non-Hodgkin lymphomas and CLL
Previous research has shown remarkable rates of complete and durable remission in patients with CLL56–59 and B-cell non-Hodgkin lymphoma (B-NHL).23,56–61 CAR T-cell therapy has been approved for the treatment of lymphoma in adults, with a lower remission rate of approximately 50–70%.6,62–64 Furthermore, antigen loss has also been observed in such patients.65,66 In a multicentre, phase II trial, 111 patients with histologically confirmed diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, or transformed follicular lymphoma were enrolled, of which 101 received Axicabtagene Ciloleucel, an autologous anti-CD19 CAR T-cell therapy. The objective response rate observed in the patients was 82%, and the CR rate was 54%. At a median follow-up of 15.4 months, 42% of the patients continued to demonstrate a response, with 40% showing CR.6 Another clinical trial enrolled 28 adult patients, wherein complete remission occurred in 6 out of 14 patients with diffuse large B-cell lymphoma and in 10 out of 14 patients with follicular lymphoma.59,66 Porter et al. treated 14 adult patients with CLL using CD19 CAR T cells, and reported CR in 4 (29%) patients.59
Underlying mechanisms of resistance
Two main mechanisms have been recognised in relapse following CAR T-cell therapy, including antigen-negative and antigen-positive relapses.
Antigen-positive relapse
Antigen-positive relapse has been assumed to be due primarily to short persistence of CAR T cells7; however, it can also occur in association with a suppressive tumour microenvironment.67 The reasons for loss of CAR-T cell persistence are complex and might be difficult to determine in individual patients. According to a trial by Park et al., shorter duration of persistence of the CD19-CD28-ζ CAR T cells employed by Memorial Sloan Kettering Cancer Center (MSKCC) could partially explain the low rate of CD19-positive relapse.2 In another study, although the CR rates in the cohort were robust, early relapse was noted in a subset of patients, associated with loss of CAR T cells in the blood due to an anti-CAR T-cell immune response to the epitopes in the murine single-chain variable fragment (scFv). This mechanism was reported to contribute to the early loss of CAR T cells in some patients after the use of a CAR containing a murine scFv.68
Antigen-negative relapse
The reason for antigen-negative relapse was unclear. Since the antigen-negative relapse has been considered a major barrier to CAR-T therapies, studies have uncovered multiple mechanisms responsible for the antigen-negative relapse, which are described below.
Epitope-masking
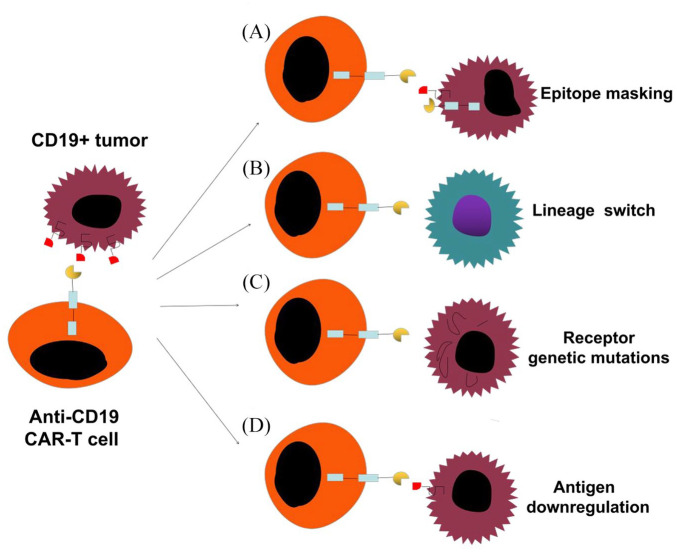
Ruella et al. described a rare case of a 20-year-old man with B-ALL who had suffered three chemotherapeutic relapses. He was enrolled in a phase I trial [ClinicalTrials.gov identifier: NCT01626495] to evaluate the safety, feasibility, and engraftment of CD19-targeted CAR T cells (CTL019) in paediatric and young adult patients with B-ALL.10 The patient was in complete remission at day 28 after infusion. However, he experienced relapse after 9 months of the CAR T-cell therapy with CD19-negative disease. Ruella et al. probed this patient’s CD19-negative relapse, ruling out CD19 mutations and splicing variants, and found that the anti-CD19 CAR had been introduced into a single leukaemic B cell during manufacturing of the CD19 CAR T cell. This tumour clone was infused into the patient alongside the CAR T-cell therapy and eventually expanded, which led to disease progression and death. The study reported that resistance to CTL019 occurred not due to loss of target by the leukaemic blasts, but due to the CAR molecule bound to adjacent CD19, which effectively masked the CD19 epitope from CAR T cells in the patient. Ruella and his team were able to create a model of this phenomenon, known as ‘epitope masking’ (Figure 2A), both in vitro and in vivo.
Figure 2.
Mechanisms of resistance to CAR-T cell therapy. (A) Lentiviral modification of a single leukemic cell allowed for joint CAR19 and CD19 expression on their cell surface, effectively masking the CD19 epitope from CAR T cells. (B) Tumour cells can switch to a genetically related but phenotypically different disease. (C) Tumour cells, through genetic mutations, can either completely lose CD19 receptor expression or modify the CD19 receptor that lack the extracellular epitopes recognised by CAR T cells. (D) Tumour cells downregulate the surface target antigen to levels below those needed for CAR T cells activation.
CAR, chimeric antigen receptor.
Lineage switch
Lineage switch occurs when a patient experiences relapse with a genetically related but phenotypically different malignancy (Figure 2B), which might be a mechanism for antigen loss after CAR T-cell therapy observed in clinical trials.9,69–72 Evans et al. reported relapse after CD19 CAR T-cell therapy in a patient with CLL with Richter’s transformation. The patient demonstrated a plasmablastic lymphoma, which is inherently CD19-negative.73 A group of researchers from Seattle reported that two of the seven patients with B-ALL harbouring rearrangement of the mixed lineage leukaemia gene experienced relapse with CD19-negative AML following treatment with CD19 CAR T cells.9
Receptor genetic mutations
Acquired mutations and alternatively spliced CD19 alleles in the malignant B cells are other mechanisms for CD19-negative relapse following CD19-targeted CAR T-cell therapy (Figure 2C). Sotillo et al. found that exon 2 of CD19 was frequently spliced, leading to the disappearance of the CD19 epitope, which is recognised by CAR T cells.8 In addition, they observed the variants Δexon-5, 6, which lack the transmembrane domain of CD19, thereby leading to loss of surface expression. Furthermore, they observed that the CD19 protein was present in some patients; however, it was truncated due to lack of the epitope required to trigger CAR T cell recognition for lysis. The Orlando trail identified mutations in CD19 in all 12 specimens at the time of relapse.74 Mutations were found throughout exons 2–5 of CD19. Encoding of the transmembrane domain begins at exon 5; therefore, variants in exons 2–5 have been predicted to lead to a truncated protein lacking membrane anchorage. Each patient in the study had at least one unique frameshift insertion or deletion and, in some cases, missense single nucleotide variants were also observed, and alternative splicing occurred with rare frequency. Therefore, acquired mutations and alternative splicing could be other possible mechanisms responsible for antigen-negative relapse.75,76
Antigen downregulation
Partial antigen loss may be considered a mechanism for resistance to CAR T-cell therapy due to antigen downregulation (Figure 2D).11–15 During the course of antigen recognition, natural TCRs produce a highly organised immune synapse that can recognise an antigen at a very low density.77,78 However, the immune synapse created during antigen recognition by CARs is less organised than that by a natural TCR.79 These distinctions are likely to significantly affect the quality of responses induced in T cells expressing CARs. Fry et al. observed that relapses in patients treated with a CD22 CAR were associated with diminished and variable CD22 site density on B-ALL cells.11 The investigators further demonstrated in animal studies that differential levels of CD22 on leukaemic cells could have a dramatic impact on the anti-cancer efficacy. Another study using a CD20 CAR demonstrated that a threshold level of around 200 antigen molecules per target cell was required to induce lysis, while approximately 10-fold higher numbers of molecules were needed to stimulate cytokine production.13 Therefore, the signal strength and effector function of CARs might be limited by density of the tumour antigens.
Overcoming resistance to CAR T-cell therapy
Improving CAR T-cell design
Selection of effector T cells
Effector T cells are the main processing plant for the biological activity of CARs, and play a crucial role in the anti-tumour effect and duration of action of CARs. Accurate detection and isolation of the most potential subpopulations of T cells before in vitro expansion can improve the outcomes of CAR T-cell immunotherapy. The cytotoxic activity and proliferative capacity of effector memory T cells are superior to that of central memory T cells (TCM) in vitro; however, TCMs have the potential to induce immune memory, as well as exert a longer lasting anti-tumour activity.80 Stem cell memory T cells (TSCM) have the property of persistent self-renewal; therefore, these cells have the potential for high proliferation and persistence.81 Thus, the process of inducing the conversion of CAR T cells into TCMs and TSCMs could be an alternative method to prevent antigen-positive recurrence by enhancing the response and persistence of the cells.
Antigen density
Numerous studies have demonstrated that antigen density on tumour cells correlates with the efficacy and remission durability of CAR T cells in patients with leukaemia and lymphoma.13,15,82–85 A recent research demonstrated that upregulation of CD22 on the cell surface improved CAR T cell functionality and long-term persistence.82 Moreover, Bryostatin 1, a drug that is being administered safely to humans, can increase the expression of CD22 in leukaemia and lymphoma cell lines, resulting in longer duration of in vivo response. Another research found that γ-secretase inhibitors could markedly increase surface levels of BCMA on myeloma cells, thereby improving tumour recognition by CAR T cells in vitro and enhancing anti-tumour efficacy of BCMA-targeted CAR T-cell therapy.86
Selection of co-stimulatory molecules
The co-stimulatory molecules in the intracellular signalling region of the CAR T cells play an important role in regulating T cell expansion, duration, and anti-tumour effects; however, the biological activities of individual costimulatory molecules are different. Common costimulatory molecules include CD28, 4-1BB, OX40, ICOS, and CD27, of which CD28 and 4-1BB can effectively promote the secretion of IL-2 and IFN-γ. Studies have shown that 4-1BB can effectively promote the expansion of memory T cells and reduce the depletion of persistent CAR T signals.45 Thus, CAR T cells incorporating a 4-1BB costimulatory molecule might lead to a reduced antigen-positive relapse. Hombach et al. proved that CAR T cells containing CD28 costimulatory molecules were more effective than those containing CD28-OX40, because CD28-OX40 are capable of promoting activation-induced cell death and reduce anti-tumour activity.87 Other studies have shown that CAR T cells with two co-stimulants (such as CD28 and 4-1BB) were more effective in improving the survival and cytotoxicity of T cells than CAR T cells with a single co-stimulatory molecule. The above studies indicate that co-stimulatory molecules greatly affect the efficacy of CAR T-cell therapy; however, further in vitro and in vivo research is necessary to determine the optimal type and number of co-stimulatory molecules.
Fully human CARs
Presently, clinical trials commonly use the CAR scFv segment of murine origin, which has high affinity and immunogenicity. CAR T cells with high affinity have poor ability to distinguish tumour cells with high levels of target antigen from normal cells with low expression. Furthermore, the human body will reject CARs with high immunogenicity, considering them foreign bodies. Reducing immunogenicity of CARs using fully human scFvs could improve the persistence of CAR T cells and their functions against tumour cells.88–90 Sommermeyer et al. reported that CARs constructed from fully human CD19-specific scFvs exhibited superior function in vitro and in vivo compared with the FMC63 CAR utilised in clinical trials.91 Specifically, fully human CD19-specific scFvs were more effective in lysing CD19+ target cells, produced higher levels of cytokines, and proliferated more after activation compared with murine scFv.
Armoured CAR T cells
Armoured CAR T cells are modified to co-express cytokines and co-stimulatory molecules in order to enhance the anti-tumour immune response by converting a suppressive tumour microenvironment into a proinflammatory one.92 For example, CAR T cells armoured to secrete IL-12 enhance the cytotoxic activity of CD8+ T and NK cells and stimulate a Th1 helper T cell response.93 CD40L expressed in armoured CAR T cells increased the cytotoxicity of these cells in vitro and prolonged survival of lymphoma-bearing mice. CAR T cells armoured with 4-1BBL has been reported to exert immunostimulatory effects.94 However, the effectiveness of this approach needs further clinical translation as the results have been predominantly proven on preclinical models.
Universal CAR T cells
Universal CAR T cells are used in genome-editing technologies such as zinc finger nuclease, transcription activator-like effector nuclease (TALEN) and CRISPR-Cas9 to knock out TCR, human leukocyte antigen and other related signaling pathway genes on donor T cells,95 thereby reducing the risk of graft-versus-host disease and immune rejection. Furthermore, simultaneously knocking out immune checkpoints such as programmed death-1 (PD-1) has been shown to enhance the function of CAR-T cells.95–98 Waseem et al. used TALEN to knock out TCR and CD52 on CAR T cells targeting CD19 for the treatment of patients with refractory ALL, and reported improvement in the condition of the patients within 28 days after treatment. The results proved the safety and effectiveness of universal CAR T-cell immunotherapy for the first time.97 Therefore, use of universal CAR T cells might be another possible strategy to overcome resistance to CAR T-cell therapy.
Multi-targeted CAR T cells
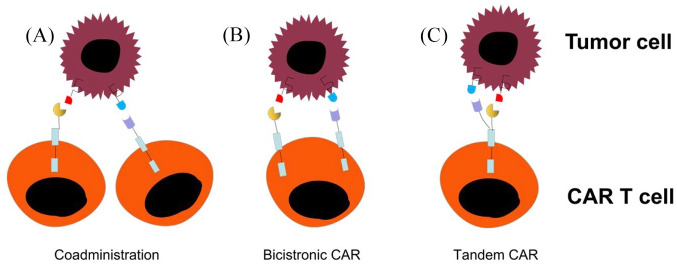
Strategies to overcome the relapse rate due to antigen loss following CAR T-cell therapy can be combined with the following approaches: (a) T-cell products that are separately transduced for different CARs can be infused together or sequentially; (b) use of a single vector that encodes two or three different CARs on a single T cell (bicistronic CAR); or (c) encode two CARs on the same chimeric protein using a single vector (tandem CAR) (Figure 3). Majzner et al. described these different approaches in a review.12 Many of these approaches are currently being investigated in clinical trials on patients with haematological malignancies.11,99–101 A recent study investigated the clinical efficacy of bispecific tandem CAR T cells directed against both CD19 and CD20 antigens in patients with relapsed/refractory (R/R) B-cell NHL.102 In addition, Ruella et al. reported that dual CAR CD19 and CD123 overcame both antigen escape and lineage switch.103 Several clinical trials are underway to test multi-specific CAR T cells; however, the effectiveness of these approaches remains to be established.
Figure 3.
Targeting more than one antigen receptor approaches. (A) Coadministration-producing two separate CAR-T cell products and infusing together or sequentially. (B) Bicistronic CAR-using a single vector that encodes two or three different CARs on a single T cell. (C) Tandem CAR-encoding two CARs on same chimeric protein using a single vector.
CAR, chimeric antigen receptor.
Improvement of tumour immune microenvironment
Improving the tumour immune microenvironment can greatly improve the immune efficacy of CAR T cells and reduce the adverse events. However, due to the complexity of the tumour microenvironment and the diversity of regulatory mechanisms, clinical efficacy cannot be achieved by monotherapy. The main regulatory mechanisms of the immune microenvironment can be combined with the following comprehensive treatments.
Studies have proved that addition of an agent blocking the PD-1 immunosuppressive pathway (anti-PD-1) greatly improved the efficacy of CAR T cells by inhibition of the interaction between PD-1 and its ligands PD-L1/PD-L2.104,105
Similarly, chemotherapy and radiotherapy can improve immunosuppression by inducing apoptosis of or specifically removing regulatory T cells (Tregs). Moreover, eradication of Tregs can enhance the cell response and increase levels of CAR T cells.106 One study found that chemotherapy based on low-dose cyclophosphamide could effectively eliminate Tregs and exert immunomodulatory effects. A combined immunotherapeutic approach has been reported to improve the prognosis.107
The cytokines TGF-β and IL-10 are the major immunosuppressive factors, and downregulate the expression of TGF-β and IL-10 receptors on T cells by genetic engineering methods, to improve the efficacy of CAR T cells. In addition, activation factors such as IL-2, IL-12, and IL-15 can promote the immune function of effector T cells by creating a microenvironment that is conducive to the survival and efficacy of T cells, resulting in more effective anti-tumour effects by inducing the secretion of activating factors by CAR T cells.47,108,109
Combination therapy
Combining CAR T-cell therapy with other agents, such as Bruton tyrosine kinase inhibitors, may reduce recurrence after infusion and improve long-term survival. Fraietta et al. reported that treatment with ibrutinib could significantly increase the implantation and expansion of CAR T cells in patients with CLL, and enhanced its targeted cytotoxic activity.110 The outcomes could be attributed to downregulation of immunosuppressive receptors and improvement in the proliferation and activation functions of T cells by ibrutinib.110,111 On the other hand, differentiation of Th2 cells could have been inhibited and immune response of Th1 cells could have been promoted by inhibiting the activity of IL-2 mediated T cell kinase.112 Other studies have shown superior effectiveness of the combined therapeutic approach than ibrutinib113,114 or CAR T-cell monotherapy,115 thus providing a new research direction to address the issue of resistance to CAR T-cell therapy.
Conclusion
In conclusion, advancements in our understanding of the mechanisms of resistance to CAR T-cell therapy are leading to new insights regarding this treatment. Novel strategies are being developed to overcome the resistance and improve clinical outcomes in patients with relapsed and refractory haematological malignancies. Various treatment approaches, such as targeting more than one antigen receptor, armoured CAR T-cells, fully human CAR T cells, CAR NK-cell therapy, and combination therapies with other immunotherapeutic agents are being explored to overcome the issue of resistance. However, the effectiveness of the aforementioned treatments remains unclear. Thus, further research is needed to maximise the duration of responses while minimising the risk of relapse.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National natural Science Foundation of China (U1904139), Department of Science and Technology of Henan province (182102310114).
ORCID iD: Mingzhi Zhang  https://orcid.org/0000-0002-4265-944X
https://orcid.org/0000-0002-4265-944X
Contributor Information
Qing Cai, Department of Oncology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan province, China.
Mingzhi Zhang, Department of Oncology, The First Affiliated Hospital of Zhengzhou University, 6th Floor, Building 10, No.1 Construction East Road, Zhengzhou, Henan Province 450052, China.
Zhaoming Li, Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China.
References
- 1. Sadelain M, Brentjens R, Rivière I, et al. CD19 CAR therapy for acute lymphoblastic leukemia. Am Soc Clin Oncol Educ Book 2015: e360–e363. [DOI] [PubMed] [Google Scholar]
- 2. Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018; 378: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turtle CJ, Hay KA, Hanafi L-A, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 2017; 35: 3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019; 20: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol 2019; 10: 153133357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015; 5: 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016; 127: 2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruella M, Xu J, Barrett DM, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med 2018; 24: 1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018; 24: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov 2018; 8: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe K, Terakura S, Martens AC, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 ζ chimeric antigen receptor-modified effector CD8+ T cells. J Immunol 2015; 194: 911–920. [DOI] [PubMed] [Google Scholar]
- 14. Hombach AA, Görgens A, Chmielewski M, et al. Superior therapeutic index in lymphoma therapy: CD30+ CD34+ hematopoietic stem cells resist a chimeric antigen receptor T-cell attack. Mol Ther 2016; 24: 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walker AJ, Majzner RG, Zhang L, et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol Ther 2017; 25: 2189–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levine BL, Miskin J, Wonnacott K, et al. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev 2017; 4: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finney HM, Lawson AD, Bebbington CR, et al. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol 1998; 161: 2791–2797. [PubMed] [Google Scholar]
- 18. Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989; 86: 10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 2012; 119: 3940–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu J, Deng Y, Benson DM, et al. CS1-specific Chimeric Antigen Receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014; 28: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuwana Y, Asakura Y, Utsunomiya N, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun 1987; 149: 960–968. [DOI] [PubMed] [Google Scholar]
- 22. Sermer D, Brentjens R. CAR T-cell therapy: full speed ahead. Hematol Oncol 2019; 37(Suppl. 1): 95–100. [DOI] [PubMed] [Google Scholar]
- 23. Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008; 112: 2261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harding FA, McArthur JG, Gross JA, et al. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992; 356: 607–609. [DOI] [PubMed] [Google Scholar]
- 25. Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996; 14: 233–258. [DOI] [PubMed] [Google Scholar]
- 26. Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 2010; 16: 1245–1256. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther 2007; 15: 825–833. [DOI] [PubMed] [Google Scholar]
- 28. Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008; 14: 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen MC, Riddell SR. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev 2014; 257: 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Stegen SJC, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 2015; 14: 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 2009; 9: 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guedan S, Chen X, Madar A, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 2014; 124: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hombach AA, Heiders J, Foppe M, et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology 2012; 1: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol 2002; 20: 70–75. [DOI] [PubMed] [Google Scholar]
- 35. Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011; 121: 1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yvon E, Del VM, Savoldo B, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res 2009; 15: 5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Enblad G, Karlsson H, Loskog AS. CAR T-cell therapy: the role of physical barriers and immunosuppression in lymphoma. Hum Gene Ther 2015; 26: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009; 106: 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen F, Fan C, Gu X, et al. Construction of anti-CD20 single-chain antibody-CD28-CD137-TCRζ recombinant genetic modified T cells and its treatment effect on B cell lymphoma. Med Sci Monit 2015; 21: 2110–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilkie S, Picco G, Foster J, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol 2008; 180: 4901–4909. [DOI] [PubMed] [Google Scholar]
- 41. Zhao Y, Wang QJ, Yang S, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol 2009; 183: 5563–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther 2007; 18: 712–725. [DOI] [PubMed] [Google Scholar]
- 43. Chang L, Chang WC, McNamara G, et al. Transgene-enforced co-stimulation of CD4+ T cells leads to enhanced and sustained anti-tumor effector functioning. Cytotherapy 2007; 9: 771–784. [DOI] [PubMed] [Google Scholar]
- 44. Wang J, Press OW, Lindgren CG, et al. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes. Mol Ther 2004; 9: 577–586. [DOI] [PubMed] [Google Scholar]
- 45. Zhong X-S, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 2010; 18: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chmielewski M, Kopecky C, Hombach AA, et al. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011; 71: 5697–5706. [DOI] [PubMed] [Google Scholar]
- 48. Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012; 119: 4133–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kerkar SP, Goldszmid RS, Muranski P, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011; 121: 4746–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res 2010; 70: 6725–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pritchard MT, Wolf SF, Kraybill WF, et al. The anti-tumor effect of interleukin-12 is enhanced by mild (fever-range) thermal therapy. Immunol Invest 2005; 34: 361–380. [DOI] [PubMed] [Google Scholar]
- 52. Zhang L, Kerkar SP, Yu Z, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 2011; 19: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013; 122: 4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turtle CJ, Hanafi L-A, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126: 2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3: 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7: 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017; 377: 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018; 379: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015; 33: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 2017; 25: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kochenderfer JN, Somerville R, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 2017; 25: 2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shalabi H, Kraft IL, Wang H-W, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica 2018; 103: e215–e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ye B, Stary CM, Li X, et al. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer 2018; 17: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ghorashian S, Pule M, Amrolia P. CD19 chimeric antigen receptor T cell therapy for haematological malignancies. Br J Haematol 2015; 169: 463–478. [DOI] [PubMed] [Google Scholar]
- 68. Hay KA, Turtle CJ. Chimeric antigen receptor (CAR) T cells: lessons learned from targeting of CD19 in B-cell malignancies. Drugs 2017; 77: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rayes A, McMasters RL, O’Brien MM. Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer 2016; 63: 1113–1115. [DOI] [PubMed] [Google Scholar]
- 70. Zoghbi A, Stadt UZ, Winkler B, et al. Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement. Pediatr Blood Cancer. Epub ahead of print 28 April 2017. DOI: 10.1002/pbc.26594. [DOI] [PubMed] [Google Scholar]
- 71. Nagel I, Bartels M, Duell J, et al. Hematopoietic stem cell involvement in BCR-ABL1-positive ALL as a potential mechanism of resistance to blinatumomab therapy. Blood 2017; 130: 2027–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jacoby E, Nguyen SM, Fountaine TJ, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun 2016; 7: 12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Evans AG, Rothberg PG, Burack WR, et al. Evolution to plasmablastic lymphoma evades CD19-directed chimeric antigen receptor T cells. Br J Haematol 2015; 171: 205–209. [DOI] [PubMed] [Google Scholar]
- 74. Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 2018; 24: 1504–1506. [DOI] [PubMed] [Google Scholar]
- 75. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother 2017; 40: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bagashev A, Sotillo E, Tang C-HA, et al. CD19 alterations emerging after CD19-directed immunotherapy cause retention of the misfolded protein in the endoplasmic reticulum. Mol Cell Biol 2018; 38: e00383-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Valitutti S, Lanzavecchia A. Serial triggering of TCRs: a basis for the sensitivity and specificity of antigen recognition. Immunol Today 1997; 18: 299–304. [PubMed] [Google Scholar]
- 78. Sykulev Y, Joo M, Vturina I, et al. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 1996; 4: 565–571. [DOI] [PubMed] [Google Scholar]
- 79. Davenport AJ, Cross RS, Watson KA, et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci U S A 2018; 115: E2068–E2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A 2005; 102: 9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 2012; 12: 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ramakrishna S, Highfill SL, Walsh Z, et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin Cancer Res 2019; 25: 5329–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weijtens ME, Hart EH, Bolhuis RL. Functional balance between T cell chimeric receptor density and tumor associated antigen density: CTL mediated cytolysis and lymphokine production. Gene Ther 2000; 7: 35–42. [DOI] [PubMed] [Google Scholar]
- 84. Turatti F, Figini M, Balladore E, et al. Redirected activity of human antitumor chimeric immune receptors is governed by antigen and receptor expression levels and affinity of interaction. J Immunother 2007; 30: 684–693. [DOI] [PubMed] [Google Scholar]
- 85. James SE, Greenberg PD, Jensen MC, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol 2008; 180: 7028–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pont MJ, Hill T, Cole GO, et al. γ-secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 2019; 134: 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hombach AA, Rappl G, Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation”. Mol Ther 2013; 21: 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Song DG, Ye Q, Poussin M, et al. A fully human chimeric antigen receptor with potent activity against cancer cells but reduced risk for off-tumor toxicity. Oncotarget 2015; 6: 21533–21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lanitis E, Poussin M, Hagemann IS, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther 2012; 20: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 2015; 7: 275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sommermeyer D, Hill T, Shamah SM, et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia 2017; 31: 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jaspers JE, Brentjens RJ. Development of CAR T cells designed to improve antitumor efficacy and safety. Pharmacol Ther 2017; 178: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kerkar SP, Leonardi AJ, van Panhuys N, et al. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther 2013; 21: 1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med 2007; 13: 1440–1449. [DOI] [PubMed] [Google Scholar]
- 95. Ren J, Liu X, Fang C, et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res 2017; 23: 2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Torikai H, Reik A, Liu PQ, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 2012; 119: 5697–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 2017; 9: eaaj2013. [DOI] [PubMed] [Google Scholar]
- 98. Liu X, Zhang Y, Cheng C, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res 2017; 27: 154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Amrolia PJ, Wynn R, Hough R, et al. Simultaneous targeting of CD19 and CD22: phase I study of AUTO3, a bicistronic chimeric antigen receptor (CAR) T-Cell therapy, in pediatric patients with relapsed/refractory B-cell Acute Lymphoblastic Leukemia (r/r B-ALL): amelia study. Blood 2018; 132(Suppl. 1): 279. [Google Scholar]
- 100. Qin H, Ramakrishna S, Nguyen S, et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics 2018; 11: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Qin H, Nguyen SM, Ramakrishna S, et al. Novel CD19/CD22 bicistronic chimeric antigen receptors outperform single or bivalent cars in eradicating CD19+CD22+, CD19-, and CD22- pre-B leukemia. Blood 2017; 130(Suppl. 1): 810. [Google Scholar]
- 102. Shah NN, Zhu F, Taylor C, et al. A phase 1 study with point-of-care manufacturing of dual targeted, tandem anti-CD19, anti-CD20 chimeric antigen receptor modified T (CAR-T) cells for relapsed, refractory, non-Hodgkin lymphoma. Blood 2018; 132: 4193. [Google Scholar]
- 103. Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest 2016; 126: 3814–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. John LB, Devaud C, Duong CP, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 2013; 19: 5636–5646. [DOI] [PubMed] [Google Scholar]
- 105. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yao X, Ahmadzadeh M, Lu Y-C, et al. Levels of peripheral CD4+FoxP3+ regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 2012; 119: 5688–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lutsiak MEC, Semnani RT, De Pascalis R, et al. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005; 105: 2862–2868. [DOI] [PubMed] [Google Scholar]
- 108. Nishio N, Dotti G. Oncolytic virus expressing RANTES and IL-15 enhances function of CAR-modified T cells in solid tumors. Oncoimmunology 2015; 4: e988098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang LX, Westwood JA, Moeller M, et al. Tumor ablation by gene-modified T cells in the absence of autoimmunity. Cancer Res 2010; 70: 9591–9598. [DOI] [PubMed] [Google Scholar]
- 110. Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016; 127: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kondo K, Shaim H, Thompson PA, et al. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia 2018; 32: 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122: 2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Winqvist M, Asklid A, Andersson PO, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish chronic lymphocytic leukemia group. Haematologica 2016; 101: 1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol 2014; 15: 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2017; 130: 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]