Abstract
Background:
Previous studies have shown low adherence to the recommendation to repeat point-of-care glucose (POCG) within 15 minutes following the treatment of inpatient hypoglycemia. We sought to evaluate whether patient and clinical factors may predict time-to-repeat (TTR) POCG following hypoglycemic events in hospitalized adult patients.
Methods:
This was a retrospective cross-sectional analysis of 22 226 index hypoglycemic (≤70 mg/dL) readings (of 993 395 total POCG samples) from 6226 hospital admissions within the Johns Hopkins Health System over three years. Time-to-repeat was defined as the difference in time (minutes) between the index POCG and the next POCG sample. Multivariable logistic regression was used to evaluate the association of TTR with clinical, patient, and hospital factors.
Results:
The median (IQR) TTR was 49 (25-119) minutes, and 14.1% of index POCGs had a TTR ≤15. Severity of hypoglycemia, intensive care unit (ICU), intermediate care (IMC) and pediatrics admissions, and dextrose or glucagon administration were associated with higher adjusted odds of TTR ≤15 minutes. Admission to community hospitals, procedural units, surgery, and labor and delivery was associated with lower adjusted odds of TTR ≤15 minutes. Age, sex, insulin on board, secretagogue use, diabetes type, nutritional status, previous POCG value, and glycemic variability were not significantly associated.
Conclusion:
There is low adherence to the recommendation to repeat a POCG within 15 minutes following the treatment of inpatient hypoglycemia, which may be mediated by both patient and hospital factors. Further studies are needed to understand the mediators and implications of this practice variability.
Keywords: point-of-care, inpatient, hypoglycemia, time-to-repeat, glucose
Introduction
Hypoglycemia is prevalent among hospitalized patients, with 6.3% and 5.7% of patient-days having at least one hypoglycemic episode in intensive care unit (ICU) and non-ICU settings, respectively.1 Within five adult hospitals in our health system (Johns Hopkins Medicine), rates of inpatient hypoglycemia, defined as a blood glucose (BG) ≤70 mg/dL range from 4.0% to 5.4% of patient-days, averaged over one year. While a definite causal relationship has not been established, both iatrogenic and spontaneous hypoglycemia are associated with adverse clinical outcomes, including increased mortality, morbidity, and length of hospital stay.2-7
Consistent with clinical practice guidelines, most hospital policies recommend the “15-15 rule” in their treatment protocols for hypoglycemia, which consists of administration of 15 g of rapid-acting carbohydrate with repeat point-of-care glucose (POCG) in 15 minutes.8-11 For patients who are unable to consume oral glucose due to their current nutritional status or because of neurocognitive impairment from hypoglycemia, administration of intravenous dextrose and/or glucagon are recommended for prompt restoration of normal glucose.9,11,12
Several studies have shown that adherence to the recommendation to repeat a POCG within 15 minutes following hypoglycemia treatment is low.13-20 Studies have shown a direct association between the hypoglycemic POCG value and the time-to-repeat (TTR) POCG,13,14,20 indicating that primary healthcare responders (typically nurses) take into account the severity of hypoglycemia in determining the acuity of their response; however, whether patient or hospital factors besides the hypoglycemic POCG value itself are associated with TTR has not been well studied. One study found that median TTR differed by diabetes type and insulin use, but a formal statistical analysis was not provided.17 We suspect that other patient and systems’ factors beyond the hypoglycemic value influence the healthcare team’s responsiveness to a hypoglycemic episode.
In this study, we sought to evaluate clinical factors that are associated with TTR in hospitalized patients with hypoglycemia. We hypothesized that the severity of hypoglycemia, the use of glucose-lowering medications, the presence of diabetes, and the patient’s nutritional status at the time of hypoglycemia would be associated with the TTR. Specifically, we postulated that there would be an inverse relationship between TTR when progressing from categories of mild/moderate hypoglycemia (BG 54-70 mg/L), clinically significant hypoglycemia (BG 40-54 mg/dL), and severe hypoglycemia (BG <40 mg/dL), and that the TTR would be lower in (a) inpatients with iatrogenic hypoglycemia (ie, due to insulin or sulfonylurea therapy) compared to spontaneous hypoglycemia, (b) patients with known diabetes, and (c) in patients who are nil per os (NPO) at the time of hypoglycemic events.
Methods
Design
This was a retrospective cross-sectional analysis of 6226 admissions of 5234 unique adult patients hospitalized at five hospitals within the Johns Hopkins Health System, which is composed of two academic and three community hospitals in the Maryland/District of Columbia region, from January 1, 2015 to July 31, 2018. The study flowchart is shown in Figure 1. A total of 993 395 POCG samples were collected from 30 425 adult patient admissions with at least five POCG readings during hospitalization. After excluding normoglycemic readings (BG >70 mg/dL), the last POCG measurement of admission (since the outcome of time-to-repeat BG could not be ascertained), possibly spurious POCG readings (defined as a BG >70 mg/dL occurring within five minutes of a hypoglycemic reading),15,21 and POCG outliers (based on manufacturer’s specified limits of detection of 10 to 600 mg/dL for the Nova StatStrip blood glucose monitoring system used throughout the health system), we included 20 226 index hypoglycemic POCG values in the analysis. The study was approved by the Institutional Review Board of the Johns Hopkins School of Medicine.
Figure 1.
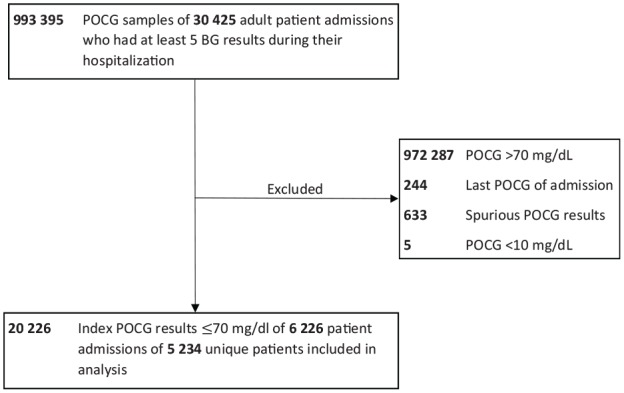
Study flowchart.
BG, blood glucose; POCG, point-of-care glucose.
Data Sources
Relevant clinical factors that can influence either glycemic control or the perceived severity or acuity of a hypoglycemic POCG reading were extracted from our health system’s common electronic health record, EpicCare (Epic Systems Corporation), by the Johns Hopkins Clinical Analytics team. Demographics, anthropometrics, admission and discharge diagnoses, admitting service, medications, glucose data, and diet orders were collected. The classification of diabetes type was based on relevant International Classification of Diseases (ICD)-9 and ICD-10 codes on the problem list, past medical history, or admission diagnoses at the time of admission (supplemental Table S1). The primary discharge diagnosis was collected and reported according to the ICD code. Since a patient could change locations during their hospitalization, admitting service was defined as to reflect the actual location at the time of the index POCG value. A similar approach was used for diet orders.
Exposure Variables
Several clinical and pharmacological exposures were obtained as potential predictors of TTR. Insulin on board was calculated based on the pharmacologic action of different insulin types and the time of the last administered dose relative to the time of the index POCG value. Specifically, four windows of 36, 24, 12, and 5 hours, respectively, following administration of subcutaneous insulins were considered to reflect active insulin on board. These four windows were used for, respectively, insulin degludec or U-300 glargine; glargine U-100 or detemir; neutral protamine Hagedorn (NPH)/regular 70/30 or NPH; and novolog or humalog. Intravenous regular insulin, whether infused in isolation or as a component of total parenteral nutrition, was considered to be on board if the start and stop time of the infusion overlapped the index POCG time. Insulin on board was treated as a binary variable if one or more units of subcutaneous or intravenous insulin was on board.
Oral secretagogues are rarely used in the inpatient setting according to our inpatient glycemic management guidelines; however, these medications are used for selected inpatients and can contribute to hypoglycemia.22 We defined secretagogue to be on board when the index POCG time overlapped with the 24-hour window following administration of a sulfonylurea or meglitinide. Glucocorticoids can contribute to significant hyperglycemia in the inpatient setting, but are also a risk factor for hypoglycemia when tapered without a concurrent reduction in the insulin dose.23,24 We collected systemic glucocorticoid doses (hydrocortisone, prednisone, prednisolone, methylprednisolone, and dexamethasone) and considered these to be active if the time of index POCG overlapped with the pharmacological duration of action of the specific glucocorticoid as follows: hydrocortisone (10 hours); prednisone, prednisolone, and methylprednisolone (24 hours); and dexamethasone (54 hours).25
Since the treatment of hypoglycemia can influence TTR, we collected dextrose and glucagon data from the medication administration record (MAR) and evaluated administration of either or both following within 15 minutes of the index POCG value. The administration of oral glucose (eg, juice) is not directly entered into our medication record by the healthcare team and we assumed that the treatment of hypoglycemic episodes that did not include dextrose or glucagon would have consisted of oral glucose administration per our health system-wide hypoglycemia policies. Considering that the availability of POCG monitoring devices in a given hospital could influence TTR POCG, we collected information about the number of glucometer devices and the number of glucometer operators at each hospital to calculate a ratio of operators per device. Of note, the nursing staffing models in the five hospitals in our health system largely follow the National Database of Nursing Quality Indicators (NDNQI) benchmarks;26 however, there is some variability in the nurse:patient ratio and nursing hours across and within hospitals that is dependent on the available clinical support, unique patient needs, and case mix index.
Previous studies have demonstrated a strong association between the severity of hypoglycemia and TTR.13,17,20 We evaluated the index POCG as a continuous measure and as a categorical variable according to the International Hypoglycemia Study Group consensus statement as follows: 54 to 70 mg/dL (mild/moderate) and 40 to 53 mg/dL (clinically significant).27 Consistent with other studies, we considered <40 mg/dL to be severe hypoglycemia.1 The coefficient of variation of POCG, a measure of glycemic variability, was calculated as the standard deviation divided by the mean of all POCG measurements from time of admission inclusive of the index POCG value.
Outcome Variable
Time-to-repeat was defined as the difference in time (minutes) between the index POCG and the next POCG value. First, we sought to compare the outcome of TTR ≤15 minutes compared to TTR >15 minutes in order to evaluate predictors related to adherence to the strict hospital policy. Then, we compared increasing time windows for TTR (16-30, 31-45, 46-60, and >60 minutes) each against the control of ≤15 minutes to understand how clinical predictors vary across increasing time windows relative to the treatment standard. We are unable to assess the lag time between the index POCG value and the treatment with oral glucose, since our MAR and/or nursing flowsheets do not capture the administration of oral glucose. Thus, we suspect that the 16- to 30-minute window would more closely capture adherence to the policy of treating and repeating POCG within 15 minutes as it would allow some time for treatment.
Given the possibility that repeated hypoglycemic POCG measurements occurring within close succession represent a single hypoglycemic episode,15 which could influence the situational awareness and responsiveness to each hypoglycemic reading, we performed sensitivity analyses in which repeated hypoglycemic POCG values occurring within 30 minutes, 60 minutes, 2 hours, and the same day were excluded in order to isolate the clinical response for distinct hypoglycemic episodes.
Statistical Analysis
Descriptive statistics were used to summarize the characteristics of the patient population at the admission level and at the time of the index hypoglycemic POCG. Normality of data were assessed using histograms and tests of skewness and kurtosis. As all continuous measures were non-normally distributed, medians and interquartile ranges (IQRs) are reported. Univariate analyses were conducted with each of the binary TTR outcome measures. For TTR ≤15 as an outcome, the control was >15 minutes. For each of the other TTR outcome measures (eg, 16-30 and 31-45 minutes), the control was TTR ≤15 minutes. The univariate analyses were used to identify clinical factors that were significantly associated with the outcome measures (supplemental Tables S2–6). To evaluate differences in nonparametric continuous variables by each outcome measure, the Wilcoxon signed rank test was used. For categorical variables, the chi-square test was performed.
Multivariable logistic regression models were developed for the outcome measures: TTR ≤15, 16-30, 31-45, 45-60, and >60 minutes. Covariates identified from the univariate logistic regression analyses (not reported) to have a P-value <.1 or felt to be clinically relevant in the association between hypoglycemia and the outcome were included in the multivariable logistic regression models. Robust standard error estimates were determined using clustering analysis at the unique patient admission level. Statistical analyses were performed using Stata Statistical Software: Release 15 (College Station, TX, United States). P < .05 was considered statistically significant.
Results
The characteristics of the patients who experienced at least one hypoglycemic POCG reading during admission (N = 6226) are shown in Table 1. The population consisted of older, predominantly white patients with a slight female predominance. Despite the higher total bed size of the two academic hospitals combined (1625 beds), compared to the three community hospitals combined (784 beds), the prevalence of hypoglycemia was not proportional to bed size, suggesting higher overall rates of hypoglycemia in community hospitals. The majority of hypoglycemic episodes (57.9%) occurred in patients who did not have diabetes (ie, spontaneous hypoglycemia), followed by type 2 diabetes (37.2%) and type 1 diabetes (3.2%). Hypoglycemia was documented as a discharge diagnosis in only 2057 (33%) of the index hypoglycemic readings. At the academic hospitals, there were 544 POCGs used by 6650 operators; at the community hospitals, there were 157 POCGs used by 2846 operators. Accordingly, the ratios of operators per glucometer at the academic and community hospitals were 12.2 and 18.1, respectively.
Table 1.
Patient Characteristics at Admission Level.
| Factor | Value |
|---|---|
| Patient admissions | 6226 |
| Total hypoglycemic POCG readings in cohort | 20 226 |
| Hypoglycemic POCG readings per patient admission, median (IQR) | 2 (1-4) |
| Discharge diagnosis of hypoglycemia, n (%) | 2057 (33.0) |
| Age (years), median (IQR) | 63.0 (49.0-74.0) |
| Sex: Male, n (%) | 2895 (46.5) |
| Race, n (%) | |
| White | 2924 (47.0) |
| Black | 2519 (40.5) |
| Other | 783 (12.6) |
| Body mass index (kg/m2), median (IQR) | 26.8 (22.7-32.2) |
| Length of stay (days), median (IQR) | 6.1 (3.5-11.7) |
| Hospital type, n (%) | |
| Academic | 3415 (54.9) |
| Community | 2811 (45.1) |
| Diagnosis of diabetes at admission, n (%) | |
| None | 3606 (57.9) |
| Type 1 diabetes | 197 (3.2) |
| Type 2 diabetes | 2316 (37.2) |
| Other types | 107 (1.7) |
| Primary discharge diagnosis, n (%) | |
| Diseases of the circulatory system | 971 (15.6) |
| Endocrine, nutritional and metabolic diseases | 966 (15.5) |
| Certain infectious and parasitic diseases | 680 (10.9) |
| Injury, poisoning, and certain other consequences of external causes | 705 (11.3) |
| Neoplasms/blood diseases | 393 (6.3) |
| Mental, behavioral, and neurodevelopmental disorders | 224 (3.6) |
| Diseases of the nervous system | 200 (3.2) |
| Diseases of the respiratory system | 329 (5.3) |
| Diseases of the digestive system | 522 (8.4) |
| Diseases of the musculoskeletal system and connective tissue | 253 (4.1) |
| Diseases of the genitourinary system | 320 (5.1) |
| Other | 654 (10.5) |
Abbreviations: IQR, Interquartile range (25th-75th percentile); POCG, point of care glucose.
Table 2 shows patient and admission characteristics for the index hypoglycemic POCG readings (N = 20 226). The majority occurred on medicine (30.6%), surgery (15.4%), or ICU (20.7%) services. There was a relatively even distribution of diet types, with approximately one-third each receiving NPO, carbohydrate controlled, and regular diets. There was a low prevalence of glucocorticoid use (6.8%). Insulin was on board for approximately (38%) of hypoglycemic readings. Consistent with clinical practice guidelines,11 very few (3.5%) of patients were on oral secretagogues at the time of hypoglycemia.
Table 2.
Patient Characteristics at Time of Index Hypoglycemic Point of Care Glucose Readings.
| Factor | N = 20 226 index POCG samples |
|---|---|
| Admitting service at time of BG results, n (%) | |
| Medicine | 6176 (30.6) |
| Emergency room | 2485 (12.3) |
| Intensive care unit | 4175 (20.7) |
| Intermediate care unit | 2238 (11.1) |
| Labor and delivery/obstetrics | 723 (3.6) |
| Pediatrics | 231 (1.1) |
| Psychiatry | 477 (2.4) |
| Operating room | 308 (1.5) |
| Procedural | 243 (1.2) |
| Surgery | 3114 (15.4) |
| Diet, n (%) | |
| Regular diet | 5770 (28.5) |
| Carbohydrate controlled | 5322 (26.3) |
| NPO or clear liquid | 5947 (29.4) |
| Other or unknown | 3187 (15.8) |
| Medications on board, n (%) | |
| Glucocorticoid | 1382 (6.8) |
| Insulin | 7674 (37.9) |
| Secretagogue | 702 (3.5) |
| Hypoglycemia treatment medications,a n (%) | |
| Dextrose oral gel | 50 (0.2) |
| Dextrose IV | 1129 (5.6) |
| Glucagon IM | 52 (0.3) |
| Dextrose and/or glucagon | 1227 (6.1) |
| POCG summary measures | |
| Index POCG value, mg/dL, median (IQR) | 61 (52-67) |
| Any previous POCG ≤70 mg/dL during admission, n (%) | 14 090 (69.7) |
| Previous POCG value, median (IQR) | 81.0 (64.0-111.0) |
| Admission coefficient of variation of POCG, median (IQR) | 0.4 (0.3-0.5) |
| Index hypoglycemic category, n (%) | |
| 54-70 mg/dL | 14 507 (71.7) |
| 40-53 mg/dL | 3954 (19.5) |
| <40 mg/dL | 1765 (8.7) |
| Outcome measure | |
| Time-to-repeat POCG, min, median (IQR) | 49 (25-119) |
Abbreviations: BG, blood glucose; IQR, Interquartile range (25th-75th percentile); IM, intramuscular; IV, intravenous; NPO, nil per os; POCG, point of care glucose.
Within 15 minutes of index POCG value.
The median (IQR) hypoglycemic value was 61 (52-67) mg/dL, with 71.7%, 19.5%, and 8.8% occurring within the mild/moderate, clinically significant, and severe hypoglycemic categories, respectively. There was a very high prevalence of antecedent hypoglycemia in relation to the index POCG, with nearly 70% having had a prior POCG ≤70 mg/dL. There was substantial glycemic variability prior to the index event, with the median (IQR) coefficient of variation of 0.4 (0.3-0.5).
Despite health-system wide policies recommending TTR within 15 minutes of hypoglycemia treatment, the median (IQR) TTR was 49 (25-119) minutes. The median (IQR) TTR for 54-60, 40-53, and <40 mg/dL were 63 (31-157), 32 (18-59), and 20 (7-36) minutes, respectively. Of the 20 226 index hypoglycemic events, 2853 (14.1%) had a TTR ≤15 minutes. Dextrose, glucagon, dextrose, and/or glucagon were administered within 15 minutes following 5.8%, 0.3%, and 6.1% of index POCG readings, respectively.
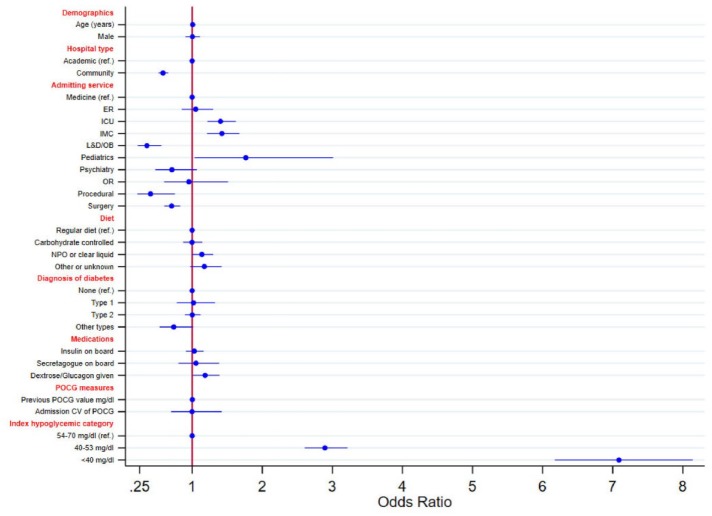
Figure 2 shows the adjusted odds ratios (aORs) for each of the clinical factors with TTR ≤15 minutes. Variables most strongly associated with this outcome measure were severity of index POCG value, with severe and clinically significant hypoglycemia being associated with 7.09 (95% CI, 6.19-8.12) and 2.89 (95% CI, 2.60-2.3.22) higher aORs, respectively, compared to mild/moderate hypoglycemia. Admission to ICU, intermediate care (IMC), and pediatric services was associated with aORs of 1.40 (95% CI, 1.22-1.62), 1.42 (95% CI, 1.20-1.68), and 1.76 (95% CI, 1.04-3.00), respectively, compared to medical service. Admission to a procedural unit was strongly associated with lower adherence to TTR ≤15 minutes (aOR 0.41; 95% CI, 0.22-0.75). The administration of dextrose and/or glucagon was also associated with aOR of 1.18 (95% CI, 1.02-1.38). Variables associated with lower odds of adherence to the TTR ≤15 minutes were admission to a procedural unit, surgery, and labor and delivery, with 60%, 30%, and 65% lower aORs compared to medicine, respectively. Admission to a community hospital was associated with a 42% lower odds ratio compared to academic hospitals. There was a nonsignificant trend (P = .051) in patients who were NPO or on a clear liquid diet at the time of the index event (aOR 1.39; 95% CI, 1.00-1.30).
Figure 2.
Adjusted odds ratio of TTR ≤15 minutes by patient and hospital characteristics.
CV, coefficient of variation; ER, emergency room; ICU, intensive care unit; IMC, intermediate care unit; L&D/OB, labor and delivery/obstetrics; NPO, nil per os; OR, operating room; POCG, point of care glucose; TTR, time-to-repeat.
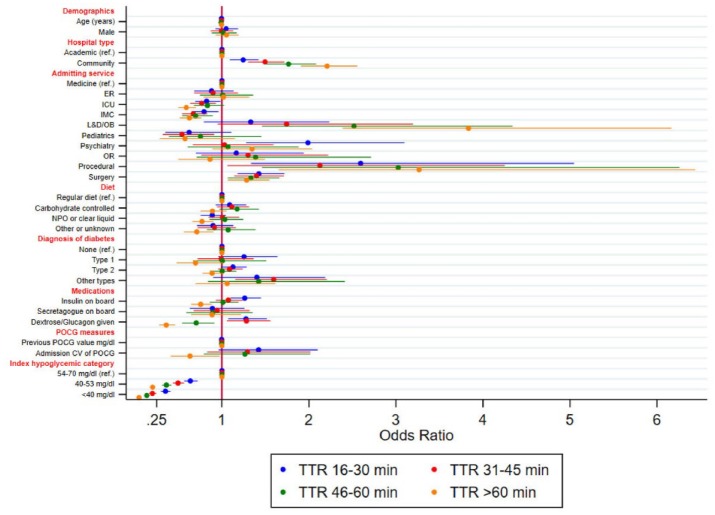
Figure 3 shows the adjusted association of each of the clinical predictors with increasing time windows relative to TTR ≤15 minutes as the control. Index hypoglycemic category, hospital type, and admitting service were strongly associated with all TTR outcome measures. Diabetes type, insulin on board, and treatment with dextrose and/or glucagon were associated with some, but not all, TTR outcome measures. Not surprisingly, severity of hypoglycemia was the strongest predictor of TTR, with a direct relationship between POCG value and increasing TTR. Compared with mild/moderate hypoglycemia, the aORs of severe hypoglycemia for the TTR outcomes of 16-30, 31-45, 46-60, and >60 minutes were 0.35 (95% CI, 0.30-0.41), 0.20 (95% CI, 0.17-0.24), 0.14 (95% CI, 0.11-0.17), and 0.05 (95% CI, 0.04-0.06), respectively. Clinically significant hypoglycemia was also strongly associated with all TTR outcome measures, with aORs ranging from 0.20 to 0.64 compared to mild/moderate hypoglycemia. Hospital type was also a strong predictor, with increasing aORs observed with increasing TTR windows. Compared to academic hospitals, admission to a community hospital was associated with aORs of 1.25, 1.50, 1.77, and 2.21, respectively, for TTR 16-30, 31-45, 46-60, and >60 minutes. Generally, admission to the IMC or ICU was associated with lower aORs for each of the TTR outcomes compared to medical services, while admission to a procedural unit was directly associated with increasing TTR windows (TTR >60 minutes: aOR 3.27; 95% CI, 1.66-6.44).
Figure 3.
Adjusted odds ratios of increasing TTR windows by patient and hospital characteristics.
CV, coefficient of variation; ER, emergency room; ICU, intensive care unit; IMC, intermediate care unit; L&D/OB, labor and delivery/obstetrics; NPO, nil per os; OR, operating room; POCG, point of care glucose; TTR, time-to-repeat.
Interestingly, we did not observe a clear association between diabetes type and the increasing TTR windows. Relative to the control of TTR ≤15 minutes, insulin on board was associated with a higher odds of TTR of 16 to 30 minutes (1.26; 95% CI 1.10-1.45), but lower odds of TTR >60 minutes (0.76; 95% CI, 0.65-0.88). With respect to nutritional status at the time of the index event, NPO or clear liquid diet was associated with a lower aOR for TTR >60 minutes (0.77; 95% CI, 0.66-0.90). Nutritional status was otherwise not associated with the other TTR outcome measures. Relative to TTR ≤15 minutes, the administration of dextrose or glucagon was associated with higher aOR for TTR 16 to 31 minutes (1.28; 95% CI, 1.08-1.51) and TTR 31 to 45 minutes (1.28; 95% CI, 1.06-1.55), and lower aOR for TTR 46 to 60 minutes (0.71; 95% CI, 0.54-0.91) and TTR >60 minutes (0.36; 95% CI 0.28-0.46).
The results of our sensitivity analyses, which excluded repeated hypoglycemic POCG measurements within varying time windows relative to the index event for all of the TTR outcome measures, demonstrated similar findings and did not substantially change the inferences of our analyses (supplemental Figures S1–8).
Discussion
In this study, we found that there was generally low adherence to the hospital policy to promptly repeat a POCG following hypoglycemia treatment, with only approximately 14% having TTR within 15 minutes. Even when allowing 5 minutes for treatment and 15 minutes for repeat, only 20% of index POCGs had a TTR within 20 minutes. The median (IQR) TTR was 49 (25-115) minutes. In this regard, our findings align with the previous studies showing very low rates of adherence to this component of the hypoglycemia treatment protocol for hospitalized patients.13-18 A study from the United Kingdom conducted from 2008 to 2013 found 8.9% had a repeat POCG within 15 minutes and a median (IQR) TTR of 80 (36-249) minutes,13 indicating even lower rates of adherence compared to our US based study. Another study found that only 9% of patients had a TTR within 10 to 20 minutes of a hypoglycemic episode.16
As we had hypothesized, the severity of hypoglycemia was a strong predictor of TTR. When moving from categories of mild/moderate to clinically significant to severe hypoglycemia, the median TTR declined from 63, 32, to 20 minutes, respectively. While it is difficult to directly compare our results to other studies given variability in definitions of severe hypoglycemia, this pattern of shorter TTR with declined BG values has been observed in other hospital settings. Bilhimer et al found that the TTR was 12 (IQR 6-27.8) minutes in emergency department patients with BG ≤50 mg/dL.28 Deetz et al found that critical action value glucose readings (<45 mg/dL) in the inpatient setting were followed by a median TTR of ~17 minutes.14
We did not confirm our hypothesis that insulin therapy, diagnosis of diabetes, and NPO status would be strong predictors of TTR. We did, however, identify other factors that we had initially suspected to be related to TTR, such as hospital type and admitting service. For the outcome of TTR ≤15 minutes, the most important factors were severity of hypoglycemia, hospital type, admitting service, and treatment with IV dextrose and glucagon. Severe hypoglycemia (POCG <40 mg/dL) was the strongest predictor, with sevenfold higher odds of TTR ≤15 minutes.
We found lower odds of TTR ≤15 minutes in community hospitals compared to academic hospitals. This finding was somewhat unexpected and may reflect less centralized glucose management programs and nursing education around the topic of hypoglycemia compared to large academic medical centers. In our academic hospitals, we have dedicated inpatient diabetes management services, which provide not only direct patient care but also extensive education and outreach to nursing staff29,30 regarding hypoglycemia prevention and management. At our community hospitals, most inpatients are managed by hospitalists who may have less expertise compared to inpatient diabetes specialists in adjusting insulin to prevent hypoglycemia. Accordingly, higher overall rates of hypoglycemia in our community hospitals may lead to lower rates of adherence to the hypoglycemia treatment policy. An alternative explanation may be that the higher ratio of operators per glucometers at the community hospital (a reflection of resources) resulted in delays in obtaining a POCG device in a timely fashion.
Not surprisingly, admission to intensive care or intermediate care units was associated with higher adherence to TTR ≤15 minutes. The nurse-to-patient ratio in intensive care and intermediate care units may explain the shorter TTR on these admitting services, as well as greater severity of illness and acuity overall (which were not directly measured in this study). The finding of longer TTR in obstetrics may reflect perceived physiological response of hypoglycemia during labor or in the peripartum period. Notably, obstetrical patients with hypoglycemia are excluded from our hospital-wide hypoglycemia policy and are managed by a specific unit-based glucose management protocol. We also found significantly lower adherence to TTR ≤15 minutes in our procedural units, which included pre-operative units and interventional radiology units. The reason for this is not readily apparent. One explanation may be competing nursing-related tasks and priorities for these patients who are often followed for shorter periods of time compared to inpatients on medical or surgical units. There could also be differential training on the hypoglycemia policy delivered to procedural or pre-op nurses compared to floor nurses.
Even after adjustment for severity of hypoglycemia, administration of dextrose or glucagon was independently associated with TTR ≤15 minutes, which may be related to (a) nursing perception that hypoglycemia may be corrected more quickly with dextrose or glucagon compared to oral glucose or (b) the fact that patients who are unable to take oral glucose may be perceived to be sicker and/or deemed to be at higher risk from hypoglycemia. As we were unable to determine whether patients were symptomatic or neurocognitively impaired at the time of the hypoglycemic episode, it is possible that the use of dextrose/glucagon is a marker of symptomatic hypoglycemia. In our analyses using increasing TTR windows, we identified insulin use and nutritional status to be associated with some but not all outcomes. Generally, our findings support the observation that insulin use or being NPO is associated with lower odds for longer TTR, consistent with the perception that such clinical factors would confer greater risk for persistent or progressive hypoglycemia if untreated.
Considering that the neuroadrenergic signs and symptoms of hypoglycemia can develop quickly with declining BG, there are multiple potential negative implications of delayed TTR in the hospital (eg, cognitive impairment, patient distress, dissatisfaction, arrhythmias, and seizures).31 Further qualitative studies using structured interviews or focused group sessions could be conducted to better understand whether and how nurses consider patient clinical factors when determining the timing of their response to hypoglycemia, and/or whether systems’ factors (eg, staffing constraints) play a role in their ability to respond promptly. Since the current inpatient guidelines recommend prompt treatment of hypoglycemia,9,10,12 quality improvement interventions are needed to increase awareness and nursing education about the importance of adhering to a TTR within 15 minutes of treatment.32 Another potential approach would be to use automated computerized alarms to page nurses within 15 minutes following a hypoglycemic POCG. Since the nurse would already be aware of the hypoglycemic reading, such an alert would improve adherence not by creating situational awareness, but rather by providing time tracking support, which is useful for a busy nurse managing multiple patients with competing priorities.
To our knowledge, we are not aware of any studies that have systematically evaluated clinical predictors of TTR in hypoglycemic hospitalized patients. The strengths of this study include the large sample size across multiple academic and community hospitals in a health system, which increases the generalizability of these findings. By processing the medication administration data around pharmacological duration of action of insulin and glucocorticoids, we were able to assess the presence of active medications at the time of the index hypoglycemic event. We were also able to assess nutrition status in relation to the index event. Our sensitivity analyses attempted to address the issue of repeated low POCGs as part of a single clinically relevant hypoglycemic episode. The main limitation of this retrospective study was the absence of administration information regarding oral glucose, which required us to make an assumption about the time required to treat prior to repeat POCG. We did not have a data about severity of illness score; however, we believe that admitting service can be used as a proxy. We were not able to confirm the source of blood for the POCG (eg, capillary, venous, and arterial), which may introduce some variability in sample measurement33; however, we anticipate that the vast majority of POCG samples are indeed fingerstick capillary samples. Finally, although our nursing staffing models are based on national benchmarks and are generally consistent throughout our five hospitals, we were unable to obtain information regarding nursing-to-patient or nursing hours by patient acuity to evaluate whether nurse staffing was directly associated with our outcome.
Conclusion
There is low adherence to the recommendation to repeat a POCG within 15 minutes of a hypoglycemic reading in hospitalized patients. Hospital factors and severity of hypoglycemia appear to be strongest predictors of TTR. Further studies involving front-line staff may be needed to better understand how and why these factors are related to TTR, and whether increased TTR is associated with adverse patient outcomes.
Supplemental Material
Supplemental material, Abusamaan_SupplementalMaterial for Predictors of Time-to-Repeat Point-of-Care Glucose Following Hypoglycemic Events in Hospitalized Patients by Mohammed S. Abusamaan, David C. Klonoff and Nestoras Mathioudakis in Journal of Diabetes Science and Technology
Acknowledgments
We would like to thank Sam Sokolinsky and Shamil Fayzullin from the Johns Hopkins Health System Quality and Clinical Analytics for their assistance with data extraction from the electronic medical record.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MSA and NM have no conflicts of interest to disclose. DCK is a consultant to Abbott, Ascensia, Eoflow, Lifecare, Merck, Roche, and Voluntis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Nestoras Mathioudakis was supported by grant 1K23DK111986-01 from the National Institute for Diabetes and Digestive and Kidney Diseases
ORCID iD: Mohammed S. Abusamaan  https://orcid.org/0000-0001-9307-1315
https://orcid.org/0000-0001-9307-1315
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853-861. [DOI] [PubMed] [Google Scholar]
- 2. Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36(5):1107-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones GC, Timmons JG, Cunningham SG, Cleland SJ, Sainsbury CAR. Hypoglycemia and clinical outcomes in hospitalized patients with diabetes: does association with adverse outcomes remain when number of glucose tests performed is accounted for? J Diabetes Sci Technol. 2017;11(4):720-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29(12):e445-e448. [DOI] [PubMed] [Google Scholar]
- 6. Akirov A, Grossman A, Shochat T, Shimon I. Mortality among hospitalized patients with hypoglycemia: insulin related and noninsulin related. J Clin Endocrinol Metab. 2017;102(2):416-424. [DOI] [PubMed] [Google Scholar]
- 7. Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108-1118. [DOI] [PubMed] [Google Scholar]
- 8. Slama G, Traynard PY, Desplanque N, et al. The search for an optimized treatment of hypoglycemia. Carbohydrates in tablets, solutin, or gel for the correction of insulin reactions. Arch Intern Med. 1990;150(3):589-593. [PubMed] [Google Scholar]
- 9. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S61-S70. [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S173-S181. [DOI] [PubMed] [Google Scholar]
- 11. Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. [DOI] [PubMed] [Google Scholar]
- 12. Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. [DOI] [PubMed] [Google Scholar]
- 13. Jones GC, Perry CG, Monaghan A, Kennon B, Sainsbury CAR. Capillary blood glucose monitoring, in-patient hypoglycaemia and quality of care. Br J Diabetes Vasc Dis. 2015;15(1):24-26. [Google Scholar]
- 14. Deetz CO, Nolan DK, Scott MG. An examination of the usefulness of repeat testing practices in a large hospital clinical chemistry laboratory. Am J Clin Pathol. 2012;137(1):20-25. [DOI] [PubMed] [Google Scholar]
- 15. Weinberg ME, Bacchetti P, Rushakoff RJ. Frequently repeated glucose measurements overestimate the incidence of inpatient hypoglycemia and severe hyperglycemia. J Diabetes Sci Technol. 2010;4(3):577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anthony M. Treatment of hypoglycemia in hospitalized adults: a descriptive study. Diabetes Educ. 2007;33(4):709-715. [DOI] [PubMed] [Google Scholar]
- 17. Jones GC, Khan J, Sainsbury CAR. Is all hypoglycaemia treated as equal? An observational study of how the type of diabetes and treatment prescribed prior to admission influences quality of treatment of inpatient hypoglycaemia. Acta Diabetol. 2017;54(3):247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maynard GA, Huynh MP, Renvall M. Iatrogenic inpatient hypoglycemia: risk factors, treatment, and prevention: analysis of current practice at an Academic Medical Center with implications for improvement efforts. Diabetes Spectrum. 2008;21(4):241-247. [Google Scholar]
- 19. Coats A, Marshall D. Inpatient hypoglycaemia: a study of nursing management. Nurs Prax N Z. 2013;29(2):15-24. [PubMed] [Google Scholar]
- 20. D’Netto M, Murphy CV, Mitchell A, Dungan K. Predictors of recurrent hypoglycemia following a severe hypoglycemic event among hospitalized patients. Hosp Pract (1995). 2016;44(1):1-8. [DOI] [PubMed] [Google Scholar]
- 21. Corl D, Yin T, Ulibarri M, et al. What can we learn from point-of-care blood glucose values deleted and repeated by nurses? J Diabetes Sci Technol. 2018;12(5):985-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deusenberry CM, Coley KC, Korytkowski MT, Donihi AC. Hypoglycemia in hospitalized patients treated with sulfonylureas. Pharmacotherapy. 2012;32(7):613-617. [DOI] [PubMed] [Google Scholar]
- 23. Mathioudakis N, Dungan K, Baldwin M, Korytkowski M, Reider J. Steroid-associated hyperglycemia. In: Draznin B. (ed) Managing Diabetes and Hyperglycemia in the Hospital Setting, A Clinician’s Guide. Alexandria, VA: American Diabetes Association; 2016. [Google Scholar]
- 24. Kim HN, Mathioudakis N. Management of glucocorticoid-induced diabetes and/or hyperglycemia. In: Matfin G. (ed) Endocrine and Metabolic Medical Emergencies: A Clinician’s Guide. 2nd ed. Hoboken, NJ: John Wiley & Sons Ltd; 2018. [Google Scholar]
- 25. Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655-670. [DOI] [PubMed] [Google Scholar]
- 26. National Database of Nursing Quality Indicators. https://nursingandndnqi.weebly.com/ndnqi-indicators.html. Accessed September 9, 2019.
- 27. Group IHS. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40(1):155-157. [DOI] [PubMed] [Google Scholar]
- 28. Bilhimer MH, Treu CN, Acquisto NM. Current practice of hypoglycemia management in the ED. Am J Emerg Med. 2017;35(1):87-91. [DOI] [PubMed] [Google Scholar]
- 29. Kemmerer T, Bashura H, Dintzis J, Mathioudakis N, Golden SH. The impact of nursing and advanced practice clinicians on the implementation and outcomes of an inpatient glucose management program. AADE Pract. 2015;3(5):16-25. [Google Scholar]
- 30. Munoz M, Pronovost P, Dintzis J, et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Jt Comm J Qual Patient Saf. 2012;38(5):195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17(5):819-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Destree L, Vercellino M, Armstrong N. Interventions to improve adherence to a hypoglycemia protocol. Diabetes Spectr. 2017;30(3):195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sagkal Midilli T, Ergın E, Baysal E, Arı Z. Comparison of glucose values of blood samples taken in three different ways. Clin Nurs Res. 2019;28(4):436-455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Abusamaan_SupplementalMaterial for Predictors of Time-to-Repeat Point-of-Care Glucose Following Hypoglycemic Events in Hospitalized Patients by Mohammed S. Abusamaan, David C. Klonoff and Nestoras Mathioudakis in Journal of Diabetes Science and Technology