Abstract
Setting:
India’s National Tuberculosis Elimination Programme (NTEP) covers diagnostic and therapeutic costs of TB treatment. However, persons living with TB (PLWTB) continue to experience financial distress due to direct costs (payment for testing, treatment, travel, hospitalization, and nutritional supplements) and indirect costs (lost wages, loan interest, and cost of domestic helpers).
Objective:
To analyze the magnitude and pattern of TB-related costs from the perspective of Indian PLWTB.
Design:
We identified relevant articles using key search terms (‘tuberculosis,’ ‘India,’ ‘cost,’ ‘expenditures,’ ‘financing,’ ‘catastrophic’ and ‘out of pocket’) and calculated variance-weighted mean costs.
Results:
Indian patients incur substantial direct costs (mean: US$46.8). Mean indirect costs (US$666.6) constitute 93.4% of the net costs. Mean direct costs before diagnosis can be up to four-fold that of costs during treatment. Treatment in the private sector can result in costs up to six-fold higher than in government facilities. As many as one in three PLWTB in India experience catastrophic costs.
Conclusion:
PLWTB in India face high direct and indirect costs. Priority interventions to realize India’s goal of eliminating catastrophic costs from TB include decreasing diagnostic delays through active case finding, reducing the need for travel, improving awareness and perception of NTEP services, and ensuring sufficient reimbursement for inpatient TB care.
Keywords: catastrophic cost, out-of-pocket, distress financing, coping, indirect costs
Abstract
Contexte :
Le programme national d’élimination de la tuberculose (NTEP) d’Inde couvre les coûts du diagnostic et du traitement de la TB. Les personnes vivant avec le TB (PLWTB) sont cependant toujours confrontées à des graves difficultés financières dues aux coûts directs (paiement des tests, du traitement, des trajets, de l’hospitalisation et des compléments nutritionnels) et aux coûts indirects (perte de revenus, intérêts du prêt, et coût des aides à domicile).
Objectif :
Analyser la magnitude et le profil des coûts liés à la TB selon la perspective des PLWTB en Inde.
Schéma :
Nous avons identifié les articles pertinents en utilisant les mots clés (« tuberculosis », « India », « cost», « expenditures », « financing », « catastrophic » et « out of pocket » [tuberculose, Inde, coût, dépense, financement, catastrophique, paiement direct]) et nous avons calculé les coûts moyens en variance pondérée.
Résultats :
Les patients indiens subissent des coûts directs substantiels (moyenne : US$46,8). Les coûts indirects moyens (US$666,6) constituent 93,4% des coûts nets. Les coûts directs moyens avant le diagnostic peuvent être quatre fois plus élevés que les coûts au cours du traitement. Le traitement dans le secteur privé peut coûter jusqu’à six fois plus que dans les structures publiques. Jusqu’à un tiers des PLWTB en Inde subissent des coûts catastrophiques.
Conclusion :
Les PLWTB en Inde font face à des coûts directs et indirects élevés. Les interventions prioritaires à mettre en œuvre pour réaliser l’objectif de l’Inde d’éliminer les coûts catastrophiques liés à la TB incluent la réduction du délai de diagnostic grâce à une recherche active des cas, la diminution des besoins de trajets, l’amélioration de la sensibilisation et de la perception des services NTEP et l’assurance d’un remboursement suffisant pour la prise en charge en hôpital de la TB.
Abstract
Marco de Referencia:
El Programa Nacional de Eliminación de la Tuberculosis de la India (NTEP) cubre los costos del diagnóstico y el tratamiento de la TB. Sin embargo, las personas con TB (PLWTB) aún afrontan dificultades económicas debido a los costos directos (pago de las pruebas, el tratamiento, los desplazamientos, la hospitalización y los suplementos nutricionales) y los costos indirectos (salarios perdidos, intereses de préstamos y remuneración de empleados domésticos).
Objetivo:
Analizar la magnitud y el tipo de costos relacionados con la TB desde la perspectiva de las PLWTB en la India.
Método:
Se encontraron artículos pertinentes utilizando términos clave de búsqueda (“tuberculosis”, “India”, “cost”, “expenditures”, “financing”, “catastrophic” y “out of pocket” [tuberculosis, India, costos, gastos, financiación, catastróficos y pago directo]) y se calcularon los costos promedio ponderados por la varianza.
Resultados:
En la India, los pacientes asumen gastos directos considerables (promedio: US$46,80). Los costos indirectos promedio (US$666,60) constituyen el 93,4% de los costos netos. Los costos directos promedio antes del diagnóstico pueden corresponder hasta cuatro veces los costos durante el tratamiento. En el sector privado, los costos del tratamiento pueden ser hasta seis veces más altos que en los establecimientos gubernamentales. Una de cada tres PLWTB en la India afronta gastos catastróficos.
Conclusión:
Las PLWTB en la India afrontan altos costos directos e indirectos. Entre las intervenciones prioritarias que contribuirían a lograr el objetivo de eliminar los costos catastróficos por TB en la India se cuentan el acortar los retrasos diagnósticos mediante la búsqueda activa de casos, disminuir la necesidad de desplazarse, promover la familiarización con los servicios del NTEP y mejorar su percepción y garantizar el reembolso adecuado de la atención hospitalaria de la TB.
Mycobacterium tuberculosis is the most lethal infectious organism worldwide.1 In 2018, an estimated 10 million individuals worldwide developed TB and 1.2 million people died from the disease. India accounts for 27% of the world’s TB cases. Historically, India’s government healthcare expenditure has been around 1% of its gross domestic product (GDP).2,3 The World Bank estimated in 2015 that 176 million Indians (13.4% of the population) continue to subsist on daily incomes less than US$1.90 per day.4 In India, TB is four times more prevalent in the bottom socio-economic quintile than in the top quintile, and 21.3% of Indian people living with TB (PLWTB) need distress financing—defined as taking loans, selling assets, or receiving contributions from friends and relatives for health care-related expenses—due to high out-of-pocket (OOP) costs.5,6
The Indian government’s National TB Elimination Program (NTEP) offers free TB diagnosis and medical care.2 Nevertheless, the private sector remains an important source of TB care: the National Family Health Survey-4 (2015–2016) found that 38.8% (95% confidence interval [CI] 36.5–41.1) of PLWTB sought care from outside the public sector.7 This rate may be higher in some parts of the country; studies from the states of Tamil Nadu and Karnataka reported that 65–70% of PLWTB treated in the public sector initially sought care in the private sector.8,9 A model based on drug sales data from 2013 to 2016 found variation between states, but nationally 64% of patients were treated in the private sector.10
India is expanding government-funded health insurance for the most impoverished, but enrollment has been incomplete and Indians—particularly those in the bottom economic quintile—continue to face high OOP costs for health care.11,12 Although the government’s Pradhan Mantri Jan Arogya Yojana provides limited insurance for inpatient therapy for some of the most economically vulnerable Indians, outpatient evaluations and therapy are not covered, and reimbursement for TB-related hospitalizations is poor.7 Furthermore, enrollment rates remain low, and the national insurance is not universally accepted by private Indian hospitals.11,12
Due to concerns of inconsistent and suboptimal management in the private sector, the NTEP is pursuing public-private mix (PPM) ventures to optimize TB care and reduce costs in an initiative called Universal Access to TB care.13,14 The PPM model has helped increase notifications. Although some pilot PPM programs such as those in Mehsana, Mumbai, Maharashtra, and Patna, Bihar, use free medicines from NTEP, there is no requirement for private providers to necessarily use free medicines provided by NTEP, which results in high, often catastrophic, costs.15
Based on the National Strategic Plan 2017–25, the Indian government aims to eliminate catastrophic costs for PLWTB by 2020 and to achieve 80% reduction in TB incidence and 90% reduction in deaths by 2025.2 With these goals in mind, we analyzed the economic impact of TB for PLWTB in India to identify 1) the average cost per PLWTB; 2) the components of direct and indirect costs, and 3) to consider costs for specific groups of PLWTB (e.g., those with drug-resistant TB). We used these cost data to identify key areas for programmatic intervention.
DESIGN AND METHODS
Search strategy
We conducted a literature search using PubMed, Embase, and Web of Science using the following key search terms: ‘tuberculosis,’ ‘India,’ ‘cost,’ ‘expenditures,’ ‘financing,’ ‘catastrophic’ and ‘out of pocket.’ Studies between January 1, 1950, and January 29, 2020, were included. We also conducted searches on Google Scholar with the same keywords and reviewed the titles and abstracts of the first 200 search results to find relevant articles. The search was limited to articles written in English. Additional references were found by reviewing the bibliographies of articles found through the search. We included studies of individuals with pulmonary TB (PTB) and extrapulmonary TB (EPTB), drug-susceptible and drug-resistant disease, and those cared for in the facilities run by the government, non-government organizations (NGOs), and the private sector.
Cost conversions
Costs were adjusted for inflation using an online calculator (www.statbureau.com) to December 2018 Indian rupees (INR) and then converted to $US using the conversion rate of US$1 = INR70.
Definitions
Direct costs were defined as costs from the perspective of PLWTB and their family unit which include OOP payments for travel related to medical care, consultation fees, expenditure on TB medicines, fees for diagnostic tests, inpatient hospital bills, and food supplements. Some studies divided direct costs into direct medical costs (consultant fees, drug costs, bills for testing, and hospitalization) and direct non-medical costs (costs of traveling and accommodation for therapy and costs of nutritional supplements). Indirect costs refer to lost wages for the PLWTB and their caregivers and includes costs of hiring domestic help due to decreased ability to perform household chores. Based on WHO definitions, TB-related direct and indirect costs that exceed 20% of the annual household income are categorized as catastrophic costs.16 Distress financing is defined as taking loans, borrowing money, or dissaving (selling items). The Figure depicts the times in the care cascade when these costs are incurred.
FIGURE.
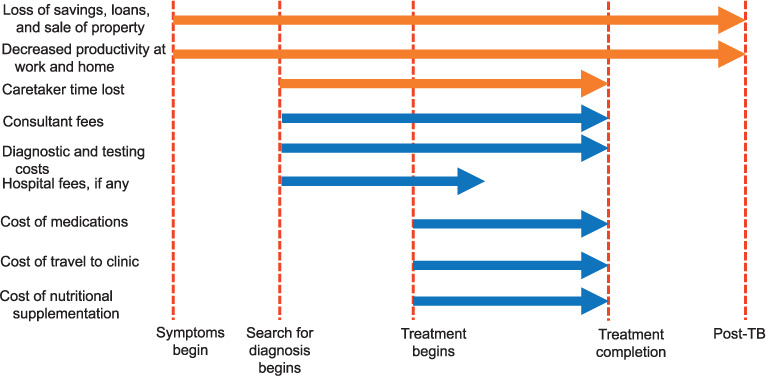
Sources and timing of direct (blue) and indirect (orange) costs for TB patients across the care continuum. TB = tuberculosis.
Data analysis
Variance-weighted means and standard deviations of direct and indirect costs for PTB were calculated from seven studies for which data were available (n = 2315) using SPSS v26.0 (IBM, Armonk, NY, USA). We contacted authors for primary data when the mean and the standard deviation values were not available.
RESULTS
Search results
Of the 87 studies identified, 34 were duplicates, 10 reported only healthcare sector costs, 8 were not relevant, and 3 were editorials or reviews; 32 studies were thus included in this review (Supplementary Figure S1). These studies were conducted in urban and rural areas across India (Supplementary Figure S2). Only six studies reported mean total direct and indirect costs for drug-susceptible TB, and we were able to obtain primary data from one study from the authors.
Ratio of direct and indirect costs
Indirect costs far exceeded the direct costs of TB. As shown in Table 1, direct costs per PLWTB ranged between US$26.5 to US$183.6 and indirect costs ranged from US$1.4 to US$673.6. Mean indirect medical costs were greater than mean direct medical costs in 6 out of 8 studies where this comparison was possible (Supplementary Figure S3). Indirect costs (US$666.6) constituted 93.3% of total costs experienced by PLWTB in India, and the mean direct costs were US$46.8 (6.7%) (Table 2).9,17–20 One observational study of 100 PLWTB in an NTEP program in Vellore, was an outlier and reported mean indirect costs of US$673.57 (standard deviation [SD] 480.12), which was 15-fold higher than the mean direct costs (US$44.62, SD 57.04). Indirect costs may have been found to be higher, because the study investigators included hours spent seeking consultation, traveling to obtain medicines, and time spent in the hospital, as well as time spent by caretakers who accompanied the PLWTB. Each hour spent was valued at US$1.82 based on the local hourly wage for unskilled laborers.
TABLE 1.
Costs of drug-susceptible TB treatment from the PLWTB perspective *
| Author, year | Study size | Setting | Direct medical costs ($US) | Error | Direct non-medical costs ($US) | Error range | Total direct costs ($US) | Error | Total indirect medical costs ($US) | Error |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug-susceptible TB | ||||||||||
| Rajeshwari, 1999 | 304 | Rural and urban | — | — | — | — | 77.1 | — | 147.8 | — |
| Ray, 2005 | 156 | Urban | — | — | — | — | 56.0 | — | 72.7 | — |
| John, 2009 | 100 | Rural | — | — | — | — | 44.6 | 60.0 | 673.6 | 480.3 |
| Pantoja, 2009 | 1106 | Urban | 148.0 | — | 35.6 | — | 183.6 | — | 102.8 | — |
| Ananthakrishan, 2012 | ||||||||||
| PTB | 219 | Urban | 25.5 | 9.1 | 9.1 | 5.8 | 35.7 | 10.7 | 71.3 | 14.9 |
| EPTB | 81 | Urban | 42.9 | 11.0 | 17.7 | 7.4 | 60.6 | 12.5 | 69.9 | 13.1 |
| Sajith, 2015 | ||||||||||
| PTB | 48 | Urban | 73.3 | 72.4 | 4.5 | 18.0 | — | — | 12.4 | 26.7 |
| EPTB | 43 | Urban | 173.2 | 54.4 | 11.7 | 29.5 | — | — | 13.9 | 31.9 |
| PTB and EPTB | 6 | Urban | 205.9 | 45.2 | 21.5 | 10.8 | — | — | 13.8 | 35.9 |
| Muniyanadi, 2015 | 220 | Rural and urban | — | — | — | — | 26.5 | 24.7 | 39.9 | 45.2 |
| Veesa, 2018 | 880 | Urban | — | — | — | — | 25.2 | 56.8 | — | — |
| Shewade, 2018 | 465 | Rural and urban | 40.9 | 87.2 | 5.6 | 31.9 | 42.9 | 88.4 | 1.4 | 4.2 |
| Prasanna, 2018† | 102 | Urban | 66.2 | 22.4—157.5 | — | — | 66.2 | 22.4—157.5 | 50.5 0.9—295.1 | |
| Sarin, 2018 | 450 | Urban | 18.5 | — | 47.3 | — | 65.7 | 79.4 | 108.0 | 392.7 |
| MDR/XDR-TB | ||||||||||
| Mullerpattan, 2019 | ||||||||||
| MDR-TB | 40 | Urban | — | — | 238.0 | — | 4754.0 | — | 968.0 | — |
| XDR-TB | 10 | Urban | — | — | 120.0 | — | 6001.0 | — | 2400.0 | — |
| Drug-resistant EPTB | 14 | Urban | — | — | 108.0 | — | 4735.0 | — | 874.0 | — |
* Costs have been adjusted for inflation through December 2018 and presented in $US.
† Studies that reported median costs and interquartile ranges instead of mean costs and standard deviations.
TB = tuberculosis; PLWTB = people living with TB; PTB = pulmonary TB; EPTB = extra-pulmonary TB; MDR-TB = multidrug-resistant TB; XDR-TB = extensively drug-resistant TB.
TABLE 2.
Components of direct and indirect costs in India for individuals with drug-susceptible TB and their contribution to overall costs at the PLWTB level, as well as possible interventions to decrease costs
| Cost-type | Components | Proportion of total costs % | Suggested interventions |
|---|---|---|---|
| Direct |
|
6.7 |
|
| Indirect |
|
93.3 |
|
TB = tuberculosis; PLWTB = people living with TB; PPM = public-private mix; NTEP = National Tuberculosis Elimination Programme.
Timing of direct costs
Direct costs were largely incurred before TB was diagnosed. A study (n = 455) from rural Tamil Nadu, India, found that the median pre-diagnostic costs (US$22.6) were almost twice the median costs during treatment (US$11.9) in 2000. Travel costs (US$1.2) were vastly reduced due to decentralization of NTEP diagnostic and therapeutic services during this period with dedicated TB units at the sub-district level.19,21 Indeed, 50% of PLWTB incurred no direct medical or non-medical costs after initiating treatment under the NTEP.
Another study from Tamil Nadu State (2012–2015) found that pre-diagnostic direct costs were approximately half the average monthly individual income in that population.8 Similarly, a study of 220 PLWTB in the Saharia tribal group in the state of Madhya Pradesh (2013–2014) reported that the bulk of indirect and direct expenditure was made while seeking a diagnosis. After initiation of therapy by the NTEP, none of the PLWTB experienced further direct medical costs.22 In Bengaluru, Karnataka, patients treated in the NTEP paid an average of US$114.2 for drugs, travel, tests, and hospitalizations before diagnosis and US$29.3 after diagnosis—an almost four-fold difference.9
Timing of indirect costs
Indirect costs were also largely concentrated in the pre-diagnostic period and intensive phase of TB therapy. Indirect costs during the intensive phase of therapy were three times those of costs in the continuation phase for PLWTB in Chennai, Tamil Nadu, and the state of Tamil Nadu.23 In Bengaluru, patients lost US$99.7 in wages, loan interest, and payments for domestic help before diagnosis, but only US$3.1 post-diagnosis for patients treated by the NTEP.9 Similarly, a study from Puducherry (2016–2017) found that indirect costs were concentrated in the pre-diagnostic period.20
By contrast, a 2018 study of 450 PLWTB treated by the NTEP in New Delhi, NCT Delhi, found that costs in the pre-diagnostic phase were lower than those incurred during treatment (US$4.8 vs. US$38).24 In this cohort, pre-diagnostic indirect costs were also lower than those during treatment (US$1.6 vs. US$106.4).
Private sector vs. public sector
Costs in the private sector exceeded those in the public sector. In a 1999 study, focus group discussions were organized among 304 PLWTB treated in government, NGO, and private health facilities in Chennai and neighboring districts in Tamil Nadu,17 according to which direct costs in government facilities (US$61.7) and NGO facilities (US$78.0) were considerably lower than in private facilities (US$406.1). Similarly, a study of PLWTB in rural Tamil Nadu (2012–2015) found that those who had visited private facilities before being treated in the public sector had 17-fold higher OOP payments.8 A prospective study from Bengaluru found that direct costs of PLWTB receiving care from private physicians were six times those of individuals treated by NTEP providers.9 Of note, as indirect costs were not accounted for in that study, the net costs may have been even higher.
Hospitalization costs
We found that hospitalization generates considerable direct and indirect costs.25 A study of a cohort of 100 PLWTB in Vellore, Tamil Nadu, reported that 25% were hospitalized with a mean length of stay of 8 days.26 The mean associated costs were US$56.54 (SD 41.29). A study of TB disease among individuals with cancer found 38% were hospitalized.27 However, a national survey suggests that of every 100 000 Indians, 50 (32 public sector, 18 private sector) were hospitalized for TB.28 The mean direct costs for hospitalized PLWTB were US$117.9 in the public sector (standard error [SE] US$9.7) and US$426.1 (SE US$35.0) in the private sector; 99.9% of hospitalization costs were paid out-of-pocket by PLWTB.
PTB vs. EPTB
Individuals with EPTB have higher expenditures than those with PTB. A prospective observational study of PLWTB treated by the NTEP in Pune, Maharashtra, in 2014–2015 found that persons with EPTB had twice the direct medical costs as persons with PTB (US$73.33, SD 72.44 vs. US$150.19, SD 47.19) due to higher costs of hospitalization, diagnostics, and treatment.29
Work days lost to TB
Work absenteeism due to TB disease and related therapy is the principal contributor to the indirect costs. In a cohort of 304 PLWTB in Tamil Nadu in 1999, the mean number of work days lost was 83 (pre-treatment 48 days, during treatment 35 days).19 Daily wage earners lost more work days than self-employed and salaried individuals. At the end of treatment, 12% were unable to return to work. Similarly, in a 2004 study from New Delhi, PLWTB lost an average of 47.1 work days before presenting to NTEP centers.18 Of note, individuals from lower socioeconomic levels missed more days than those in higher socioeconomic levels (average of 181.6 days and 24.1 days, respectively).
In addition to work absenteeism, TB disease can often mean complete loss of employment for PLWTB. In Bengaluru, TB disease led to loss of 30 working days and 15 of 320 PLWTB (4.6%) lost their jobs.9 A quantitative and qualitative study in Puducherry (2016–2017) found that the leading cause of indirect costs was job loss;20 of the 101 PLWTB studied, 65 (64%) lost their jobs. Of these 65 PLWTB, 40 attributed their job loss to TB.
Other sources of indirect costs
In addition to lost income from disruption of employment, studies have reported additional impacts with socioeconomic ramifications. For instance, in one study from Tamil Nadu, 79% of women carried out household activities, including cooking, cleaning, washing and serving food, but only 38% carried out such activities during their illness.17 Similarly, the capacity to provide childcare decreased from 69% to 34%.
Multidrug-resistant and extensively drug-resistant TB costs
Costs of drug-resistant TB treatment were substantially higher than costs of drug-susceptible TB. In a prospective observational study of 50 people with multidrug-resistant (MDR-) and pre-extensively drug-resistant (pre-XDR) TB from a private tertiary hospital in Mumbai, patients spent an average of US$4754 in direct costs. Individuals with XDR-TB had higher mean direct costs (US$6001). Direct costs constituted 80% of the total costs, with drugs (37%) and hospitalization (16%) representing the largest components of direct costs.30 A 2015 study found that drugs for MDR-TB therapy cost 135-fold more than a standard course of drugs for drug-susceptible TB.31
Although indirect costs constituted 20% of the total costs in PLWTB with drug-resistant TB, the mean indirect costs for PLWTB with MDR-TB (US$968) exceeded previous estimates for indirect costs of drug-susceptible TB.30 PLWTB with XDR-TB had even higher mean indirect costs (US$2400).
The costs of drug-resistant TB treatment reported in this urban private sector hospital are not representative of costs PLWTB face for drug-resistant TB treatment in the public sector.30 A study from Chattisgarh State found that treatment in the private sector was associated with 33-fold higher direct costs. This was largely driven by the absorption of drug and diagnosis costs by the NTEP.31
Catastrophic costs
A survey of PLWTB in Chennai from 2005 found that individuals most at risk for catastrophic costs are those in the lowest income brackets.19 In Puducherry, 32.4% of families in one study experienced catastrophic costs in 2016–2017.20 In a prospective 2011–2012 study that collected income and cost data from PLWTB being treated by the NTEP in Bengaluru, 762/891 (86%) respondents reported costs that exceeded 20% of their annual income, and 661 (74%) had costs that exceeded 40% of annual income.32 A 2018 study from Delhi reported a lower incidence of catastrophic costs (7%). This low rate of catastrophic costs was attributed to robust and decentralized NTEP services, which led to the reduction in direct medical and non-medical costs, as well as a low hospitalization rate (4%).24
A 2017 modeling study estimated that 20–22 million Indian households would incur TB-related catastrophic costs over 20 years between 2016 and 2035.33 The prevalence of catastrophic costs was concentrated in the two bottom income quintiles. Elderly individuals without formal education were also at increased risk of catastrophic costs.34 In New Delhi, annual incomes less than US$1428, family size of three or fewer, and being sole earner were associated with a higher risk of catastrophic costs.24
Hospitalization is a major risk for catastrophic costs. A 2014 national survey found that 42% of PLWTB hospitalized for TB experienced catastrophic costs.28 Overall, 26.7% of hospitalized PLWTB experienced distress financing compared to 3.5% of PLWTB who were managed in the ambulatory setting.6 Distress financing may be a good marker of catastrophic costs.28,32
Coping strategies
According to a 1999 study from Tamil Nadu, the median amount of loan for those treated in the private sector (US$275.4) was nine-fold higher than for PLWTB treated in government facilities (US$32.5), and six-fold higher than for PLWTB treated in NGO facilities (US$43.4).17 A 2014 national survey showed that 24% (95%CI 19.7–28.3) of PLWTB hospitalized in public sector facilities experienced distress financing as opposed to 41% (95%CI 34.6–47.4) in private hospitals.28
In 2005, a study found that 71% of PLWTB borrowed money, with 50% borrowing upwards of US$76.3.19 The rate of borrowing was similar in urban and rural areas, but the loan sums were twice as high in urban settings.35 A study from Chennai in 2007, however, found a lower rate of borrowing, with 7% taking loans, 6% mortgaging their property, and 23.4% of individuals experiencing a decline in savings. The median amount of loan was US$100.1.36 Other coping strategies included discontinuation of education, which was observed in 11% of children of PLWTB in a 1999 Chennai survey.17 Furthermore, 8% of children took up employment to support their families.
Measures to reduce costs
An observational study conducted across 18 districts in India in 2016–2017 found that active case finding with the assistance of community volunteers who spread TB awareness door-to-door, conducted symptom screens, and referred PLWTB to local NTEP diagnostic centers was able to significantly reduce the prevalence of catastrophic costs (adjusted prevalence ratio 0.68, 95%CI 0.69–0.97).37 Similarly, a 2019 study from Tamil Nadu reported that PLWTB identified and treated through passive case finding had higher mean overall costs (US$227, SE 19.5) compared to those who benefited from active case finding (US$69, SE 18.0).38 The greatest savings were in the pre-diagnostic period.
Access to medical insurance can also reduce catastrophic costs. Institution of insurance policies in the states of Chhattisgarh and Gujarat have both demonstrated reductions in catastrophic costs.31,39 Universal healthcare may play a role in reducing catastrophic costs for the most economically disadvantaged Indians.40 However, doing so alone without addressing accessibility and awareness is unlikely to be sufficient. Modeling studies suggest that an aggressive expansion of the access to treatment was the most cost-effective strategy for preventing loss of disability-adjusted life years from TB and reducing costs incurred by PLWTB.41
DISCUSSION
TB inflicts financial hardships on millions of Indians. Understanding the timing and sources of these costs is important to create health care delivery models that minimize these costs. We reviewed the literature and performed a cost analysis. In our analysis, patients with drug-susceptible TB paid a mean of US$46.8 in direct costs and US$666.5 in indirect costs. Costs for the treatment of drug-resistant TB were considerably higher. Based on the literature, the location of patients, their use of private sector services, and need for hospitalization, among other factors, determine costs. Indirect costs constituted a striking 93.3% of the costs from the perspective of PLWTB. Studies largely defined indirect costs as lost wages due to TB. Future economic studies should include elements such as discontinuation of education, as well as decreased ability to perform household work and childcare, as these constitute an economic loss.
Both direct and indirect costs tend to be highest before TB is diagnosed. Decreasing diagnostic delays through active case finding, standardized diagnostic approach, and novel triage tests may reduce costs.37,38 Increasing public awareness of TB symptoms and fighting TB stigma may also empower PLWTB to seek diagnosis earlier.42,43 Expanding access to robust primary and TB care, decentralizing health care services, and providing community-based services through community health workers may decrease indirect and direct costs of travel for TB care as has been noted in Tamil Nadu and New Delhi.19,24,41 TB care should be designed not to disrupt the livelihood of PLWTB, as excessive clinic visits and adherence support can lead to loss of wages and employment. It is clear that this should be balanced against the need to ensure adherence and minimize the development of drug resistance. In remote areas, reliable disbursement of travel subsidies is crucial for impoverished Indians who have to travel long distances to obtain care from NTEP centers. Paid sick leave programs for PLWTB would mitigate the financial burden of lost employment. Policy and operational research will be crucial in assessing these interventions and implementing them effectively.
Costs in the private sector can be six times those in the public sector. PPM ventures have been shown to reduce direct costs for PLWTB.44 The NTEP is already attempting to engage private providers to standardize approach to TB diagnosis. These efforts are laudable but there is room to grow.45 Mandating the use of free drugs available through NTEP by PPM programs will reduce drug costs. Increasing awareness of NTEP services, continued decentralization of services, and dispelling negative attitudes about the NTEP may encourage impoverished Indians to avail of free care. Social determinants of health, such as poverty, malnutrition, and crowded living conditions inform TB risk.46,47 Modeling studies support the importance of addressing sustainable development goals to attain the goals of the End TB Strategy.48 The NTEP should also partner with welfare programs such as the targeted public distribution system and the Pradhan Mantri Awas Yojana to provide comprehensive services to PLWTB.49,50
Inpatient care is often necessary for PLWTB. As detailed above, hospitalization markedly increases risk of catastrophic costs, particularly in the private sector. Improving inpatient insurance coverage for impoverished Indians is crucial. It should be noted that hospitalization costs were one of the top contributors to the direct costs of treating drug-resistant TB. Studies in South Africa have demonstrated that outpatient therapy for MDR-TB using community-based programs is not only feasible and less expensive, but also sometimes more effective.51,52 Similar programs could be instituted in India.
This review found high rates of catastrophic costs in both rural and urban settings. Economic evaluations that do not consider pre-diagnostic expenses and focus only on costs incurred during treatment underestimate the number of PLWTB facing catastrophic costs. Also, economic evaluations should routinely account for coping mechanisms such as loans and dissaving, which are indicators of catastrophic costs. Given that indirect costs constitute such a large component of health care costs, they should be included in the definition of catastrophic costs. The impact of children dropping out of school must also be considered, given the important long-term socioeconomic ramifications.
Based on our analysis of the source, timing, and magnitude of economic costs faced by PLWTB in India, we composed a list of strategies that may help mitigate the financial hardship associated with TB. While our analysis was limited to India, these conclusions and strategies may also be applicable to other low- and middle-income countries with high TB burdens.
We acknowledge some limitations in this analysis. The majority of available data refer to costs incurred by individuals who were treated through the NTEP. Given that models have suggested that the majority of Indian TB cases are treated in the private sector, this underrepresentation is a major gap.10 Estimation and reporting of costs were not consistent across studies, particularly the estimation of indirect costs due to missed work or lost unemployment. The wide range of cost estimates (Table 1 and Supplementary Figure S3) reflects marked heterogeneity in the way direct and indirect costs were measured. None of the studies used for cost analysis reported the proportion of insured patients or the fraction of payments made by insurance companies. In the future, standardization of cost analysis studies will be important. Furthermore, given the cultural heterogeneity of India, these costs may not represent all communities. Rural studies were underrepresented in this analysis. Furthermore, there are differences in government subsidies available to PLWTB in different states. Tribal communities in particular may have a different pattern of costs. Coping mechanisms were underrepresented in the literature we identified through this search strategy. Finally, we used a national deflator tool to standardize results from studies over two decades. As disaggregated data for one multi-state study was not available, we used the mean costs derived from across states. Using state-specific costs and inflation rates may have enhanced accuracy of cost estimates.
There may also be both publication and selection biases that could limit the representativeness of the findings. As studies were restricted to publications in English, inclusion bias may have occurred. In addition, studies only included people diagnosed with TB. Approximately 4.4% of the poorest Indians develop TB disease but are not diagnosed or do not seek treatment.5 Costs incurred by these undiagnosed PLWTB or those who drop out of treatment may be significantly different.
The high costs associated with TB entrap Indians into a vicious cycle of poverty and disease. Breaking the cycle will yield rich dividends in the form of a healthier population and a healthier economy.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH), Bethesda, MD, USA (5T32AI052074-13); National Science Foundation, Alexandria, VA, USA (OISE-9531011); Federal funds from the Government of India’s Department of Biotechnology, New Delhi, India; the Indian Council of Medical Research, New Delhi, India; and the Office of AIDS Research (NIH) and distributed in part by CRDF Global, Arlington, VA, USA (USB-31150-XX-13); grants from Warren Alpert Foundation, Providence, RI, USA; and Boston University School of Medicine, Boston, MA, USA; and Boston University Clinical and Translation Science Institute, Boston, MA, USA (1UL1TR001430).
Footnotes
The funders had no role in study design, analysis, or reporting.
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2018. WHO/CDS/TB/2018.20. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 2.Pai M, Bhaumik S, Bhuyan S S. India’s plan to eliminate tuberculosis by 2025: converting rhetoric into reality. BMJ Glob Health. 2017;2:e000326. doi: 10.1136/bmjgh-2017-000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey A, Ploubidis G B, Clarke L, Dandona L. Trends in catastrophic health expenditure in India: 1993 to 2014. Bull World Health Organ. 2018;96(1):18. doi: 10.2471/BLT.17.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Bank. Poverty & equity data portal, India 2019. Washington DC, USA: World Bank; 2019. http://povertydata.worldbank.org/poverty/country/IND [Google Scholar]
- 5.Mazumdar S, Satyanarayana S, Pai M. Self-reported tuberculosis in India: evidence from NFHS-4. BMJ Glob Health. 2019;4(3):e001371. doi: 10.1136/bmjgh-2018-001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav J, John D, Menon G. Out of pocket expenditure on tuberculosis in India: Do households face hardship financing? Indian J Tuberc. 2019;66(4):448–460. doi: 10.1016/j.ijtb.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Pardeshi G, Deluca A, Agarwal S, Kishore J. Tuberculosis patients not covered by treatment in public health services: findings from India’s National Family Health Survey 2015–16. Trop Med Int Health. 2018;23(8):886–895. doi: 10.1111/tmi.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veesa K S, et al. Diagnostic pathways and direct medical costs incurred by new adult pulmonary tuberculosis patients prior to anti-tuberculosis treatment–Tamil Nadu, India. PloS One. 2018;13(2):e0191591. doi: 10.1371/journal.pone.0191591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantoja A, et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part I. Socio-economic profile and costs among tuberculosis patients. Int J Tuberc Lung Dis. 2009;13(6):698–704. [PubMed] [Google Scholar]
- 10.Arinaminpathy N, et al. Tuberculosis treatment in the private healthcare sector in India: an analysis of recent trends and volumes using drug sales data. BMC Infect Dis 2019. 2019;19(1):539. doi: 10.1186/s12879-019-4169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karan A, Yip W, Mahal A. Extending health insurance to the poor in India: An impact evaluation of Rashtriya Swasthya Bima Yojana on out of pocket spending for healthcare. Soc Sci Med. 2017;181:83–92. doi: 10.1016/j.socscimed.2017.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khetrapal S, Acharya A. Expanding healthcare coverage: An experience from Rashtriya Swasthya Bima Yojna. Indian J Med Res. 2019;149(3):369–375. doi: 10.4103/ijmr.IJMR_1419_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bronner Murrison L, et al. How do urban indian private practitioners diagnose and treat tuberculosis? A cross-sectional study in Chennai. PLoS One. 2016;11(2):e0149862. doi: 10.1371/journal.pone.0149862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwan A, et al. Variations in the quality of tuberculosis care in urban India. PLoS Med. 2018;15(9):e1002653. doi: 10.1371/journal.pmed.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand T, Babu R, Jacob A G, Sagili K, Chadha S S. Enhancing the role of private practitioners in tuberculosis prevention and care activities in India. Lung India. 2017;34(6):538–544. doi: 10.4103/0970-2113.217577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Protocol for survey to determine direct and indirect costs due to TB and to estimate proportion of TB-affected households experiencing catastrophic total costs due to TB field testing version. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 17.Rajeswari R, Balasubramanian R, Muniyandi M, Geetharamani S, Thresa X, Venkatesan P. Socio-economic impact of tuberculosis on patients and family in India. Int J Tuberc Lung Dis. 1999;3(10):869–877. [PubMed] [Google Scholar]
- 18.Ray T, Sharma N, Singh M, Ingle G. Economic burden of tuberculosis in patients attending DOT centres in Delhi. J Communicable Dis. 2005;37(2):93–98. [PubMed] [Google Scholar]
- 19.Muniyandi M, Ramachandran R, Balasubramanian R. Costs to patients with tuberculosis treated under DOTS programme. Indian J Tuberc. 2005;52(4):188–196. [Google Scholar]
- 20.Prasanna T, Jeyashree K, Chinnakali P, Bahurupi Y, Vasudevan K, Das M. Catastrophic costs of tuberculosis care: a mixed methods study from Puducherry, India. Glob Health Action. 2018;11(1):1477493. doi: 10.1080/16549716.2018.1477493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. A brief history of tuberculosis control in India. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 22.Muniyandi M, Rao V, Bhat J, Yadav R, Sharma R. Household catastrophic health expenditure due to tuberculosis. Global J Med Res. 2015;2:9. [Google Scholar]
- 23.Ananthakrishnan R, Muniyandi M, Jeyaraj A, Palani G, Sathiyasekaran B. Expenditure pattern for TB treatment among patients registered in an urban government DOTS program in Chennai City, South India. Tuberc Res Treat. 2012;2012:747924. doi: 10.1155/2012/747924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarin R, Vohra V, Singla N, Thomas B, Krishnan R, Muniyandi M. Identifying costs contributing to catastrophic expenditure among TB patients registered under RNTCP in Delhi metro city in India. Indian J Tuberc. 2019;66(1):150–157. doi: 10.1016/j.ijtb.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Laurence Y V, Griffiths U K, Vassall A. Costs to health services and the patient of treating tuberculosis: a systematic literature review. Pharmacoeconomics. 2015;33(9):939–955. doi: 10.1007/s40273-015-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John K, Daley P, Kincler N, Oxlade O, Menzies D. Costs incurred by patients with pulmonary tuberculosis in rural India. Int J Tuberc Lung Dis. 2009;13(10):1281–1287. [PubMed] [Google Scholar]
- 27.Bennett Z, et al. The economics of managing tuberculosis in cancer patients in an oncology center in eastern India. Infect Contr Hosp Epidemiol. 2019;40(1):122–124. doi: 10.1017/ice.2018.284. [DOI] [PubMed] [Google Scholar]
- 28.Kastor A, Mohanty S K. Disease-specific out-of-pocket and catastrophic health expenditure on hospitalization in India: do Indian households face distress health financing? PLoS One. 2018;13(5):e0196106. doi: 10.1371/journal.pone.0196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sajith M, Thomas A, Kothia J J, Chandrakar B, Pawar A, Bargaje M D. Cost of therapy incurred for tuberculosis patients receiving directly observed therapy (DOT) Int J Pharm Pharm Sci. 2015;7(10):141–144. [Google Scholar]
- 30.Mullerpattan J B, Udwadia Z Z, Banka R A, Ganatra S R, Udwadia Z F. Catastrophic costs of treating drug resistant TB patients in a tertiary care hospital in India. Indian J Tuberc. 2019;66(1):87–91. doi: 10.1016/j.ijtb.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Kundu D, et al. Innovative social protection mechanism for alleviating catastrophic expenses on multidrug-resistant tuberculosis patients in Chhattisgarh, India. WHO South-East Asia J Public Health. 2015;4(1):69–77. doi: 10.4103/2224-3151.206624. [DOI] [PubMed] [Google Scholar]
- 32.Madan J, Lönnroth K, Laokri S, Squire S B. What can dissaving tell us about catastrophic costs? Linear and logistic regression analysis of the relationship between patient costs and financial coping strategies adopted by tuberculosis patients in Bangladesh, Tanzania and Bangalore, India. BMC Health Serv Res. 2015;15(1):476. doi: 10.1186/s12913-015-1138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verguet S, et al. Catastrophic costs potentially averted by tuberculosis control in India and South Africa: a modelling study. Lancet Global Health. 2017;5(11):e1123–e1132. doi: 10.1016/S2214-109X(17)30341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinda E M, Kowal P, Attermann J, Enemark U. Health service use, out-of-pocket payments and catastrophic health expenditure among older people in India: The WHO Study on global AGEing and adult health (SAGE) J Epidemiol Community Health. 2015;69(5):489–494. doi: 10.1136/jech-2014-204960. [DOI] [PubMed] [Google Scholar]
- 35.Muniyandi M, Ramachandran R, Balasubramanian R, Narayanan P. Socio-economic dimensions of tuberculosis control: review of studies over two decades from Tuberculosis Research Center. J Communicable Dis. 2006;38(3):204. [PubMed] [Google Scholar]
- 36.Ananthakrishnan R, Jeyaraj A, Palani G, Sathiyasekaran B. Socioeconomic impact of TB on patients registered within RNTCP and their families in the year 2007 in Chennai, India. Lung India. 2012;29(3):221. doi: 10.4103/0970-2113.99103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shewade H D, et al. Active case finding among marginalised and vulnerable populations reduces catastrophic costs due to tuberculosis diagnosis. Glob Health Action. 2018;11(1):1494897. doi: 10.1080/16549716.2018.1494897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muniyandi M, et al. Catastrophic costs due to tuberculosis in South India: comparison between active and passive case finding. Trans R Soc Trop Med Hyg 2020. 114(3):185–192. doi: 10.1093/trstmh/trz127. [DOI] [PubMed] [Google Scholar]
- 39.Ranson M K. Reduction of catastrophic health care expenditures by a community-based health insurance scheme in Gujarat, India: current experiences and challenges. Bull World Health Organ. 2002;80:613–621. [PMC free article] [PubMed] [Google Scholar]
- 40.Verguet S, Laxminarayan R, Jamison D T. Universal public finance of tuberculosis treatment in India: an extended cost-effectiveness analysis. Health Econ. 2015;24(3):318–332. doi: 10.1002/hec.3019. [DOI] [PubMed] [Google Scholar]
- 41.Menzies N A, et al. Cost-effectiveness and resource implications of aggressive action on tuberculosis in China, India, and South Africa: a combined analysis of nine models. Lancet Global Health. 2016;4(11):e816–e826. doi: 10.1016/S2214-109X(16)30265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ngamvithayapong J, Winkvist A, Diwan V. High AIDS awareness may cause tuberculosis patient delay: results from an HIV epidemic area, Thailand. AIDS. 2000;14(10):1413–1419. doi: 10.1097/00002030-200007070-00015. [DOI] [PubMed] [Google Scholar]
- 43.van't Hoog A H, et al. Optimal triage test characteristics to improve the cost-effectiveness of the Xpert MTB/RIF assay for TB diagnosis: a decision analysis. PLoS One. 2013;8(12):e82786. doi: 10.1371/journal.pone.0082786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantoja A, et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part II. Cost and cost-effectiveness. Int J Tuberc Lung Dis. 2009;13(6):705–712. [PubMed] [Google Scholar]
- 45.Nautiyal R G, Singh R K. Public private mix in tuberculosis control: is it really working in India? Int J Community Med Public Health. 2018;5(2):728–733. [Google Scholar]
- 46.Sinha P, Davis J, Saag L, Wanke C, Salgame P, Mesick J et al. Undernutrition and tuberculosis: public health implications. J Infect Dis. 2019;219(9):1356–1363. doi: 10.1093/infdis/jiy675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hargreaves J R, Boccia D, Evans C A, Adato M, Petticrew M, Porter J D H. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654–662. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter D J, et al. The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of Sustainable Development Goal 1. Lancet Global Health. 2018;6(5):e514–e522. doi: 10.1016/S2214-109X(18)30195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharya S, Falcao V L, Puri R. The public distribution system in India: policy evolution and program delivery trends. In: Alderman H, Gentilini U, Yemtsov R, editors. The 1.5 billion people question: food, vouchers, or cash transfers. Washington, D.C., USA: World Bank; 2017. pp. 43–105. [Google Scholar]
- 50.Gohil J, Gandhi Z H. Pradhan Mantri Awas Yojana (PMAY) Scheme—an emerging prospect of affordable housing in India. Int Res J Eng Technol. 2019;6(12):2546–2550. [Google Scholar]
- 51.Brust J C, et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012;16(8):998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loveday M, et al. Community-based care vs. centralised hospitalisation for MDR-TB patients KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2015;19(2):163–171. doi: 10.5588/ijtld.14.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]


