Abstract
Background:
The Revised National Tuberculosis Control Programme (RNTCP) in Andhra Pradesh, India, introduced TrueNat™ MTB/Rif, a rapid molecular test for detecting Mycobacterium tuberculosis (MTB) and rifampicin (RIF) resistance at 193 TB units (TUs) in October 2018. We evaluated its impact on TB diagnosis and assessed the operational feasibility of its deployment at point-of-care (POC) settings.
Methods:
We compared the number of presumptive TB cases tested and the number (proportion) of microbiologically positive before (January–August 2018) and after (January–August 2019) the deployment of TrueNat. We interviewed laboratory technicians and Senior TB Laboratory Supervisor from 25 randomly selected TUs to assess operational feasibility.
Results:
In 2018, 10.5% (range 8.9–13.1) of 245,989 presumptive cases tested were positive. In 2019, of the 185,435 presumptive cases tested, 13.7% (range 9.6–18.9) were positive. The proportion of presumptive TB cases in whom MTB was detected using TrueNat was 14.4% (range 10.0–21.2). TrueNat significantly increased case detection (incidence rate ratio [IRR] 1.30; 95%CI 1.15–1.46), yielding an additional 18 TB cases per 100 000 population. Laboratory technicians became comfortable in performing TrueNat after a median of 10 tests (interquartile range 5–17.5). Invalid reports declined from 6.8% to 3.6%.
Conclusion:
The deployment of TrueNat as POC diagnostic test improved case detection and was operationally feasible under RNTCP.
Keywords: Andhra tuberculosis programme, point-of-care, presumptive TB, molecular diagnosis, operational research, India
Abstract
Contexte :
Le Programme national tuberculose (NTP) de l’Andhra Pradesh, Inde, avait introduit, en octobre 2018, TrueNat® MTB/Rif, un test moléculaire rapide de détection de Mycobacterium tuberculosis et de résistance à la rifampicine dans 193 unités tuberculose (TU). Nous avons évalué son impact sur le diagnostic de TB et évalué la faisabilité opérationnelle de son déploiement dans des lieux d’intervention (POC).
Méthode :
Nous avons comparé le nombre de cas de TB présumés qui ont été testés et le nombre (proportion) de cas positifs en microbiologie, avant (janvier–août 2018) et après (janvier–août 2019) le déploiement de TrueNat. Nous avons interviewé des techniciens de laboratoire (LT) et des superviseurs de laboratoire TB (STLS) de 25 TU sélectionnées au hasard pour évaluer la faisabilité opérationnelle.
Résultats :
En 2018, 10,5% (fourchette 8,9–13,1) de 245 989 cas présumés testés étaient positifs. En 2019, sur les 185 435 cas présumés testés, 13,7% (fourchette 9,6–18,9) ont été positifs. La proportion de cas de TB présumés parmi lesquels M. tuberculosis a été détecté par TrueNat a été de 14,4% (fourchette 10,0–21,2). TrueNat a significativement augmenté la détection des cas (ratio d’incidence 1,30 ; IC 95% 1,15–1,46), amenant 18 cas de TB supplémentaires par 100 000 habitants. Les LT se sont sentis à l’aise dans la réalisation de TrueNat après avoir réalisé une médiane de 10 (IQR 5–17,5) tests. Les rapports invalides/les erreurs ont décliné de 6,8% à 3,6%.
Conclusion :
Le déploiement de TrueNat comme test de diagnostic de POC a amélioré la détection des cas et a été opérationnellement faisable au sein du NTP.
Abstract
Marco de referencia:
El Programa Nacional de Tuberculosis (NTP) de Andhra Pradesh, en la India introdujo la prueba molecular rápida, TrueNat® MTB/Rif, para detectar el Mycobacterium tuberculosis y la resistencia a la rifampicina en 193 unidades de TB (TU) en octubre del 2018. Se evaluó su impacto en el diagnóstico de la TB y la factibilidad operativa de desplegarla en el lugar de la consulta.
Método:
Se comparó el número de casos con presunción de TB examinados con la prueba y el número (la proporción) de casos positivos microbiológicamente, antes (de enero a agosto del 2018) y después (de enero a agosto del 2019) del despliegue de la prueba TrueNat. Se entrevistaron los técnicos de laboratorio (LT) y el supervisor principal del laboratorio de TB (STLS) de 25 TU escogidas de manera aleatoria, con el fin de evaluar la factibilidad operativa.
Resultados:
En el 2018, el 10,5% (intervalo 8,9–13,1) de los 245 989 casos con presunción de TB examinados con la prueba, obtuvieron un resultado positivo. En el 2019, de los 185 435 casos presuntos que realizaron la prueba, el 13,7% (intervalo 9,6–18,9) fue positivo. La proporción de casos con presunción de TB en los cuales se detectó el M. tuberculosis mediante la prueba TrueNat fue 14,4% (intervalo 10,0–21,2). TrueNat aumentó en forma considerable la detección de casos (cociente de tasas de incidencia [IRR] 1,30; CI 95% 1,15–1,46) y aportó 18 casos adicionales de TB por 100 000 habitantes. Los LT realizaban con confianza la prueba TrueNat después de una mediana de diez pruebas (IQR 5–17,5). Los informes de prueba inválida o error disminuyeron de 6,8% a 3,6%.
Conclusión:
El despliegue de la prueba TrueNat como medio diagnóstico en el lugar de la consulta mejoró la detección de casos y fue factible en el marco del NTP.
TB is the leading cause of death due to a single infectious agent.1 In 2018, the estimated incidence of TB in India was 204 per 100 000 population,2 with a mortality of around 31 per 100 000.1 It has been estimated that a rapid and widely available diagnostic test for TB with sensitivity of ⩾85% for smear-positive and smear-negative cases, and a specificity of 97% can save ∼400 000 lives annually.3
Sputum smear microscopy (SSM), which is the cornerstone of TB diagnosis in India, has several limitations, including low sensitivity.4 TrueNat™ MTB/Rif (Molbio Diagnostics, Verna, India) is a rapid molecular test launched by Molbio, a Make In India Company, as a commercial product for diagnosis of active TB. It is a portable, battery-operated, chip-based test which detects Mycobacterium tuberculosis (MTB) in approximately 1 h and rifampicin (RIF) resistance in another 40–60 min.
TrueNat MTB has high sensitivity (91.1%) and specificity (100%) compared with a composite reference standard consisting of smear and culture results, clinical treatment and follow-up, and radiology findings.5 There is high agreement (92.7%) between Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) and TrueNat in MTB detection.6 TrueNat had significantly higher sensitivity than Xpert (84.1% vs. 81%; P < 0.001).7 Compared to SSM, TrueNat has been shown to increase life expectancy by 0.39 years and was also found to be cost-effective.8 TrueNat is less resource-intensive than Xpert.7,9 Deploying TrueNat at point-of-care (POC) eliminates the need for sample transportation, as is required for Xpert thus adding cost benefit, besides detecting RIF resistance during the patient’s first visit. The WHO’s rapid evaluation of evidence, which is expected to inform the updated 2020 Consolidated Guidelines on TB, suggests that the accuracy of TrueNat is comparable to that of Xpert.10
Based on the evidence supporting the POC use of TrueNat, the Government of Andhra Pradesh, a state in India, introduced TrueNat under its Revised National Tuberculosis Control Programme (RNTCP) at TB unit (TU) level for TB diagnosis in October 2018. After almost a year of its deployment, we evaluated its impact on microbiologically confirmed TB case detection by comparing the case notification rate of microbiologically positive TB before (January–August 2018) and after (January–August 2019) the implementation of TrueNat under the RNTCP, and assessed the operational feasibility of deploying TrueNat at POC settings.
METHODS
Study settings
Andhra Pradesh, a state in southern India, is divided into 13 districts, and is home to a population of over 49 million, 70% of which resides in rural areas. The state has a sex ratio of 996 females per 1000 males and a literacy rate of 67.4%. Under the RNTCP, designated microscopy centres (DMCs) are the most peripheral units delivering TB care where TB diagnosis and treatment is available. At the next level, TUs oversee the DMCs and report to the district TB centres, which in turn report to the State TB centre. Diagnosis and treatment for TB is offered free of cost under the RNTCP. Besides the public healthcare system, patients can also avail of TB care from the private sector.
SSM is available from the level of DMCs and upwards. Cartridge-based nucleic acid amplification test (CBNAAT), as the Xpert is referred to in India, is available at the level of all districts and also at some TUs, based on the load of presumptive TB cases. Until October 2018, only SSM was offered as the first line of TB diagnosis, when TrueNat was implemented at the TU level for diagnosis (243 TrueNat sites spread across 193 TUs).
Study design
We compared TB case notification rates before and after the implementation of TrueNat using programmatic data from RNTCP.
Data accuracy checking
Before extracting data for analysis, we evaluated the accuracy and consistency of the data reported across all recording and reporting formats under RNTCP in the years 2018 and 2019 (Table 1). We randomly selected 10% of the TrueNat sites (n = 25). A data extraction form consisting of variables to be tallied across and within the reporting formats was prepared using Open Data Kit (ODK) for tablet-based data collection.
TABLE 1.
List of records and reports under NTP in Andhra Pradesh that were verified for data accuracy
| Year | Time points | Summary data verified across records/reports | Record/report |
|---|---|---|---|
| 2018 | January, February, April, May, July, August |
|
|
| 2019 | April, May, July, August* |
|
|
* Between January and March 2019, Annexure M was used to report TrueNat test results. From April 2019, the CBNAAT reporting format was adapted by the TrueNat sites for their monthly reporting to be able to effectively capture all information relevant to TrueNat.
† Due to delays in printing or other operational delays, some of the sites continued to use laboratory registers to record TrueNat testing.
RNTCP = Revised National TB Control Programme; TB = tuberculosis; MTB = Mycobacterium tuberculosis; CBNAAT = cartridge-based nucleic acid amplification test.
Impact of TrueNat deployment at POC for TB diagnosis
We used the following monthly RNTCP reports to extract data from January to August 2018 and January to August 2019: 1) Annexure M (monthly report on SSM), 2) TrueNat reports (report on TrueNat testing), and 3) CBNAAT reports (report on CBNAAT testing) (Supplementary Table S1). We compared the number of presumptive TB cases tested, and the number of microbiologically positive TB cases detected before and after TrueNat implementation. The period between October to December 2018 was not considered for analysis, as the sites were transitioning from SSM to TrueNat.
Operational feasibility
We interviewed laboratory technicians (LTs) and Senior TB Laboratory Supervisors (STLSs) at the 25 TUs using a semi-structured questionnaire to collect information about operational issues in TrueNat implementation. The operational feasibility was broadly assessed under the following domains: initiation of TrueNat testing in the centre, logistics, sample preparation and testing, training, time required for TrueNat testing, reporting, waste disposal, breakdown and troubleshooting.
Data analysis
Data accuracy checking
The data points generated from laboratory/culture and drug susceptibility testing (CDST) registers were considered as reference points against which other records were evaluated. For 2018, the agreement was quantified in two comparisons: 1) laboratory/CDST register vs. monthly laboratory register summary; and 2) laboratory/CDST register vs. Annexure M. For 2019, the agreement was quantified by comparing laboratory/CDST register and TrueNat monthly reports.
Impact on TB case notification rate
Case notification rate was operationally defined as the number of TB cases notified per 100 000 population within the RNTCP over a specific time period.11 The total number of presumptive cases tested and the proportion of microbiologically positive cases among these were compared between January–August 2018 and January–August 2019. We used interrupted time series analysis (ITSA) to measure the impact of TrueNat on the detection of positive TB cases. We hypothesised a priori that the introduction of TrueNat would produce a level change in the number of TB cases detected.12 We proposed a regression equation with 1) time elapsed since the beginning of the study period (January 2018), and 2) dummy variable identifying a given time point as pre- or post-implementation as independent variables and 3) the number of positive cases detected as the outcome variable. Since in most of the count data models, variance tends to be greater than mean (over-dispersion), we used negative binomial regression instead of Poisson to adjust the standard error. The incidence rate ratio (IRR) and its 95% confidence intervals (CIs) were calculated to indicate the additional yield of positive cases after introduction of TrueNat. Data were analysed using Stata v14.0 (StataCorp, College Station, TX, USA; 2015).
Operational feasibility
The operational issues faced by LTs and STLSs were given as frequencies and percentages or medians and interquartile ranges.
Ethics statement
As the study involved secondary data analysis of data obtained using record review, ethics approval was not sought. The data collected did not have any identifiers and confidentiality was ensured.
RESULTS
Data accuracy
The average accuracy of 2018 data reported was 96.4% (range 77.0–100.0) when data in laboratory/CDST registers were compared with those in the monthly laboratory summary (Table 2). Data accuracy was 94.9% (range 77.0–100.0) when data in the laboratory/CDST registers were compared with Annexure M. In 2019, data accuracy was 90.9% (range 48.7–100.0), when data in CDST/laboratory register were compared with TrueNat monthly report.
TABLE 2.
Accuracy of data on presumptive TB cases tested as recorded in the RNTCP records and reports in 25 selected TB Units in Andhra Pradesh, India, 2018 and 2019
| Date accuracy | Presumptive TB cases, n (%) | Positives, n (%) | ||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | |||
| Laboratory register vs. monthly laboratory summary | Laboratory register vs. Annexure M | Laboratory register vs. monthly TrueNat report | Laboratory register vs. monthly laboratory summary | Laboratory register vs. Annexure M | Laboratory register vs. monthly TrueNat report | |
| 100% | 6 (24.0) | 3 (12.0) | 3 (12.0) | 13 (52.0) | 14 (56.0) | 10 (40.0) |
| 95–99% | 15 (60.0) | 16 (64.0) | 9 (36.0) | 4 (16.0) | 5 (20.0) | 7 (28.0) |
| 90–94% | 1 (4.0) | 1 (4.0) | 7 (28.0) | 3 (12.0) | 2 (8.0) | 3 (12.0) |
| <90% | 3 (12.0) | 5 (20.0) | 6 (24.0) | 5 (20.0) | 4 (16.0) | 5 (20.0) |
RNTCP = Revised National TB Control Programme; TB = tuberculosis.
Impact assessment
In the 193 TUs studied, a total of 245 989 presumptive cases were tested using SSM during January–August 2018, of which 25 726 (10.5%; range 8.9–13.1) tested positive. During January–August 2019, of the total 185 435 presumptive cases, 32 876 cases were tested using SSM and 152 559 using TrueNat. The positivity of SSM and TrueNat were respectively 10.4% (range 6.4–14.5) and 14.4% (range 10.0–21.2). A total of 25 359 (13.7%; range 9.6–18.9) tested microbiologically positive in 2019. TrueNat positivity exhibited a wide range across districts, with East Godavari (10.0%) reporting the lowest and Kadapa (21.2%) reporting the highest. Seven districts reported a positivity less than the state average of 14.4% (Table 3).
TABLE 3.
Presumptive cases tested and proportion of positive cases detected in Andhra Pradesh, India, between January to August 2018 and 2019 (total and by district)
| District | SSM, 2018 | SSM, 2019 | TrueNat, 2019 | SSM+TrueNat, 2019 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total presumptive cases tested | Presumptive cases that tested positive | Total presumptive cases tested | Presumptive cases that tested positive | Total presumptive cases tested | Presumptive cases that tested positive | Invalid results | Errors | Presumptive cases | Presumptive cases that tested positive | ||||
| n | n | % | n | n | % | n | n | % | n | n | n | n | |
| Anantapur | 17,043 | 2,209 | 13.0 | 2,623 | 358 | 13.7 | 7,032 | 1,463 | 20.8 | 285 | 120 | 9,655 | 1,821 |
| Chittoor | 23,601 | 2,738 | 11.6 | 1,538 | 152 | 9.9 | 14,293 | 1,941 | 13.6 | 713 | 276 | 15,831 | 2,093 |
| East Godavari | 31,584 | 2,869 | 9.1 | 4,801 | 406 | 8.5 | 17,627 | 1,755 | 10.0 | 491 | 202 | 22,428 | 2,161 |
| Guntur | 20,819 | 2,724 | 13.1 | 3,646 | 323 | 8.9 | 10,636 | 1,929 | 18.1 | 239 | 80 | 14,282 | 2,252 |
| Kadapa | 16,657 | 1,511 | 9.1 | 3,686 | 379 | 10.3 | 8,315 | 1,764 | 21.2 | 248 | 82 | 12,001 | 2,143 |
| Krishna | 17,384 | 1,963 | 11.3 | 417 | 44 | 10.6 | 12,247 | 1,791 | 14.6 | 361 | 163 | 12,664 | 1,835 |
| Kurnool | 25,047 | 2,481 | 9.9 | 3,623 | 272 | 7.5 | 12,513 | 2,158 | 17.3 | 291 | 125 | 16,136 | 2,430 |
| Nellore | 17,565 | 1,568 | 8.9 | 2,755 | 225 | 8.2 | 10,422 | 1,209 | 11.6 | 211 | 120 | 13,177 | 1,434 |
| Prakasam | 11,916 | 1,269 | 10.7 | 840 | 54 | 6.4 | 11,411 | 1,580 | 13.9 | 434 | 118 | 12,251 | 1,634 |
| Srikakulam | 11,683 | 1,056 | 9.0 | 479 | 41 | 8.6 | 10,030 | 1,421 | 14.2 | 477 | 121 | 10,509 | 1,462 |
| Visakhapatnam | 24,422 | 2,198 | 9.0 | 5,139 | 743 | 14.5 | 18,721 | 2,254 | 12.0 | 528 | 182 | 23,860 | 2,997 |
| Vizianagaram | 10,742 | 1,135 | 10.6 | 860 | 81 | 9.4 | 8,755 | 1,291 | 14.8 | 188 | 87 | 9,615 | 1,372 |
| West Godavari | 17,526 | 2,005 | 11.4 | 2,469 | 332 | 13.5 | 10,557 | 1,393 | 13.2 | 280 | 88 | 13,026 | 1,725 |
| State | 245,989 | 25,726 | 10.5 | 32,876 | 3,410 | 10.4 | 152,559 | 21,949 | 14.4 | 4,746 | 1,764 | 185,435 | 25,359 |
SSM = sputum smear microscopy.
ITSA indicated that there was a significant increase in the proportion of presumptive TB cases testing positive for TB (IRR 1.30, 95% CI 1.15–1.46) after implementation of TrueNat. There were 30% more TB cases detected post-TrueNat implementation than pre-implementation. In contrast to the actual scenario where TrueNat was used for TB diagnosis in 2019, we constructed a hypothetical scenario in which only SSM is used for TB diagnosis in 2019. There was a change in level in the solid line representing positive cases, before and after the implementation of TrueNat (Figure). The proportion of presumptive TB cases who would have tested positive in this counterfactual scenario is represented by a dashed line in the Figure.
FIGURE.
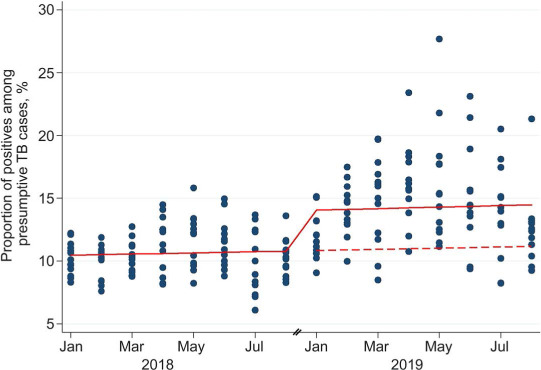
Interrupted time series analysis of the impact of implementing TrueNat on the proportion testing positive among all presumptive TB cases using a level-change regression model in Andhra Pradesh, India (2018–2019). Solid line = predicted line based on negative binomial regression model; dashed line = counterfactual scenario if TrueNat had not been implemented.
Between January and August 2018, the case notification rate of microbiologically positive TB in Andhra Pradesh was 49/100 000. We estimated that, had we tested all presumptive cases in 2018 using TrueNat instead of SSM, there would have been 35 422 microbiologically positive cases instead of the 25 726 cases detected using only SSM. This would translate into a case notification rate of 67 positives cases/100 000 and would yield an additional 18 positive cases/100 000.
Feasibility assessment
Of the 25 LTs interviewed, 17 were RNTCP staff, while 8 were staff from other programmes who had been given the additional responsibility of RNTCP laboratory testing. The LTs and STLSs had a median experience of respectively 14 (interquartile range [IQR] 9.5–16.5) and 10 years (IQR 6.0–15.0). Twenty of the 25 LTs interviewed had undergone training in TrueNat testing. Eight of the 24 STLSs felt that they had inadequate number of trained staff to perform TrueNat testing. It took LTs a median of 10 tests (IQR 5.0–17.5) to become comfortable in performing TrueNat. Between April and August 2019, the percentage of invalid results reported by TrueNat sites showed a decline from 6.8% to 3.6%.
There was no external quality assurance mechanism for TrueNat testing. Two LTs expressed difficulty in any one of the steps—setting up, installation, daily start-up, handling, portability, entering and editing patient details or cleaning of TrueNat equipment. Sample preparation, especially transfer of elute to the chip well was the only step where four LTs faced difficulty. All except two STLSs felt that they were able to process as many or even more samples per day compared to SSM. Of the 24 STLSs, 21 perceived that TrueNat was comparable or superior to SSM in accuracy, and 17 (70.8%) felt that it was easier to perform than SSM. Fifteen (62.5%) felt that the accuracy of TrueNat was as high or higher than CBNAAT, while 7 (29.2%) found it to be more difficult to perform than CBNAAT.
All laboratories had received TrueNat logistics within 1–2 days of requesting. Only one site reported stock out of MTB kits in the previous quarter. Seven sites had their sample preparation device replaced at least once and six sites had their TrueLab device replaced at least once since installation. Written guidelines about biomedical waste management for TrueNat were not available at 16 of the 25 sites. STLS reported facing technical issues in sample preparation and transfer and higher workload due to inadequate manpower. The suggestions given by LTs/STLSs for improving implementation of TrueNat are presented in Table 4.
TABLE 4.
Suggestions provided by LTs and STLS for the improvement of TrueNat implementation under RNTCP
| Domain | Suggestion |
|---|---|
| Instrument-related |
|
| Technique/training related |
|
| Monitoring and evaluation |
|
LT = laboratory technician; STLS = Senior TB Laboratory Supervisor; RNTCP = Revised National TB Control Programme; DMC = designated microscopy centre; TB = tuberculosis.
DISCUSSION
Our analysis of data on 431 424 presumptive TB cases tested under RNTCP in Andhra Pradesh, India, indicated that TrueNat improved case notification rates by 30%. The programmatic implementation of TrueNat was operationally feasible.
The overall accuracy of the data reported was satisfactory. This reflects on the quality of the training of the LTs/STLSs and the monitoring and evaluation methods used in the programme. However, six sites reported <90% data accuracy in TrueNat recording and reporting. This could have been due to the delay in making logistics such as new registers and reporting formats available to the TrueNat sites, because of which LTs made informal entries in the existing laboratory register or maintained additional records to capture this information. This practice could have led to erroneous reporting. This emphasises the need for uniform recording and reporting mechanisms to be used at all TrueNat sites. LT-generated reports could also be validated by machine-generated reports from the TrueNat device.
The number of presumptive cases tested had decreased in 2019 from 2018, likely due to a temporary suspension of SSM for diagnosis in DMCs (other than the TrueNat sites) between February 2019 and August 2019. The suspension of SSM was based on a state-level policy decision to make molecular diagnosis available to every presumptive TB case. All samples from DMCs were to be transported to the TrueNat sites for testing using TrueNat. In view of the decline in the number of presumptive TB cases tested, the state had resumed SSM for diagnosis in all DMCs from September 2019, until TrueNat was available at DMC level. Thus, the drop in the number of presumptive cases observed in January–August 2019 was temporary and is expected to improve.
TrueNat positivity observed in our analysis (14.4%) was lower than the positivity reported earlier (18.1%).7 This difference may be attributed to the programmatic conditions under which the current evaluation was conducted, as against the experimental, controlled situations in which the earlier assessment had been done (using staff trained for the purpose under rigorous monitoring).
The inter-district variation in TrueNat positivity could be due to varying disease load across districts. Another reason could be the varying levels of adherence to the definition of presumptive cases, leading to differences in the number of presumptive cases tested in different districts. Variations in TrueNat testing practices and reporting across districts could also influence the number of positives detected. There is currently no quality assurance mechanism in place for TrueNat reports. A periodic quality assurance mechanism in the form of blinded rechecking of results should be built into the programme, as is currently in place for SSM.
The decline in the number of invalid reports could be attributed to the newer batch of TrueNat chips which had replaced the faulty earlier ones or the better technique employed by the LTs with increasing experience with TrueNat.
Inadequate manpower and higher workload were reported as issues after the introduction of TrueNat, mostly because of the redistribution of LTs from other programmes to fill vacant positions. This highlights the need for the vacant positions of LTs to be filled and training of all LTs to be completed prior to the proposed country-wide expansion of TrueNat as POC.
CONCLUSIONS
Deployment of TrueNat can significantly improve TB case detection as compared to SSM. While some minor issues are to be anticipated in the initial stabilisation period, proper logistics planning, manpower management and an inbuilt external quality assurance mechanism can help successfully integrate TrueNat as a POC diagnostic test under the RNTCP. Future research on the impact of such an integration on TB detection, treatment outcomes and TB burden as a whole can provide important lessons for other low- and middle-income countries battling high TB burden.
Acknowledgments
This study was funded by the Indian Council of Medical Research, New Delhi, India.
Footnotes
Conflicts of interest: none declared.
References
- 1.Bernal JL, et al. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2016;46(1):348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Central TB Division, Ministry of Health and Family Welfare, Government of India India TB report, 2019. New Delhi, India: MoHFW; 2019. [Google Scholar]
- 3.Keeler AE, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;2:49–58. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 4.Ngabonziza JCS, et al. Diagnostic performance of smear microscopy and incremental yield of Xpert in detection of pulmonary tuberculosis in Rwanda. BMC Infect Dis. 2016;16(1):660. doi: 10.1186/s12879-016-2009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikam C, et al. Rapid Diagnosis of Mycobacterium tuberculosis with Truenat MTB: a near-care approach. PLoS ONE. 2013;8(1):e51121. doi: 10.1371/journal.pone.0051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikam C, et al. Evaluation of the Indian TrueNAT micro RT-PCR device with GeneXpert for case detection of pulmonary tuberculosis. Int J Mycobacteriol. 2014;3(3):205–210. doi: 10.1016/j.ijmyco.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy S, et al. Final report on operational feasibility and performance of TrueNat MTB Rif assays in field settings under the Revised National Tuberculosis Control Program. New Delhi, India: Indian Council of Medical Research; 2016. [Google Scholar]
- 8.Lee DJ, et al. Rapid, point-of-care diagnosis of tuberculosis with novel Truenat Assay: cost-effectiveness and budgetary impact analysis for India’s public sector. Open Forum Infect Dis. 2018;5(Suppl 1):S582. doi: 10.1371/journal.pone.0218890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajsekar K, Tyagi K, Singh M. Rapid Health Technology Assessment for incorporating TrueNat as a diagnostic tool for tuberculosis under RNTCP in India. New Delhi, India: Indian Council of Medical Research; 2019. [Google Scholar]
- 10.World Health Organization. Rapid Communication. Molecular assays as initial tests for the diagnosis of tuberculosis and rifampicin resistance. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 11.World Health Organization. TB notification rate. Geneva, Switzerland: WHO; 2015. https://www.who.int/healthinfo/indicators/2015/chi_2015_45_tb_notification.pdf [Google Scholar]
- 12.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]


