Abstract
Global HIV program stakeholders, including the US President’s Emergency Plan for AIDS Relief (PEPFAR), are undertaking efforts to ensure that eligible people living with HIV (PLHIV) receiving antiretroviral treatment (ART) receive a course of TB preventive treatment (TPT). In PEPFAR programming, this effort may require providing TPT not only to newly diagnosed PLHIV as part of HIV care initiation, but also to treatment-experienced PLHIV stable on ART who may not have been previously offered TPT. TPT scale-up is occurring at the same time as a trend to provide more person-centered HIV care through differentiated service delivery (DSD). In DSD, PLHIV stable on ART may receive less frequent clinical follow-up or receive care outside the traditional clinic-based model. The misalignment between traditional delivery of TPT and care delivery in innovative DSD may require adaptations to TPT delivery practices for PLHIV. Adaptations include components of planning and operationalization of TPT in DSD, such as determination of TPT eligibility and TPT initiation, and clinical management of PLHIV while on TPT. A key adaptation is alignment of timing and location for TPT and ART prescribing, monitoring, and dispensing. Conceptual examples of TPT delivery in DSD may help program managers operationalize TPT in HIV care.
Keywords: tuberculosis preventive treatment, differentiated service delivery, HIV treatment
Abstract
Les parties prenantes du programme mondial VIH, notamment le plan américain PEPFAR (US President’s Emergency Plan for AIDS Relief), entreprennent des efforts afin de s’assurer que les personnes vivant avec le VIH (PLVIH), éligibles, recevant un traitement antirétroviral (TAR), reçoivent également un traitement préventif TB (TPT). Dans la programmation PEPFAR, cet effort pourrait nécessiter de fournir le TPT non seulement aux PLVIH nouvellement diagnostiquées dans le cadre de l’initiation de la prise en charge du VIH, mais également aux PLVIH stables déjà traités par TAR à qui on n’aurait pas encore offert le TPT. L’expansion du TPT survient au même moment comme une tendance à offrir une prise en charge du VIH davantage centrée sur la personne à travers une prestation de services différenciée (DSD). Dans la DSD, les PLVIH stables sous TAR bénéficient d’un suivi clinique moins fréquent ou sont soignés hors du modèle traditionnel en structures de santé. Le décalage entre la prestation traditionnelle du TPT et la prestation de soins dans des DSD innovantes peut nécessiter des adaptations aux pratiques de prestation du TPT pour les PLVIH. Ces adaptations incluent des éléments de planification et d’opérationnalisation du TPT dans la DSD, comme la détermination de l’éligibilité au TPT et sa mise en route et la prise en charge clinique des PLVIH sous TPT. Une adaptation majeure est l’alignement en termes de temps et de lieu pour la prescription, le suivi et la délivrance du TPT et du TAR. Des exemples conceptuels de délivrance du TPT dans la DSD aideraient les gestionnaires de programme à rendre opérationnel le TPT au sein de la prise en charge du VIH.
Abstract
Los interesados directos del Programa Mundial del VIH, incluido el Plan de Emergencia del Presidente (de los Estados Unidos) para el Alivio del Sida (PEPFAR), emprenden ahora esfuerzos encaminados a garantizar que las personas con infección por el VIH (PLVIH), que siguen un tratamiento antirretrovírico (TAR) y que reúnen las condiciones, reciban un ciclo de tratamiento preventivo de la TB (TPT). En la programación del PEPFAR esta iniciativa puede necesitar la provisión de TPT no solo a las personas con un diagnóstico reciente de infección por el VIH, como parte del inicio de la atención del VIH, sino también a las PLVIH, con experiencia de tratamiento y que se encuentran estables recibiendo el TAR, a quienes tal vez no se haya propuesto antes el TPT. La ampliación del TPT ocurre de manera simultánea con la tendencia a ofrecer una atención del VIH más centrada en la persona mediante la prestación diferenciada de servicios (DSD). En la DSD, las PLVIH, estables con el TAR, pueden tener encuentros de seguimiento clínico menos frecuentes o recibir atención por fuera del modelo tradicional en los consultorios. La discordancia entre la provisión tradicional del TPT y la prestación de atención en el marco innovador de la DSD exige adaptaciones de las prácticas de prestación del TPT a las PLVIH. Las adaptaciones incluyen componentes de planeación y puesta en práctica del TPT en la DSD, como la determinación de los criterios para recibir el TPT, el inicio del mismo y el manejo clínico de las PLVIH mientras reciben el TPT. Una adaptación primordial es la coordinación del ritmo y el lugar de prescripción, supervisión y suministro del TPT y el TAR. La presentación de ejemplos teóricos de provisión del TPT en el marco de la DSD puede ayudar a los gerentes de programas a poner en práctica el TPT en la atención del VIH.
TB is the leading cause of death among people living with HIV (PLHIV), making TB preventive treatment (TPT) an essential component of care for this population. The WHO estimated that in 2017, 9% of the 10 million (95% uncertainty interval 9.0–11.1) people who developed TB disease were PLHIV,1 and in 2018, 251 000 (95% uncertainty interval 223 000–281 000) PLHIV died due to TB disease.2 Isoniazid preventive therapy, the most common TPT regimen, has been shown to reduce TB-related mortality among PLHIV by 37% at 5 years, independent of protection from antiretroviral treatment (ART).3 Despite this evidence, the WHO estimated that only 36% of PLHIV who received HIV treatment across 59 countries in 2017 also received TPT.1 A recent review of barriers to TPT implementation in 35 countries found that only 21 (60%) reported nationwide programmatic TPT implementation.4 To jumpstart treatment of PLHIV with TPT, global stakeholders made a pledge to ensure six million PLHIV receive TPT by 2022 during a 2018 United Nations High-Level Meeting on TB.5
The US President’s Emergency Plan for AIDS Relief (PEPFAR) is one of the major stakeholders in HIV care delivery, supporting 15.7 million PLHIV on antiretroviral therapy (ART) as of September 2019.6 In 2017, PEPFAR introduced a program indicator to track the number of PLHIV completing TPT, and is aiming to provide TPT to all eligible PLHIV on ART in its supported country programs by 2021. Reaching that goal requires offering TPT not only to newly diagnosed PLHIV as part of the initiation of HIV care, but also to treatment-experienced PLHIV stable in HIV care and on ART who may not have been previously offered TPT. Between October 2019 and September 2020, 18 of 29 PEPFAR-supported programs targeted a higher number of PLHIV to receive TPT than the number of PLHIV targeted to newly initiate ART (Figure 1). In these programs, TPT target achievement relies critically on provision for PLHIV already established on ART.
FIGURE 1.
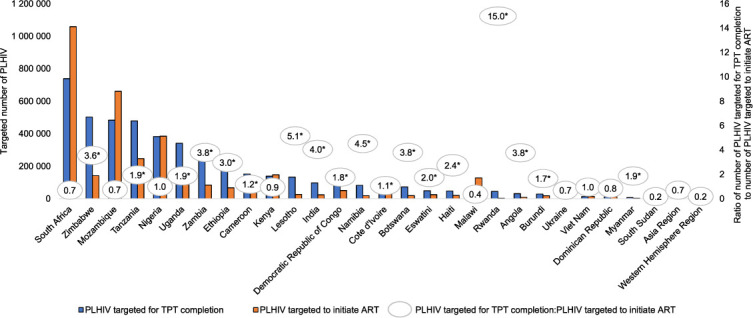
Ratio of the number of PLHIV targeted for TPT completion to the number of PLHIV targeted to initiate ART—29 PEPFAR programs, October 2019–September 2020.*† * 18 PEFPAR-supported country or regional programs in which the ratio of number of PLHIV targeted for TPT completion to number of PLHIV targeted to initiate ART is >1, indicating more PLHIV are targeted for TPT completion than PLHIV initiating ART. In these programs, achieving TPT targets may require provision of TPT to PLHIV already stable on ART and who may be receiving HIV care through differentiated service delivery. † Data source: PEPFAR Data for Accountability, Transparency, and Impact fiscal year 2020 targets, except for PLHIV targeted for TPT completion from Kenya and Nigeria, which come from Country Operational Plan 2019 planning level letters for fiscal year 2020 targets. PLHIV = people living with HIV; TPT = tuberculosis preventive treatment; ART = antiretroviral treatment; PEPFAR = US President’s Emergency Plan for AIDS Relief.
Global TPT implementation and scale-up in HIV programs is occurring simultaneously with an ongoing trend in the HIV treatment delivery landscape, in which programs are providing more person-centered HIV care and treatment through differentiated service delivery (DSD). In this context, DSD describes the adaptations that can be made to HIV services, including ART delivery, to meet the care needs and preferences of PLHIV, while also streamlining care in the context of limited human resources and infrastructure.7 DSD varies across domains of HIV care delivery of what service is delivered (ART, clinical monitoring, blood sampling for laboratory testing), when it is delivered (frequency of encounter), where it is delivered (in a facility, in the community), and by whom it is delivered (physician, other healthcare provider, community health worker, peer). DSD may allow PLHIV stable on ART, such as those who have achieved documented viral load suppression and have no clinical evidence of opportunistic infections, to receive less frequent clinical follow-up or receive care outside the traditional clinic-based model, which may improve their satisfaction and long-term ART adherence and retention. DSD may also free up space and time in the clinic for health professionals to focus on newly diagnosed PLHIV or those with high viral load or opportunistic infections.8
In practice, DSD models such as multi-month medication prescribing and dispensing, ‘fast-tracking’ for ART refills, or community ART distribution allow PLHIV on ART to receive facility-based clinical monitoring once every 3–6 months, or receive ART refills or clinical monitoring monthly but in the community. DSD for stable PLHIV should offer all recommended TB-HIV services, including regular TB screening and TPT. The less frequent facility-based clinical monitoring inherent in DSD for PLHIV stable in care, however, may not align with current WHO guidelines or clinical practices for monthly monitoring of PLHIV initiating TPT.9 This misalignment between traditional delivery of TPT and how care is delivered in many DSD models may contribute to the slow implementation and scale-up of TPT among PLHIV who are stable on ART. Thus, for TPT to be delivered to all PLHIV as part of a comprehensive package of HIV care, PEPFAR-supported programs and public health practitioners may need to consider adapting existing TPT delivery practices to accommodate the care models of PLHIV receiving DSD.
Programmatic considerations for implementation of TPT in DSD models for PLHIV have been described within a broader context of TB care within DSD,8 and in recent framework documents on integrating TPT within DSD.10,11 This commentary builds upon previous work by further addressing real-world implementation of TPT in DSD. This includes describing components of planning and operationalization of TPT within DSD, including determination of TPT eligibility and TPT initiation, and clinical management of PLHIV while on TPT. Conceptual and real-world examples of TPT integration in DSD are also presented.
PLANNING FOR TB PREVENTIVE TREATMENT WITHIN DIFFERENTIATED SERVICE DELIVERY IMPLEMENTATION
In planning for TPT in DSD implementation, PEPFAR country programs and implementing partners, along with the country Ministries of Health (MOH) they support, may consider several elements. First, they should understand the extent of TPT needs among PLHIV currently in care by estimating the number of PLHIV on ART who have yet to be offered a course of TPT. Next, they should understand the extent of DSD in their HIV programs, by mapping which DSD models for stable PLHIV are available and at which treatment sites, as well as the number of PLHIV receiving ART in each model who have yet to be offered a course of TPT. Third, they may consider which TPT regimens are available in the country (6H, 3HP, or both), and how the length of treatment, timing of dosing, and method of administration (directly observed therapy or self-administered therapy) may affect frequency and types of encounters with health workers. Fourth, they may consider whether the frequency and types of encounters with health workers for purposes of TPT delivery can be modified to align with frequency and types of encounters already established within existing DSD models for stable PLHIV, or if the frequency and types of encounters for PLHIV in DSD during a course of TPT must instead be temporarily modified to accommodate those desired during the course of TPT. Finally, program managers should consider which clinical tasks in TPT delivery, including determination of TPT eligibility, initiation of TPT, screening for presumptive TB, monitoring for TPT-related adverse events (AEs), and monitoring of adherence, may be shifted to non-physician healthcare providers, community health workers, or peers.
Another important element to consider is how to manage TPT when considering transitioning stable PLHIV from standard HIV care to DSD. To ensure newly diagnosed PLHIV initiate a course of TPT upon or soon after ART initiation, program managers could consider adding the completion of 6 months of isoniazid, or an alternate short-course regimen if available, as a prerequisite for clinical ‘stability’, and thus eligibility for DSD. TPT could be provided to PLHIV as they move toward meeting other prerequisites for eligibility for DSD, such as demonstrating viral load suppression. Alternatively, among PLHIV who have initiated but not completed TPT and are otherwise eligible for transition to DSD, program managers may consider including the continuation of TPT within DSD to ensure TPT completion.
An additional consideration in planning for TPT in DSD is to incorporate the concept in national HIV and TB guidelines. National standardized operating procedures endorsed by the MOH and included in national guidelines are crucial for making TPT a part of standard HIV care and codifying policies for its use in DSD. Equally important is to include TPT-related indicators, such as TPT initiation and completion, in national monitoring and evaluation frameworks.
OPERATIONALIZATION OF TUBERCULOSIS PREVENTIVE TREATMENT IN DIFFERENTIATED SERVICE DELIVERY
Determination of TPT eligibility (including TB screening) and TPT initiation
It is critical to evaluate PLHIV to determine eligibility prior to offering TPT. Evaluation tools and checklists are available to identify common symptoms of TB disease and contraindications to TPT.12 For those in DSD, this evaluation could take place at the next scheduled facility visit, or for stable PLHIV receiving ART in a community DSD model, in the community. Evaluation for TPT eligibility could provide an opportunity to implement other components of quality HIV care delivery, including viral load testing or transition to newly recommended ART regimens. Health workers could be trained to administer a checklist to review specific TPT contraindications and symptoms of TB disease. Ruling out TB disease is crucial and can be done using the WHO four-symptom TB screen. Thereafter, if no contraindications to TPT or symptoms of TB disease are present, the health worker should counsel and educate on benefits of TPT and potential TPT-related AEs using standardized, comprehensive materials, before offering TPT. At TPT initiation, health workers and PLHIV should review how to recognize symptoms of TB disease and potential TPT-related AEs and be empowered to report these should they occur.
Clinical management of PLHIV while on TPT
Ongoing screening for and management of presumptive TB, TPT-related AEs, and adherence are important components of TPT clinical management. At follow-up encounters with health workers during a TPT course, standardized, comprehensive education and counseling on symptoms of TB disease and TPT-related AEs can be provided. Screening could be done in a facility, pharmacy, or in the community, and in fact, where the screening takes place could change during the TPT course, such as requiring the first follow-up visit to occur in a facility, but subsequent encounters to occur in the community. An important consideration is to define procedures for alerting and managing potential presumptive TB or TPT-related AEs, to include referral and linkage to TB investigations or clinical evaluation, especially when a non-clinician, such as a pharmacist, community health worker, or peer, is conducting the screening. Similarly, standardizing procedures for clinicians and epidemiologists to conduct clinical evaluation and investigation of potential TPT-related AEs is a critical part of AE surveillance.
Another important component of TPT clinical management is management of prescribing and dispensing of TPT. Alignment of TPT prescribing and dispensing with ART prescribing and dispensing is critical,13 because in both programmatic and cohort study settings, this alignment has been associated with higher TPT completion rates.14,15 Adherence monitoring can be aligned with TPT dispensation and screening for presumptive TB and TPT-related AEs. Documenting TPT completion is an important programmatic indicator, including within PEPFAR programs. TPT completion may be defined as picking up the final package of TPT doses, or upon having a health system encounter after receiving the final package of TPT doses. These activities could take place in a facility, pharmacy, or in the community, at the convenience of the recipient of care. Offering flexible treatment in DSD should not undermine program surveillance or monitoring and evaluation, so adaptations in monitoring tools and protocols to document PLHIV initiating and completing TPT in DSD may be needed and incorporated in national MOH reporting guidelines. Again, health workers can be trained to conduct standardized adherence monitoring and document TPT completion.
CONCEPTUAL EXAMPLES OF TUBERCULOSIS PREVENTIVE TREATMENT INTEGRATION IN DIFFERENTIATED SERVICE DELIVERY
TPT could be delivered in a facility-based DSD model for stable ART patients (Figure 2). In this conceptual example of a facility-based model, PLHIV stable on ART are enrolled in a DSD model that consists of 3-month ART prescribing (with fast-track medication pick-up and screening for presumptive TB, AEs, and adherence at the pharmacy) and 6-month clinic visits. When these PLHIV start TPT, after appropriate TB screening and evaluation, as well as counseling on benefits of TPT and on potential TPT-related AEs using standardized education materials, a 1-month supply of TPT is given at their routine 6-month clinic visit. They return 1 month later for a TPT assessment (screening for presumptive TB, AEs, and adherence) at the facility. If no presumptive TB, serious AEs, or adherence concerns are identified, PLHIV are given a 2-month supply of TPT. They then return 2 months later for their regularly scheduled ART fast-track visit, where they undergo a TPT assessment by the pharmacist in addition to collecting 3 months of ART and TPT (if no presumptive TB, serious AEs, or adherence concerns are identified). At the next 6-month clinic visit (3 months after their fast-track visit), a full TPT assessment and evaluation for completion is conducted by the clinician. The advantage of this model is that the existing DSD model is only ‘broken’ once: at an extra clinic visit 1 month after initiating TPT.
FIGURE 2.
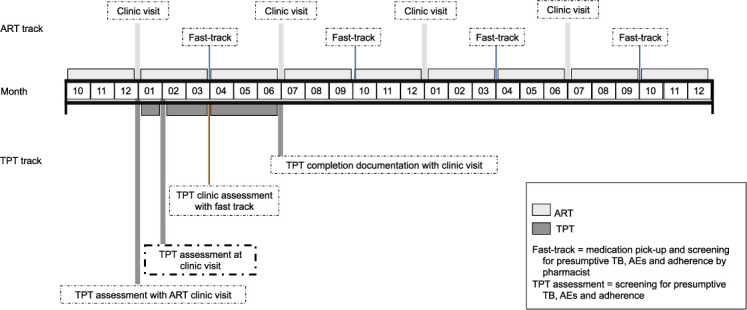
TPT delivery for stable PLHIV in DSD: conceptual example of a facility-based model. PLHIV stable on ART are enrolled in a DSD model that consists of 3-month ART prescribing (fast-track medication pick-up and screening for presumptive TB, AEs, and adherence at the pharmacy) and 6-month clinic visits. When these PLHIV start TPT after appropriate TB screening and evaluation, as well as counseling on benefits of TPT and on potential TPT-related AEs using standardized education materials, a 1-month supply of TPT is given at their routine 6-month clinic visit. They return 1 month later for a TPT assessment (screening for presumptive TB, AEs, and adherence) at the facility. If no presumptive TB, serious AEs, or adherence concerns are identified, PLHIV are given a 2-month supply of TPT. They then return 2 months later for their regularly scheduled ART fast-track visit, where they undergo a TPT assessment by the pharmacist in addition to collecting 3 months of ART and TPT (if no presumptive TB, serious AEs, or adherence concerns are identified). At the next 6-month clinic visit, a TPT assessment and evaluation for completion is conducted by the clinician. This model has the following benefits: stable PLHIV on ART only ‘break’ their regular cycle of 6-month facility visits once, 1 month after initiating TPT. ART = antiretroviral therapy; TPT = TB preventive treatment; TB = tuberculosis; AE = adverse event; PLHIV = people living with HIV; DSD = differentiated service delivery.
Of note, reduced frequency of visits to pick up TPT medication may be less important to TPT completion than other components of DSD models for stable PLHIV. A cross-sectional study of PLHIV on ART receiving TPT in Uganda in a DSD model vs. a standard HIV care model found a higher proportion in the DSD model completed TPT, although TPT was still dispensed monthly.16
TPT could also be delivered in a community-based model (Figure 3). In this conceptual example, PLHIV stable on ART are enrolled in a DSD model that consists of 6-month clinic visits with TPT dispensed monthly in the community. At the routine 6-month clinic visit, PLHIV are counseled on benefits of TPT and on potential TPT-related AEs using standardized education materials, then given a 1-month supply of TPT. They receive a TPT assessment (screening for presumptive TB, AEs, and adherence) 1 month later at a clinic visit or a community-based group meeting. If no presumptive TB, serious AEs, or adherence concerns are identified, they are given another 1-month supply of TPT along with ART. They continue to receive a TPT assessment at each of their group meetings at Months 2–5. At the next 6-month clinic visit, a TPT assessment and evaluation for completion is conducted by the clinician. As with the facility-based example, the care model of the existing DSD is only ‘broken’ once: at an extra clinic visit 1 month after initiating TPT. This model can be adapted for community ART groups as well, with all eligible PLHIV in the group potentially initiating and completing TPT together, which may reinforce adherence and reporting of possible presumptive TB or TPT-related AEs. Community-based DSD for TPT initiation with multi-month prescribing and dispensing among PLHIV initiating ART in the community in South Africa demonstrated that 355 of 388 (91%) of PLHIV eligible for TPT initiated TPT.17
FIGURE 3.
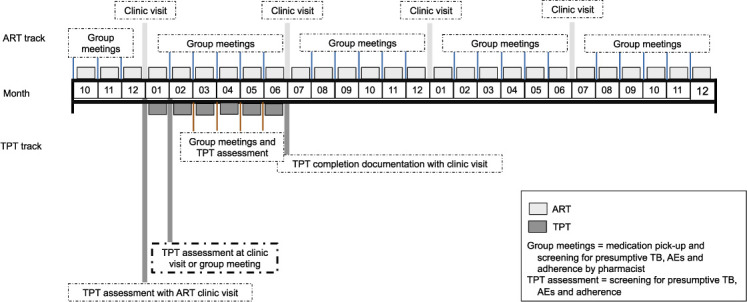
TPT delivery for stable PLHIV in DSD: conceptual example of a community-based model. PLHIV stable on ART are enrolled in a DSD model that consists of 6-month clinic visits with TPT dispensed monthly in the community. At the routine 6-month clinic visit, PLHIV are counseled on benefits of TPT and on potential TPT-related AEs using standardized education materials, then given a 1-month supply of TPT. They receive a TPT assessment (screening for presumptive TB, AEs, and adherence) 1 month later at a clinic visit or a community-based group meeting. If no presumptive TB, serious AEs, or adherence concerns are identified, they are given another 1-month supply of TPT along with ART. They continue to receive a TPT assessment at each of their group meetings at Months 2–5. At the next 6-month clinic visit, a TPT assessment and evaluation for completion is conducted by the clinician. The advantage of this model is that stable PLHIV on ART only ‘break’ their regular cycle of 6-month facility visits once if the program decides they must go to a clinic for their 1-month TPT assessment. Of note, this model can be adapted for community ART groups as well, with all eligible PLHIV in the group potentially initiating and completing TPT together, which may reinforce adherence and reporting of possible presumptive TB or TPT-related AEs. ART = antiretroviral therapy; TPT = TB preventive treatment; TB = tuberculosis; AE = adverse event; PLHIV = people living with HIV; DSD = differentiated service delivery.
CONCLUSIONS
TPT is an essential part of HIV care and integral to reducing morbidity and mortality among PLHIV. There is substantial momentum, both globally and within PEPFAR-supported country programs, to rapidly provide a full TPT course to all PLHIV. Reaching that goal may require providing TPT not only to PLHIV initiating ART but also to PLHIV considered stable on ART and already receiving care in DSD. Aligning TPT delivery with the less frequent facility-based encounters of DSD means modifying how key components of TPT delivery, including determination of eligibility, TPT medication dispensation, screening for TB disease, monitoring for TPT-related AEs and adherence, and documenting outcomes, are traditionally done. Adapting TPT to deliver it within DSD, and incorporating those adaptations in national MOH HIV and TB guidelines, acknowledges and accommodates PLHIV preferences and empowers community health workers and PLHIV themselves to monitor and take charge of their health. Early implementation of TPT in DSD models suggests that this approach improves TPT completion rates.16 Gathering best practices and data on programmatic implementation of TPT in DSD is an important next step in further integrating TPT in person-centered HIV care.
Acknowledgments
The writing of this manuscript was supported by the President’s Emergency Plan for AIDS Relief (Washington DC, USA) through the US Centers for Disease Control and Prevention (Atlanta, GA, USA).
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies.
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2018. WHO/CDS/TB/2018.20. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 2.World Health Organization. Global tuberculosis report, 2019. WHO/CDS/TB/2019.15. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 3.Badje A, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health. 2017;5(11):e1080–e1089. doi: 10.1016/S2214-109X(17)30372-8. [DOI] [PubMed] [Google Scholar]
- 4.Surie D, et al. Policies, practices and barriers to implementing tuberculosis preventive treatment, 35 countries, 2017. Int J Tuberc Lung Dis. 2019;23(12):1308–1313. doi: 10.5588/ijtld.19.0018. [DOI] [PubMed] [Google Scholar]
- 5.United Nations. Political declaration of the United Nations General Assembly High-Level Meeting on the fight against tuberculosis. New York, NY, USA: UN; 2018. [Google Scholar]
- 6.President’s Emergency Fund for AIDS Relief. PEPFAR 2020 Country Operational Plan Guidance for all PEPFAR Countries. Washington DC, USA: PEPFAR; 2019. [Google Scholar]
- 7.International AIDS Society. Differentiated service delivery. Geneva, Switzerland: International AIDS Society; 2020. http://www.differentiatedcare.org/ [Google Scholar]
- 8.Pathmanathan I, Pevzner E, Cavanaugh J, Nelson L. Addressing tuberculosis in differentiated care provision for people living with HIV. Bull World Health Organ. 2017;95(1):3. doi: 10.2471/BLT.16.187021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, Switzerland: WHO; 2018. [PubMed] [Google Scholar]
- 10.New York, NY, USA: ICAP Columbia University Mailman School of Public Health; 2020. Integrating intensive TB case-finding and TB preventive treatment services into differentiated ART models, 2019.https://cquin.icap.columbia.edu/wp-content/uploads/2020/01/CQUIN-TPT-Toolkit_Jan-2020_Final_Cover.pdf [Google Scholar]
- 11.Rabkin M, et al. Leveraging differentiated HIV service delivery to expand tuberculosis preventive treatment: a call to action. Int J Tuberc Lung Dis. 2020;24(2):165–169. doi: 10.5588/ijtld.19.0595. [DOI] [PubMed] [Google Scholar]
- 12.Office of the US Global AIDS Coordinator. TB preventive treatment (TPT): Implementation Tools. Washington DC, USA: Office of the US Global AIDS Coordinator; 2020. https://www.pepfarsolutions.org/tools-2/2018/9/25/tpt-implementation-tools [Google Scholar]
- 13.Pathmanathan I, et al. TB preventive therapy for people living with HIV: key considerations for scale-up in resource-limited settings. Int J Tuberc Lung Dis. 2018;22(6):596–605. doi: 10.5588/ijtld.17.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takarinda KC, Choto RC, Mutasa-Apollo T, Chakanyuka-Musanhu C, Timire C, Harries AD. Scaling up isoniazid preventive therapy in Zimbabwe: has operational research influenced policy and practice? Public Health Action. 2018;8(4):218–224. doi: 10.5588/pha.18.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams LV, et al. High completion rates of isoniazid preventive therapy among persons living with HIV in Swaziland. Int J Tuberc Lung Dis. 2017;21(10):1127–1132. doi: 10.5588/ijtld.16.0946. [DOI] [PubMed] [Google Scholar]
- 16.Tram KH, et al. Predictors of isoniazid preventive therapy completion among HIV-infected patients receiving differentiated and non-differentiated HIV care in rural Uganda. AIDS Care. 2020;32(1):119–127. doi: 10.1080/09540121.2019.1619661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro A. Community-based TB preventive therapy in the DO ART study, 2018. New York, NY, USA: ICAP Columbia University Mailman School of Public Health; 2018. http://cquin.icap.columbia.edu/wp-content/uploads/2019/04/ICAP_CQUIN_Shapiro_Final.pdf [Google Scholar]


