Summary box.
After many years of very low measles incidence, a measles outbreak began in the central region of Madagascar in September 2018.
The outbreak reached all 22 regions of the island, causing nearly 1000 deaths, with more than 100 000 cases reported.
The magnitude of the outbreak, the age profile of cases and the history of incidence and vaccination in Madagascar align with a core expectation from epidemiological theory, the concept of a ‘posthoneymoon period’ outbreak where large outbreaks can occur following years of (apparently) successful control.
An emergent important public health challenge is how to characterise the risk of post-honeymoon outbreaks for measles and other vaccine preventable infections, and how to learn from this outbreak to build preparedness for future outbreak prevention and response strategies.
Madagascar’s experience indicates that investment in relevant data streams (from case surveillance, to vaccination deployment and serology) alongside efforts to develop national capacity for integrative analysis of such diverse data could help enable deployment of timely targeted vaccination campaigns to prevent such outcomes in the future.
Measles and outbreak risk
Measles vaccination is often referred to as a ‘best buy’ in public health, because of the high case fatality rate associated with infection, alongside the existence of a safe and inexpensive vaccine. The current WHO recommendation is that all children have access to two doses of the measles vaccine.1 In 2011, countries in the WHO African region adopted a measles elimination goal to be reached by 2020. In the last decades, substantial gains have been made in numbers of cases and deaths averted. Yet, an important feature of measles epidemiology is that large outbreaks can occur following years of (apparently) successful control. This phenomenon is known as a ‘posthoneymoon period’ outbreak.2 The ‘honeymoon’ consists of the period following vaccine introduction where cases drop substantially. This ‘honeymoon’ is at risk of ending in an outbreak if vaccination coverage is subsequently suboptimal. Every year, children born into the population that are left unvaccinated are also unlikely to experience immunisation by natural infection, because measles incidence is low. These children, thus, remain susceptible to measles, and over the years, as children are born and left unvaccinated, susceptible individuals accumulate in the population, across the range of ages reflecting cohorts born during the low incidence years. Once the size of the susceptible pool exceeds the threshold for herd immunity (defined as the proportion susceptible in the population exceeding 1/R0 where R0 is the number of new infections per susceptible individual in a completely susceptible population, which may be as high as 20 for measles3), a new outbreak can take hold, and it will grow at a speed defined by the effective reproductive number RE=R0S where S is the proportion of the population that is susceptible.
The context of the outbreak
Since 2004, the number of measles cases reported in Madagascar had plummeted from tens of thousands of annual cases to fewer than 20 confirmed cases per year.4 This drop in cases followed a successful expansion of the immunisation programme (figure 1A) in which consistent (although low) routine measles vaccination coverage (between 55% and 85%) was combined with vaccination campaigns targeting ages from 9 months to 14 years (in 2004) or 9 months to 4 years (in 2007, 2010, 2013, 2016).5 The combination of low incidence and the potential for incomplete vaccination coverage over a number of years suggested that Madagascar might have a large susceptible population distributed across a wide age range, yielding potential for a wide age range, fast-growing outbreak.
Figure 1.
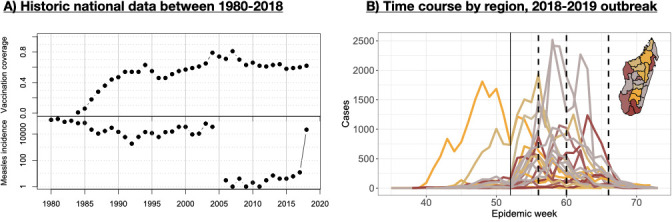
Historical context of measles in Madagascar and the recent outbreak (A) measles first dose vaccination coverage estimates from UNICEF (y-axis, top) and measles incidence from who (y-axis, log scale, bottom) from 1980 to 2018 (x-axis), showing a sharp decline in cases in 2005 with 10 or less cases in each years following 2005 until the start of the focal outbreak in 2018 when more than 21 000 cases reported; (B) time series of suspected cases (y-axis) against time (indicated epidemic week starting in 2018, thin vertical line shows the separation between 2018 and 2019) from the 2018 to 2019 line-list data in each of the 22 regions, coloured by the timing of the peaks (orange is earliest, grey intermediate, and brown latest); dashed vertical lines indicate the approximate timing of the three waves of vaccination (different districts were targeted in each wave, see text); inset shows the regions of Madagascar coloured as for the time series (orange is the earliest peaks, grey intermediate and brown the latest).
The scale, age range and early growth of the outbreak
In September 2018, a cluster of laboratory-confirmed measles cases was detected via Madagascar’s national febrile/rash surveillance system for measles and rubella, whereby local health centres send samples taken from suspected cases in to the national reference lab in Antananarivo.6–8 At that time, the positivity rate of measles suspected cases reached 2.12% every year compared with an average of 0.54% during the last previous 5 years (ranged from 0.22% to 0.95%). By October 2018, increasing numbers of measles cases were detected by the health system (figure 1B). Subsequently, over 100 000 suspected cases were reported, with 37% of the 2930 tested individuals being confirmed measles cases.8 The outbreak began on the highlands in the region of Analamanga (province of Antananarivo) and spread to every region in the country (online supplemental figure S1).
bmjgh-2020-003153supp001.pdf (702.6KB, pdf)
Typically, in the absence of vaccination, the average age of measles infection ranges from 2 to 5 years. In the 2018–2019 Madagascar outbreak, the average age of infection in suspected cases was 9 years (and rates of laboratory confirmation did not vary over age, see online supplemental text). Regionally, there was a significant positive correlation between the average age of infection and vaccination coverage in 2009 (figure 2A, ). Regions with higher vaccination coverage should have smaller numbers of infected individuals, and, as a result, encounters between susceptible individuals and infected individuals in the years preceding the outbreak will also have been rare. Unvaccinated (and unimmunised) individuals could thus remain susceptible for longer in regions with historically high vaccination rates, leading to pools of older susceptible individuals and a higher average age of infection (figure 2A). Following the logic of a ‘honeymoon’ described above, the balance between immunisation achieved by vaccination, and reduced immunisation resulting from declines in natural infection over the years preceding the outbreak, might even be tilted such that regions with higher vaccination coverage might also have larger pools of susceptible individuals. This could allow faster epidemic growth in these regions. Comparing the early growth of the epidemic estimated using RE (or the number of new infections per infectious individual6) indicates that, indeed, the outbreak grew faster in some regions with historically high levels of vaccine coverage (figure 2B).
Figure 2.
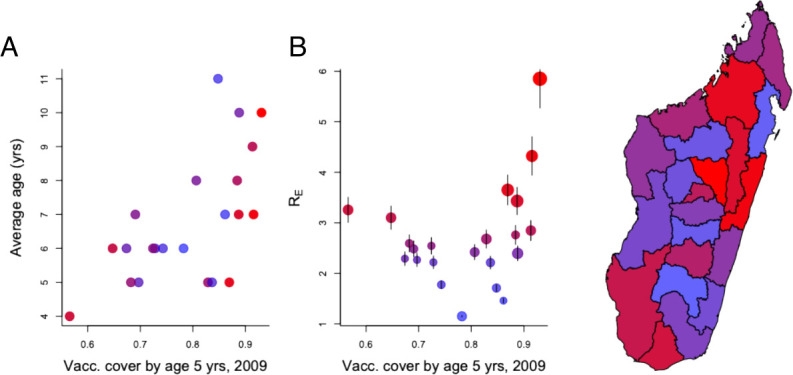
Outbreak characteristics. (A) Measles vaccination coverage at 5 years in each of the 22 regions estimated from Madagascar’s 2009 Demographic and Health Survey (x-axis) plotted against (A) the median age distribution of cases (y-axis) and (B) the RE of the outbreak in each region (y-axis). On both plots, regions with higher rE are shaded from blue to red; and these are also shown in the MAP, to indicate fastest early growth of the outbreak in central/norther regions. Two regions (Androy and Atsimo Andrefana) have high rE despite low vaccination coverage (red points to the left of B). Regional connectivity might be sufficiently low in these remote locations that even at low vaccination coverage, local extinction occurs, allowing susceptible build-up, or vaccination coverage in 2009 might provide a poor proxy for changes over the intervening decade.
The outbreak response
In October 2018, rapidly growing case numbers in the Analamanga region (where the capital city of Antananarivo is located) prompted deployment of a vaccination campaign across a limited spatial extent (four health districts of the urban community of Antananarivo) targeting children up to 5 years of age. The history of incidence (figure 1A) and the age profile of cases in the first weeks further suggested that the age range at risk might extend up to 15 years. However, the resources necessary to deploy an outbreak response of the scale indicated (national vaccination, reaching up to 15 years old) were lacking. Coordinating funds from across the donor community (including the Measles and Rubella Initiative, the African Development Bank Group, the Central Emergency Response Fund, etc) took time, even as the outbreak was progressing. Given these delays, the decision was made to initially target for vaccination individuals up to 10 years of age, and 25 of the most urban and highly connected districts (out of a total 114 districts across the country); and then to follow-up as resources could be mobilised. In all, the outbreak response included three waves of vaccination (14–18 January 2019, 18–22 February 2019 and 25 March–5 April 2019) (figure 1B, online supplemental figure S1 and table S1), eventually reaching all districts.
Three things determine the success of outbreak response vaccination campaigns: (1) the coverage achieved; (2) the timing of the campaign relative to the time course of the epidemic9 and (3) the age range targeted.10 The outbreak response aimed to achieve high coverage, and was deployed as fast as possible along lines shaped by the practical considerations detailed above. Nevertheless, time series of suspected cases suggest that in many regions, waves of vaccination occurred after susceptible depletion had started to reduce spread (figure 1, online supplemental figure S1). The decision was made to target children up to 10 years of age because of a lack of funds, which may have missed a fraction of the susceptible population (online supplemental figure S3, across the country 36% of cases occurred in children aged over 10 years of age).
Strengthening the response
Many lines of evidence suggest that Madagascar experienced a ‘posthoneymoon’ measles outbreak. An obvious and critical public health question is whether we can identify that a country is experiencing a ‘honeymoon period’. This would allow the public health sector to anticipate, and perhaps even avert the outbreak by laying the groundwork for vaccination campaigns, or outbreak response vaccination. The experience of Madagascar underscores that there are rich opportunities to leverage existing data to this end. This outbreak reflects an important missed opportunity—existing data could have been better exploited to position human, financial and health resources ahead of the response. First, data on vaccination coverage,7 historical incidence4 and local demography11 can be combined with mathematical models to define outbreak risk, building increased specificity into existing approaches. Critically, such efforts should occur in tandem with approaches to strengthen the quality and spatial and temporal resolution of these sources of information. Indeed, in 2017, analysis via the WHO Measles Programmatic Risk Assessment tool (which can be explored here12), which integrates spatially resolved indicator scores on population immunity, surveillance quality and programme performance in the last 3 years had first suggested that the risk of an outbreak was large, a concern amplified by various lines of evidence suggesting generalised system weaknesses. However, part of this system weakness was associated with data quality issues, which complicated interpreting the results and launching a response. Second, existing convenience serological samples (eg, fever-rash surveillance for which samples are already available) could be leveraged to test for population immunity to measles. This approach had been applied to existing fever-rash surveillance in Madagascar, and did indeed suggest important outbreak risk in Madagascar prior to 2018 outbreak,6 as a result of accumulation of susceptible individuals resulting from a combination of low circulation and incomplete vaccination coverage. However, convenience samples of this type are generally spatially variable, and thus may be non-representative. Thus, third, small-scale targeted serological surveys designed to be representative and requiring novel sample collection to ground-truth local population immunity (eg, as currently done in Madagascar to evaluate the HIV situation among specific groups) could provide an important further line of evidence. Such surveys could either be deployed in particular scenarios (eg, towards suspected regions of vulnerability), or routinely (eg, associated with ‘vaccination weeks’, brief campaigns occurring biannually and an important part of the vaccine delivery system in Madagascar7). Mathematical models suggest that obtaining data on the immunity status of even a small fraction of the population could yield critical information as to outbreak risk,13 noting, however, that the added value of a serological survey relative to existing information should be seriously evaluated.14 Finally, the themes addressed so far focus on approaches to anticipating and thus mitigating the outbreak by ensuring population immunity. Another important aspect is in readiness for outbreak response. On the logistical side, an important barrier to the response was lack of coordination across core partners. In the wake of both this outbreak and the recent plague outbreak, Madagascar has taken steps to address this with the development of a special committee including governmental, non-governmental and technical partners with remit to manage outbreak responses.
Measles control in Madagascar: next steps
Madagascar experienced up to a decade of low measles circulation prior to this outbreak (figure 1). Most cities and towns in Madagascar are below the ‘Critical Community Size’—a threshold population size that measles requires to persist without stochastic extinction15—making local measles extinction likely, particularly with growing vaccination coverage (figure 1). These features make measles elimination in Madagascar seem relatively tractable. Furthermore, as an island, risks of measles reintroduction following elimination are also reduced.16 Beyond these general features, the current context may be particularly propitious for measles elimination. Following the 2018–2019 measles outbreak, population immunity will be high (since so many people were infected, and with the large-scale vaccine catch up efforts). The population is also likely to be sensitised to the risks of measles given the high burden recently imposed. It could be a propitious time to add a second dose of measles-containing vaccine to Madagascar’s childhood immunisation schedule (as suggested by WHO1).
However, it is increasingly recognised that effective control or elimination efforts need community support, particularly for communities whose experience of vaccination efforts may otherwise seem divorced from the realities of their needs (eg, following polio elimination efforts). Given this context, expanding local capacity to integrate available data streams and generate high-resolution maps of risk will make it possible to deploy much more effectively vaccination efforts by targeting specific regions and/or age groups rather than as homogeneous national campaigns.17 As well as reducing costs, such targeting could limit repeated revaccination of already immunised individuals, and are likely be easier to communicate to communities. Developing national capacity for such analyses will have benefits for pathogens beyond measles, and is an important direction for future investment and innovation in Madagascar. The foundations for this are being laid, as currently evidenced by integration of public health data-streams within the District Health Information System 2, to innovation emerging from academic and non-governmental organisations partners in Madagascar like the Malagasy-led development of a COVID-19 dashboard (www.covid19mg.org). Although there have been large global and national gains in measles control and elimination, delayed vaccination campaigns, interrupted routine services and downscaling of laboratory/virological surveillance, especially in the context of the current COVID-19 pandemic, make developing strategies to strengthen prediction of outbreaks and identify optimal strategies to combat them a policy priority.
bmjgh-2020-003153supp002.pdf (92.9KB, pdf)
Footnotes
Handling editor: Seye Abimbola
Twitter: @CJEMetcalf, @ProfRAKOT1
Contributors: All authors provided extensive discussion of the core issues; AR and CJM wrote the first draft; AWi and AWe provided technical assistance on plotting; all authors edited and approved the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.World Health Organization Measles vaccines: WHO position paper, April 2017 - recommendations. Vaccine 2019;37:219–22. 10.1016/j.vaccine.2017.07.066 [DOI] [PubMed] [Google Scholar]
- 2.McLean AR, Anderson RM. Measles in developing countries. part II. The predicted impact of mass vaccination. Epidemiol Infect 1988;100:419–42. 10.1017/S0950268800067170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RM, May RM. Infectious diseases of humans. Oxford: Oxford University Press, 1991. [Google Scholar]
- 4.World Health Organization WHO vaccine-preventable diseases: monitoring system. 2017 global summary - Incidence time series for Madagascar (MDG), 2017. [Google Scholar]
- 5.World Health Organization Immunization, vaccines and biologicals, 2019. Available: http://www.who.int/immunization_monitoring/data/data_subject/en/index.html
- 6.Winter AK, Wesolowski AP, Mensah KJ, et al. . Revealing measles outbreak risk with a nested immunoglobulin G serosurvey in Madagascar. Am J Epidemiol 2018;187:2219–26. 10.1093/aje/kwy114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mensah K, Heraud JM, Takahashi S, et al. . Seasonal gaps in measles vaccination coverage in Madagascar. Vaccine 2019;37:2511–9. 10.1016/j.vaccine.2019.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimpa MM, Andrianirinarison JC, Sodjinou VD, et al. . Measles outbreak in 2018-2019, Madagascar: epidemiology and public health implications. Pan Afr Med J 2020;35:84. 10.11604/pamj.2020.35.84.19630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grais RF, Conlan AJK, Ferrari MJ, et al. . Time is of the essence: exploring a measles outbreak response vaccination in Niamey, niger. J R Soc Interface 2008;5:67–74. 10.1098/rsif.2007.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prada JM, Metcalf CJE, Takahashi S, et al. . Demographics, epidemiology and the impact of vaccination campaigns in a measles-free world - Can elimination be maintained? Vaccine 2017;35:1488–93. 10.1016/j.vaccine.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari MJ, Grenfell BT, Strebel PM. Think globally, act locally: the role of local demographics and vaccination coverage in the dynamic response of measles infection to control. Philos Trans R Soc Lond B Biol Sci 2013;368:20120141. 10.1098/rstb.2012.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Measles programmatic risk assessment tool, 2018. Available: https://www.who.int/immunization/monitoring_surveillance/routine/measles_assessment/en/
- 13.Lessler J, Metcalf CJE, Cutts FT, et al. . Impact on epidemic measles of vaccination campaigns triggered by disease outbreaks or serosurveys: a modeling study. PLoS Med 2016;13:e1002144. 10.1371/journal.pmed.1002144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter AK, Martinez ME, Cutts FT, et al. . Benefits and challenges in using seroprevalence data to inform models for measles and rubella elimination. J Infect Dis 2018;218:355–64. 10.1093/infdis/jiy137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett MS. The critical community size for measles in the United States. J R Stat Soc Ser A 1960;123:37–44. 10.2307/2343186 [DOI] [Google Scholar]
- 16.Metcalf CJE, Hampson K, Tatem AJ, et al. . Persistence in epidemic metapopulations: quantifying the rescue effects for measles, mumps, rubella and whooping cough. PLoS One 2013;8:e74697 10.1371/journal.pone.0074696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowell SF, Blazes D, Desmond-Hellmann S. Four steps to precision public health. Nature 2016;540:189–91. 10.1038/540189a [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-003153supp001.pdf (702.6KB, pdf)
bmjgh-2020-003153supp002.pdf (92.9KB, pdf)


