Abstract
Background
Veneto is one of the first Italian regions where the COVID-19 outbreak started spreading. Containment measures were approved soon thereafter. The present study aims at providing a first look at the impact of the containment measures on the outbreak progression in the Veneto region, Italy.
Methods
A Bayesian changepoint analysis was used to identify the changing speed of the epidemic curve. Then, a piecewise polynomial model was considered to fit the data in the first period before the detected changepoint. In this time interval, that is, the weeks from 27 February to 12 March, a quadratic growth was identified by a generalised additive model (GAM). Finally, the model was used to generate the projection of the expected number of hospitalisations at 2 weeks based on the epidemic speed before the changepoint. Such estimates were then compared with the actual outbreak behaviour.
Results
The comparison between the observed and predicted hospitalisation curves highlights a slowdown on the total COVID-19 hospitalisations after the onset of containment measures. The estimated daily slowdown effect of the epidemic growth is estimated as 78 hospitalisations per day as of 27 March (95% CI 75 to 81).
Conclusions
The containment strategies seem to have positively impacted the progression of the COVID-19 epidemic outbreak in Veneto.
Keywords: PUBLIC HEALTH, COVID-19, Italy
INTRODUCTION
Given the earlier onset of the epidemic in Italy compared with the rest of Europe, we would like to share some data on the likely impact of the measures taken in our Veneto region, a western, well-industrialised, high-income area in the North-Eastern part of Italy. Veneto has been one of the first areas in the country where COVID-19 has been spreading. Based on official data,1 the number of positive cases at the COVID-19 test, as of 26 March, is 6935, with 287 deaths and 1773 hospitalisations (326 in the intensive care units). Veneto region was one of the first in Italy to react to the outbreak by adopting severe actions in line with recommendations based on previous experiences,2 3 like the adoption of community-based containment strategies. Veneto region started by quarantining the city of Vo’ and further implementing such a plan with the spreading of the outbreak (Table S1, Supplementary Material).
jech-2020-214209s001.pdf (179.4KB, pdf)
This research work aims at identifying the possible effect of the policies implemented in the Veneto region (Italy) to contain the COVID-19 epidemic outbreak.
METHODS
A Bayesian changepoint detection method (BCPDM) based on the procedure proposed by Barry and Hartigan4 was used to identify when the epidemic changed speed as compared with what was to be expected. The posterior distribution of the changepoints was obtained combining the prior distributions and the likelihood derived from the hospitalisation data. The prior distribution of the mean μij of hospitalisations beginning at time ti and ending at time tj was chosen as a weakly informative normal distribution. The posterior distribution was estimated via Markov chain Monte Carlo method (MCMC) with 500 iterations and 50 burn-in replications. The time series were modelled assuming an underlying sequence of parameters partitioned into contiguous blocks; the beginning of each block was a changepoint. The time-series observation was then assumed to be independent in different blocks, given the sequence of parameters. The initial partition considered only a changepoint at the last timepoint and, at each step of MCMC, the probability of a changeoint at the time i was updated.
Then, a piecewise polynomial model was implemented to fit the data in the period before the detected changepoint, that was, the weeks from 27 February to 12 March. A quadratic growth (two degrees of the polynomials) was identified by a local polynomial regression model with 0.75° of smoothing.5 The McFadden Pseudo-R squared6 was calculated to assess the goodness of fit. The 95% confidence bounds for the predicted total hospitalisation series were computed using estimates of Standard Errors (SE) at each time point.
The comparison between the curves led to the calculation of the following indicators:
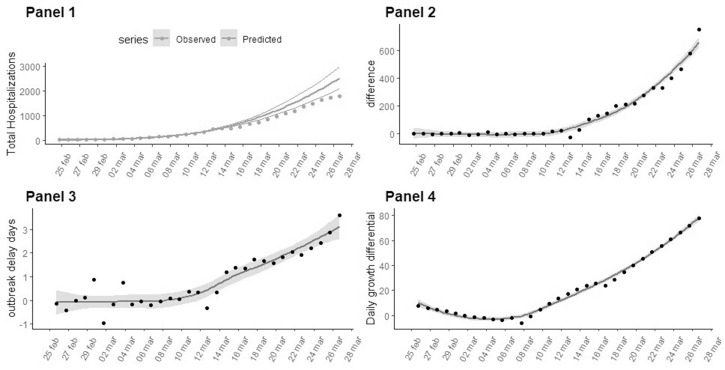
the time series of avoided COVID-19 hospitalisations calculated as the difference between the predicted and observed COVID-19 hospitalisations. The predictions assumed that the epidemic growth pattern followed the trend estimated until 12 March (figure 1, panel 2).
the time series of gained days of hospitalisations. The time-series values were calculated as the difference between the number of days needed to obtain the same level of hospitalisations between observed and The prior distribution of the estimated curves (figure 1, panel 3).
the time series of the difference between the number of COVID-19 hospitalisations per day estimated as the daily derivative of the predicted cases minus the observed ones (figure 1, panel 4).
Figure 1.
Time series for the indicators related to the COVID-19 epidemic outbreak in Italy reported and estimated from 24 February to 27 March. For the estimated measures, the grey areas indicate the 95% confidence bounds. Panel 1 Estimated total hospitalisations (bold curve with 95% confidence bounds) based on the outbreak trend until 12 March (changepoint day). The observed trend of COVID-19 cases is represented with a dotted line. Panel 2 The number of avoided COVID-19 hospitalised cases in the Veneto region compared with the trend expected as of 12 March. Panel 3 Days of delay estimated by taking the difference between the days needed to achieve an equal level of hospitalisations between the estimated total hospitalisations and the observed data. Panel 4 The difference in daily growth (number of daily hospitalisations) between the estimated and observed epidemic curves.
The analyses were performed using R v.3.6.37 powered by the bcp v.4.038 and VGAM v.1.1–19 packages.
RESULTS
A specific changepoint in the hospitalisation growth was identified on the 12th of March, 17 days after the first decree law that established a lockdown in some towns in Veneto. BPCDM approach has been proved to be robust in detecting changepoints concerning autocorrelation in time series (coverage rate equal to 80% when autocorrelation lies between −0.5 and 0.5).10 In our hospitalisation case time series, autocorrelation was estimated equal to 0.45 at a lag of 5 days.
The comparison between the observed and predicted hospitalisation curves highlights a slowdown on the series of the total COVID-19 cases after the 12th of March. This day represents a changepoint which was detected by BCPDM (figure 1, panel 1). The trend of the epidemic curve reveals a quadratic shape in the early stages of diffusion (McFadden Pseudo R2=0.9). This is in line with recent studies, indicating that COVID-19 does not exhibit exponential growth, especially in the early stages11 where a quadratic model is a better fit.
As of 27 March, 658 (95% CI 618 to 698) hospitalisations were avoided as was shown by performing a comparison of the observed total COVID-19 hospitalised cases with the projected cases estimated, assuming a consistent growth with pre-changepoint growth pattern (figure 1, panel 2).
The difference between the days needed to achieve an equal level of hospitalised cases between the observed and predicted growth curve on 27 March is equal to 3.11 days (95% CI 2.59 to 3.63)(figure 1, panel 3). This delay effect seems to grow until 27 March.
The estimated daily slowdown effect of the epidemic growth on 12 March is equal to 78.01 hospitalisations per day as of 27 March (95% CI 75.09 to 80.94) (figure 1, panel 4).
DISCUSSION
Changepoint analysis methods have already been proposed in the surveillance systems to monitor changes in influenza-like illness.10 In this work, we proposed to use this methodology combined with a piecewise polynomial model of the hospitalisation case time series to address the assessment of the impact of public health interventions on COVID-19 hospitalisations.
As a study limitation, the projection of expected hospitalisation was not extended beyond 2 weeks from the detected changepoint. This was decided not only for avoiding exceedingly long-term extrapolation of the pre-changepoint model, but also to account for the estimated range of the COVID-19 incubation period, which lies between 2 and 14 days.12
This study reveals that 658 hospitalisations were avoided, thanks to a slowing down in the evolution of the epidemic compared with what was expected, based on an initial model that assumes an absence of intervention. Such data represent a reduction of 34.2% compared with the foreseen cases on 27 March. Indeed, the outbreak slowed down, with 78.01 hospitalisations per day as of 12 March.
More importantly for the management of the healthcare system, such slowdown allowed to ‘gain’ about 3.11 days before reaching the same levels of hospitalisation as predicted by the model, giving the opportunity, in particular to the ICUs working in the regional area, to get better organised.13
Based on the Veneto region experience, the containment strategies would indicate a positive impact on the management of the outbreak.
What is already known on this subject.
Veneto region has been one of the first areas in Italy where COVID-19 has been spreading; but it is also one of the first in Italy to react to the outbreak by adopting severe containment measures.
What this study adds.
The containment strategies would indicate a positive impact on the management of the outbreak, with 658 hospitalisations avoided at the 27th of March.
Footnotes
Correction notice: This article has been corrected since it first published. The end statement heading ‘Open access’ has been removed, as this article is not open access. Instead, this article has been made freely available for use in accordance with BMJ’s website terms and conditions for the duration of the COVID-19 pandemic or until otherwise determined by BMJ.
Contributors: Conceptualisation, DG: methodology; PB: formal analysis; DA: investigation; ND and CL: data curation; CL. and IP: writing—original draft preparation; GL: writing—review and editing, supervision DG.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
REFERENCES
- 1.{covid19ita} [Internet ]. covid19ita. [citato ( 28 Marzo 2020)]. Available at: https://r-ubesp.dctv.unipd.it/shiny/covid19ita/
- 2.Xiao Y, Torok ME, Taking the right measures to control COVID-19. Lancet Infect Dis [Internet] 2020. Available https://www.thelancet.com/journals/laninf/article/PIIS1473-30992030152-3/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RM, Heesterbeek H, Klinkenberg D et al. , How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020. 10.1016/S0140-6736(20)30567-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry D, Hartigan JA. A Bayesian analysis for change point problems. J Am Stat Assoc Marzo 1993;88:309. [Google Scholar]
- 5.Chambers JM, Hastie TJ, curatori. Statistical models in S. New York: Chapman & Hall, 1992: 608 pag. [Google Scholar]
- 6.McFadden D. Quantitative methods for analyzing travel behaviour of individuals: some recent developments [Internet]. Cowles Foundation for Research in Economics, Yale University, 1977. Available https://EconPapers.repec.org/RePEc:cwl:cwldpp:474 [Google Scholar]
- 7.R Core Team R: a Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing, 2015. Available https://www.R-project.org/ [Google Scholar]
- 8.Erdman C, Emerson JW, bcp: an R Package for performing a Bayesian analysis of change point problems. J Stat Softw 2007; 23 10.18637/jss.v023.i03 [DOI] [Google Scholar]
- 9.Yee TW. Vector generalized linear and additive models: with an implementation in R. New York: Springer, 2015: 589 pag. Springer series in statistics. [Google Scholar]
- 10.Kass-Hout TA, Xu Z, McMurray P et al. , Application of change point analysis to daily influenza-like illness emergency department visits. J Am Med Inform Assoc Novembre 2012;19:1075–81. 10.1136/amiajnl-2011-000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandenburg A. Quadratic growth during the 2019 novel coronavirus epidemic. ArXiv Prepr 2020;ArXiv200203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei S, Jiang F, Su W et al. , Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clin Med Aprile 2020;100331 10.1016/j.eclinm.2020.100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzoni G, Lanera C, Azzolina D et al. , Is a more aggressive COVID-19 case detection approach mitigating the burden on ICUs? Some reflections from Italy. Critical Care 2020; 10.1186/s13054-020-02881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jech-2020-214209s001.pdf (179.4KB, pdf)