Abstract
Acute ischaemic stroke is a major public health priority and will become increasingly relevant to neurologists of the future. The cornerstone of effective stroke care continues to be timely reperfusion treatment. This requires early recognition of symptoms by the public and first responders, triage to an appropriate stroke centre and efficient assessment and investigation by the attending stroke team. The aim of treatment is to achieve recanalisation and reperfusion of the ischaemic penumbra with intravenous thrombolysis and/or endovascular thrombectomy in appropriately selected patients. All patients should be admitted directly to an acute stroke unit for close monitoring for early neurological deterioration and prevention of secondary complications. Prompt investigation of the mechanism of stroke allows patients to start appropriate secondary preventative treatment. Future objectives include improving accessibility to endovascular thrombectomy, using advanced imaging to extend therapeutic windows and developing neuroprotective agents to prevent secondary neuronal damage.
Keywords: STROKE, CEREBROVASCULAR DISEASE, NEUROANATOMY, NEUROEPIDEMIOLOGY, NEURORADIOLOGY, REHABILITATION, DYSPHAGIA
INTRODUCTION
Stroke is the fourth leading cause of death and the largest cause of adult neurological disability in the UK.1 2 The associated socioeconomic burden is huge; the aggregate cost of stroke, including long-term healthcare, rehabilitation and loss of employment, is estimated to be £25.6 billion per year.3 As such, it is one of the key diseases targeted by the National Health Service (NHS) Long Term Plan in England and Wales.4
In contrast to most other countries around the world, stroke medicine in the UK is not the sole preserve of neurologists; indeed, most stroke consultants in the NHS are geriatricians. While stroke medicine is indisputably multidisciplinary, appropriately trained neurologists are well placed to manage stroke and its mimics. In the UK, the new neurology training curriculum will produce consultants trained in stroke medicine, with the potential to expand the stroke workforce.4 Here, we review the diagnosis and management of acute ischaemic stroke and transient ischaemic attack (TIA) for the practising neurologist.
Service design
The introduction of intravenous thrombolysis with recombinant tissue-type plasminogen activator (rtPA, alteplase) to treat acute ischaemic stroke required a revolution in the organisation of stroke care. Recognition that ‘time is brain’ drove effective public and prehospital awareness campaigns, such as the ‘Face, Arm, Speech, Time’ (FAST) test5 and rapid prehospital triage to designated centres.
The organisation of stroke care depends upon local geography, but the implementation of dedicated acute stroke pathways varies widely in the UK. Comprehensive stroke centres provide all aspects of acute stroke care. Triage of patients eligible for endovascular thrombectomy directly to a comprehensive stroke centre (the ‘mothership’ model) may improve the likelihood of good outcome, even if other hospitals are closer. Primary stroke centres are usually smaller centres that initiate intravenous thrombolysis and transfer patients eligible for endovascular thrombectomy to a comprehensive stroke centre, the so-called ‘drip-and-ship’ model.6 Rural hospitals without a stroke team can be linked with stroke centres by telemedicine for thrombolysis calls.7 8 The key aspect of any stroke service model is that patients can access specialist expertise, neuroimaging and stroke unit care without delay.9
The distinction between TIA and stroke cannot be made while the patient remains symptomatic; therefore, all patients should be assessed rapidly. Patients with a completed TIA (symptom resolution within 24 hours) or minor, non-disabling, stroke require prompt mechanistic investigation and secondary preventative treatment, with expert review within 24 hours recommended for all suspected cases.10 Organisational models to achieve this commonly include rapid-access clinics (figure 1). The remainder of this article focuses on the assessment and treatment of acute disabling ischaemic stroke.
Figure 1.
Eligibility, investigations, diagnosis and treatment in a rapid-access TIA clinic.
DVLA, Driver and Vehicle Licensing Agency; TIA, transient ischaemic attack.
Diagnosis
Initial history
As with all aspects of neurology, the history is crucial for diagnosis. However, in the setting of acute stroke, details need to be acquired efficiently and focused on answering a few key questions. Collateral history from witnesses or family members is essential as the nature of the deficit commonly prevents patients themselves from giving a reliable history.
‘When was the patient last seen to be well?’ Early determination of whether the patient is within the reperfusion therapy treatment window sets the pace of subsequent investigations and aids the triage of simultaneous referrals. Symptom onset should be documented as a clock time to avoid confusion. The time recorded for unwitnessed events or ‘wake-up’ strokes should be when the patient was definitely last well (rather than when found); the surrogate use of an activity can be useful, for example, waking to go to the toilet or successfully using a mobile phone.
‘How quickly did the symptoms develop?’ Stroke symptom onset is usually sudden, although notable exceptions include the stuttering nature of capsular warning syndrome, or prodromal symptoms of basilar artery occlusion. Fluctuating severity is common in the early hours after stroke, and initial improvement may be followed by deterioration, especially among those with intracranial vessel occlusion. More gradual evolution of symptoms may suggest alternative diagnoses.
‘Is there any significant past medical and drug history?’ A brief overview of the patient’s background, especially vascular risk factors, will influence the diagnostic decision process; these details can sometimes be obtained from electronic medical records before the patient’s arrival. Risk factors associated with ischaemic stroke include cigarette smoking, hypertension, hypercholesterolaemia, diabetes mellitus, cardiac or peripheral vascular disease, and drugs of abuse. A history of carotid stenosis or atrial fibrillation may suggest a cause.11 Reviewing the list of medication helps to screen for known relevant diagnoses, risk factors for stroke, and whether the patient is taking oral anticoagulation therapy as a potential contraindication to thrombolysis.
Stroke mimics account for at least 20–25% of acute presentations and many of them can be suspected from the history. In one study, the five most frequent stroke mimics were seizure, syncope, sepsis, migraine and brain tumours12; detailed reviews can be found in Practical Neurology.13 14
Posterior circulation strokes are misdiagnosed three times more often than anterior circulation strokes, as they frequently present with non-specific symptoms, including isolated ‘dizziness’ (vertigo or disequilibrium) or headache.15 Acute onset vertigo or disequilibrium with an additional posterior circulation symptom should necessitate further assessment.
Examination
An overview of the patient can be made immediately and should focus on the level of consciousness, head and/or gaze deviation, and laterality of purposeful movements. As in any emergency situation, an initial screen of the airway, breathing and circulation and vital signs will establish cardiovascular stability and suitability to go to scan.
Up to 80% of patients with acute ischaemic stroke have an elevated blood pressure (BP) (≥140 mmHg systolic),16 which spontaneously improves over the following week17 –19 and is associated with poorer outcomes in both ischaemic stroke and intracerebral haemorrhage.20 21 The cause of transient post-stroke hypertension is unknown, but potential mechanisms include disturbed cerebral autoregulation or non-stroke causes such as urinary retention or psychological stress.22 Pyrexia is also common and could reflect aspiration pneumonia, urinary tract infection or infective endocarditis.23
A focused, rather than extensive, neurological examination should be performed in order to identify the affected vascular territory and to quantify physical impairment using the National Institutes of Health Stroke Scale (NIHSS). Limitations to clinical examination in the hyperacute setting include the immaturity of physical signs (such as hypertonia or brisk reflexes) and the degree of patient cooperation. In agitated or dysphasic patients, there is a greater reliance on careful observation when assessing limb paresis, eye movements or visual fields.
The NIHSS is the most commonly used neurological deficit rating scale, with a maximum score of 42 (hypothetical due to several mutually exclusive items). Its advantages include an accredited training and certification system (http://www.nihstrokescale.org/), quick completion time (≤10 min24) and facilitation of communication between team members. It may be used to monitor deficit severity, to identify neurological deterioration and to select patients for reperfusion therapy. Its limitations include the underrepresentation of non-dominant hemisphere deficits,25 such as apraxia or anosognosia (which may be subtle but potentially significantly disabling), and low sensitivity for posterior circulation deficits.26
Quick recognition of common stroke syndromes increases diagnostic confidence and facilitates an efficient neurological examination. Despite limited use in the hyperacute setting, stroke syndromes often suggest the underlying cause. Large-vessel stroke syndromes (table 1) suggest an atheroembolic cause, whereas lacunar syndromes are classically associated with cerebral small-vessel disease. Lacunar syndromes include contralateral pure motor, pure sensory and sensorimotor impairment, the clumsy hand–dysarthria syndrome (which can also be cortical) and ataxic hemiparesis.
Table 1.
Large-vessel stroke syndromes (assumes left hemispheric dominance)
| Vascular territory | Signs and symptoms |
|---|---|
| Internal carotid artery | Combined anterior cerebral artery/middle cerebral artery syndromes; ipsilateral monocular visual loss secondary to transient central retinal artery occlusion (amaurosis fugax); branch retinal artery occlusions may present as ipsilesional altitudinal field cuts. |
| Anterior cerebral artery | Contralateral leg numbness and weakness, possibly ipsilateral (‘sympathetic’) or contralateral ideomotor apraxia, (L) transcortical motor aphasia, (R) motor neglect. Occasionally urinary incontinence (medial micturition centre), ipsilateral eye deviation and paratonic rigidity. |
| Middle cerebral artery | Superior division (lateral frontal and superior parietal lobes): contralateral face/arm (more than leg) numbness and weakness, contralateral homonymous hemianopia (lower fields), cortical hand syndrome*, ipsilateral gaze preference, [dom] expressive aphasia, [non-dom] contralateral hemispatial neglect, agraphaesthesia, astereognosis. Inferior division (lateral temporal and inferior parietal lobes): contralateral homonymous hemianopia (upper fields), [dom] receptive aphasia, [non-dom] constructional apraxia. |
| Posterior cerebral artery† | Complete or partial contralateral homonymous hemianopia, if midbrain involvement ipsilateral third nerve palsy with mydriasis and contralateral hemiparesis (Weber syndrome), (L with splenium of corpus callosum) alexia without agraphia. |
| Superior cerebellar artery | Ipsilateral limb and gait ataxia. |
| Anterior inferior cerebellar artery | Vertigo and ipsilateral deafness, possibly also ipsilateral facial weakness and ataxia. |
| Vertebral/posterior inferior cerebellar artery |
Ipsilateral limb and gait ataxia; if lateral medullary involvement, may have ipsilateral fifth cranial nerve, cerebellar, nucleus ambiguous (hoarseness and dysphagia), vestibular nucleus dysfunction, Horner’s syndrome and contralateral hemisensory loss to pain and temperature (Wallenberg syndrome). |
| Basilar artery | Pontine localisation with impaired lateral gaze, horizontal diplopia and disconjugate gaze, non-localised hemiparesis, dysarthria; ‘locked-in syndrome’ with bilateral pontine infarction (intact vertical eye movements, anarthria, quadriplegia). |
Adapted from Southerland et al.27
*Targeted infarct of the precentral motor hand cortex (‘hand knob’) often associated with ipsilateral internal carotid stenosis, causing deficit involving only the contralateral hand, several fingers, or just the thumb.28
†Note the potential for paradoxical embolisation from the anterior to posterior territory in patients with fetal-origin posterior circulation arteries (posterior cerebral arteries arising from the distal internal carotid artery—a normal anatomical variant) and for a detailed review of the vascular supply of the thalamus, see Powell et al.29
L, left hemisphere; R, right hemisphere.
The three-step ‘HINTS’ (Head-Impulse-Nystagmus-Test-of-Skew) bedside examination is often used to assess patients presenting with acute vestibular syndromes and has a high sensitivity (100%) and specificity (96%) for detecting a central cause.30 31 As its positive predictive value is only 69%, an isolated abnormal head impulse test (suggesting unilateral peripheral vestibulopathy) should be interpreted with caution.32
Investigations
Pre-imaging
Rapid neuroimaging is essential for patients with acute stroke. The American Stroke Association guidelines advise that the only necessary prior investigation is a capillary blood glucose,33 which in practice is obtained by paramedics. An intravenous cannula is often required for contrast or perfusion imaging sequences, allowing a blood panel to be obtained simultaneously. This would usually include a screen for infection, renal function and, if the patient takes anticoagulants, a coagulation screen. Although many radiology departments require a recent renal function before giving contrast,34 recent studies have questioned the concept of contrast-induced nephropathy.35 36
Imaging
Stroke centres should establish protocols to eliminate delays to neuroimaging, for example, protocolled stroke imaging sequences and priority use of a designated scanner near to the emergency department.
Neuroimaging in the hyperacute acute stroke setting remains predominantly CT-based.37 A non-contrasted CT scan of head is quick, sensitive and cost-effective at ruling out intracranial haemorrhage, which is usually sufficient for making thrombolysis decisions.38 However, CT scanning has much lower sensitivity and specificity for acute ischaemia because the net tissue water content (and therefore visual change in parenchymal attenuation) changes over hours after the onset of ischaemia. Specificity is compromised by the high prevalence of existing ischaemic changes or old established infarcts. Signs of acute ischaemia on non-contrast CT include loss of grey–white matter differentiation (eg, at the insular ribbon), hemispheric sulcal effacement, loss of integrity of the lentiform nucleus or hyperdensity within an intracranial artery (the ‘dense artery sign’). Early ischaemic changes can be quantified to assess the extent of parenchymal damage using the 10-point Alberta Stroke Program Early CT Score (ASPECTS).39
Multimodal CT imaging comprises CT-perfusion and/or CT-angiography, as well as non-contrast CT, aiming to improve and broaden case selection for reperfusion therapy. Rapid multimodal CT can be performed in acute stroke care pathways. Stroke centres need clear protocols for efficient interpretation to prevent unnecessary delays in giving rtPA.40
CT-angiography of the cervicocranial and intracranial arteries should be performed urgently to detect intracranial large artery occlusion when endovascular thrombectomy is available. Intracranial large artery occlusion is a marker of poor prognosis in minor stroke and TIA41 and observational evidence suggests that patients with non-disabling symptoms due to intracranial large artery occlusion may benefit from thrombolysis,42 but a randomised trial is ongoing.43
CT-perfusion sequences can assess various aspects of cerebral perfusion (see discussion below), often with automated software, such as MIStar (Apollo Medical Imaging Technology) or Rapid Processing of Perfusion and Diffusion (RAPID) CT-perfusion (iSchemaView); these technologies ease interpretation by increasing inter-observer reproducibility and ensuring use of validated thresholds. A comprehensive review of CT-perfusion interpretation has recently been published in Practical Neurology.44
MRI has much greater sensitivity for ischaemia than CT, particularly in minor stroke where it can predict poor short- and long-term outcomes.45 Moreover, comparing different sequences offers an approximate indication of time since onset.46 Rapid stroke MRI protocols typically include diffusion-weighted imaging (DWI), time-of-flight MR-angiogram of the intracranial arteries, T2-fluid-attenuated inversion recovery (FLAIR) and a blood-sensitive sequence such as gradient-recalled echo or susceptibility-weighted imaging.47
Principles of acute stroke care
The main objective of acute ischaemic stroke treatment is to salvage ischaemic, but viable, brain tissue by recanalising occluded cerebral arteries and reperfusing the ischaemic penumbra.48 The penumbra is a region of electrically inexcitable, hypoperfused parenchyma surrounding the irreversibly damaged core49 that is temporarily supported by leptomeningeal collateral flow. Failure to recruit or maintain collaterals underlies the highly variable individual speed of evolution of the core; the mechanisms of collateral failure are currently poorly understood.50 Rapidly declining benefit from reperfusion therapies (‘time is brain’)51 reflects the average pathophysiological status of failure of collateral support over several hours. Some people, identified by imaging, maintain collaterals for longer periods, and later reperfusion is beneficial. Figure 2 offers a structured approach to acute stroke reperfusion; it is an overview of ‘best practice’ and should be used in conjunction with local protocols tailored to available services where necessary.
Figure 2.
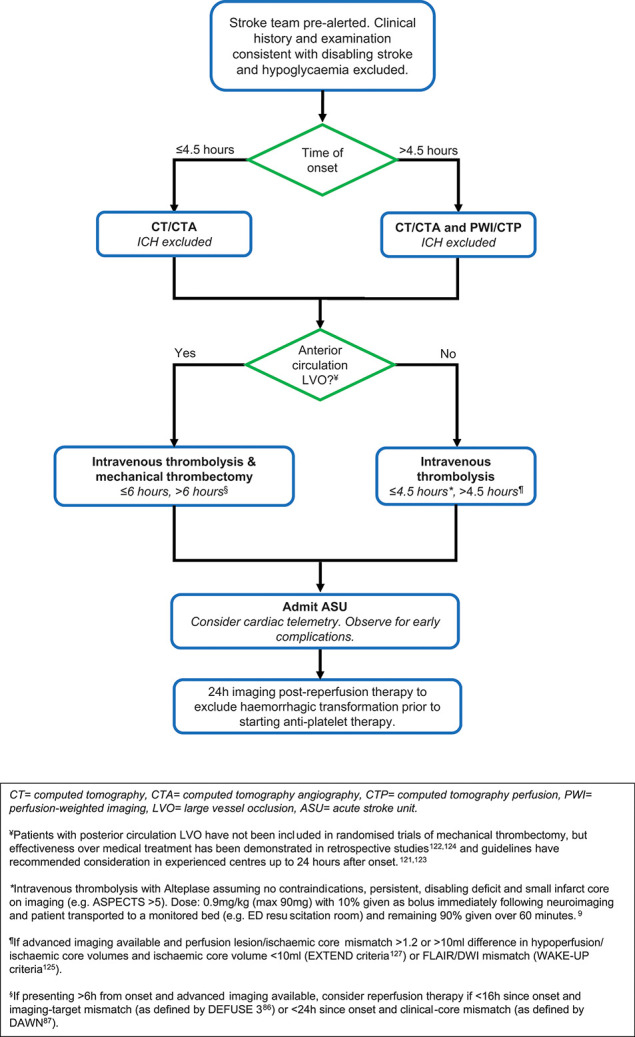
Process of acute ischaemic stroke reperfusion therapy: an overview of ‘best practice’.
Patients with severely elevated BP (≥185 mmHg systolic or ≥110 mmHg diastolic) are precluded from thrombolysis due to alteplase licensing restrictions; they may require intravenous antihypertensive therapy9 (eg, intravenous labetalol 5–10 mg or glyceryl trinitrate 50 mg in 50 mL starting at 1.5 mL/hour). However, the BP threshold is based on the original alteplase trial inclusion criteria52 and there is no evidence that reducing BP in this context helps clinically; indeed, recent data suggest a complex interaction between reperfusion status, BP and patient outcome, with one study suggesting that lowering BP before reperfusion treatment may be inappropriate.53
Acute reperfusion strategies
Intravenous thrombolysis
Tissue-type plasminogen activator (tPA) cleaves plasminogen on the surface of thrombi to form plasmin, a powerful endogenous fibrinolytic enzyme.54 Intravenous rtPA (alteplase) is proven and licenced to improve functional outcome in acute ischaemic stroke up to 4.5 hours after symptom onset.10 55 The treatment effect is heavily time-dependent: the number needed to treat for excellent functional outcome at 1.5 hours is five, compared with nine at 3.0–4.5 hours.56 The relative benefit of rtPA is not modified by baseline stroke severity or by age.56 57
UK guidelines recommend all patients with disabling symptoms should be considered for rtPA treatment within 3 hours of symptom onset, and up to 4.5 hours in those aged under 80. Patients presenting at 4.5–6 hours should be considered on an individual basis for treatment, recognising that the benefits are smaller than if treated earlier, but that the risks of a worse outcome, including death, are not increased.58 The UK performs poorly compared with other countries, both in the proportion of patients receiving rtPA (12% for the past 6 years59) and mean door-to-needle times (52 min last year in England and Wales60); considerable improvements in outcome are achievable if these could be bettered.
Informed consent is rarely possible and should not delay treatment. In one registry of nearly 2000 patients, a median door-to-needle time of only 20 min included a consent discussion of less than a minute61; however if unavailable, treatment should proceed in the patient’s best interests.
Currently, there is little evidence to support thrombolysis in patients with non-disabling ischaemic stroke.62 Table 2 shows the relative and absolute contraindications to rtPA. Symptomatic intracerebral haemorrhage is the most feared adverse effect of rtPA but haemorrhage associated with significant neurological deterioration occurs in only approximately 1.9% of treated patients.63 64 Radiological haemorrhagic transformation occurs due to reperfusion and is more common in people with larger infarcts (who therefore more severe baseline deficits). Neurological deterioration after rtPA infusion is common but usually reflects the initial ischaemic injury; in one recent case series, only 1 of 511 patients deteriorated during the rtPA infusion due to intracerebral haemorrhage. Most deterioration related to intracerebral haemorrhage occurred after the complete rtPA infusion, and deterioration was four times more likely to be due to initial ischaemia rather than to intracerebral haemorrhage.65 Deteriorating patients need urgent repeat neuroimaging to clarify the cause and rtPA infusion is usually suspended pending imaging.
Table 2.
American Heart Association/American Stroke Association absolute and relative contraindications to treatment of acute ischaemic stroke with alteplase
| Absolute contraindications | Comments regarding alteplase (rtPA) use |
|---|---|
| Systolic blood pressure >185 mmHg or diastolic blood pressure >110 mmHg | Treatment recommended if blood pressure can be lowered |
| International normalised ratio (INR) >1.7 | Must be tested if the patient is taking Warfarin, rapid point of care testing can be used in the hyperacute setting. |
| Direct oral anticoagulant (DOAC) use within 48 hours | Safety of thrombolysis in patients on DOACs is not well studied. Direct factor Xa assays may become a fast and reliable method for measuring direct factor Xa inhibitor activity (apixaban and rivaroxaban) and dilute thrombin time is sensitive to the presence of dabigatran activity, but more research is required.* |
| Platelets <100 000/mm3 | Serum platelet level not required before rtPA unless low platelet count is suspected. |
| Active internal bleeding | Low bleeding risk in those with past (>21 days) gastrointestinal bleeding. |
| Intracranial or intraspinal surgery or severe head trauma within 3 months | No high-quality evidence. but the location of potential surgical site bleeding may limit benefits of rtPA compared to general surgical patients. |
| Intracerebral vascular malformations | Including cavernous angioma, capillary telangiectasia, developmental venous anomalies and arteriovenous malformations. Insufficient data and large variation in haemorrhage risk. |
| Intracranial malignancy | rtPA should be safe with extra-axial but not recommended in those with intra-axial malignancy. |
| Previous intracerebral haemorrhage | ‘Recent’ history of previous intracerebral haemorrhage in recent FDA label. Limited data but increased risk of developing symptomatic intracerebral haemorrhage seems related to volume of encephalomalacia from the previous haemorrhage, same vascular territory as ischaemic event and how recent. Cerebral microbleeds have not been shown to increase risk of symptomatic intracerebral haemorrhage. |
| Ischaemic stroke within 3 months | Limited data to suggest increased risk of adverse events. Contraindication removed from updated FDA licence. |
| Arterial puncture at non-compressible site within 7 days | Including subclavian or jugular catheterisation. |
| Infective endocarditis | Cerebral infarcts caused by septic emboli are prone to haemorrhagic transformation due to septic arteritis. |
| Relative contraindications | |
| Rapidly improving symptoms | rtPA recommended if symptom improvement but remains disabled. |
| Pregnancy and early postpartum (<14 days) | Insufficient data, case-by-case risk decision with obstetric team. |
| Unruptured intracranial aneurysm | Small (<10 mm) unsecured unruptured intracranial aneurysm should not preclude rtPA, insufficient data on larger unruptured intracranial aneurysms. |
| Seizure at onset | Concerns of diagnostic uncertainty (post-ictal neurological deficits). |
| Major extracranial trauma within 14 days | Limited data. One small meta-analysis of rtPA use in cervical artery dissection (including some trauma-related) reported no safety concerns. |
| Major surgery within 14 days or gastrointestinal or genitourinary surgery within 21 days | Including coronary artery bypass grafting, organ biopsy or childbirth and relates to increased risk of surgical site haemorrhage. Limited data, so not an absolute contraindication, but case-by-case risk decision. |
| Acute myocardial infarction (MI) within 3 months | rtPA recommended if concurrent myocardial infarction and ischaemic stroke. If history of myocardial infarction within the last 3 months, rtPA is reasonable if non-STEMI or right/inferior myocardial STEMI. Lower class of evidence for left anterior myocardial STEMI. |
Abridged from Demaerschalk et al66.
NSTEMI, non-ST-elevation myocardial infarction; rtPA= recombinant tissue-type plasminogen activator (alteplase); STEMI, ST-elevation myocardial infarction.
*The European Heart Rhythm Association recommends considering thrombolysis if DOAC plasma level is below the lower limit of detection (or <30 ng/mL for Xa inhibitors if >4 hours after intake) or last dose within 24–48 hours and normal renal function.67 One meta-analysis has indicated no increased risk of sICH with prior DOAC use patients treated with IVT and reports successful thrombolysis after dabigatran reversal with idarucizumab.68 Andexanet alfa is FDA-approved for Xa inhibitor reversal but is not currently licensed in the UK.
FDA, Food and Drug Administration; IVT, intravenous thrombolysis; NSTEMI, non-ST-elevation myocardial infarction; rtPA, recombinant tissue-type plasminogen activator (alteplase); sICH, symptomatic intracranial hemorrhage; STEMI, ST-elevation myocardial infarction.
Orolingual angioedema is a recognised complication of rtPA; while most cases are mild and self-limiting, severe attacks requiring airway management can occur in up to 1% of treated patients; people taking ACE inhibitors or those with insular ischaemia are at increased risk.69 Stroke centres should develop local protocols with the anaesthetic department for assessing and urgently managing angioedema. Although management in this setting is not evidence based, treatment should be consistent with that of other drug reactions (figure 3).
Figure 3.
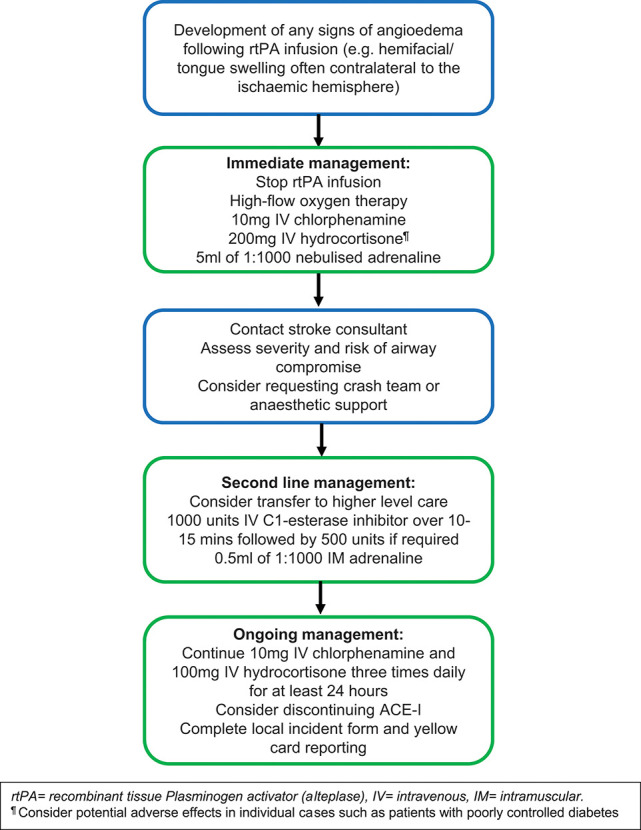
Suggested treatment algorithm for rtPA-associated angioedema.
rtPA, recombinant tissue-type plasminogen activator.
Endovascular thrombectomy
Despite the overall benefit of rtPA, the subgroup of patients with large proximal intracranial vessel occlusion (large artery occlusion; carotid, proximal middle cerebral arteries) have low rates of recanalisation with thrombolysis and only a 25% chance of a good outcome.70 71 Endovascular thrombectomy in addition to best medical therapy has been proven in nine randomised trials as superior to best medical therapy alone (including intravenous rtPA in the majority of patients) for patients with anterior circulation large artery occlusion.72 –80 The number needed to treat to achieve a reduction of one or more points on modified Rankin Scale (mRS) is 2.6.81 A detailed guide has recently been published in Practical Neurology.82
Unfortunately, the UK has been slow to provide this service; current endovascular thrombectomy rates are 5.5 per 1000 ischaemic strokes in the UK, versus 50 in the US and Western Europe.59 Parts of the UK, notably Scotland, have no access to thrombectomy at all. In England and Wales, the NHS Long Term Plan aims for a 10-fold increase by 2022, in part by expanding endovascular thrombectomy training to specialities other than interventional neuroradiology.4
Guidelines from the UK’s National Institute of Health and Care Excellence (NICE) recommend endovascular thrombectomy for patients with disabling acute ischaemic stroke (arbitrarily defined as NIHSS ≥6) due to imaging-proven anterior circulation large-vessel occlusion up to 6 hours, and posterior circulation (basilar or posterior cerebral artery) large-vessel occlusion up to 24 hours after symptom onset.10 Patients with lower NIHSS but functionally disabling symptoms may also be considered due to the high risk of deterioration associated with large-vessel occlusion.83
As with rtPA, the benefit of endovascular thrombectomy is highly time-dependent.84 However, several clinical trials showed favourable outcome of endovascular thrombectomy versus medical management in anterior circulation large-vessel occlusion beyond 6 hours, although based on small numbers of patients.73 76 85 Two trials have extended the therapeutic window even further: up to 16 hours in DEFUSE 386 and 24 hours in DAWN87 with CT perfusion or DWI-perfusion imaging with clinical mismatch. These trials demonstrated that imaging can select patients with large artery occlusion whose good collateral supply makes them likely to benefit from endovascular thrombectomy.
The optimal mode of anaesthesia during endovascular thrombectomy has yet to be determined; retrospective data suggested that general anaesthesia may be harmful (although potentially biased by patient selection),88 whereas single-centre randomised trials have shown neutral or beneficial effects.89 Multicentre randomised trials are ongoing.82
The complication rates of endovascular thrombectomy are in keeping with other emergency procedures and serious adverse events are rare.90 Although adverse events occur in approximately 15% of patients (including vasospasm, arterial perforation or dissection, device misplacement, symptomatic intracerebral haemorrhage or embolisation to new or target vessel territory), clinical outcome is not affected overall; the number needed to treat of 2.6 includes these complications.91
Acute stroke unit and early complications
Guidelines recommend that everyone with acute ischaemic stroke is admitted directly to an acute stroke unit.9 Stroke unit care has an number needed to treat of 17 to avoid death or disability, a benefit that is sustained over time without lengthening hospital stays.92 93 Key features of the acute stroke unit include stroke-specific multidisciplinary care (physiotherapy, speech and language therapy, occupational therapy) and high nursing ratios.94 95 However, for the past 5 years, only 58% of patients in England and Wales were admitted to an acute stroke unit within 4 hours.60
Key functions of an acute stroke unit are the prevention of secondary brain insults by maintaining physiological homeostasis (table 3) and monitoring of neurological status.96 The patient should also undergo bedside cardiac telemetry if atrial fibrillation has not been confirmed.
Table 3.
Targets for maintaining homeostasis in acute ischaemic stroke patients
| Variable | Target/intervention |
|---|---|
| Oxygen saturation | Oxygen supplementation if saturation <95% |
| Hydration | Assessed within 4 hours using multiple tools |
| Swallowing | Screen for dysphagia with validated tool within 4 hours and before any oral intake (including medication) |
| Plasma glucose | 5–15 mmol/L |
| Blood pressure | No target. Indication for treatment:
|
Adapted from National Clinical Guideline for Stroke 2016.9
Neurological deterioration should prompt urgent repeat neuroimaging; early neurological complications include recurrent ischaemia, cerebral oedema or haemorrhagic transformation. Repeat brain imaging around 24 hours following rtPA administration is widely undertaken to inform on intracerebral haemorrhage incidence as a quality of care metric, and visualisation of an infarct may provide prognostic and mechanistically relevant information, but the role for routine repeat imaging is debatable. Once haemorrhagic complications have been excluded at 24 hours, antiplatelet therapy should start, most often 300 mg aspirin daily for 2 weeks followed by lifelong clopidogrel monotherapy.
Patients with large volume hemispheric infarcts from acute occlusion of the proximal middle cerebral artery or internal carotid artery are particularly vulnerable to ‘malignant’ cerebral oedema, with a mortality rate of up to 78%.97 Decompressive hemicraniectomy increases the chance of survival (number needed to treat of 2), but patients are often left with significant disability (mRS 4–5 at 1 year in 43% with decompressive hemicraniectomy vs 17% with medical management)98; however, the great majority rate their quality of life as being satisfactory despite disability.99 Updated NICE guidance10 has removed the upper age limit for consideration of decompressive hemicraniectomy, in line with trial evidence. The current eligibility criteria are as follows:
Surgery may be performed 48 hours from stroke onset
Clinical deficits that suggest middle cerebral artery infarction with NIHSS >15
Decreased level of consciousness (≥1 on level of consciousness on NIHSS)
Infarction of ≥50% of middle cerebral artery territory as seen on CT scanning or infarct volume >145 cm3 on DWI
The high incidence of dysphagia after stroke is a risk factor for aspiration pneumonia and is associated with increased mortality and disability.100 Guidelines recommend that patients receive a bedside swallowing assessment and appropriate adaptation of oral intake to prevent aspiration.10 Although there are no randomised studies to determine whether screening methods improve outcomes,101 observational data suggest that delayed assessment is associated with a higher risk of aspiration pneumonia.102 Prophylactic antibiotics have not proven effective.103
Non-ambulatory patients with ischaemic stroke are at high risk of deep vein thrombosis.104 Prophylaxis with low-molecular-weight heparin is not recommended due to the risk of haemorrhagic transformation,9 although some studies have shown no significant additional risk.105 Intermittent pneumatic compression devices are effective (compared to compression stockings) at reducing the risk of deep vein thrombosis and are recommended for all non-ambulatory stroke patients.9 106
Future directions
There is a wealth of active clinical research in stroke medicine, driven by the significant public health implications of this common and socioeconomically impactful disease. A particular priority in the UK is to improve systems that reduce onset-to-needle times, increase access to endovascular thrombectomy and admission rates to acute stroke units. Audits, including the Sentinel Stroke National Audit Programme (SSNAP), measure the processes and structure of stroke care and use these data to drive improvements.
Mobile stroke units with in-built CT scanners and telemedicine links with stroke centres are associated with earlier thrombolytic delivery and improved clinical outcome in urban settings but are resource intensive and their optimal deployment depends on accurate prehospital triage.107
Alternatives to alteplase that are more fibrin-specific may be safer, more effective and may increase the therapeutic window. However, desmoteplase did not improve functional outcome compared with placebo in acute ischaemic patients 3–9 hours after symptom onset.108 Although tenecteplase has not proven superior to alteplase in minor ischaemic stroke patients109 (a trial in patients with non-disabling symptoms due to large-vessel occlusion is ongoing43), it doubled recanalisation rates in pre-endovascular treatment of strokes from large-vessel occlusion with improved functional outcome.110 In addition, the single bolus administration of tenecteplase may be advantageous for drip-and-ship thrombectomy service pathways.
Ongoing trials are investigating the efficacy of endovascular thrombectomy in patient subgroups, including basilar artery occlusion (BASICS111), low NIHSS (MOSTE112 and ENDOLOW113), or low ASPECTS score (TESLA,114 TENSION115 and IN EXTREMIS112). The optimal prehospital service pathway is another unanswered question, and mothership and drip-and-ship models are also being compared in a multicentre trial.116
Multiple preclinical and clinical studies to prevent secondary neuronal injury following ischaemic stroke have been unsuccessful, and to date there are no evidence-based neuroprotective agents.117 Although the neuroprotectant nerinetide did not improve outcomes in endovascular-treated patients compared with placebo in one recent randomised trial, secondary subgroup analyses suggest further investigation may be warranted in patients not treated with alteplase.118 Translational studies of neuroprotective therapies may be aided by novel tissue banking of thrombi extracted by endovascular thrombectomy.119 The CHARM trial aims to assess whether glibenclamide (BIIB093) improves functional outcome in patients with malignant brain oedema.120
CONCLUSION
Stroke medicine is a varied and rapidly developing field that provides the opportunity to offer life-changing treatments to patients affected by the leading cause of neurological disability. Stroke care will have increasing relevance for neurologists of the future and as a specialty we have a lot to offer, in particular with diagnostic expertise. Equally, we may need to develop our skills further, for example, through managing acutely unwell patients with general medical problems on the acute stroke unit or by learning how to perform mechanical thrombectomy.
Key points.
Stroke is a public health priority and prompt specialist intervention significantly reduces the burden of death and disability.
We need effective systems in place for prehospital recognition and appropriate triage of suspected acute stroke.
Patients with non-disabling stroke or TIA should be assessed within 24 hours.
Reperfusion with intravenous thrombolysis and/or endovascular thrombectomy are highly effective therapies, but time-dependent.
All patients should be treated in an acute stroke unit, for monitoring to detect and act on physiological insults including brain oedema, and for investigating promptly to allow initiation of mechanism-appropriate secondary preventative treatments.
Footnotes
Contributors: RH drafted the manuscript. Other authors revised the manuscript.
Acknowledgement: The authors would like to thank Mr Philip Baker for his assistance in designing the figures.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned externally reviewed by John Fink, Christchurch, New Zealand and Michael O’Sullivan, Brisbane, Australia.
Further Reading
- 1.Fernandes PM, Whiteley WN, Hart SR. et al. Strokes: mimics and chameleons. Pract Neurol. 2013;13:21–8. [DOI] [PubMed] [Google Scholar]
- 2.Nadarajan V, Perry RJ, Johnson J. et al. Transient ischemic attacks: mimics and chameleons. Pract Neurol. 2014;14:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans MRB, White P, Cowley P. et al. Revolution in acute ischaemic stroke care: a practical guide to mechanical thrombectomy. Pract Neurol. 2017;17:252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Stroke Association State of the nation: stroke statistics [Internet]. 2018. Available https://www.stroke.org.uk/sites/default/files/state_of_the_nation_2018.pdf (accessed 22 Feb 2020).
- 2.Global, regional, and national burden of neurological disorders. 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459–80. 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroke Association Current, future and avoidable costs of stroke in the UK [Internet]. 2015. Available https://www.stroke.org.uk/sites/default/files/costs_of_stroke_in_the_uk_report_-executive_summary_part_2.pdf (accessed 24 Feb 2020).
- 4.The NHS Long Term Plan [Internet]. 2019. Available https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (accessed 22 Feb 2020).
- 5.Wolters FJ, Paul NLM, Li L, et al. Sustained impact of UK FAST-test public education on response to stroke: a population-based time-series study. Int J Stroke 2015;10:1108–14. 10.1111/ijs.12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milne MSW, Holodinsky JK, Hill MD, et al. Drip ‘n ship versus mothership for endovascular treatment: modeling the best transportation options for optimal outcomes. Stroke 2017;48:791–4. 10.1161/STROKEAHA.116.015321 [DOI] [PubMed] [Google Scholar]
- 7.Hurford R, Tyrrell PJ. Stroke thrombolysis: where are we and where are we going? Clin Med 2013;13:s20–3. 10.7861/clinmedicine.13-6-s20 [DOI] [PubMed] [Google Scholar]
- 8.Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol 2008;7:787–95. 10.1016/S1474-4422(08)70171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Party intercollegiate stroke working party. National Clinical Guideline for Stroke [Internet]. 2016. Available https://www.strokeaudit.org/SupportFiles/Documents/Guidelines/2016-National-Clinical-Guideline-for-Stroke-5t-1.aspx (accessed 22 Feb 2020).
- 10.National Institute for Health and Clinical Excellence (NICE). Clinical guideline (NG128) Stroke and transient ischaemic attack in over 16s: diagnosis and initial management [Internet]. 2019. Available http://www.nice.org.uk/guidance/CG68 [PubMed]
- 11.Hankey GJ. Stroke. Lancet (London, England) 2017;389:641–54. 10.1016/S0140-6736(16)30962-X [DOI] [PubMed] [Google Scholar]
- 12.Gibson LM, Whiteley W. The differential diagnosis of suspected stroke: a systematic review. J R Coll Physicians Edinb 2013;43:114–18. 10.4997/JRCPE.2013.205 [DOI] [PubMed] [Google Scholar]
- 13.Fernandes PM, Whiteley WN, Hart SR, et al. Strokes: mimics and chameleons. Pract Neurol 2013;13:21–8. 10.1136/practneurol-2012-000465 [DOI] [PubMed] [Google Scholar]
- 14.Nadarajan V, Perry RJ, Johnson J, et al. Transient ischaemic attacks: mimics and chameleons. Pract Neurol [Internet] 2014;14:23–31. 10.1136/practneurol-2013-000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee G, Stone SP, Werring DJ. Posterior circulation ischaemic stroke. BMJ 2018;361:k1185 10.1136/bmj.k1185 [DOI] [PubMed] [Google Scholar]
- 16.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007;25:32–8. 10.1016/j.ajem.2006.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoda K, Okada Y, Fujimoto S, et al. Blood pressure changes during the initial week after different subtypes of ischemic stroke. Stroke 2006;37:2637–9. 10.1161/01.STR.0000242781.80832.cc [DOI] [PubMed] [Google Scholar]
- 18.Wallace JD, Levy LL. Blood pressure after stroke. JAMA 1981;246:2177–80. 10.1001/jama.1981.03320190035023 [DOI] [PubMed] [Google Scholar]
- 19.McManus M, Liebeskind DS. Blood pressure in acute ischemic stroke. J Clin Neurol 2016;12:137–46. 10.3988/jcn.2016.12.2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogelholm R, Avikainen S, Murros K. Prognostic value and determinants of first-day mean arterial pressure in spontaneous supratentorial intracerebral hemorrhage. Stroke 1997;28:1396–400. 10.1161/01.STR.28.7.1396 [DOI] [PubMed] [Google Scholar]
- 21.Okumura K, Ohya Y, Maehara A, et al. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens 2005;23:1217–23. 10.1097/01.hjh.0000170385.76826.4a [DOI] [PubMed] [Google Scholar]
- 22.Fischer U, Rothwell PM. Blood pressure management in acute stroke: does the Scandinavian Candesartan Acute Stroke Trial (SCAST) resolve all of the unanswered questions? Stroke 2011;42:2995–8. 10.1161/STROKEAHA.111.619346 [DOI] [PubMed] [Google Scholar]
- 23.Wrotek SE, Kozak WE, Hess DC, et al. Treatment of fever after stroke: conflicting evidence. Pharmacotherapy 2011;31:1085–91. 10.1592/phco.31.11.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brott T, Adams HPJ, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 25.Lyden P, Claesson L, Havstad S, et al. Factor analysis of the National Institutes of Health Stroke Scale in patients with large strokes. Arch Neurol 2004;61:1677–80. 10.1001/archneur.61.11.1677 [DOI] [PubMed] [Google Scholar]
- 26.Sato S, Toyoda K, Uehara T, et al. Baseline NIH Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology 2008;70:2371–7. 10.1212/01.wnl.0000304346.14354.0b [DOI] [PubMed] [Google Scholar]
- 27.Southerland AM. Clinical evaluation of the patient with acute stroke. Continuum (Minneap Minn) 2017;23:40–61. [DOI] [PubMed] [Google Scholar]
- 28.Peters N, Muller-Schunk S, Freilinger T, et al. Ischemic stroke of the cortical “hand knob” area: stroke mechanisms and prognosis. J Neurol 2009;256:1146–51. 10.1007/s00415-009-5104-8 [DOI] [PubMed] [Google Scholar]
- 29.Powell R, Hughes T. A chamber of secrets. The neurology of the thalamus: lessons from acute stroke. Pract Neurol 2014;14:440–5. 10.1136/practneurol-2014-000852 [DOI] [PubMed] [Google Scholar]
- 30.Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009;40:3504–10. 10.1161/STROKEAHA.109.551234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan K, Bassilious K, Eriksen E, et al. Posterior circulation stroke diagnosis using HINTS in patients presenting with acute vestibular syndrome: a systematic review. Eur Stroke J 2019;4:233–9. 10.1177/2396987319843701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wren D, Moynihan B, Pereira A. Responses to a practical approach to acute vertigo. Pract Neurol 2009;9:52–3. 10.1136/jnnp.2008.162545 [DOI] [PubMed] [Google Scholar]
- 33.Jauch EC, Saver JL, Adams HPJ, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 34.Faculty of Clinical Radiology. Standards for intravascular contrast administration to adult patients [Internet]. 2014. Available https://www.rcr.ac.uk/sites/default/files/Intravasc_contrast_web.pdf (accessed 22 Feb 2020).
- 35.McDonald JS, McDonald RJ, Carter RE, et al. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology 2014;271:65–73. 10.1148/radiol.13130775 [DOI] [PubMed] [Google Scholar]
- 36.McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014;273:714–25. 10.1148/radiol.14132418 [DOI] [PubMed] [Google Scholar]
- 37.Brazzelli M, Shuler K, Quayyum Z, et al. Clinical and imaging services for TIA and minor stroke: results of two surveys of practice across the UK. BMJ Open 2013;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wardlaw JM, Seymour J, Cairns J, et al. Immediate computed tomography scanning of acute stroke is cost-effective and improves quality of life. Stroke 2004;35:2477–83. 10.1161/01.STR.0000143453.78005.44 [DOI] [PubMed] [Google Scholar]
- 39.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet (London, England) 2000;355:1670–4. 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 40.Salottolo KM, Fanale CV, Leonard KA, et al. Multimodal imaging does not delay intravenous thrombolytic therapy in acute stroke. AJNR Am J Neuroradiol 2011;32:864–8. 10.3174/ajnr.A2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coutts SB, Modi J, Patel SK, et al. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke 2012;43:1013–17. 10.1161/STROKEAHA.111.637421 [DOI] [PubMed] [Google Scholar]
- 42.Heldner MR, Jung S, Zubler C, et al. Outcome of patients with occlusions of the internal carotid artery or the main stem of the middle cerebral artery with NIHSS score of less than 5: comparison between thrombolysed and non-thrombolysed patients. J Neurol Neurosurg Psychiatry 2015;86:755–60. 10.1136/jnnp-2014-308401 [DOI] [PubMed] [Google Scholar]
- 43.A randomized controlled trial of TNK-tPA versus standard of care for minor ischemic stroke with proven occlusion (TEMPO-2) [Internet]. 2015. Available https://clinicaltrials.gov/ct2/show/NCT02398656 (accessed 24 Feb 2020). [DOI] [PMC free article] [PubMed]
- 44.Wing SC, Markus HS. Interpreting CT perfusion in stroke. Pract Neurol 2019;19:136–42. 10.1136/practneurol-2018-001917 [DOI] [PubMed] [Google Scholar]
- 45.Hurford R, Li L, Lovett N, et al. Prognostic value of “tissue-based” definitions of TIA and minor stroke: population-based study. Neurology 2019;92:e2455–e2461. 10.1212/WNL.0000000000007531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vilela P, Rowley HA. Brain ischemia: CT and MRI techniques in acute ischemic stroke. Eur J Radiol 2017;96:162–72. 10.1016/j.ejrad.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 47.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. AJNR Am J Neuroradiol 2008;29:e23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 1981;12:723–5. 10.1161/01.STR.12.6.723 [DOI] [PubMed] [Google Scholar]
- 49.Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry 2004;75:353–61. 10.1136/jnnp.2003.025825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2017;48:2621–7. 10.1161/STROKEAHA.117.017673 [DOI] [PubMed] [Google Scholar]
- 51.Saver JL. Time is brain: quantified. Stroke 2006;37:263–6. 10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 52.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–7. 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 53.Hong L, Cheng X, Lin L, et al. The blood pressure paradox in acute ischemic stroke. Ann Neurol 2019;85:331–9. [DOI] [PubMed] [Google Scholar]
- 54.Medcalf RL, Davis SM. Plasminogen activation and thrombolysis for ischemic stroke. Int J Stroke 2012;7:419–25. 10.1111/j.1747-4949.2012.00783.x [DOI] [PubMed] [Google Scholar]
- 55.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 56.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet (London, England) 2014;384:1929–35. 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lansberg MG, Schrooten M, Bluhmki E, et al. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified Rankin Scale. Stroke 2009;40:2079–84. 10.1161/STROKEAHA.108.540708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet (London, England) 2012;379:2364–72. 10.1016/S0140-6736(12)60738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J 2019;4:13–28. 10.1177/2396987318786023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.SSNAP Move the dial of stroke care: the sixth Sentinel Stroke National Audit Programme annual report [Internet]. 2019. Available https://www.strokeaudit.org/Documents/National/Clinical/Apr2018Mar2019/Apr2018Mar2019-AnnualReport.aspx
- 61.Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012;79:306–13. 10.1212/WNL.0b013e31825d6011 [DOI] [PubMed] [Google Scholar]
- 62.Khatri P, Kleindorfer DO, Devlin T, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA 2018;320:156–66. 10.1001/jama.2018.8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seet RCS, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis 2012;34:106–14. 10.1159/000339675 [DOI] [PubMed] [Google Scholar]
- 64.Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke 2007;38:2279–83. 10.1161/STROKEAHA.107.487009 [DOI] [PubMed] [Google Scholar]
- 65.James B, Chang AD, McTaggart RA, et al. Predictors of symptomatic intracranial haemorrhage in patients with an ischaemic stroke with neurological deterioration after intravenous thrombolysis. J Neurol Neurosurg Psychiatry 2018;89:866–9. 10.1136/jnnp-2017-317341 [DOI] [PubMed] [Google Scholar]
- 66.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:581–641. 10.1161/STR.0000000000000086 [DOI] [PubMed] [Google Scholar]
- 67.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–93. [DOI] [PubMed] [Google Scholar]
- 68.Shahjouei S, Tsivgoulis G, Goyal N, et al. Safety of intravenous thrombolysis among patients taking direct oral anticoagulants: a systematic review and meta-analysis. Stroke 2020;51:533–41. 10.1161/STROKEAHA.119.026426 [DOI] [PubMed] [Google Scholar]
- 69.Hurford R, Rezvani S, Kreimei M, et al. Incidence, predictors and clinical characteristics of orolingual angio-oedema complicating thrombolysis with tissue plasminogen activator for ischaemic stroke. J Neurol Neurosurg Psychiatry 2015;86:520–3. 10.1136/jnnp-2014-308097 [DOI] [PubMed] [Google Scholar]
- 70.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010;41:2254–8. 10.1161/STROKEAHA.110.592535 [DOI] [PubMed] [Google Scholar]
- 71.Mishra SM, Dykeman J, Sajobi TT, et al. Early reperfusion rates with IV tPA are determined by CTA clot characteristics. AJNR Am J Neuroradiol 2014;35:2265–72. 10.3174/ajnr.A4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 73.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 74.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 75.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 76.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 77.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138–47. 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 78.Mocco J, Zaidat OO, von Kummer R, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke 2016;47:2331–8. 10.1161/STROKEAHA.116.013372 [DOI] [PubMed] [Google Scholar]
- 79.Muir KW, Ford GA, Messow C-M, et al. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry 2017;88:38–44. 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khoury NN, Darsaut TE, Ghostine J, et al. Endovascular thrombectomy and medical therapy versus medical therapy alone in acute stroke: a randomized care trial. J Neuroradiol 2017;44:198–202. 10.1016/j.neurad.2017.01.126 [DOI] [PubMed] [Google Scholar]
- 81.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England) 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 82.Evans MRB, White P, Cowley P, et al. Revolution in acute ischaemic stroke care: a practical guide to mechanical thrombectomy. Pract Neurol 2017;17:252–65. 10.1136/practneurol-2017-001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mokin M, Ansari SA, McTaggart RA, et al. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS Standards and Guidelines Committee. J Neurointerv Surg 2019;11:215–20. 10.1136/neurintsurg-2018-014640 [DOI] [PubMed] [Google Scholar]
- 84.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 85.Evans JW, Graham BR, Pordeli P, et al. Time for a time window extension: insights from late presenters in the ESCAPE trial. AJNR Am J Neuroradiol 2018;39:102–6. 10.3174/ajnr.A5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 88.Davis MJ, Menon BK, Baghirzada LB, et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology 2012;116:396–405. 10.1097/ALN.0b013e318242a5d2 [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y, Jia L, Fang F, et al. General anesthesia versus conscious sedation for intracranial mechanical thrombectomy: a systematic review and meta-analysis of randomized clinical trials. J Am Heart Assoc 2019;8:e011754 10.1161/JAHA.118.011754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Behme D, Gondecki L, Fiethen S, et al. Complications of mechanical thrombectomy for acute ischemic stroke-a retrospective single-center study of 176 consecutive cases. Neuroradiology 2014;56:467–76. 10.1007/s00234-014-1352-0 [DOI] [PubMed] [Google Scholar]
- 91.Balami JS, White PM, McMeekin PJ, et al. Complications of endovascular treatment for acute ischemic stroke: prevention and management. Int J Stroke 2018;13:348–61. 10.1177/1747493017743051 [DOI] [PubMed] [Google Scholar]
- 92.Candelise L, Gattinoni M, Bersano A, et al. Stroke-unit care for acute stroke patients: an observational follow-up study. Lancet (London, England) 2007;369:299–305. 10.1016/S0140-6736(07)60152-4 [DOI] [PubMed] [Google Scholar]
- 93.Stroke Unit Triallists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013;(9)CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paley L, Williamson E, Bray BD, et al. Associations between 30-day mortality, specialist nursing, and daily physician ward rounds in a national stroke registry. Stroke 2018;49:2155–62. 10.1161/STROKEAHA.118.021518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bray BD, Ayis S, Campbell J, et al. Associations between stroke mortality and weekend working by stroke specialist physicians and registered nurses: prospective multicentre cohort study. PLoS Med 2014;11:e1001705 10.1371/journal.pmed.1001705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Middleton S, McElduff P, Ward J, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet (London, England) 2011;378:1699–706. 10.1016/S0140-6736(11)61485-2 [DOI] [PubMed] [Google Scholar]
- 97.Hacke W, Schwab S, Horn M, et al. “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996;53:309–15. 10.1001/archneur.1996.00550040037012 [DOI] [PubMed] [Google Scholar]
- 98.Alexander P, Heels-Ansdell D, Siemieniuk R, et al. Hemicraniectomy versus medical treatment with large MCA infarct: a review and meta-analysis. BMJ Open 2016;6:e014390 10.1136/bmjopen-2016-014390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Middelaar T, Nederkoorn PJ, van der Worp HB, et al. Quality of life after surgical decompression for space-occupying middle cerebral artery infarction: systematic review. Int J Stroke 2015;10:170–6. 10.1111/ijs.12329 [DOI] [PubMed] [Google Scholar]
- 100.Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–63. 10.1161/01.STR.0000190056.76543.eb [DOI] [PubMed] [Google Scholar]
- 101.Smith EE, Kent DM, Bulsara KR, et al. Effect of dysphagia screening strategies on clinical outcomes after stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke 2018;49:e123–e128. [DOI] [PubMed] [Google Scholar]
- 102.Bray BD, Smith CJ, Cloud GC, et al. The association between delays in screening for and assessing dysphagia after acute stroke, and the risk of stroke-associated pneumonia. J Neurol Neurosurg Psychiatry 2017;88:25–30. 10.1136/jnnp-2016-313356 [DOI] [PubMed] [Google Scholar]
- 103.Kalra L, Irshad S, Hodsoll J, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet (London, England) 2015;386:1835–44. 10.1016/S0140-6736(15)00126-9 [DOI] [PubMed] [Google Scholar]
- 104.Kelly J, Rudd A, Lewis RR, et al. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke 2004;35:2320–5. 10.1161/01.STR.0000140741.13279.4f [DOI] [PubMed] [Google Scholar]
- 105.Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet (London, England) 2007;369:1347–55. 10.1016/S0140-6736(07)60633-3 [DOI] [PubMed] [Google Scholar]
- 106.Dennis M, Sandercock P, Graham C, et al. The Clots in Legs Or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess 2015;19:1–90. 10.3310/hta19760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ebinger M, Siegerink B, Kunz A, et al. Effects of pre-hospital acute stroke treatment as measured with the modified Rankin Scale; the Berlin - Pre-hospital Or Usual care Delivery (B_PROUD) trial [Internet] In: International Stroke Conference. Los Angeles, 2020. Available https://www.abstractsonline.com/pp8/#!/7927/presentation/5405 [Google Scholar]
- 108.Albers GW, von Kummer R, Truelsen T, et al. Safety and efficacy of desmoteplase given 3-9 h after ischaemic stroke in patients with occlusion or high-grade stenosis in major cerebral arteries (DIAS-3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Neurol 2015;14:575–84. 10.1016/S1474-4422(15)00047-2 [DOI] [PubMed] [Google Scholar]
- 109.Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 2017;16:781–8. 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 110.Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 2018;378:1573–82. 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 111.van der Hoeven EJRJ, Schonewille WJ, Vos JA, et al. The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials 2013;14:200 10.1186/1745-6215-14-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.In extremis/MOSTE-LASTE homepage [Internet]. 2020. Available https://www.inextremis-study.com
- 113.Endovascular Therapy for Low NIHSS Ischemic Strokes (ENDOLOW) [Internet]. 2019. Available https://clinicaltrials.gov/ct2/show/NCT04167527 (accessed 22 Feb 2020).
- 114.The TESLA Trial: Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke (TESLA) [Internet]. Available: https://clinicaltrials.gov/ct2/show/NCT03805308
- 115.Bendszus M, Bonekamp S, Berge E, et al. A randomized controlled trial to test efficacy and safety of thrombectomy in stroke with extended lesion and extended time window. Int J Stroke 2019;14:87–93. 10.1177/1747493018798558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abilleira S, Perez de la Ossa N, Jimenez X, et al. Transfer to the local stroke center versus direct transfer to endovascular center of acute stroke patients with suspected large vessel occlusion in the catalan territory (RACECAT): study protocol of a cluster randomized within a cohort trial. Int J Stroke 2019;14:734–44. [DOI] [PubMed] [Google Scholar]
- 117.Patel RAG, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis 2017;59:542–8. 10.1016/j.pcad.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 118.Hill MD, Goyal M, Menon BK, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet [Internet] 2020. Available http://www.sciencedirect.com/science/article/pii/S0140673620302580 [DOI] [PubMed] [Google Scholar]
- 119.Fraser JF, Collier LA, Gorman AA, et al. The Blood And Clot Thrombectomy Registry And Collaboration (BACTRAC) protocol: novel method for evaluating human stroke. J Neurointerv Surg 2019;11:265–70. 10.1136/neurintsurg-2018-014118 [DOI] [PubMed] [Google Scholar]
- 120.Sheth KN, Kimberley WT, Albers GW, et al. Design of a Phase III Study of Intravenous Glibenclamide (BIIB093) for Large Hemispheric Infarction: the CHARM study (P2.3-036). Neurology [Internet] 2019;92:P2.3–036. Available http://n.neurology.org/content/92/15_Supplement/P2.3-036.abstract [Google Scholar]
- 121.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg 2019;11:535–8. 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 122.Gory B, Mazighi M, Blanc R, et al. Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first-line strategy (ADAPT vs stent retriever). J Neurosurg 2018;129:1482–91. 10.3171/2017.7.JNS171043 [DOI] [PubMed] [Google Scholar]
- 123.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 124.Mohlenbruch M, Stampfl S, Behrens L, et al. Mechanical thrombectomy with stent retrievers in acute basilar artery occlusion. AJNR Am J Neuroradiol 2014;35:959–64. 10.3174/ajnr.A3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thomalla G, Boutitie F, Fiebach JB, et al. Stroke with unknown time of symptom onset: baseline clinical and magnetic resonance imaging data of the first thousand patients in WAKE-UP (efficacy and safety of MRI-based thrombolysis in wake-up stroke: a randomized, double-blind, placebo-controlled trial. Stroke 2017;48:770–3. 10.1161/STROKEAHA.116.015233 [DOI] [PubMed] [Google Scholar]
- 126.Prasad K, Siemieniuk R, Hao Q, et al. Dual antiplatelet therapy with aspirin and clopidogrel for acute high risk transient ischaemic attack and minor ischaemic stroke: a clinical practice guideline. BMJ 2018;363:k5130 10.1136/bmj.k5130 [DOI] [PubMed] [Google Scholar]
- 127.Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 2019;380:1795–803. 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]



