Abstract
Background
Of the side effects of prostaglandin analogues (PGAs), uveitis and cystoid macular oedema (CME) have significant potential for vision loss based on postmarket reports. Caution has been advised due to concerns of macular oedema and uveitis. In this report, we researched and summarised the original data suggesting these effects and determined their incidence.
Methods
Preferred Reporting Items for Systematic review and Meta-Analyses guidelines were followed. Studies evaluating topical PGAs in patients with ocular hypertension or open angle glaucoma were included. MEDLINE, PubMed, EMBASE, CINAHL, Web of Science, Cochrane Library, LILACS and ClinicalTrials.gov were searched between 1946 and 2019. Experimental studies, animal studies and randomised studies with other intraocular pressure-lowering eye drops were excluded.
Results
214 studies (28 232 patients) met the inclusion criteria. Using prospective data, the incidence of uveitis and CME among PGA users were 62/28 232 (0.22%) and 25/28 232 (0.09%), respectively. A higher frequency of both uveitis and CME were found among latanoprost users compared with bimatoprost. There were 21 case studies reporting CME including 48 eyes in 43 patients. 47 of 48 eyes (97.9%) had previous incisional ocular surgery. 8 eyes were re-challenged, of which 7 (87.5%) recurred. 7 case studies reported uveitis in 15 eyes of 10 patients. 7 of 15 eyes (46.7%) were either pseudophakic or aphakic. 6 eyes were re-challenged, and all 6 (100%) recurred.
Conclusions
Cases of uveitis or CME revealed a confounding effect of ocular surgery, aphakia or subluxed intraocular lens. PGAs may be used in non-surgical patients without concern of causing CME or uveitis. The incidences of PGA-associated CME and uveitis are rare with limited prospective studies on the cause-effect relationship.
Keywords: glaucoma, macula, pharmacology, inflammation
Introduction
Endogenous prostaglandins (PGs) have a role in inflammatory mediation in the eyes, as they do in other parts of the body.1 In 1977, Camras et al found that low doses of topical prostaglandin analogues (PGAs) decreased intraocular pressure (IOP).1 2 In contrast, large quantities of PGAs infused into animal eyes led to ocular inflammation with breakdown of the blood-aqueous barrier. This prompted further studies, leading to the first PGA, latanoprost, being approved by the United States Food and Drug Administration in 1996. Clinical trials did not yield any serious complications and PGAs became popular due to having once-daily administration and few side effects. PGAs have emerged as the most potent IOP-lowering topical medication with bimatoprost reported as the most effective and unoprostone as the least effective.3
The specific mechanism of action of PGAs is not completely understood. It is known that they increase uveoscleral outflow and there is growing evidence that they also increase conventional outflow through Schlemm’s canal.4 The proposed mechanism is that PGAs bind to E-type prostanoid receptors and prostaglandin F receptors in ‘the ciliary muscle, resulting in ciliary muscle relaxation and increased aqueous humour outflow’. They also ‘induce[s] (matrix metalloproteinases) MMP-1, MMP-3 and MMP-9 expression in ciliary smooth muscle cells’ that break down collagens and other connective tissue structures in the ‘extracellular matrix in the ciliary muscle, iris root and sclera, reducing outflow resistance to fluid flow’.5 After being on the market, cases of uveitis and CME were reported after PGA use. Due to these studies, PGA drug labels include 1) intraocular inflammation (uveitis) and 2) CME, especially in aphakic patients, pseudophakic patients with a torn posterior lens capsule or in patients with known risk factors for macular oedema.6
The retrospective review of 94 patients by Warwar et al revealed 6.4% and 2.1% of patients developed anterior uveitis and cystoid macular oedema (CME), respectively, while being treated with latanoprost.7 We found few randomised controlled trials (RCTs) that study the relationship between PGs, CME and uveitis. As well, CME and uveitis are rarely reported as complications in RCTs. In RCT by Gandolfi et al comparing bimatoprost (n=119) and latanoprost (n=113) in patients with glaucoma and ocular hypertension, uveitis was reported in only one patient in each group.8 We compiled a systematic literature review to assess the relationship between PGAs, uveitis and CME to address the gap in evidence of these side effects. Current teaching advises caution in using PGAs in patients with uveitis and CME and further knowledge would allow clinicians to better weigh the benefits and risks of PGA administration across different patient profiles. Our primary study outcome is the incidence of uveitis or CME after PGA use in patients treated for ocular hypertension (OH) and open angle glaucoma (OAG).
Methods
The methodology we used closely adheres to the recommendations found in the Cochrane Handbook.9 The Preferred Reporting Items for Systematic review and Meta-Analyses guidelines were followed, including a 27-item checklist and a 4-phase flow diagram to optimise transparency and clarity of reporting.10 A meta-analysis was not completed. Our study adhered to the Declaration of Helsinki and informed consent was not required for our study.
Eligibility criteria for considering studies for this review
In order to be considered for this systematic review, all potentially eligible studies had to evaluate the use of topical PGAs in patients with OH or OAG. Definitions for OH and OAG in this study follow the American Academy of Ophthalmology (AAO) preferred practice patterns. Experimental studies were excluded from this review. To isolate the side-effect profile of PGAs, studies that evaluate topical PGA in combination with other topical medications such as carbonic anhydrase inhibitors, beta-blockers, miotics and alpha-agonists were excluded. This restriction was not applied for case studies. No restrictions were placed with regard to age of study population, study design and type of publication. For convenience, only English articles were used.
Search methods for identifying studies
A comprehensive search strategy was developed with the help of a medical librarian using varying combinations of OH, OAG, PGA, CME and uveitis (see online supplementary appendix 1). We searched MEDLINE, PubMed, EMBASE, CINAHL, Web of Science, Cochrane Library, LILACS and ClinicalTrials.gov from 1946 to 2019. The initial search for 1946 to 2016 was performed on 22 April 2016. The updated search for 2016–2019 was performed on 17 June 2019.
bjophthalmol-2019-315280s001.pdf (1.1MB, pdf)
Study selection
For studies published between 1946 and 2016, the title, abstract and full-text screening were conducted by two authors (JH and BH) independently. Any disagreements were resolved with consensus of all authors. For studies published between 2016 and 2019, the title, abstract and full-text screening were conducted by one author (JTV).
Data collection
Data from all eligible articles were extracted by two independent authors (JH and BH) using a predefined data extraction form for articles obtained from 1946 to 2016 (see online supplementary appendix 2). The data extraction for articles from 2016 to 2019 was collected by JTV. A sample of eligible studies was piloted to test the completeness of the data extraction form. Further data extraction was performed by JH and JTV for case studies using a different template (see online supplementary appendix 3). Any changes were reflected in the final manuscript.
Summary measures, data synthesis and analysis
Information summarised included study design distribution, source of funding and length of follow-up (table 1). The agents used in these studies were tabulated (table 2). Occurrences of uveitis and CME were summarised for prospective RCTs and their incidences were calculated (table 3). A Fischer’s exact test was performed to assess the significance of uveitis and CME frequencies among latanoprost and bimatoprost users. A summary of each case study was created that included the PGA used, the number of eyes and patients, clinical history, time of onset to CME or uveitis, postoperative status, whether the agent was re-challenged and if so whether the adverse event recurred and the reversibility of the adverse event (online supplementary appendices 4, 5).
Table 1.
Summary of studies included in the systematic review, including description of study design, number of studies, number of patients, source of funding and length of follow-up
| Study design | |||
| RCT | Observational study | Case study | |
| Number of included studies n (%) | 99 (46.3) | 87 (40.6) | 28 (13.1) |
| Number of patient n (%) | 28 232 (33.8) | 55 250 (66.1) | 53 (0.1) |
| Source of funding n (%) | |||
| None | 5 (0.05) | 7 (0.08) | 5 (0.18) |
| Industry | 54 (0.55) | 41 (0.47) | 0 |
| Public | 0 | 1 (0.01) | 4 (0.14) |
| Unspecified | 40 (0.40) | 38 (0.44) | 19 (0.68) |
| Length of follow-up in months | |||
| Mean (SD) | 4.85 (5.34) | 8.26 (11.58) | 5.16 (4.28) |
| Range | 0.2–36 | 0.5–60 | 0.5–19 |
RCT, randomised controlled trial.
Table 2.
Agents used in the included studies (99 RCTs, 87 observational studies and 28 case studies)
| Agent | Number of groups (%) |
| Latanoprost | 145 (46.5) |
| Bimatoprost | 74 (23.7) |
| Travoprost | 52 (16.7) |
| Tafluprost | 21 (6.7) |
| Latanoprostene Bunod | 9 (2.9) |
| Unoprostone | 7 (2.2) |
| ONO 9054 | 4 (1.3) |
Number does not add to a total of 136 because there may be multiple groups in a single study (total=183).
RCT, randomised controlled trial.
Table 3.
Overall estimate of incidence of anterior uveitis and CME based on prospective RCT data
| Prostaglandin agent | Number at risk | Uveitis n (%) |
CME n (%) |
Surgical-related CME n (%) |
| Latanoprost | 12 170 | 59 (0.48) | 17 (0.14) | 6 (0.05) |
| Bimatoprost | 6746 | 1 (0.01) | 0 | 0 |
| Tafluprost | 4605 | 0 | 0 | 0 |
| Travoprost | 2830 | 0 | 0 | 0 |
| Latanoprostene Bunod | 1391 | 0 | 0 | 0 |
| Unoprostone | 118 | 0 | 0 | 0 |
| ONO 9054 | 93 | 2 (2.15) | 0 | 0 |
| Bimatoprost+travoprost | 81 | 0 | 0 | 0 |
| Latanoprost+travoprost | 75 | 0 | 0 | 2 (2.67) |
| Latanoprost+tafluprost | 67 | 0 | 0 | 0 |
| Latanoprost+unoprostone | 56 | 0 | 0 | 0 |
Using prospective data, the incidence of uveitis and CME among PGA users was 62/28 232 (0.22%) and 25/28 232 (0.09%), respectively.
Bimatoprost 0.3 mg/mL was used in the patient who developed uveitis.
CME, cystoid macular oedema; PGA, prostaglandin analogue; RCT, randomised controlled trial.
Results
The largest number of studies in the systematic review were RCTs followed by observational studies and case studies. About half of these studies (46.3%) were RCTs and a significant portion were funded by industry (55.0% of the RCTs). The follow-up time was the shortest for RCTs (4.9 months), followed by case studies (5.2 months) and observational studies (8.3 months).
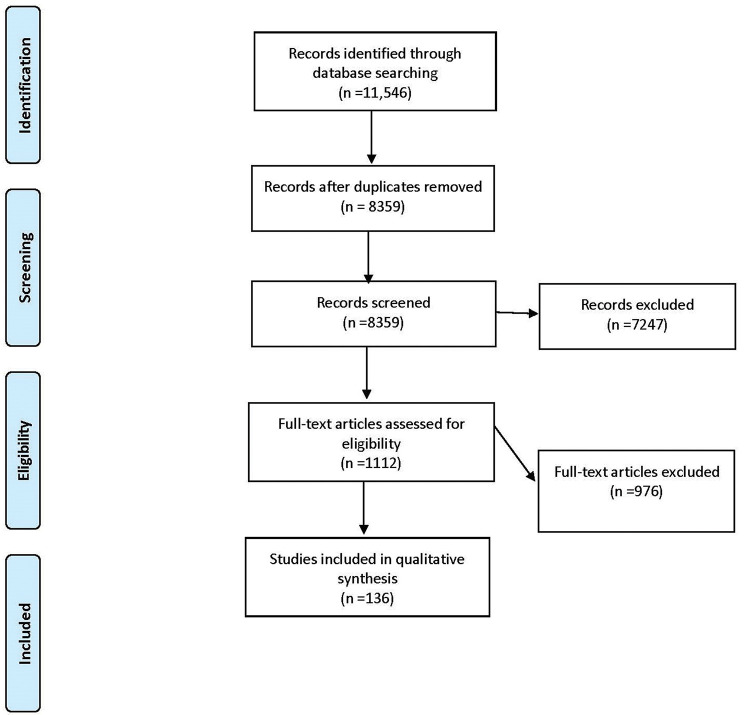
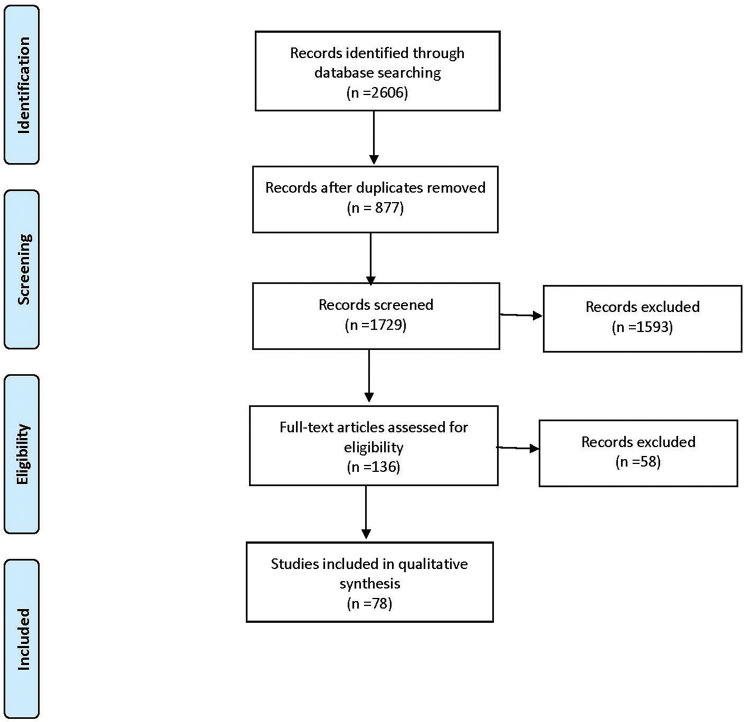
A total of 214 studies and 28 232 patients met the inclusion criteria (figures 1 and 2). Basic information is shown in table 1. This study included 99 (46.3%) RCTs, 87 (40.6%) observational studies and 28 (13.1%) case studies. Funding for these studies was from industry for 54 (55.0%) of RCTs, 41 (47.0%) of observational studies and 0 case studies. The mean follow-up time was 4.85 months for RCTS, 8.26 months for observational studies and 5.16 months for case studies. The PGA used in these studies were bimatoprost (74), latanoprost (145), latanoprostene bunod (9), ONO 9054 (4), tafluprost (21), travoprost (52), and unoprostone (7) as shown in table 2.
Figure 1.
Flow diagram of systematic review based on the Preferred Reporting Items for Systematic review and Meta-Analyses guidelines (database search performed on 22 April 2016).15
Figure 2.
Flow diagram of systematic review based on the Preferred Reporting Items for Systematic review and Meta-Analyses guidelines (database search performed on 17 June 2019).15
Using prospective data, the incidence of uveitis and CME among PGA users in this specific population was 62/28 232 (0.22%) and 25/28 232 (0.09%), respectively as shown in table 3. Specifically, there were 62 cases of uveitis following bimatoprost (1), latanoprost (59) and ONO 9054 (2) use. There were 25 cases of CME, following use of latanoprost (23) and latanoprost with travoprost (2). The incidence of uveitis was 0.48% among latanoprost users and 0.01% among bimatoprost users. A Fischer’s exact test demonstrated a significant difference (p<0.05) in uveitis rates between latanoprost and bimatoprost (0.48% and 0.01%) shown in online supplementary appendix 4. The results were also significant (p<0.05) for CME between latanoprost and bimatoprost (0.19% and 0.00%) shown in online supplementary appendix 5. The median follow-up duration for latanoprost was 13 weeks (ranging from 4 to 156 weeks) and for bimatoprost was 16 weeks (ranging from 8 to 52 weeks).
There were 21 case studies reporting CME including 48 eyes in 43 patients (online supplementary appendix 4). Forty-seven of 48 eyes (97.9%) were either pseudophakic, aphakic or had a subluxed intraocular lens (IOL). Forty-seven of 48 eyes (97.9%) had previous incisional ocular surgery. The median time of onset of CME after introduction of the PGA was 30 days (range 7–365 days). Ten of 48 (20.8%) eyes were postoperative findings. Eight eyes were re-challenged, of which seven of eight (87.5%) recurred. All cases were reversible.
There were 7 case studies reporting uveitis, including 15 eyes in 10 patients (online supplementary appendix 5). Seven of 15 eyes (46.7%) were either pseudophakic or aphakic, but no patients had recent surgery. Eight of 15 eyes (53.3%) did not have incisional surgery, 5 of which had no ocular interventions aside from medication and 3 of which had laser surgery (2 had laser trabeculoplasty and 1 had laser photocoagulation for macroaneurysm). The median time of onset of uveitis after introduction of the PGA was 6 days (range 1–61 days). Six eyes were re-challenged, of which six of six (100%) recurred. All cases were reversible. Full details of each case study can be found in online supplementary appendices 6 and 7.
Discussion
In our systematic review, the prevalence of uveitis and CME in the context of using a PGA was determined and the relationship between PGAs, CME and uveitis was explored. Using prospective data, the incidence of uveitis or CME among PGA users was very low, 62/28 232 (0.22%) and 25/28 232 (0.09%), respectively. The studies that reported uveitis did not comment on whether the affected eyes were pseudophakic or phakic. For the patients with CME, 6/17 (35.2%) patients were pseudophakic, 4/17 (23.5) had previous history of uveitis and 2/17 (11.8%) patients had previous retinal vein occlusions. These factors may contribute to a higher susceptibility of developing CME.
Of note, the frequency was significant (p<0.05) for uveitis comparing latanoprost and bimatoprost (0.48% and 0.01%, respectively). The frequency was also significant (p<0.05) for CME between latanoprost and bimatoprost (0.19% and 0.00%). The results suggest that the uveitis and CME rates for latanoprost are higher compared with other PGAs. The median follow-up duration for latanoprost (13 weeks, ranging from 4 to 156 weeks) and bimatoprost (16 weeks, ranging from 8 to 52 weeks) were similar. The duration of follow-up is unlikely to account for the difference in complications between latanoprost and bimatoprost since the lengthier studies of latanoprost did not report a greater proportion of complications. The cause for this difference is unclear and further studies are required to control for confounding factors that may contribute to this finding, such as previous ocular surgery, pseudophakic eyes or history of previous inflammation.
After being on the market, several studies raised concerns of uveitis and CME associated with PGA use. These included animal studies that showed ocular inflammation when large quantities of prostaglandins were infused into rabbit eyes as well as numerous case reports of CME and uveitis.1 It is unclear of whether PG caused complications or developed de novo. The incidences may reflect the rate of uveitis and CME in the general population.
In the case reports, a very high percentage of patients who developed CME had recent ocular surgery, aphakia or subluxed IOL (98%). This suggests that surgery may be the cause of CME rather than PGA use. Alternatively, pseudophakic eyes may have increased risk of PGA-induced CME due to a reduction of physical barrier and increase in posterior spread of the PGA.
The median time of onset from initiation of PGA and macular oedema was long (30 days), which reduces the likelihood of PGA use being the main contributing factor.
In the 7 cases of reported uveitis, 7 of 15 eyes (46.7%) were pseudophakic or aphakic and none of the patients had recent surgery. The median time of onset was 6 days after PGA use. Surgery appears to be less of a confounding factor for these cases. A direct association with PGAs and uveitis is more likely compared with CME, given the shorter time of onset and lack of recent surgical history. The higher rate of incidence and rate of recurrence on re-challenge test supports this, however, the data come from only seven case studies. In the 21 case studies reporting CME after PGA use, 8 eyes were re-challenged, of which 87.5% recurred. In the studies reporting uveitis, six eyes were re-challenged, of which 100% recurred.
Recent studies consistently cite retrospective review of 94 patients by Warwar et al, which revealed 6.4% and 2.1% of patients developed anterior uveitis and CME, respectively while being treated with latanoprost.7 Although this study raised concerns, the study sample has few patients compared with large RCTs. There also may be selection bias in the study since a continuous sample was not obtained for the patient population. In a study by Markomichelakis et al, latanoprost was compared with fixed combination timolol plus dorzolamide and found no significant differences in the relapse rates of uveitis between the groups.11 The results of the study do not support a causal relationship between PGA and uveitis.
The Preferred Practice Pattern from AAO lists active uveitis as a potential contraindication of PGA.12 However, there is a lack of evidence to establish a cause-effect relationship of PGA use and worsening uveitis or CME. Furthermore, the Canadian Ophthalmology Society (COS) lists side effects of PGAs to include CME in aphakic and pseudophakic patients and possible anterior uveitis, which is more consistent with our findings in this study.13
The strength of the study is the inclusion of all RCTs, case series and case reports with pooled statistics from 1946 to 2019. A limitation of the study is that most of the data are based on retrospective studies and could include bias. Enrolment biases of original RCTs and observational studies cannot be controlled for. Reporting bias in the literature is another limitation of this study. Most studies had durations between a few months to a year. The systematic review was conducted in only English language literature.
Based on this systematic review, current evidence from all available RCTs, observational studies and case reports reveals a paucity of published reports to show a cause-effect relationship of CME or uveitis associated with PGA use. PGAs could be used in the majority of non-surgical patients without concern of causing CME or uveitis, and re-challenge could be considered to help establish a cause-effect relationship. The case studies reported reversibility in all eyes affected with CME or uveitis either after discontinuation of PGAs alone or with medical treatment. A retrospective comparative case series by Chang et al of 84 consecutive patients with uveitis and raised IOP demonstrated there were no significant difference in frequency of anterior uveitis or visually significant CME during PGA treatment compared with non-PGA treatment.14 This raises the question of whether we should continue to teach that PGAs should be avoided in patients with active uveitis. Further prospective studies are needed to establish whether CME or uveitis are associated with PGAs.
Footnotes
Correction notice: This article has been corrected since it was published Online First. Minor formatting has been corrected.
Contributors: Conception and design: CG. Data collection: Hu, Hong, Vu. Analysis and interpretation: Hu, Hong, Vu. Overall responsibility: Hu, Hong, Gottlieb, Vu.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Eakins KE. Prostaglandin and non-prostaglandin mediated breeakdown of the blood-aqueous barrier. Exp Eye Res 1977;25(Suppl):483–98. 10.1016/s0014-4835(77)80043-2 [DOI] [PubMed] [Google Scholar]
- 2.Camras CB, Bito LZ, Eakins KE. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Invest Ophthalmol Vis Sci 1977;16:1125–34. [PubMed] [Google Scholar]
- 3.Li T, Lindsley K, Rouse B, et al. . Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology 2016;123:129–40. 10.1016/j.ophtha.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim KS, Nau CB, O'Byrne MM, et al. . Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology 2008;115:790–5. 10.1016/j.ophtha.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther 2014;30:102–9. 10.1089/jop.2013.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pifer Xalatan. Woodstock, IL: Catalent pharma solutions, 2012. [Google Scholar]
- 7.Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. experience and incidence in a retrospective review of 94 patients. Ophthalmology 1998;105:263–8. 10.1016/s0161-6420(98)92977-3 [DOI] [PubMed] [Google Scholar]
- 8.Gandolfi S, Simmons ST, Sturm R, et al. . Three-Month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther 2001;18:110–21. 10.1007/BF02850299 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Green S, Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2011. [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markomichelakis NN, Kostakou A, Halkiadakis I, et al. . Efficacy and safety of latanoprost in eyes with uveitic glaucoma. Graefes Arch Clin Exp Ophthalmol 2009;247:775–80. 10.1007/s00417-009-1036-3 [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Ophthalmology Glaucoma Panel Preferred practice pattern guidelines. primary open-angle glaucoma. San Francisco, CA: American Academy of Ophthalmology, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee, Canadian Ophthalmological Society . Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol 2009;44 Suppl 1:S7–54. 10.3129/cjo44s1 [DOI] [PubMed] [Google Scholar]
- 14.Chang JH, McCluskey P, Missotten T, et al. . Use of ocular hypotensive prostaglandin analogues in patients with uveitis: does their use increase anterior uveitis and cystoid macular oedema? Br J Ophthalmol 2008;92:916–21. 10.1136/bjo.2007.131037 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2019-315280s001.pdf (1.1MB, pdf)