Four Providencia rettgeri isolates and one Providencia stuartii isolate were obtained from urine samples of five patients in 2018 in Japan. All of the isolates were resistant to imipenem and meropenem, and three were highly resistant to both carbapenems, with MICs of 512 μg/ml. The three highly carbapenem-resistant isolates harbored blaIMP-70, encoding a variant of IMP-1 metallo-β-lactamase with two amino acid substitutions (Val67Phe and Phe87Val), and the other two harbored blaIMP-1 and blaIMP-11, respectively.
KEYWORDS: Providencia rettgeri, Providencia stuartii, IMP-70, metallo-β-lactamase
ABSTRACT
Four Providencia rettgeri isolates and one Providencia stuartii isolate were obtained from urine samples of five patients in 2018 in Japan. All of the isolates were resistant to imipenem and meropenem, and three were highly resistant to both carbapenems, with MICs of 512 μg/ml. The three highly carbapenem-resistant isolates harbored blaIMP-70, encoding a variant of IMP-1 metallo-β-lactamase with two amino acid substitutions (Val67Phe and Phe87Val), and the other two harbored blaIMP-1 and blaIMP-11, respectively. Whole-genome sequencing revealed that an isolate harbored two copies of blaIMP-1 on the chromosome and that the other four harbored a copy of blaIMP-11 or blaIMP-70 in a plasmid. Expression of blaIMP-70 conferred carbapenem resistance in Escherichia coli. Recombinant IMP-70 and an IMP-1 variant with Val67Phe but without Phe87Val had significant higher hydrolytic activities against meropenem than recombinant IMP-1, indicating that an amino acid substitution of Val67Phe affects increased activities against meropenem in IMP-70. These results suggest that Providencia spp. become more highly resistant to carbapenems by acquisition of two copies of blaIMP-1 or by mutation of blaIMP genes with amino acid substitutions, such as blaIMP-70.
INTRODUCTION
Providencia is a genus of Gram-negative bacteria belonging to the family Enterobacteriaceae. Among Providencia spp., Providencia rettgeri and Providencia stuartii are the common causes of hospital-acquired infections (1, 2). They mainly cause travelers’ diarrhea and catheter-related urinary tract infections (3, 4). However, there are several case reports of other infections with P. rettgeri and P. stuartii, including neonatal sepsis caused by P. rettgeri (5) and conjunctivitis (6), meningitis after neurosurgery (7), and endocarditis (8) caused by P. stuartii, indicating that these pathogens have the potency to cause serious infections.
P. rettgeri clinical isolates producing IMP-1 metallo-β-lactamase (MBL) were first obtained in 2000 during a laboratory-based surveillance in western Japan (9). A P. stuartii clinical isolate producing VIM-19 MBL was first reported in 2008 in Algeria (10). Since then, there have been at least 12 reports of carbapenem-resistant P. rettgeri isolates and 5 reports of carbapenem-resistant P. stuartii isolates (Table 1). All of these isolates produced MBLs, and the majority of carbapenem-resistant P. rettgeri isolates produced IMP-type or NDM-type MBLs (Table 1), i.e., IMP-type MBL-producing isolates were detected in Japan, the Republic of Korea, and the United States, and NDM-type MBL-producing isolates were detected worldwide. The majority of carbapenem-resistant P. stuartii isolates, however, produced VIM-type MBLs, and these were detected in Algeria and Greece (Table 1). There is a report describing an NDM-type MBL-producing P. stuartii isolate in Afghanistan (Table 1).
TABLE 1.
Previous reports of P. rettgeri and P. stuartii isolates producing MBLs
| Species | Metallo-β-lactamase | Location of MBL-encoding genea | Yr(s) of isolation | Country of isolation | Reference |
|---|---|---|---|---|---|
| P. rettgeri | IMP-1 | – | 2000 | Japan | 9 |
| IMP-1 | Plasmid (2.5 kb) | 2002 | Japan | 30 | |
| IMP-27 | Plasmid | 2016 | USA | 31 | |
| IMP-27 | – | 2018 | Republic of Korea | 32 | |
| NDM-1 | – | 2008, 2011 | Israel | 33 | |
| NDM-1 | Plasmid | 2012 | Nepal | 34 | |
| NDM-1 | Plasmid (178 kb) | 2012 | Mexico | 35 | |
| NDM-1 | Plasmid (190 kb) | 2014 | China | 36 | |
| NDM-1 | – | 2017 | Bulgaria | 37 | |
| NDM | – | 2013 | Brazil | 38 | |
| NDM-18 | Plasmid | 2017 | South Africa | 39 | |
|
P. stuartii |
VIM-2 | – | 2004 | Republic of Korea | 40 |
| NDM-1 | Plasmid (178kb) | 2012 | Afghanistan | 41 | |
| VIM-1 | – | 2011 | Greece | 42 | |
| VIM-1 | Plasmid (180 kb, IncA/C) | 2012 | Greece | 43 | |
| VIM-1 | Plasmid | 2013 | Greece | 44 | |
| VIM-19 | Plasmid (180 kb) | 2008 | Algeria | 10 |
A dash (–) indicates that the location of MBL-encoding genes was unspecified.
IMP-type MBL was first detected in a clinical isolate of Pseudomonas aeruginosa in Japan in 1991 (11). Since then, IMP-type MBL producers have been spreading nationwide in Japan and in east and southeast Asian countries, although they have been globally detected (12).
This report describes the detection of 5 clinical isolates of carbapenem-resistant P. rettgeri and P. stuartii producing IMP-type metallo-β-lactamase in 3 hospitals located in different regions in Japan.
RESULTS
Drug susceptibility.
As shown by the drug susceptibility profiles of 5 Providencia sp. isolates shown in Table 2, P. rettgeri BML2496 was susceptible to amikacin and aztreonam but resistant to amoxicillin, amoxicillin-clavulanate, ceftazidime, cephradine, ciprofloxacin, and meropenem. The MICs of imipenem and meropenem were 16 and 64 μg/ml, respectively. P. rettgeri BML2531 showed a similar profile of drug susceptibility to that of BML2496 (Table 2). However, against P. rettgeri BML2526 and BML2576, MICs of imipenem and meropenem (512 μg/ml) were much higher than those against BML2496 and BML2531 (Table 2). BML2526 and BML2576 were highly resistant to ciprofloxacin (MICs of 512 μg/ml and 256 μg/ml, respectively). P. stuartii BML2537 was intermediate to amikacin but resistant to amoxicillin, amoxicillin-clavulanate, aztreonam, ceftazidime, cephradine, ciprofloxacin, and meropenem, and MICs of imipenem and meropenem (>512 μg/ml and 512 μg/ml, respectively) were much higher (Table 2). For all isolates tested, arbekacin MICs were low (2 to 8 μg/ml), but colistin MICs were high (64 μg/ml) (Table 2).
TABLE 2.
Drug susceptibility profiles of Providencia sp. clinical isolates
| Antibiotic(s) | MIC (μg/ml) of antibiotic against: |
||||
|---|---|---|---|---|---|
|
P. rettgeri |
P. stuartii |
||||
| BML2496 | BML2531 | BML2526 | BML2576 | BML2537 | |
| Arbekacin | 4 | 8 | 2 | 8 | 2 |
| Amikacin | 8 | 4 | 32 | 32 | 32 |
| Amoxicillin | 512 | >512 | >512 | >512 | >512 |
| Amoxicillin-clavulanatea | 256 | 128 | 128 | 128 | 512 |
| Aztreonam | 2 | <0.25 | 8 | 8 | 32 |
| Ceftazidime | 128 | 256 | 128 | 128 | 512 |
| Cephradine | >512 | >512 | >512 | >512 | >512 |
| Ciprofloxacin | 32 | 64 | 512 | 256 | 16 |
| Colistin | >64 | >64 | >64 | >64 | >64 |
| Imipenem | 16 | 32 | 512 | 512 | >512 |
| Meropenem | 64 | 32 | 512 | 512 | 512 |
The compound ratio of amoxicillin versus clavulanate is 1:5 (wt/wt).
Drug-resistant genes.
All five isolates had several genes and mutations associated with resistance to aminoglycosides, β-lactams, and fluoroquinolones (Table 3). All had one or two of five genes encoding aminoglycoside-modifying enzymes, namely aac(2′)-Ia, aac(6′)-Iae, aac(6′)-Ib4, aac(6′)-Il, and/or aadA1 (Table 3). The aac(6′)-Iae gene may be associated with the increased susceptibility to amikacin of BML2526, BML2537, and BML 2576 (Table 3). Isolates had one of three genes encoding metallo-β-lactamases, namely blaIMP-1, blaIMP-11, or blaIMP-70 (Table 3). In addition, they had one or two of four other genes encoding β-lactamases, namely blaCTX-M-2, blaOXA-1, blaOXA-2, and/or blaTEM-1B (Table 3). All isolates had three or four mutations in the quinolone resistance-determining regions of gyrA, parC, and gyrB with amino acid substitutions that included Ser83Ile, Asp87Ala, and Asp87Glu in gyrA; Ser87Ile and Ser87Arg in parC; and Glu468Asp in gyrB (Table 3).
TABLE 3.
Genetic characterization of carbapenem-resistant Providencia sp. isolates
| Isolate | Genome | Plasmid type | Size (bp) | GC content (%) | Antibiotic resistance gene(s) encoding: |
Amino acid substitution(s) in quinolone resistance gene: |
|||
|---|---|---|---|---|---|---|---|---|---|
| Aminoglycoside(s) | β-lactam(s) | GyrA | ParC | GyrB | |||||
| P. rettgeri BML2496 | Chromosome | 4,651,003 | 49.6 | aac(6')-Ib4 | blaIMP-1, blaOXA-1 | Ser83Ile, Asp87Ala | Ser87Ile | ||
| P. rettgeri BML2526 | Chromosome | 4,342,905 | 51.0 | aadA1 | |||||
| Plasmid | IncA/C2 | 204,791 | 50.2 | aac(6')-Iae | blaIMP-70, blaCTX-M-2, blaTEM-1B | Ser83Ile, Asp87Glu | Ser87Ile | Glu468Asp | |
| Plasmid | Nontypeable | 71,575 | 50.5 | ||||||
| P. rettgeri BML2531 | Chromosome | 4,696,377 | 50.1 | ||||||
| Plasmid | IncT | 84,930 | 50.3 | aac(6')-Il | blaIMP-11, blaOXA-2 | Ser83Ile, Asp87Glu | Ser87Ile | ||
| P. rettgeri BML2576 | Chromosome | 4,351,300 | 51.0 | aadA1 | |||||
| Plasmid | IncA/C2 | 204,791 | 50.2% | aac(6')-Iae | blaIMP-70, blaCTX-M-2, blaTEM-1B | Ser83Ile, Asp87Glu | Ser87Ile | Glu468Asp | |
| Plasmid | Nontypeable | 71,575 | 50.5% | ||||||
| P. stuartii BML2537 | Chromosome | 4,418,649 | 49.8% | aac(2')-Ia | |||||
| Plasmid | IncA/C | 152,754 | 50.0% | aac(6')-Iae | blaIMP-70 | Ser83Ile, Asp87Glu | Ser87Arg | ||
| Plasmid | Nontypeable | 30,292 | 50.9% | blaCTX-M-2 | |||||
Complete whole-genome sequences.
Analysis using MiSeq and MinION platforms revealed the complete whole-genome sequences of all five isolates (Table 3). Four isolates of P. rettgeri had a 4.3- to 4.7-Mbp chromosome with a GC content of 49.6 to 51.0%, and an isolate of P. stuartii had a 4.4-Mbp chromosome with a GC content of 49.8% (Table 3). BML2496 did not have any plasmid, but the others had one or two plasmid(s) of 30 to 200 kbp (Table 3). BML2526 had two plasmids of 70 kbp and 200 kbp, and BML2576 had two with the same sizes as those of BML2526. The sequences of the two 70-kbp and 200-kbp plasmids were identical between the two isolates.
Locations of blaIMP genes and their genetic environments.
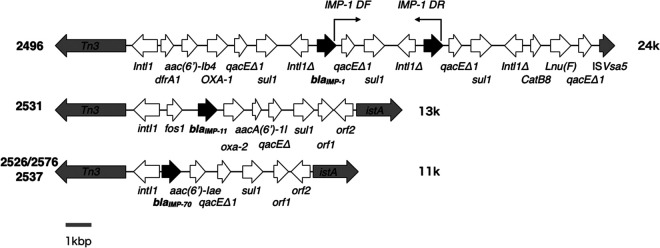
The blaIMP-1 gene was located on the chromosome of BML2496 (Table 3). The blaIMP-11 gene was located in an 85-kbp plasmid of BML2531. The blaIMP-70 gene was located in a 200-kbp plasmid of BML2526/BML2576 and in a 150-kbp plasmid of BML2537 (Table 3).
Two copies of blaIMP-1 were detected on the chromosome of BML2496, and the two copies existed in a row at nucleotide positions 3,384,049 to 3,384,789 and 3,387,611 to 3,387,958, i.e., the following genetic structure of int1Δ-blaIMP-70-qacEΔ1-sul1 was repeated (Fig. 1). To confirm the existence of the two copies of blaIMP-1, PCR was conducted using a primer set (IMP-1 DF and IMP-1 DR) targeting the two copies, as shown in Fig. 1. Consequently, a 3.5-kbp PCR product was detected as expected based on the whole-genome sequence of BML2496 (Fig. 1).
FIG 1.
Genomic environments of blaIMP-1 and blaIMP-70 in P. rettgeri and P. stuartii clinical isolates. Genes are represented as arrows, which indicate their transcription orientations and relative lengths. MBL genes, tnp genes, and truncated genes are shown as black arrows, gray arrows, and Δ, respectively. orf1 is a gene encoding a hypothetical protein, and orf2 is a gene encoding an ATP-binding protein.
Drug susceptibility of Escherichia coli DH5α expressing blaIMP-1, blaIMP-70, and two variants of blaIMP-1.
IMP-70 is a variant of IMP-1 with two amino acid substitutions, Val67Phe and Phe87Val (GenBank accession no. LC348383.1). A variant of blaIMP-1 encoding an amino acid substitution of Val67Phe was blaIMP-10 (accession no. NG_049173). Another variant of blaIMP-1 encoding an amino acid substitution of Phe87Val [blaIMP-1(Phe87Val)] was prepared as described in Materials and Methods. E. coli DH5α expressing blaIMP-1, blaIMP-10, blaIMP-1(Phe87Val), and blaIMP-70 showed significantly increased MICs for all antibiotics tested, including four carbapenems, compared with those of a vector control (Table 4). Compared with E. coli expressing blaIMP-1, E. coli expressing blaIMP-70 showed slightly, but not significantly, increased MICs of doripenem and meropenem, while it showed the same MICs of imipenem and panipenem (Table 4). However, E. coli expressing blaIMP-10 showed a significantly higher MIC of doripenem and a slightly increased MIC of meropenem (Table 4). E. coli expressing blaIMP-1(Phe87Val), in contrast, showed no increased MIC for all carbapenems tested (Table 4).
TABLE 4.
Drug susceptibility profiles of E. coli isolates expressing IMP-1, IMP-10, a variant of IMP-1 with an amino acid substitution (Phe87Val), and IMP-70
| Antibiotic(s) | Antibiotic MIC (μg/ml) against: |
||||
|---|---|---|---|---|---|
| E. coli DH5α (pHSG398) | E. coli DH5α (pHSG398/IMP-1) | E. coli DH5α (pHSG398/IMP-10)a | E. coli DH5α [pHSG398/IMP-1(Phe87Val)] | E. coli DH5α (pHSG398/IMP-70)b | |
| Amoxicillin | 4 | 512 | 32 | 32 | 16 |
| Amoxicillin-clavulanatec | 4 | 128 | 32 | 16 | 8 |
| Ceftazidime | ≤0.25 | 128 | 256 | 16 | 64 |
| Cephradine | 16 | 256 | 128 | 128 | 64 |
| Doripenem | ≤0.06 | 2 | 8 | 1 | 4 |
| Imipenem | 0.125 | 1 | 1 | 1 | 1 |
| Meropenem | ≤0.06 | 4 | 8 | 2 | 8 |
| Panipenem | 0.125 | 2 | 2 | 1 | 2 |
IMP-10 is a variant of IMP-1 with an amino acid substitution of Val67Phe.
IMP-70 is a variant of IMP-1 with two amino acid substitutions, Val67Phe and Phe87Val.
The compound ratio of amoxicillin versus clavulanate is 1:5 (wt/wt).
Enzymatic activities of IMP-1, IMP-70, and two variants of IMP-1.
IMP-70 is a variant of IMP-1 with Val67Phe and Phe87Val. Two variants of IMP-1 with an amino acid substitution of Val67Phe (IMP-10) and that of Phe87Val [IMP-1(Phe87Val)] were prepared as described in Materials and Methods. Enzymatic activities of recombinant IMP-70, IMP-10, and IMP-1(Phe87Val) were compared with those of recombinant IMP-1. IMP-70 and IMP-10 showed greater hydrolytic activities than IMP-1 against meropenem, i.e., the kcat/Km values of IMP-70 and IMP-10 were 2.3- and 3.4-fold higher, respectively, than those of IMP-1. However, IMP-70 showed similar enzyme activities against doripenem, imipenem, and panipenam to those of IMP-1 (Table 5). IMP-1(Phe87Val) showed similar or reduced enzymatic activities against all carbapenems tested (Table 5).
TABLE 5.
Kinetic parameters of β-lactamases IMP-1 and IMP-70 with substrates
| Substrate | Kinetic parameter for β-lactamase: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Km (μM)a
|
Kcat (s−1)a
|
Kcat/Km (μM−1 · s−1)a
|
||||||||||
| IMP-1 | IMP-10 | IMP-1(F87V) | IMP-70 | IMP-1 | IMP-10 | IMP-1(F87V) | IMP-70 | IMP-1 | IMP-10 | IMP-1(F87V) | IMP-70 | |
| Penicillin G | 941 ± 53 | 305 ± 17 | 818 ± 48 | 203 ± 6 | 390 ± 11 | 14 ± 1 | 13 ± 1 | 7 ± 0 | 0.42 | 0.047 | 0.016 | 0.036 |
| Ceftazidime | 71 ± 3 | 259 ± 5 | 363 ± 5 | 106 ± 6 | 7 ± 0 | 43 ± 1 | 4 ± 0 | 2 ± 0 | 0.1 | 0.17 | 0.0097 | 0.021 |
| Doripenem | 32 ± 3 | 10 ± 1 | 2626 ± 61 | 27 ± 0 | 4 ± 0 | 8 ± 0 | 239 ± 5 | 5 ± 0 | 0.13 | 0.82 | 0.091 | 0.18 |
| Imipenem | 205 ± 14 | 472 ± 18 | 534 ± 26 | 88 ± 6 | 46 ± 2 | 112 ± 3 | 92 ± 3 | 21 ± 0 | 0.23 | 0.24 | 0.17 | 0.24 |
| Meropenem | 40 ± 2 | 79 ± 3 | 1799 ± 67 | 36 ± 2 | 6 ± 0 | 40 ± 2 | 310 ± 11 | 13 ± 0 | 0.15 | 0.51 | 0.17 | 0.35 |
| Panipenem | 60 ± 6 | 46 ± 2 | 744 ± 19 | 83 ± 6 | 24 ± 1 | 16 ± 0 | 178 ± 4 | 19 ± 1 | 0.4 | 0.35 | 0.24 | 0.23 |
Km and kcat were calculated as means ± SD from three independent experiments.
Production of IMP-type MBL in clinical isolates of P. rettgeri and P. stuartii.
As shown by Western blotting in Fig. 2, all five isolates tested (BML2496, BML2526, BML2531, BML2537, and BML2576) produced IMP-type MBLs (IMP-1 was produced by BML2496, and IMP-70 was produced by the other three isolates). P. rettgeri BML2496, an IMP-1 producer, produced the largest amount of IMP-type MBL among all isolates tested (Fig. 2). P. rettgeri BML2526 and BML2576 and P. stuartii BML2537 produced relatively large amounts of IMP-type MBLs, whereas BML2531 produced a smaller amount of IMP-type MBL (IMP-11) than did the other four isolates tested. IMP-type MBL (IMP-11) produced by BML2531 was detected as a band with a lower molecular weight than those of other bands produced by the remaining four isolates, which correspond to their molecular weights calculated with amino acid sequences (27.06 kDa and 27.12 kDa, respectively).
FIG 2.
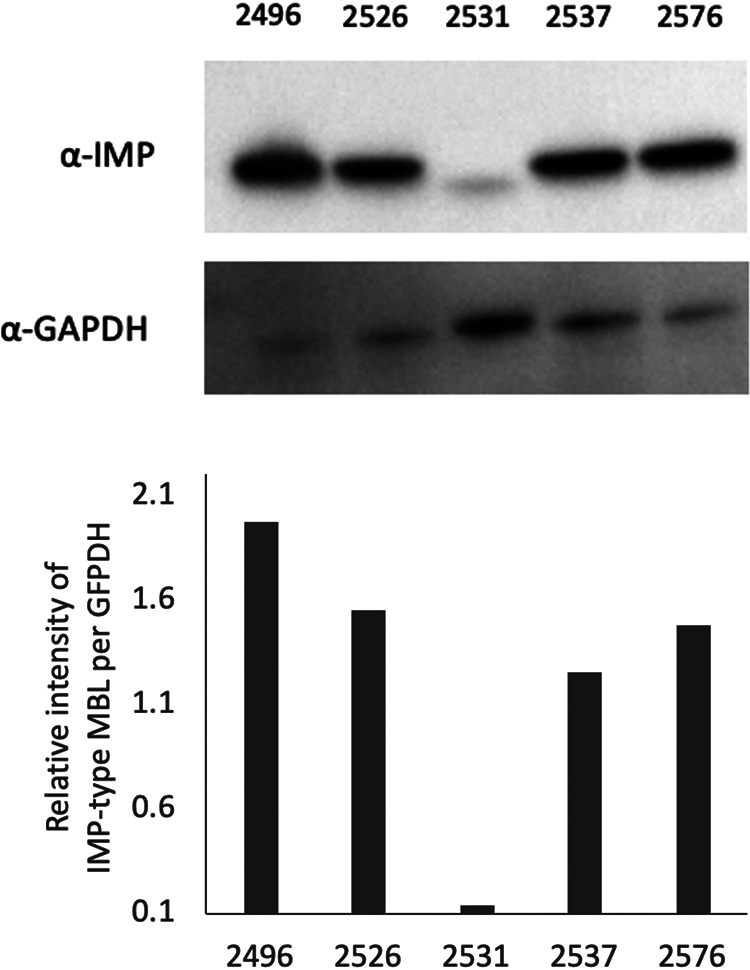
IMP-type metallo-β-lactamase expression in carbapenem-resistant clinical isolates of P. rettgeri and P. stuartii. Bacterial cells of P. rettgeri BML2496, BML2526, BML2531,and BML2576 and of P. stuartii BML2537 were solubilized in 2 × 107 CFU, which was applied to an SDS-PAGE gel and transferred onto Immobilon-P. IMP-type MBL and GAPDH were visualized using monoclonal antibodies (upper panel), respectively, as described in Materials and Methods. Relative intensity of IMP-type MBL per GAPDH was calculated using NIH ImageJ software (https://imagej.nih.gov/ij/) (lower).
DISCUSSION
Two copies of blaIMP-1 in P. rettgeri BML2496 may be necessary for effective IMP-1 production and high hydrolytic activities against carbapenems. Western blot analysis revealed that BML2496 produced a relatively high level of IMP-1 (Fig. 2). There are at least four reports describing carbapenem-resistant Gram-negative pathogens harboring two copies of genes encoding carbapenemases (13–16). An isolate of P. aeruginosa obtained from a patient in Serbia harbored two blaNDM-1 copies in the genome on the chromosome (13). Carbapenem-resistant P. aeruginosa harboring two copies of blaIMP-34 on the chromosome was isolated from a patient in Japan (14). A clinical isolate of E. coli obtained from a patient in China harbored blaNDM-1 on the chromosome along with two tandem copies of an ISCR1 element (15). An E. coli isolate obtained from a patient in China had an IncF plasmid carrying two copies of blaNDM-5 (16). Our present study, together with these reports, strongly suggests that acquisition of two copies of a gene encoding carbapenemase is a strategy of Gram-negative pathogens to obtain high resistance to carbapenems.
The amino acid substitution of Val67Phe, but not that of Phe87Val, in IMP-70 seems to play a crucial role in a significant increase compared with IMP-1 of enzymatic activities against meropenem. Amino acid residue 67 in IMP-1 is located at the end of a loop close to the active site that consists of residues 60 to 66 (17). This loop is a major determinant for the tight binding of substrates in the active site (17). A Val67Phe amino acid substitution in IMP-43, a variant of IMP-7, increased catalytic activities against imipenem and meropenem (18). Amino acid substitutions at position 67 in IMP-1 MBLs affect the ability to hydrolyze and confer resistance to β-lactams (19). Residue 67 was important for substrate binding in VIM-type MBLs (20). Residue 87 plays a critical role in the stability and folding of VIM-2 (21).
IMP-44, a variant of IMP-11 with two substitutions (Val67Phe and Phe87Ser), had more efficient catalytic activities against carbapenems than those of IMP-11 (18), suggesting that coexistence of two amino acid substitutions at positions 67 and 87 may be necessary to increase enzymatic activities and change substrate profiles in IMP-44. However, a substitution of Phe87Val in IMP-70 did not affect enzymatic activities (Table 5). A substitution of Phe87 to a polar amino acid such as Ser, but not to a hydrophobic amino acid such as Val, may affect enzymatic activities.
Regarding the naming of IMP-70, two sequences of IMP-70 were registered at the NCBI database (GenBank accession no. LC348383 and AUM56801). One sequence (accession no. LC348383) was registered in December 2017, and the other (accession no. AUM56801) in January 2018. We describe here IMP-70, a variant of IMP-1 in P. rettgeri and P. stuartii isolates, the sequences of which were deposited in the NCBI database (accession no. LC507075 and AP022377, respectively). These sequences correspond to that of IMP-70 (accession no. LC348383). To avoid confusion, IMP-70 sequences should be mentioned with their accession numbers, such as IMP-70 (accession no. LC348383).
MATERIALS AND METHODS
Bacterial strains and identification.
Four P. rettgeri isolates and a P. stuartii isolate were obtained from urine samples of five patients (76.6 ± 7.6 years old) collected from October to September 2018 in 3 hospitals in Japan (BML Biomedical Laboratories R&D Center, Kawagoe, Saitama, Japan). P. rettgeri BML2496 and BML2531 were isolated at two hospitals in Saitama and Kochi, Japan, respectively. Isolates of P. rettgeri BML2526 and BML 2576 and of P. stuartii BML2537 were obtained at a hospital in Osaka, Japan. Bacterial species were identified by WalkAway System (Beckman Coulter, Brea, CA), and confirmed by 16S RNA sequencing. A clinical isolate of Pseudomonas aeruginosa harboring blaIMP-10 was used to clone the blaIMP-10 gene (18).
Drug susceptibility testing.
MICs of ceftazidime, aztreonam, imipenem, meropenem, arbekacin, amikacin, and cephalosporin were determined using the microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute (22). MICs of colistin were determined using the microdilution method according to the guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (23).
Whole-genome sequencing.
DNA of these isolates was extracted using DNeasy blood and tissue kits (Qiagen, Tokyo, Japan), and their complete genomes were sequenced using the MiSeq platform (Illumina, San Diego, CA). The raw reads were assembled using CLC Genomics Workbench v10.0.1 (CLC bio, Aarhus, Denmark). The sequences of drug resistance genes, including those encoding β-lactamase and aminoglycoside resistance, were determined using ResFinder v3.1 (https://cge.cbs.dtu.dk/services/ResFinder/). The complete genome sequences of all 5 isolates were determined using MinION (Oxford Nanopore Technologies, Oxford, UK). The raw data were base called by Albacore v2.3.1, and adapters were removed by Porechop v0.2.3 (https://github.com/rrwick/Porechop). The long reads generated by MinION and the short reads generated by MiSeq were assembled using Unicycler. Drug resistance genes encoding β-lactamases, aminoglycoside modification enzymes, and 16S rRNA methylases (24) were detected using ResFinder 2.1 (https://cge.cbs.dtu.dk/services/ResFinder/). The sequences of the quinolone resistance genes (25) gyrA, parC, and gyrB were determined using CLC Genomics Workbench v8.0. Multilocus sequence types (MLSTs) were deduced as described in the protocols of the PubMLST database (https://pubmlst.org/paeruginosa/).
PCR.
PCR was conducted using a set of primers, IMP-1 DF (5′-GTAAGCCTCAGCATTTACAAGAACC-3′) and IMP-1DR (5′-CAAGTCACAGTGAAGTTGGAGAC-3′), to determine a distance between these sequences of blaIMP-1 on the genome of an isolate (BML2496).
Cloning of blaIMP-1, blaIMP-10, and blaIMP-70.
The open reading frames (ORFs) of blaIMP-1, blaIMP-10, and blaIMP-70 were PCR amplified using the primers EcoRI-IMP-1/70-F (5′-GGGGAATTCATGAGCAAGTTATCTGTATTC-3′) and PstI-IMP-1/70-R (5′-AAACTGCAGTTAGTTAGTTGCTTGGTTTTGATGG-3′), and the PCR products of each were digested with EcoRI and PstI and ligated into pHSG398 (TaKaRa Bio, Shiga, Japan). The plasmids were used to transform DH5α, transformants were selected on LB agar containing 100 μg/ml of chloramphenicol, and their susceptibility to various β-lactams was assayed. A mutant clone of blaIMP-1 (TTT→GTT at nucleotide positions 205 to 207) encoding IMP-1with an amino acid substitution of Phe87Val [IMP-1(Phe87Val)] was introduced into plasmid pHSGS398-IMP-1 and pET28a-IMP-1 using the KOD Plus mutagenesis kit (Toyobo, Osaka, Japan) using a set of primers, Phe87Val F (5′-TAATTGACACTCCAGTTACGGCTAAAGAT-3′) and Phe87Val R (5′-ATCTTTAGCCGTAACTGGAGTGTCAATTA-3′).
Purification of recombinant IMP-1, IMP-70, IMP-10, and IMP-1(Phe87Val).
To determine the kinetic parameters of these IMP-type enzymes, the ORFs of IMP-1, IMP-70, IMP-10, and IMP-1(Phe87Val) without signal peptide regions were amplified by PCR. IMP-1, IMP-70, IMP-10, and IMP-1(Phe87Val) were amplified using the primers BamHI-IMP-1/70-F (5′-ATGGATCCGAAAACCTGTATTTCCAAGGCGCAGAGTCTTTGCCAGATTTAA-3′) and XhoI-IMP-1/70-R (5′-ATCTCGAGTTAGTTGCTTGGTTTTGATGGT-3′). These PCR products were digested with BamHI and XhoI and ligated into pET28a (Novagen, Inc., Madison, WI). The plasmids were used to transform E. coli BL21-CodonPlus (DE3)-RIP (Agilent Technologies, Santa Clara, CA), and transformants were selected on LB agar containing 20 μg/ml of kanamycin. The transformants were incubated overnight in 200 ml of LB broth containing 20 μg/ml of kanamycin, and the progeny were transferred to 2 liters of the same broth, and further incubated for 3 h. Then, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the bacteria at a final concentration of 0.5 mM/ml, and further incubation was allowed for 2 h. The bacterial cells were collected and lysed by sonication, and the recombinant IMP proteins were purified from the soluble fraction on nickel-nitrilotriacetic acid (Ni-NTA) agarose according to the manufacturer's instructions (Qiagen, Tokyo, Japan). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed that each target protein was over 90% pure. During the purification procedures, the presence of β-lactamase activities was monitored with 100 μM nitrocefin (Oxoid Ltd., Basingstoke, UK). These enzymes were prepared in 50 mM Tris-HCl buffer (pH 7.5) in the range of 0.41 to 0.51 mg/ml. Kinetic analysis was performed in 50 mM Tris-HCl buffer (pH 7.5) containing 50 μM Zn(NO3)2 (Nakalai Tesque, Inc., Kyoto, Japan) at 37°C using a UV-visible spectrophotometer (V-730; Jasco, Tokyo, Japan). The Km, kcat, and kcat/Km ratio of each enzyme were determined by analyzing β-lactam hydrolysis under initial rate conditions using Lineweaver-Burk plots (26–28).
Western blot analysis.
Bacterial cells of P. rettgeri BML2496, BML2526, BML2531, and BML2576 and of P. stuartii BML2537 growing in the log phase were collected and solubilized in 2 × 107 CFU per 10 μl of SDS-PAGE sample buffer. These solubilized bacterial cells were applied to an SDS-PAGE gel and transferred onto Immobilon-P (Merk Millipore Ltd., Cork, Ireland). IMP-type MBL and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on Immobilon-P were visualized using a rat monoclonal antibody against IMP-type MBL (4E7) (29) and a mouse monoclonal antibody against GAPDH (Proteintech, Cambridge, UK), respectively. As secondary antibodies, horseradish peroxidase (HRP)-linked goat anti-rat IgG (SouthernBiotech, Birmingham, Alabama) and goat anti-mouse IgG (Abcam, London, UK), respectively, were used and were detected by chemiluminescence (Western BLoT chemiluminescence HRP substrate; TaKaRa Bio, Shiga, Japan). Relative intensities of IMP-type MBL per GAPDH (log relative intensity values) were calculated using NIH ImageJ software (https://imagej.nih.gov/ij/).
Ethics approval.
The study protocol was approved by the ethics committee of Juntendo University (approval number 809) and by the Biosafety Committee, Juntendo University (approval number BSL2/29-1). Allowed information about patients included age, gender, and sample tissues.
Data availability.
The genome sequence data generated by MiSeq and MinION were deposited in the DNA Data Bank of Japan (DDBJ). The data of assembled and annotated nucleotide sequences were deposited in the GenBank database with the accession numbers AP022371 to AP022377 and LC507075, respectively.
ACKNOWLEDGMENTS
This study was supported by grants from the Japan Society for the Promotion of Science (grants 18K07120 and 19K16652) and from the Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (grant 20fk0108061h0303). S.I. was supported by the Training Program for Medical Students in Basic Research, Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (grant 2117016).
Miho Ogawa are Masahiro Shimojima are employed by BMI, Inc. There are no patents, products in development, or marketed products to declare.
REFERENCES
- 1.Stock I, Wiedemann B. 1998. Natural antibiotic susceptibility of Providencia stuartii, P. rettgeri, P. alcalifaciens and P. rustigianii strains. J Med Microbiol 47:629–642. doi: 10.1099/00222615-47-7-629. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah M, Balshi A. 2018. First literature review of carbapenem-resistant Providencia. New Microbes New Infect 25:16–23. doi: 10.1016/j.nmni.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoh M, Matsuyama J, Ohnishi M, Takagi K, Miyagi H, Mori K, Park KS, Ono T, Honda T. 2005. Importance of Providencia species as a major cause of travellers’ diarrhoea. J Med Microbiol 54:1077–1082. doi: 10.1099/jmm.0.45846-0. [DOI] [PubMed] [Google Scholar]
- 4.O’Hara CM, Brenner FW, Miller JM. 2000. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol Rev 13:534–546. doi: 10.1128/CMR.13.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma D, Sharma P, Soni P. 2017. First case report of Providencia rettgeri neonatal sepsis. BMC Res Notes 10:536. doi: 10.1186/s13104-017-2866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane ES, Shum M, Chu DS. 2016. Case report: Providencia stuartii conjunctivitis. J Ophthalmic Inflamm Infect 6:29. doi: 10.1186/s12348-016-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sipahi OR, Bardak-Ozcem S, Ozgiray E, Aydemir S, Yurtseven T, Yamazhan T, Tasbakan M, Ulusoy S. 2010. Meningitis due to Providencia stuartii. J Clin Microbiol 48:4667–4668. doi: 10.1128/JCM.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krake PR, Tandon N. 2004. Infective endocarditis due to Providenca stuartii. South Med J 97:1022–1023. doi: 10.1097/01.smj.0000141308.19657.ba. [DOI] [PubMed] [Google Scholar]
- 9.Nishio H, Komatsu M, Shibata N, Shimakawa K, Sueyoshi N, Ura T, Satoh K, Toyokawa M, Nakamura T, Wada Y, Orita T, Kofuku T, Yamasaki K, Sakamoto M, Kinoshita S, Aihara M, Arakawa Y. 2004. Metallo-β-lactamase-producing gram-negative bacilli: laboratory-based surveillance in cooperation with 13 clinical laboratories in the Kinki region of Japan. J Clin Microbiol 42:5256–5263. doi: 10.1128/JCM.42.11.5256-5263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robin F, Aggoune-Khinache N, Delmas J, Naim M, Bonnet R. 2010. Novel VIM metallo-β-lactamase variant from clinical isolates of Enterobacteriaceae from Algeria. Antimicrob Agents Chemother 54:466–470. doi: 10.1128/AAC.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mojica MF, Bonomo RA, Fast W. 2016. B1-metallo-β-lactamases: where do we stand? Curr Drug Targets 17:1029–1050. doi: 10.2174/1389450116666151001105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovcić B, Lepsanović Z, Begović J, Rakonjac B, Perovanović J, Topisirović L, Kojić M. 2013. The clinical isolate Pseudomonas aeruginosa MMA83 carries two copies of the blaNDM-1 gene in a novel genetic context. Antimicrob Agents Chemother 57:3405–3407. doi: 10.1128/AAC.02312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tada T, Miyoshi-Akiyama T, Shimada K, Shiroma A, Nakano K, Teruya K, Satou K, Hirano T, Shimojima M, Kirikae T. 2016. A carbapenem-resistant Pseudomonas aeruginosa isolate harboring two copies of blaIMP-34 encoding a metallo-β-lactamase. PLoS One 11:e0149385. doi: 10.1371/journal.pone.0149385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen P, Yi M, Fu Y, Ruan Z, Du X, Yu Y, Xie X. 2017. Detection of an Escherichia coli sequence type 167 strain with two tandem copies of blaNDM-1 in the chromosome. J Clin Microbiol 55:199–205. doi: 10.1128/JCM.01581-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Liu L, McNally A, Zong Z. 2018. Coexistence of two blaNDM-5 genes on an IncF plasmid as revealed by nanopore sequencing. Antimicrob Agents Chemother 62:e00110-62. doi: 10.1128/AAC.00110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moali C, Anne C, Lamotte-Brasseur J, Groslambert S, Devreese B, Van Beeumen J, Galleni M, Frère JM. 2003. Analysis of the importance of the metallo-β-lactamase active site loop in substrate binding and catalysis. Chem Biol 10:319–329. doi: 10.1016/s1074-5521(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 18.Tada T, Miyoshi-Akiyama T, Shimada K, Shimojima M, Kirikae T. 2013. IMP-43 and IMP-44 metallo-β-lactamases with increased carbapenemase activities in multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:4427–4432. doi: 10.1128/AAC.00716-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaCuran AE, Pegg KM, Liu EM, Bethel CR, Ai N, Welsh WJ, Bonomo RA, Oelschlaeger P. 2015. Elucidating the role of residue 67 in IMP-type metallo-β-lactamase evolution. Antimicrob Agents Chemother 59:7299–7307. doi: 10.1128/AAC.01651-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Jin W, Matsunaga K, Ikemizu S, Yamagata Y, Wachino J, Shibata N, Arakawa Y, Kurosaki H. 2007. Crystallographic investigation of the inhibition mode of a VIM-2 metallo-β-lactamase from Pseudomonas aeruginosa by a mercaptocarboxylate inhibitor. J Med Chem 50:6647–6653. doi: 10.1021/jm701031n. [DOI] [PubMed] [Google Scholar]
- 21.Borgianni L, Vandenameele J, Matagne A, Bini L, Bonomo RA, Frère JM, Rossolini GM, Docquier JD. 2010. Mutational analysis of VIM-2 reveals an essential determinant for metallo-β-lactamase stability and folding. Antimicrob Agents Chemother 54:3197–3204. doi: 10.1128/AAC.01336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed Approved standard M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.EUCAST. 2016. Recommendations for MIC determination of colistin (polymyxin E), as recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf.
- 24.Galimand M, Courvalin P, Lambert T. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother 47:2565–2571. doi: 10.1128/aac.47.8.2565-2571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akasaka T, Tanaka M, Yamaguchi A, Sato K. 2001. Type II topoisomerase mutations in fluoroquinolone-resistant clinical strains of Pseudomonas aeruginosa isolated in 1998 and 1999: role of target enzyme in mechanism of fluoroquinolone resistance. Antimicrob Agents Chemother 45:2263–2268. doi: 10.1128/AAC.45.8.2263-2268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boschi L, Mercuri PS, Riccio ML, Amicosante G, Galleni M, Frère JM, Rossolini GM. 2000. The Legionella (Fluoribacter) gormanii metallo-β-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 β-lactamases. Antimicrob Agents Chemother 44:1538–1543. doi: 10.1128/aac.44.6.1538-1543.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowder MW, Walsh TR, Banovic L, Pettit M, Spencer J. 1998. Overexpression, purification, and characterization of the cloned metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 42:921–926. doi: 10.1128/AAC.42.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Queenan AM, Shang W, Flamm R, Bush K. 2010. Hydrolysis and inhibition profiles of β-lactamases from molecular classes A to D with doripenem, imipenem, and meropenem. Antimicrob Agents Chemother 54:565–569. doi: 10.1128/AAC.01004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitao T, Miyoshi-Akiyama T, Tanaka M, Narahara K, Shimojima M, Kirikae T. 2011. Development of an immunochromatographic assay for diagnosing the production of IMP-type metallo-β-lactamases that mediate carbapenem resistance in Pseudomonas. J Microbiol Methods 87:330–337. doi: 10.1016/j.mimet.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Shiroto K, Ishii Y, Kimura S, Alba J, Watanabe K, Matsushima Y, Yamaguchi K. 2005. Metallo-β-lactamase IMP-1 in Providencia rettgeri from two different hospitals in Japan. J Med Microbiol 54:1065–1070. doi: 10.1099/jmm.0.46194-0. [DOI] [PubMed] [Google Scholar]
- 31.Potter RF, Wallace MA, McMullen AR, Prusa J, Stallings CL, Burnham CAD, Dantas G. 2018. blaIMP-27 on transferable plasmids in Proteus mirabilis and Providencia rettgeri. Clin Microbiol Infect 24:1019.e5–1019.e8. doi: 10.1016/j.cmi.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathirana HNKS, Shin GW, Wimalasena SHMP, Hossain S, De Silva BCJ, Dahanayake PS, Heo GJ. 2018. Incidence of antibiogram, antibiotic resistance genes and class 1 and 2 integrons in tribe Proteeae with IMP27 gene for the first time in Providencia sp. isolated from pet turtles. Lett Appl Microbiol 67:620–627. doi: 10.1111/lam.13077. [DOI] [PubMed] [Google Scholar]
- 33.Gefen-Halevi S, Hindiyeh MY, Ben-David D, Smollan G, Gal-Mor O, Azar R, Castanheira M, Belausov N, Rahav G, Tal I, Mendelson E, Keller N. 2013. Isolation of genetically unrelated blaNDM-1-positive Providencia rettgeri strains in Israel. J Clin Microbiol 51:1642–1643. doi: 10.1128/JCM.00381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tada T, Miyoshi-Akiyama T, Dahal RK, Sah MK, Ohara H, Shimada K, Kirikae T, Pokhrel BM. 2014. NDM-1 metallo-β-lactamase and ArmA 16S rRNA methylase producing Providencia rettgeri clinical isolates in Nepal. BMC Infect Dis 14:56. doi: 10.1186/1471-2334-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrios H, Garza-Ramos U, Reyna-Flores F, Sanchez-Perez A, Rojas-Moreno T, Garza-Gonzalez E, Llaca-Diaz JM, Camacho-Ortiz A, Guzmán-López S, Silva-Sanchez J. 2013. Isolation of carbapenem-resistant NDM-1-positive Providencia rettgeri in Mexico. J Antimicrob Chemother 68:1934–1936. doi: 10.1093/jac/dkt124. [DOI] [PubMed] [Google Scholar]
- 36.Zhou G, Guo S, Luo Y, Ye L, Song Y, Sun G, Guo L, Chen Y, Han L, Yang J. 2014. NDM-1-producing strains, family Enterobacteriaceae, in hospital, Beijing, China. Emerg Infect Dis 20:340–342. doi: 10.3201/eid2002.121263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeifer Y, Trifonova A, Pietsch M, Brunner M, Todorova I, Gergova I, Wilharm G, Werner G, Savov E. 2017. Clonal transmission of gram-negative bacteria with carbapenemases NDM-1, VIM-1, and OXA-23/72 in a Bulgarian hospital. Microb Drug Resist 23:301–307. doi: 10.1089/mdr.2016.0059. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho-Assef AP, Pereira PS, Albano RM, Berião GC, Chagas TP, Timm LN, Da Silva RC, Falci DR, Asensi MD. 2013. Isolation of NDM-producing Providencia rettgeri in Brazil. J Antimicrob Chemother 68:2956–2957. doi: 10.1093/jac/dkt298. [DOI] [PubMed] [Google Scholar]
- 39.Ntshobeni NB, Allam M, Ismail A, Amoako DG, Essack SY, Chenia HY. 2019. Draft genome sequence of Providencia rettgeri APW139_S1, an NDM-18-producing clinical strain originating from hospital effluent in South Africa. Microbiol Resour Announc 8:e00259-19. doi: 10.1128/MRA.00259-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HW, Kang HY, Shin KS, Kim J. 2007. Multidrug-resistant Providencia isolates carrying blaPER-1, blaVIM-2, and armA. J Microbiol 45:272–274. [PubMed] [Google Scholar]
- 41.McGann P, Hang J, Clifford RJ, Yang Y, Kwak YI, Kuschner RA, Lesho EP, Waterman PE. 2012. Complete sequence of a novel 178-kilobase plasmid carrying blaNDM-1 in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 56:1673–1679. doi: 10.1128/AAC.05604-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douka E, Perivolioti E, Kraniotaki E, Fountoulis K, Economidou F, Tsakris A, Skoutelis A, Routsi C. 2015. Emergence of a pandrug-resistant VIM-1-producing Providencia stuartii clonal strain causing an outbreak in a Greek intensive care unit. Int J Antimicrob Agents 45:533–536. doi: 10.1016/j.ijantimicag.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 43.Drieux L, Decré D, Frangeul L, Arlet G, Jarlier V, Sougakoff W. 2013. Complete nucleotide sequence of the large conjugative pTC2 multireplicon plasmid encoding the VIM-1 metallo-β-lactamase. J Antimicrob Chemother 68:97–100. doi: 10.1093/jac/dks367. [DOI] [PubMed] [Google Scholar]
- 44.Galani L, Galani I, Souli M, Karaiskos I, Katsouda E, Patrozou E, Baziaka F, Paskalis C, Giamarellou H. 2013. Nosocomial dissemination of Providencia stuartii isolates producing extended-spectrum β-lactamases VEB-1 and SHV-5, metallo-β-lactamase VIM-1, and RNA methylase RmtB. J Glob Antimicrob Resist 1:115–116. doi: 10.1016/j.jgar.2013.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome sequence data generated by MiSeq and MinION were deposited in the DNA Data Bank of Japan (DDBJ). The data of assembled and annotated nucleotide sequences were deposited in the GenBank database with the accession numbers AP022371 to AP022377 and LC507075, respectively.