MRX-8 is a novel polymyxin analogue in development for the treatment of infections caused by Gram-negative pathogens, including those resistant to other antibiotic classes. In the present study, we examined the pharmacodynamic activity of MRX-8 against a variety of common Gram-negative pathogens in the neutropenic mouse thigh and lung models. Additionally, we examined polymyxin B (PMB) as a comparator. Plasma pharmacokinetics of MRX-8 and PMB were linear over a broad dosing range of 0.156 to 10 mg/kg of body weight and had similar AUC0–∞ (area under the drug concentration-time curve from 0 h to infinity) exposures of MRX-8, 0.
KEYWORDS: MRX-8, polymyxin, pharmacodynamics, Gram negative
ABSTRACT
MRX-8 is a novel polymyxin analogue in development for the treatment of infections caused by Gram-negative pathogens, including those resistant to other antibiotic classes. In the present study, we examined the pharmacodynamic activity of MRX-8 against a variety of common Gram-negative pathogens in the neutropenic mouse thigh and lung models. Additionally, we examined polymyxin B (PMB) as a comparator. Plasma pharmacokinetics of MRX-8 and PMB were linear over a broad dosing range of 0.156 to 10 mg/kg of body weight and had similar AUC0–∞ (area under the drug concentration-time curve from 0 h to infinity) exposures of MRX-8, 0.22 to 12.64 mg · h/liter, and PMB, 0.12 to 13.22 mg · h/liter. Dose fractionation was performed for MRX-8 using a single Escherichia coli isolate, and the results demonstrated that both Cmax (maximum concentration of drug in serum)/MIC and AUC/MIC ratios were strongly associated with efficacy. In the thigh model, dose-ranging studies included strains of E. coli (n = 3), Pseudomonas aeruginosa (n = 2), Klebsiella pneumoniae (n = 3), and Acinetobacter baumannii (n = 1). Both MRX-8 and PMB exhibited increased effects with increasing doses. MRX-8 and PMB free AUC/MIC exposures for net stasis were similar for E. coli and K. pneumoniae at 20 to 30. Notably, for P. aeruginosa and A. baumannii, the free AUC/MIC ratio for stasis was numerically much smaller for MRX-8 at 6 to 8 than for PMB at 16 to 37. In the lung model, MRX-8 was also more effective than PMB when dosed to achieve similar free-drug AUC exposures over the study period. MRX-8 is a promising novel polymyxin analogue with in vivo activity against many different clinically relevant species in both the mouse thigh and lung models.
INTRODUCTION
Resistance in Gram-negative bacteria is a worldwide public health threat (1). While new therapies such as novel β-lactamase inhibitors and enhanced tetracycline derivatives have been developed, bacteria continue to evolve, necessitating the development of novel therapeutic agents in many different mechanistic classes (2). One such class is the polymyxins. Unfortunately, toxicity and limited potency have largely led to the clinical relegation of this class as an agent of last resort and/or in combination (3). Novel polymyxin analogues that combine lower toxicity risk with improved efficacy would be a welcome addition to the armamentarium.
Pharmacokinetic/pharmacodynamic (PK/PD) studies are an integral step early in the drug development process (4). These studies integrate pharmacokinetic drug measures, organism susceptibility to the target compound, and efficacy over time (5). One advantage of PK/PD studies in the animal model is the ability to examine the impact of the infection site. The goal of these studies is to determine exposure-response relationships for optimal efficacy, which in turn allows for predicting which organisms and sites of infection the compound is likely to be clinically efficacious and designing rational dosing regimens for human study. In this study, we report the PK/PD results of a novel polymyxin analogue, MRX-8, using polymyxin B (PMB) as a comparator in the neutropenic mouse thigh and lung models.
RESULTS
Organisms and in vitro susceptibility testing.
The study organisms and susceptibility testing results are shown in Table 1. The MRX-8 MIC varied 4-fold (range, 0.5 to 2 mg/liter) while the PMB MIC did not vary between each organism (2 mg/liter).
TABLE 1.
Study organisms and MRX-8 and PMB susceptibility results
| Organism | MRX-8 MIC (mg/liter) | PMB MIC (mg/liter) |
|---|---|---|
| E. coli ATCC 25922 | 0.5 | 2 |
| E. coli 1-894-1 | 1 | 2 |
| E. coli 1-741-1 | 0.5 | 2 |
| P. aeruginosa ATCC 27853 | 2 | 2 |
| P. aeruginosa 3076 | 2 | 2 |
| K. pneumoniae ATCC 43816 | 2 | 2 |
| K. pneumoniae ATCC 4110 | 2 | 2 |
| K. pneumoniae 81-1269A | 1 | 2 |
| A. baumannii 3495 | 2 | 2 |
Pharmacokinetics of MRX-8 and PMB in mice.
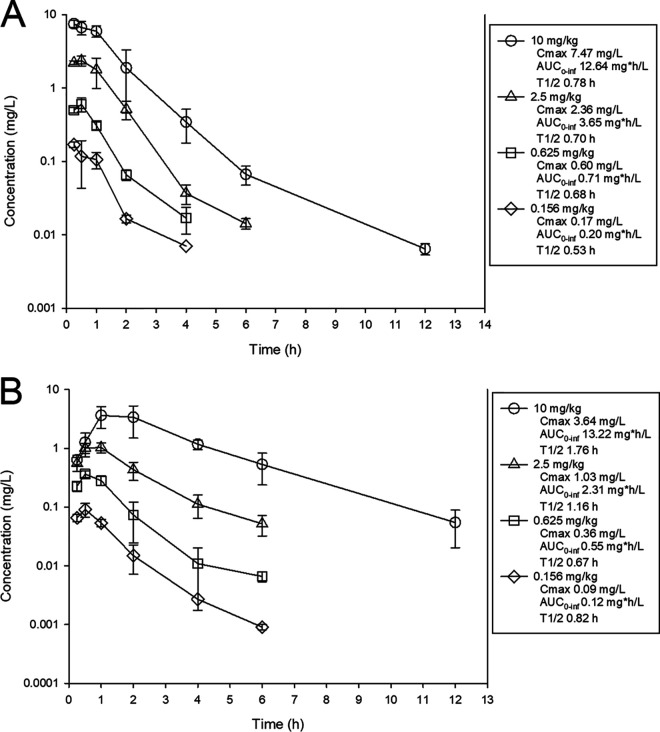
The time courses of plasma drug levels of MRX-8 and PMB are shown in Fig. 1. After subcutaneous (s.c.) administration of MRX-8 at 0.156, 0.625, 2.5, and 10 mg/kg of body weight, the maximum concentration of drug in serum (Cmax) ranged from 0.17 to 7.47 mg/liter, the area under the drug concentration-time curve from 0 h to infinity (AUC0–∞) ranged from 0.22 to 12.64 mg · h/liter, and the elimination half-life (t1/2) ranged from 0.53 to 0.78 h. After subcutaneous administration of PMB at 0.156, 0.625, 2.5, and 10 mg/kg, the Cmax ranged from 0.09 to 3.64 mg/liter, the AUC0–∞ ranged from 0.12 to 13.22 mg · h/liter, and the elimination half-life ranged from 0.82 to 1.76 h. For both drugs, the observed Cmax and AUC0–∞ values over the dose range were linear (R2 > 0.99).
FIG 1.
Single-dose plasma pharmacokinetics of MRX-8 (A) and PMB (B) in mice. Four different doses of MRX-8 were administered to mice by the subcutaneous route. Groups of three mice were sampled at each time point. Each symbol represents the mean from three animals; error bars show standard deviations. Shown in the symbol key are the maximum plasma concentration (Cmax), the area under the concentration-time curve from 0 h to infinity (AUC0–inf), and the beta elimination half-life (t1/2).
Dose fractionation studies with MRX-8.
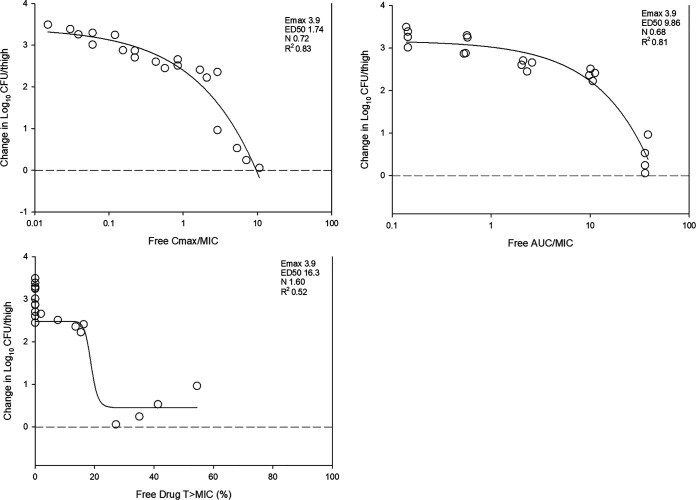
The dose-response relationships for the fractionation study of MRX-8 against Escherichia coli ATCC 25922 are shown in Fig. 2. At the start of therapy, mice had 6.5 ± 0.1 log10 CFU/thigh, and the burden increased over 24 h to 10.3 ± 0.1 log10 CFU/thigh in untreated controls. Each fractionated regimen produced relatively equivalent results for each total dose, with the exception of the highest dose. Each of three PK/PD indices was fit to the response data using nonlinear multivariate regression (Hill equation) and is shown in Fig. 3. Based on visual fit and R2 analysis, both the Cmax/MIC and AUC/MIC ratios fit the data well.
FIG 2.
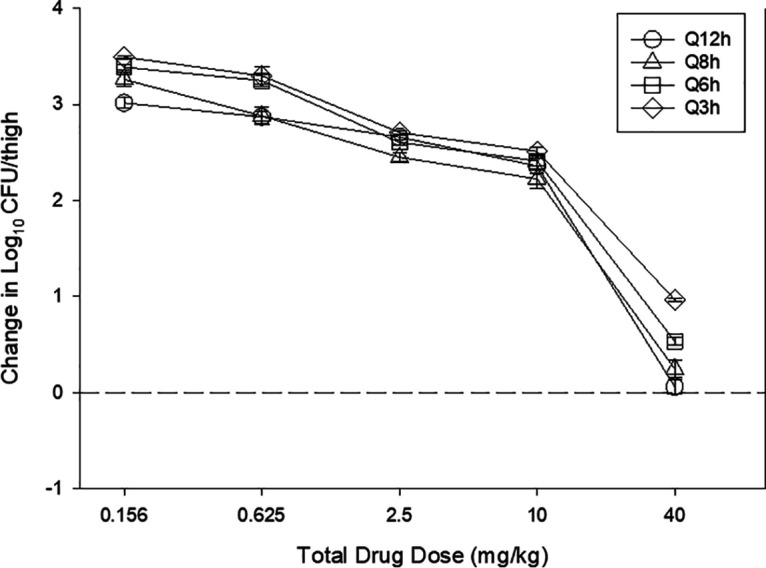
In vivo dose fractionation with MRX-8 using a neutropenic mouse thigh model. Each symbol represents the mean and standard deviation from four thighs infected with E. coli ATCC 25922. The error bars represent the standard deviations. The burden of organisms was measured at the start and end of therapy. The study period was 24 h. Five total drug (milligrams per kilogram per 24 h) dose levels were fractionated into one of four dosing regimens and are shown on the x axis. The y axis represents the change in the organism burden from the start of therapy. The dashed horizontal line represents net stasis over the treatment period.
FIG 3.
Relationship between the PK/PD indices free-drug Cmax/MIC (top left), AUC/MIC (top right), and time above the MIC (T>MIC) (bottom) and the therapeutic effect for MRX-8. Five total dose levels (0.156 to 40 mg/kg) were fractionated into q12h, q8h, q6h, and q3h dosing regimens and administered to thigh-infected (E. coli ATCC 25922) mice. The change in the burden was assessed over the 24-h treatment duration. Points above the dashed line represent the net increase in the bacterial burden, whereas those below the dashed line represent a net decrease in the bacterial burden. The PK/PD parameters Emax (maximum effect), ED50 (50% maximum effect), N (slope of the best-fit line), and R2 (coefficient of determination) are shown for each PK/PD index.
PK/PD magnitude determination for MRX-8 and PMB in the thigh model.
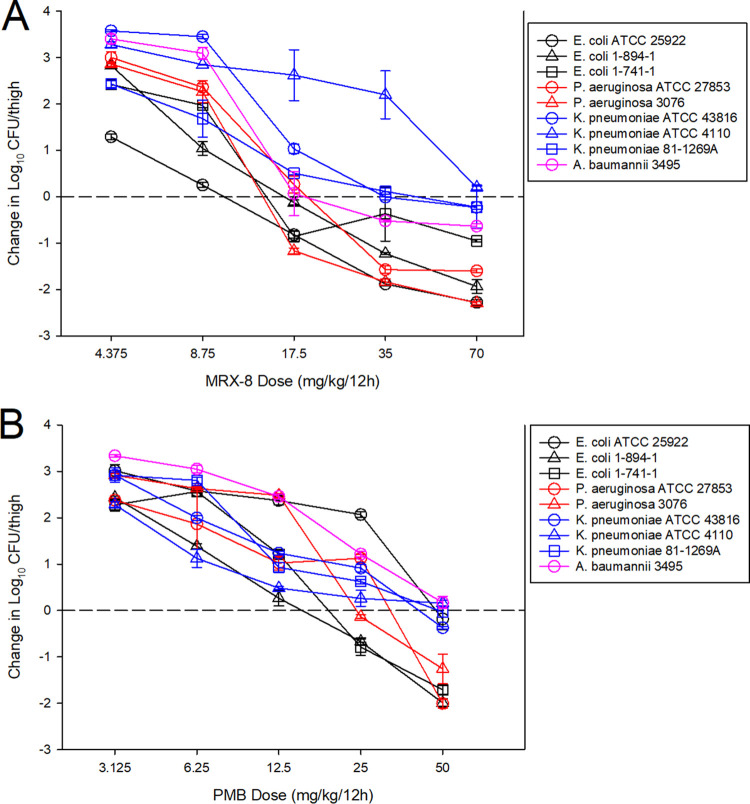
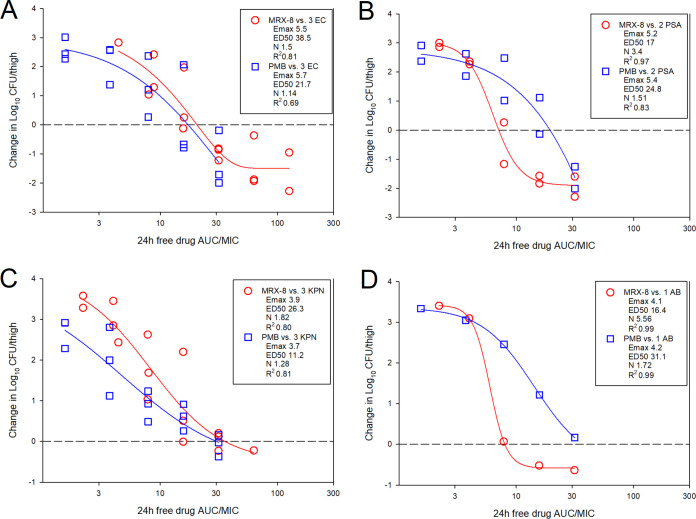
The dose-response curves for MRX-8 and PMB against multiple Gram-negative pathogens in the murine thigh model are shown in Fig. 4. At the start of therapy, mice had 6.4 ± 0.1 log10 CFU/thigh, and growth in untreated controls was relatively similar for all pathogens in each study arm at >3 log10 CFU/thigh (Tables 2 and 3). For both antibiotics, increasing doses exhibited increased effects, with the highest doses achieving net stasis or log reduction endpoints against nearly all strains. The dosing regimens for each drug were then converted into free-drug AUC estimates based on the animal pharmacokinetic data and protein binding values. The free-drug AUC/MIC ratio was calculated for each dose level-organism combination and is plotted by pathogen group in Fig. 5. The free-drug AUC/MIC ratio was a robust predictor of efficacy, in particular for MRX-8, for all organism groups, with high R2 values when fit to the sigmoid Hill maximum-effect (Emax) model. Shown in Tables 2 and 3 are the MRX-8 and PMB, respectively, static and 1-log-kill AUC/MIC exposures for each organism tested. The results between the two drugs for stasis were quite congruent for E. coli and Klebsiella pneumoniae; however, in contrast, the results were numerically quite different for Pseudomonas aeruginosa and Acinetobacter baumannii. Specifically, the fAUC (AUC for the free, unbound fraction of the drug)/MIC ratio for net stasis for E. coli against MRX-8 was 13.9 to 27.7, and for PMB, it was 8.7 to 33.9; for K. pneumoniae against MRX-8, it was 13.8 to 34.5, and for PMB, it was 25.3 to 29.7. However, for P. aeruginosa against MRX-8, the fAUC/MIC ratio for net stasis was only 6.1 to 8.3, and for PMB, it was higher at 15.8 to 16.9; for A. baumannii, it was similarly low for MRX-8 at 8.0 and much higher for PMB at 36.8. The small number of strains tested precludes a robust statistical analysis of these differences, but the results suggest that enhanced MRX-8 pharmacodynamic activity may be present for the latter two species.
FIG 4.
MRX-8 dose-response curves (A) and PMB dose-response curves (B). Five total dose levels were administered subcutaneously in a 12-hourly regimen to thigh-infected mice. The change in the burden was assessed over a 24-h treatment duration. Points above the dashed line represent a net increase in the bacterial burden, whereas those below the dashed line represent a net decrease in the bacterial burden.
TABLE 2.
MRX-8 pharmacodynamic targets associated with net stasis and 1-log kill in the neutropenic murine thigh infection model for E. coli, P. aeruginosa, K. pneumoniae, and A. baumanniia
| Organism | MIC (mg/liter) | Growth in untreated controls (log10 CFU/thigh) | Stasis |
1-log kill |
||||
|---|---|---|---|---|---|---|---|---|
| Static dose (q12h) (mg/kg) | 24-h total drug AUC/MIC ratio | 24-h free-drug AUC/MIC ratio | 1-log-kill dose (q12h) (mg/kg) | 24-h total drug AUC/MIC ratio | 24-h free-drug AUC/MIC ratio | |||
| E. coli | ||||||||
| ATCC 25922 | 0.5 | 3.46 | 10.00 | 50.57 | 17.95 | 18.97 | 95.90 | 34.05 |
| 1-894-1 | 1 | 3.54 | 15.47 | 39.10 | 13.88 | 27.02 | 68.30 | 24.25 |
| 1-741-1 | 0.5 | 3.55 | 15.45 | 78.09 | 27.72 | NA | ||
| P. aeruginosa | ||||||||
| ATCC 27853 | 2 | 3.27 | 18.47 | 23.34 | 8.29 | 26.92 | 34.02 | 12.08 |
| 3076 | 2 | 3.24 | 13.65 | 17.25 | 6.12 | 16.99 | 21.47 | 7.62 |
| K. pneumoniae | ||||||||
| ATCC 43816 | 2 | 3.63 | 30.73 | 38.83 | 13.79 | NA | ||
| ATCC 4110 | 2 | 3.48 | NA | NA | ||||
| 81-1269A | 1 | 3.62 | 38.45 | 97.17 | 34.50 | NA | ||
| A. baumannii 3495 | 2 | 3.53 | 17.93 | 22.66 | 8.04 | NA | ||
NA, not achieved.
TABLE 3.
PMB pharmacodynamic targets associated with net stasis and 1-log kill in the neutropenic murine thigh infection model for E. coli, P. aeruginosa, K. pneumoniae, and A. baumanniia
| Organism | MIC (mg/liter) | Growth in untreated controls (log10 CFU/thigh) | Stasis |
1-log kill |
||||
|---|---|---|---|---|---|---|---|---|
| Static dose (q12h) (mg/kg) | 24-h total drug AUC/MIC ratio | 24-h free-drug AUC/MIC ratio | 1-log kill dose (q12h) (mg/kg) | 24-h total drug AUC/MIC ratio | 24-h free-drug AUC/MIC ratio | |||
| E. coli | ||||||||
| ATCC 25922 | 2 | 3.46 | 53.68 | 70.57 | 33.87 | NA | ||
| 1-894-1 | 2 | 3.56 | 13.82 | 18.16 | 8.72 | 26.78 | 35.21 | 12.50 |
| 1-741-1 | 2 | 3.51 | 18.43 | 24.23 | 11.63 | 32.51 | 42.74 | 15.17 |
| P. aeruginosa | ||||||||
| ATCC 27853 | 2 | 3.23 | 25.08 | 32.97 | 15.83 | 62.57 | 82.27 | 29.21 |
| 3076 | 2 | 3.50 | 26.79 | 35.22 | 16.90 | 40.97 | 53.86 | 19.12 |
| K. pneumoniae | ||||||||
| ATCC 43816 | 2 | 3.21 | 47.06 | 61.87 | 29.70 | NA | ||
| ATCC 4110 | 2 | 3.44 | NA | NA | ||||
| 81-1269A | 2 | 3.28 | 40.14 | 52.78 | 25.33 | NA | ||
| A. baumannii 3495 | 2 | 3.45 | 58.25 | 76.58 | 36.76 | NA | ||
NA, not achieved.
FIG 5.
MRX-8 and PMB PK/PD exposure-response relationships against E. coli (EC) (A), P. aeruginosa (PSA) (B), K. pneumoniae (KPN) (C), and A. baumannii (AB) (D) in the neutropenic thigh model. The x axis shows the PK/PD index 24-h total drug AUC/MIC, and the y axis shows the therapeutic effect. The PK/PD parameters Emax (maximum effect), ED50 (50% maximum effect), N (slope of the best-fit line), and R2 (coefficient of determination) are shown.
Comparative efficacies of MRX-8 and PMB in the murine pneumonia model.
Shown in Fig. 6 are the dose-response results for MRX-8 and PMB in the murine pneumonia model against a single strain each of E. coli and P. aeruginosa. In each study, the organisms exhibited similar growth in untreated control animals with >3-log10 growth. MRX-8 had much more activity against these two isolates, yielding almost 2-log kill after 24 h, whereas PMB failed to achieve net stasis (P < 0.001). It should be noted that the dosing was designed to achieve plasma free-drug AUCs that were equivalent between the drugs (31.5 mg · h/liter), and therefore, the fAUC/MIC ratios associated with these doses were ∼15 for both organisms in the PMB study and ∼15 for the P. aeruginosa strain and ∼60 for the E. coli strain in the MRX-8 study.
FIG 6.
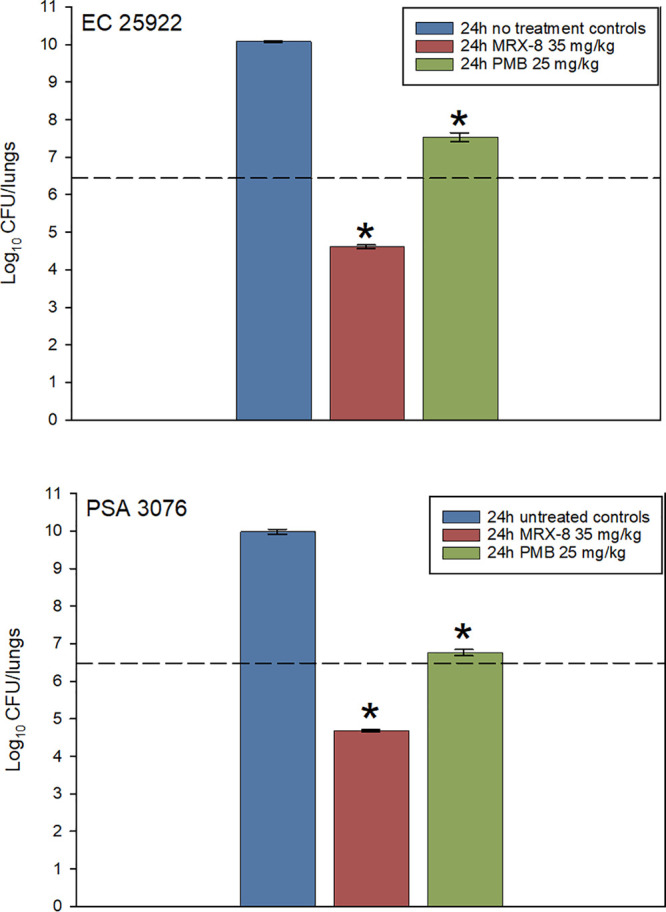
MRX-8 and PMB against E. coli ATCC 25922 (top) and P. aeruginosa 3076 (bottom) in the neutropenic mouse pneumonia model. Groups of 3 mice were infected with the select organism and treated for 24 h with MRX-8 at 35 mg/kg/12 h or PMB at 25 mg/kg/12 h by the subcutaneous route. The dashed horizontal line is the initial burden. Blue vertical bars represent growth in the untreated control. The difference at 24 h between MRX-8- and PMB-treated mice was statistically significant (*, P < 0.001 by Student’s t test).
DISCUSSION
MRX-8 is a novel polymyxin antibiotic designed to treat multidrug-resistant Gram-negative bacterial infections, including Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii. MRX-8 was developed using a “soft drug design,” which represents a new approach aimed at designing safer drugs with an increased therapeutic index by integrating metabolism and detoxification factors into the drug design process (6). Specifically, MRX-8 contains a fatty acyl tail attached via an ester bond, which allows for deesterification to a less toxic metabolite (7).
Our study examines MRX-8 efficacy in vivo to determine the pharmacodynamic properties of this novel agent. We demonstrated that both the AUC/MIC and Cmax/MIC ratios were robust PD indices predicting MRX-8 efficacy. This aligns well with previous in vitro and in vivo studies of the polymyxin class demonstrating concentration-dependent killing with relatively quick regrowth once drug concentrations fall below the MIC (8–14). This pattern of activity explains why, in studies that have utilized a dose fractionation design, both AUC/MIC and Cmax/MIC PK/PD indices are strongly associated with efficacy. The magnitude of MRX-8 PK/PD exposure for a therapeutic effect is also similar to the results of previous work within the polymyxin class. In previous studies, against select Gram-negative isolates, the fAUC/MIC ratio associated with net stasis in the mouse thigh model was 5 to 17 (9–11, 13). These PK/PD target exposures correlate well with results from the present study, in which most strains studied had stasis target exposures in this range. However, it is likely that protein binding determination and biological variability between species and strains explain some of the small differences in PK/PD target exposures between studies.
We also identified differences between MRX-8 and PMB in terms of pharmacodynamic activity that may be clinically relevant. PMB demonstrated quite similar PK/PD activities across the heterogeneous organism groups in our study. If one examines Fig. 5, it is noteworthy that nearly all 4 organism groups have a best-fit line based on the Hill equation crossing net stasis at an fAUC/MIC value close to 20 for PMB. In contrast, there was a clear division in the MRX-8 data whereby we saw 2- to 4-fold-lower PK/PD exposures associated with efficacy for P. aeruginosa and A. baumannii than for E. coli and K. pneumoniae. This enhanced effect will need to be verified with a larger group of isolates and further explored mechanistically. Similarly, we found a lesser effect, based on Emax, for K. pneumoniae than for the other organism groups. This has been demonstrated previously and, while not completely understood mechanistically, could be related to the abundant extracellular capsule made by many K. pneumoniae strains (13).
Previous lung infection model studies have also been performed with other polymyxins (9–11, 13), although they have demonstrated somewhat mixed results. Cheah and colleagues demonstrated that a significantly higher fAUC/MIC exposure was necessary for colistin efficacy in the lung model than in thigh model against P. aeruginosa but found a lack of an effect against A. baumannii (9). Landersdorfer and colleagues demonstrated a lack of an effect of polymyxin B against K. pneumoniae in the mouse lung model (13). Finally, in two studies, Dudhani and colleagues demonstrated relatively preserved fAUC/MIC target exposures for colistin against P. aeruginosa and A. baumannii in the lung model compared with thigh model results (10, 11). We found evidence of a mild effect of PMB against a single strain each of E. coli and P. aeruginosa, as the treatment almost achieved net stasis and decreased the burden from ∼2 log10 units from untreated control animals. However, the difference in efficacy for MRX-8 in the lung model was striking, as it achieved close to a 2-log10 kill (>4-log10 decrease from untreated controls), and importantly, this was determined using equivalent 24-h AUC exposures compared to PMB. These data are encouraging, and a dedicated dose-ranging PK/PD study in the mouse pneumonia model with increased numbers of strains is clearly warranted.
There are a few limitations worth noting. First, given that a heterogeneous group of Gram-negative organisms was pursued and that this was the first PK/PD evaluation of this novel compound, studies were limited in the number of strains for each species group, especially for the lung model. Studies of increased numbers of strains within each species group and studies in the thigh and lung models should be pursued to validate these findings and make the PK/PD target exposures more robust. Second, we did not examine toxicity, which is very important for this class, although we did not observe any external physical signs of toxicity in the mice. Finally, for MRX-8, these data must be interpreted in the context of human PK and organism susceptibility distributions, which have yet to be fully elucidated for this compound, in order to better understand the clinical potential for this agent.
In summary, we have established that the novel polymyxin analogue MRX-8 demonstrated reproducible, linear pharmacokinetics over a broad dose range in mice. The PK/PD indices AUC/MIC and Cmax/MIC were highly correlated with its therapeutic effect. In vivo activity was noted for MRX-8 against a diverse group of pathogens in the mouse thigh model, including E. coli, P. aeruginosa, K. pneumoniae, and A. baumannii. MRX-8 fAUC/MIC stasis target exposures against P. aeruginosa and A. baumannii were only 6 to 8, which were 2- to 4-fold lower than the MRX-8 fAUC/MIC exposures against E. coli and K. pneumoniae and 2- to 4-fold lower than the PMB fAUC/MIC exposures against all species groups. Promising in vivo activity was noted in the lung model for MRX-8 against E. coli and P. aeruginosa. Based on these results, further development and preclinical evaluation of MRX-8 are warranted.
MATERIALS AND METHODS
Organisms, media, and antibiotics.
Three E. coli, 2 P. aeruginosa, 3 K. pneumoniae, and 1 A. baumannii isolate were utilized (Table 1). Strains were chosen to vary the susceptibility to MRX-8 as much as possible while ensuring similar growth in untreated controls. All organisms were grown, subcultured, and quantified using Mueller-Hinton broth (MHB) and agar (Difco Laboratories, Detroit, MI). The drug compounds used for in vitro and in vivo studies were supplied by MicuRx Pharmaceuticals, Inc.
In vitro susceptibility studies.
The MICs for MRX-8 and PMB were determined using Clinical and Laboratory Standards Institute microdilution methods (15). All MIC assays were performed in duplicate on three separate occasions. The median MIC from replicate assays is reported and was utilized for PK/PD analysis.
Murine thigh and lung models.
Animals were maintained in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (16). All animal studies were approved by the Animal Research Committees of the William S. Middleton Memorial VA Hospital and the University of Wisconsin. Six-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 24 to 27 g were used for all studies (Harlan Sprague-Dawley, Indianapolis, IN). Mice were rendered neutropenic (<100 neutrophils/mm3) by cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) injection subcutaneously 4 days (150 mg/kg) and 1 day (100 mg/kg) before thigh infection. Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance at 580 nm of 0.3 (Spectronic 88; Bausch and Lomb, Rochester, NY). After a 1:10 dilution into fresh Mueller-Hinton broth, bacterial counts of the inoculum ranged from 106.9 to 107.1 CFU/ml. Thigh infections with each of the isolates were produced by the injection of 0.1 ml of the inoculum into the thighs of isoflurane-anesthetized mice. Therapy with MRX-8 or PMB was initiated 2 h after the induction of infection, and therapy continued for 24 h, at which point the treatment groups and controls were sacrificed for CFU enumeration. The organism burden was quantified by CFU counts from serial dilutions of thigh homogenates.
The lung model of infection was similar in that 6-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 24 to 27 g were used for all studies. Mice were rendered neutropenic by cyclophosphamide injection, and logarithmic-phase cultures were used to infect the mice as described above. After a 1:10 dilution, bacterial counts of the inoculum ranged from 107.7 to 107.9 CFU/ml. Lung infection was produced by the intranasal administration of 50 μl of the inoculum to isoflurane-anesthetized mice held upright to produce aspiration into the lungs. Therapy with MRX-8 or PMB was initiated 2 h after the induction of infection, and therapy continued for 24 h, at which point the treatment groups and controls were sacrificed for CFU enumeration. The organism burden was quantified by CFU counts from serial dilutions of lung homogenates.
Drug pharmacokinetics in mice for MRX-8 and PMB.
Single-dose plasma pharmacokinetics of MRX-8 and PMB were performed in mice. Dose levels of 0.156, 0.625, 2.5, and 10 mg/kg of MRX-8 and 0.156, 0.625, 2.5, and 10 mg/kg of PMB were administered subcutaneously. Plasma was collected from groups of three mice for drug concentration determinations at 0.25, 0.5, 1, 2, 4, 6, and 12 h. Plasma was obtained from each animal by centrifugation of anticoagulated blood obtained by cardiac puncture. Plasma was stored at −70°C. Drug concentrations were determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Charles River Laboratories, Wilmington, MA). Pharmacokinetic parameters, including the elimination half-life (t1/2), 24-h area under the drug concentration-time curve (AUC), and maximum concentration of drug in serum (Cmax), were calculated using a noncompartmental model. The half-life was determined by linear least-squares regression. The AUC was calculated from the mean concentrations using the trapezoidal rule. The pharmacokinetic estimates for dose levels that were not measured were calculated using linear interpolation for dose levels between those with measured kinetics and linear extrapolation for dose levels greater than or less than the highest and lowest dose levels with kinetic measurements. Murine protein binding evaluation by ultrafiltration and ultracentrifugation was performed by the sponsor (data on file), and values of 64.5% for MRX-8 and 52% for PMB were utilized to calculate free-drug concentrations.
PK/PD parameter determination for MRX-8.
A dose fractionation study was undertaken to determine the PK/PD index (AUC/MIC ratio, Cmax/MIC ratio, or time above the MIC) that was predictive of efficacy for MRX-8. Fourfold-increasing doses (range, 0.156 mg/kg to 40 mg/kg) of MRX-8 were fractionated into every-3-h (q3h), q6h, q8h, and q12h dosing regimens. Thigh infections with E. coli ATCC 25922 were performed as described above, and MRX-8 was administered by subcutaneous injection according to the dosing regimen prescribed in the fractionation design. Each dosing group consisted of four thigh replicates as well as 0-h and no-treatment control groups. After 24 h, the mice were euthanized, and CFU counts were determined in the thighs. To determine which PK/PD index was most closely linked with efficacy, the change in the number of bacteria in the thigh from 0 h to 24 h of therapy was correlated with (i) the free-drug Cmax/MIC ratio, (ii) the free-drug 24-h AUC/MIC ratio, and (iii) the percentage of the dosing interval during which plasma free-drug levels exceeded the MIC (%T>MIC) for each of the dosing regimens studied. The correlation between efficacy and each of the three PK/PD indices was determined by nonlinear least-squares multivariate regression derived from the Hill equation, E = (Emax × DN)/(ED50N − DN), where E is the effector, in this case, the log change in CFU between treated mice and untreated controls after the 24-h period of study; Emax is the maximum effect; D is the 24-h PK/PD index magnitude (i.e., AUC/MIC ratio, Cmax/MIC ratio, or %T>MIC); ED50 is the PK/PD exposure associated with 50% of Emax; and N is the slope of the exposure-response curve (SigmaPlot version 13; Systat Software, San Jose, CA). The values for Emax, ED50, and N were calculated using nonlinear least-squares regression. The coefficient of determination (R2) was used to estimate the variance that might be due to regression with each of the PK/PD indices.
PK/PD magnitude studies for MRX-8 and PMB.
Dose-response experiments using the thigh infection model were performed for 3 Staphylococcus aureus, 2 P. aeruginosa, 3 K. pneumoniae, and 1 A. baumannii strain as described above. The dose range consisted of 4-fold increases (ranges, 4.375 to 70 mg/kg/12 h for MRX-8 and 3.125 to 50 mg/kg/12 h for PMB) in drug concentrations administered by the s.c. route. Each dosing group included four thigh replicates as well as 0-h and no-treatment controls. After 24 h, the mice were euthanized, and CFU counts were determined in the thighs. The exposure-response relationships were examined using nonlinear least-squares multivariate regression (Hill equation) correlating the AUC/MIC ratio and treatment efficacy as described in the equation above. These PK/PD relationships were examined utilizing plasma total and free-drug concentrations from pharmacokinetic studies. The coefficient of determination (R2) from this model was used to numerically quantify the strength of this relationship. This coefficient represents the percentage of the variance in numbers of bacteria that can be attributed to the PK/PD parameter. The 24-h AUC/MIC ratios required for a static effect and 1-log kill were determined by utilizing the plasma drug concentrations.
A single-dose comparative mouse pneumonia model study was also performed. Groups of 3 neutropenic mice were infected with E. coli ATCC 25922 and P. aeruginosa 3076 and treated with MRX-8 at 35 mg/kg/12 h or PMB at 25 mg/kg/12 h. Doses were selected to provide similar free-drug AUC exposures (∼31.5 mg · h/liter) over the dosing period based on differential protein binding. No-treatment and 0-h controls were included for both strains. The treatment effect at 24 h was enumerated in the lungs, and results were compared by Student’s t test.
ACKNOWLEDGMENTS
This study was funded by CARB-X and MicuRx Pharmaceuticals, Inc.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response.
Funding Statement
CarbX
REFERENCES
- 1.CDC. 2019. Antibiotic resistance threats in the United States, 2019. CDC, Atlanta, GA. [Google Scholar]
- 2.Paterson DL, Bonomo RA. 2019. Multidrug-resistant Gram-negative pathogens: the urgent need for ‘old’ polymyxins. Adv Exp Med Biol 1145:9–13. doi: 10.1007/978-3-030-16373-0_2. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10; quiz, 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 5.Andes DR, Lepak AJ. 2017. In vivo infection models in the pre-clinical pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Curr Opin Pharmacol 36:94–99. doi: 10.1016/j.coph.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Bodor N, Buchwald P. 2000. Soft drug design: general principles and recent applications. Med Res Rev 20:58–101. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Gordeev MF. 2018. Polymyxin soft drug MRX-8 with potential to address the class nephrotoxicity, abstr 6 Abstr 3rd Int Conf Polymyxins, Madrid, Spain, 25 to 26 April 2018.
- 8.Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. 2010. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother 54:3783–3789. doi: 10.1128/AAC.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 10.Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, Nation RL. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother 54:1117–1124. doi: 10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudhani RV, Turnidge JD, Nation RL, Li J. 2010. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother 65:1984–1990. doi: 10.1093/jac/dkq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan DD, Friberg LE, Nielsen EI. 2016. A pharmacokinetic-pharmacodynamic (PKPD) model based on in vitro time-kill data predicts the in vivo PK/PD index of colistin. J Antimicrob Chemother 71:1881–1884. doi: 10.1093/jac/dkw057. [DOI] [PubMed] [Google Scholar]
- 13.Landersdorfer CB, Wang J, Wirth V, Chen K, Kaye KS, Tsuji BT, Li J, Nation RL. 2018. Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J Antimicrob Chemother 73:462–468. doi: 10.1093/jac/dkx409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, Lewis RE. 2005. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:3624–3630. doi: 10.1128/AAC.49.9.3624-3630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing; twenty-ninth informational supplement. M100-S29. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]