Remdesivir has reported efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro and in vivo. Drug-drug interactions limit therapeutic options in transplant patients. Remdesivir and its metabolite GS-441524 are excreted principally in urine. In intensive care unit (ICU) settings, in which multiple-organ dysfunctions can occur rapidly, hemodialysis may be a viable option for maintaining remdesivir treatment, while improving tolerance, by removing both remdesivir’s metabolite (GS-441524) and sulfobutylether β-cyclodextrin sodium (SEBCD).
KEYWORDS: hemodialysis, pharmacokinetics, remdesivir
ABSTRACT
Remdesivir has reported efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro and in vivo. Drug-drug interactions limit therapeutic options in transplant patients. Remdesivir and its metabolite GS-441524 are excreted principally in urine. In intensive care unit (ICU) settings, in which multiple-organ dysfunctions can occur rapidly, hemodialysis may be a viable option for maintaining remdesivir treatment, while improving tolerance, by removing both remdesivir’s metabolite (GS-441524) and sulfobutylether β-cyclodextrin sodium (SEBCD). Additional studies may prove informative, particularly in the evaluations of therapeutic options for coronavirus disease 2019 (COVID-19).
TEXT
Since the start of the COVID-19 outbreak, multiple off-label drugs (e.g., lopinavir-ritonavir and hydroxychloroquine) (1, 2) have been evaluated against this illness, with controversial results. Remdesivir (GS-5734) is an investigational nucleotide analogue with broad-spectrum antiviral activity, both in vitro (3) and in vivo (4) in animal models, against multiple emerging viral pathogens, including Ebola virus, Marburg virus, Middle East respiratory syndrome (MERS) coronavirus (CoV), and severe acute respiratory syndrome CoV 2 (SARS-CoV-2). About 5% of coronavirus disease 2019 (COVID-19) patients present an acute respiratory distress syndrome requiring intensive care (3). These patients may experience septic shock, cytokine storm, and multiple-organ dysfunction, with an increase in the risk of death. Knowledge about the possibility of eliminating nonapproved drugs through hemodialysis is essential in critical care medicine (3).
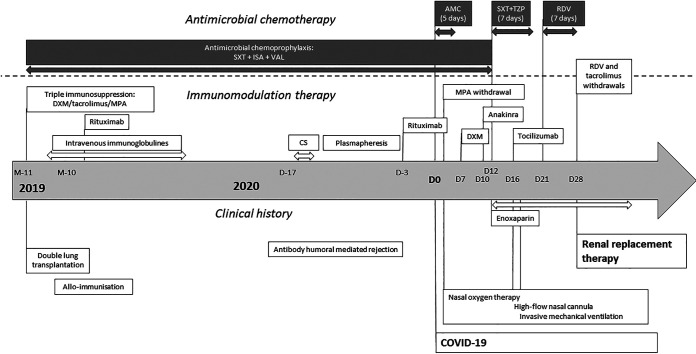
We report here the case of a man in his 40s (44 kg; 1.62 m; body mass index [BMI], 16.8 kg/m2) who had undergone a double-lung transplant 1 year before his admission to the pulmonology department for fever (38°C) and eupnea requiring 4 liters/min oxygen, with no signs of severe respiratory distress syndrome. His medical history is summarized in Fig. 1. His immunosuppressive treatment was based on tacrolimus and mycophenolate mofetil and associated with antimicrobial chemoprophylaxis (isavuconazole, valganciclovir, and sulfamethoxazole-trimethoprim). He presented with a resting respiratory rate of 22 cycles/min, with 92% oxygen saturation, blood pressure of 109/67 mm Hg, a plasma C-reactive protein (CRP) concentration of 13 mg/liter, and creatininemia of 64 μmol/liter. A chest computed tomography (CT) scan showed typical ground-glass opacities with mild lesions (25%), and a nasopharyngeal sample tested positive for SARS-CoV-2 by real-time PCR (RT-PCR) (cycle threshold [CT] = 20.93). No antiviral treatment with lopinavir-ritonavir or hydroxychloroquine was initiated due to the likelihood of deleterious drug-drug interactions (DDI) with immunosuppressants. Furthermore, due to shortages, even for compassionate use, it was not possible to administer remdesivir at an early stage of infection. Instead, empirical antibiotic treatment (amoxicillin-clavulanic acid) was initiated for 5 days. Dexamethasone (20 mg every 24 h [q24h]) was initiated on day 7, following an increase in the CRP concentration to 56 mg/liter. On day 10, in the absence of signs of improvement, the recombinant interleukin 1 (IL-1) receptor antagonist anakinra was added. Despite an enhancement of anti-inflammatory treatment, the patient’s oxygen need increased to 9 liters/min on day 12. A chest CT scan revealed segmental and subsegmental bilateral embolism, with an increase in ground-glass opacities to 70%, requiring enoxaparin. Antibiotherapy was changed to piperacillin-tazobactam and sulfamethoxazole-trimethoprim (curative dose) and continued for 7 days. Following an increase in CRP concentration (175 mg/liter on day 12), a progressive deterioration of kidney function (creatininemia, 123 μmol/liter on day 12), the persistence of nasopharyngeal viral replication (CT = 22.03), and an increase in oxygen requirement to 15 liters/min (on day 16), the IL-6 blocker tocilizumab (8 mg/kg of body weight) was administered and the patient was referred to the intensive care unit (ICU).
FIG 1.
Timeline of the clinical endpoints, immunomodulation therapy, and antimicrobial chemotherapy for our patient. AMC, amoxicillin-clavulanic acid; CS, corticosteroid pulses; DXM, dexamethasone; ISA, isavuconazole; MPA, mycophenolate; RDV, remdesivir; SXT, sulfamethoxazole-trimethoprim; TZP, piperacillin-tazobactam; VAL, valganciclovir.
On day 21, intravenous (i.v.) remdesivir infusion was finally started (200 mg and then 100 mg q24h). From day 24, a progressive deterioration of renal function (tubular necrosis) was observed, culminating in a need for intermittent hemodialysis and the discontinuation of remdesivir after 7 days of treatment (day 28) and tacrolimus. In the ICU, about 20% of COVID-19 patients develop acute kidney impairment (AKI) (3). At that time, we had no argument to suspect a new sepsis or inflammatory reaction. On the following day (day 29), plasma concentrations of remdesivir and GS-441524, its predominant circulating metabolite, were determined 24 h after the last drug intake and then again after a 4-h hemodialysis session (ultrahigh-performance liquid chromatography–tandem mass spectrometry [UPLC-MS/MS]; Waters Xevo; lower limit of quantification of both remdesivir and GS-441524, 1 ng/ml) (4). Before the hemodialysis session, trough plasma concentrations of remdesivir and GS-441524 were <1 ng/ml and 563 ng/ml, respectively. After the hemodialysis session, plasma concentrations of remdesivir and GS-441524 were <1 ng/ml and 226 ng/ml, respectively.
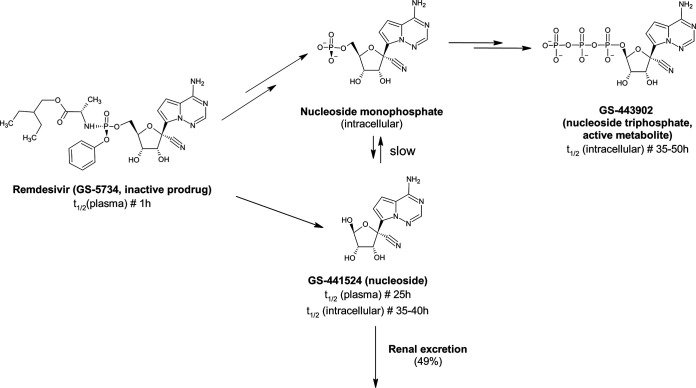
To our knowledge, this is the first report of the impact of intermittent hemodialysis on remdesivir and GS-441524 plasma exposures. Remdesivir is a monophosphoramidate prodrug of the C-adenosine nucleoside analogue GS-441524 (Fig. 2). Pharmacokinetic experiments in cynomolgus monkeys showed that the first-pass effect of oral remdesivir resulted in low bioavailability of the drug. Following i.v. administration, remdesivir is rapidly distributed in peripheral blood mononuclear cells and tissues, where it is rapidly activated to the nucleoside triphosphate (GS-443902) (5).
FIG 2.
Biotransformation of remdesivir (inactive prodrug) into GS-441524 and then GS-443902 (active metabolite, triphosphate analogue).
Its principal metabolite (GS-441524) is eliminated mainly by renal excretion (49%) (6). Given the transient nature of exposure to remdesivir itself (plasma elimination half-life [t1/2], 1 h) and due to the rapid transformation of this prodrug into GS-441524 (plasma t1/2, 25 h), avoiding the cytochrome P450 pathways, remdesivir is unlikely to be involved in deleterious DDI. It may therefore be of particular interest for the treatment of solid-organ transplant patients receiving both immunosuppressive and antifungal prophylactic treatments that might be affected by or cause DDI. For its galenic formulation for i.v. use, remdesivir is combined with sulfobutylether β-cyclodextrin sodium (SEBCD) as a solubility enhancer. This excipient is also renally cleared and accumulates if renal function is impaired (7). The use of remdesivir is not recommended in patients with an estimated glomerular filtration rate of <30 ml/min, but SEBCD is efficiently removed by intermittent dialysis (8). In our patient, before hemodialysis, the remdesivir trough plasma concentration was <1 ng/ml despite AKI, whereas the trough plasma concentration of GS-441524 was about four times the maximum concentration (Cmax) (mean [percent coefficient of variation], 142 ng/ml [30.3%]) observed in healthy adults (6) and higher than those reported in ICU patients with AKI (9). Remdesivir showed an in vitro 50% effective concentration (EC50) of 460 ng/ml against SARS-CoV-2, whereas no EC50 data have yet been reported for GS-441524 (10). The 4-h hemodialysis session removed about 59% of GS-441524, suggesting that hemodialysis might be a viable option if GS-441524 is suspected of being related to AKI. These results were consistent with the low molecular weight (291.26 g/mol), low level of plasma protein binding (<15%), and rapid transformation of remdesivir into GS-441524, which accumulated with AKI (6). Considering the long t1/2 of GS-441524 in normal renal function patients, we considered the physiological clearance in our AKI patient to be negligible facing the hemodialysis.
Unfortunately, 15 days after the persistent AKI event, ventilator-associated pneumonia due to Streptococcus haemolyticus was detected on bronchoalveolar lavage fluid analysis. The patient subsequently died from vasoplegic shock and sepsis. This clinical case highlights the difficulty of treating COVID-19 in a lung transplant patient, in a complicated context of immunosuppression, and with multiple additional coinfections and AKI.
In ICU settings, in which multiple-organ dysfunctions can occur rapidly, hemodialysis may be a viable option for maintaining remdesivir treatment, while improving tolerance, by removing both remdesivir’s metabolite (GS-441524) and SEBCD. Additional studies may prove informative on safety or a possible dose adjustment, particularly in the context of evaluations of therapeutic options for COVID-19.
ACKNOWLEDGMENTS
We thank Gilead Sciences for providing GS-441524 reference standards. We also thank Julie Sappa (Alex Edelman Associates) for her editorial support.
We have no funding to declare. B.V. has received travel grants from Gilead and Janssen and lecture fees from Gilead. D.D. has received fees for participation on the advisory boards of Gilead-Sciences, ViiV Healthcare, Janssen-Cilag, and Merck. J.M. received congress reimbursement fees from Fishe & Paykel and CSL Behring. J.-F.T. received fees for participation on the advisory boards of Astellas, Nabriva, Paratek, 3M, Becton-Dickinson, Merck, Pfizer, Bayer Parma, MedImmune, and Gilead and for giving lectures for Merck, Pfizer, 3M, and bioMérieux. His research group has received grants from Astellas, Merck, Pfizer, and 3M. G.P. has received travel grants, consultancy fees, honoraria, or study grants from various pharmaceutical companies, including Gilead Sciences, Merck, Pharmatechnologie, and ViiV Healthcare. None of the authors has any other competing interests to declare.
REFERENCES
- 1.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, et al. 2020. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehra MR, Ruschitzka F, Patel AN. 2020. Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy S, Gomersall CD, Fowler RA. 2020. Care for critically ill patients with COVID-19. JAMA 323:1499. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 4.Avataneo V, de Nicolò A, Cusato J, Antonucci M, Manca A, Palermiti A, Waitt C, Walimbwa S, Lamorde M, di Perri G, D’Avolio A. 2020. Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: a tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J Antimicrob Chemother 75:1772–1777. doi: 10.1093/jac/dkaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y-C, Deng Q-X, Dai S-X. 2020. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis 35:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Medicines Agency. 2020. Summary on compassionate use. Remdesivir. Gilead. European Medicines Agency, Amsterdam, The Netherlands: https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf. [Google Scholar]
- 7.Hoover RK, Alcorn H, Lawrence L, Paulson SK, Quintas M, Luke DR, Cammarata SK. 2018. Clinical pharmacokinetics of sulfobutylether-β-cyclodextrin in patients with varying degrees of renal impairment. J Clin Pharmacol 58:814–822. doi: 10.1002/jcph.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luke DR, Wood ND, Tomaszewski KE, Damle B. 2012. Pharmacokinetics of sulfobutylether-β-cyclodextrin (SBECD) in subjects on hemodialysis. Nephrol Dial Transplant 27:1207–1212. doi: 10.1093/ndt/gfr472. [DOI] [PubMed] [Google Scholar]
- 9.Tempestilli M, Caputi P, Avataneo V, Notari S, Forini O, Scorzolini L, Marchioni L, Bartoli TA, Castilletti C, Lalle E, Capobianchi MR, Nicastri E, D’Avolio A, Ippolito G, Agrati C. 1 July 2020. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother doi: 10.1093/jac/dkaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]