Resistance to beta-lactams has created a major clinical issue. QPX7728 is a novel ultrabroad-spectrum cyclic boronic acid beta-lactamase inhibitor with activity against both serine and metallo-beta-lactamases developed to address this resistance for use in combination with beta-lactam antibiotics. The objective of these studies was to evaluate the activity of QPX7728 in combination with multiple beta-lactams against carbapenem-resistant Klebsiella pneumoniae isolates in a neutropenic mouse thigh infection model.
KEYWORDS: beta-lactams, QPX7728, CRE
ABSTRACT
Resistance to beta-lactams has created a major clinical issue. QPX7728 is a novel ultrabroad-spectrum cyclic boronic acid beta-lactamase inhibitor with activity against both serine and metallo-beta-lactamases developed to address this resistance for use in combination with beta-lactam antibiotics. The objective of these studies was to evaluate the activity of QPX7728 in combination with multiple beta-lactams against carbapenem-resistant Klebsiella pneumoniae isolates in a neutropenic mouse thigh infection model. Neutropenic mice were infected with strains with potentiated beta-lactam MICs of ≤2 mg/liter in the presence of 8 mg/liter QPX7728. Two strains of carbapenem-resistant K. pneumoniae were tested with aztreonam, biapenem, cefepime, ceftazidime, ceftolozane, and meropenem alone or in combination with 12.5, 25, or 50 mg/kg of body weight of QPX7728 every 2 hours for 24 hours. Treatment with all beta-lactams alone either was bacteriostatic or allowed for bacterial growth. The combination of QPX7728 plus each of these beta-lactams produced bacterial killing at all QPX7728 doses tested. Overall, these data suggest that QPX7728 administered in combination with different partner beta-lactam antibiotics may have utility in the treatment of bacterial infections due to carbapenem-resistant K. pneumoniae.
INTRODUCTION
The beta-lactam class of antibiotics is one of the most valuable and widely utilized classes of antimicrobial agents used for the treatment of nosocomial and community-acquired bacterial infections (1, 2). This class of antibiotics not only has an excellent safety profile but also exhibits broad-spectrum activity against both Gram-positive and Gram-negative bacteria. Over the last three decades, resistance to beta-lactams has increased globally, threatening the utility of this important class of antibiotics (3–7).
Resistance to beta-lactams is primarily mediated by bacterial enzymes called beta-lactamases. These enzymes hydrolyze the beta-lactam ring which eliminates the antibacterial activity of the beta-lactam. Beta-lactam activity can be diminished by beta-lactamases from one or multiple enzymes classes. Beta-lactamases have been categorized into four classes (Ambler classifications) known as A, B, C, and D. The hydrolysis of beta-lactams by the enzymes of classes A, C, and D relies on an active site serine residue, whereas those of class B are metalloenzymes that use one or two zinc ions to coordinate hydrolysis. While serine enzymes from different classes may share some structural similarity, metalloenzymes are structurally dissimilar (9, 10). Given the differences in enzyme structure and binding sites, finding a single molecule that can inhibit both serine and metallo-beta-lactamases has been a daunting challenge.
As resistance to carbapenems, the most active and beta-lactamase-stable beta-lactams, increased, new programs were initiated to develop novel beta-lactamase inhibitors with activity against carbapenemases. These programs were highly successful and led to the development and approval of Avycaz (ceftazidime-avibactam), Recarbrio (imipenem-cilastatin/relebactam), and Vabomere (meropenem-vaborbactam). These agents have broad activity against class A (TEM, CTX-M, SHV, and KPC) and class C (AmpC) enzymes. Avycaz has some activity against class D (OXA-48) enzymes. However, none of these agents have activity against metallo-beta-lactamases, such as NDM, VIM, and IMP or against class D enzymes found in Acinetobacter baumannii, such as OXA-23 (11–15). Consequently, there is a continued effort to discover and develop new beta-lactamase inhibitors that can inhibit both serine and metallo-beta-lactamases in Enterobacteriaceae as well as in nonfermenters (Pseudomonas aeruginosa and A. baumannii).
As the part of this effort, our team discovered and initiated the development of QPX7728, an ultrabroad-spectrum beta-lactamase inhibitor with activity against all four beta-lactamase classes and the ability to restore activity to cephalosporins, carbapenems, penicillins, and monobactams (16). QPX7728 in combination with multiple different beta-lactams has in vitro activity against resistant organisms considered serious or urgent threats by the CDC, such as carbapenem-resistant Enterobacteriaceae, P. aeruginosa, and A. baumannii (17–19). The objective of these studies was to evaluate the in vivo activity of QPX7728 administered in combination with multiple beta-lactams in a neutropenic mouse thigh infection model of carbapenem-resistant K. pneumoniae.
RESULTS
Susceptibility studies.
The susceptibilities of carbapenem-resistant K. pneumoniae KP1096 and K. pneumoniae KP1223 to aztreonam, biapenem, cefepime, ceftazidime, ceftolozane, and meropenem were determined alone and combined with a fixed concentration of 8 mg/liter QPX7728. These strains produced various beta-lactamases as well as other non-beta-lactamase-mediated resistance mechanisms, including porin mutation(s) that are known to affect the potency of beta-lactams. As shown in Table 1, the in vitro potency of these beta-lactams was markedly improved when combined with QPX7728 at 8 mg/liter.
TABLE 1.
Susceptibility of K. pneumoniae strains to beta-lactams alone and in combination with QPX7728
| Strain | Beta-lactamases | MIC (mg/liter) ofa
: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATM |
BIA |
FEP |
CAZ |
TOL |
MEM |
||||||||
| Alone | QPX7728b | Alone | QPX7728 | Alone | QPX7728 | Alone | QPX7728 | Alone | QPX7728 | Alone | QPX7728 | ||
| KP1096 | KPC-2, TEM, SHV-11 | >256 | 0.25 | >256 | 0.13 | 128 | 1 | 128 | 1 | 64 | 1 | >256 | 0.5 |
| KP1223 | KPC-2, SHV, TEM | >256 | 2 | >256 | ≤0.06 | >256 | 0.5 | >256 | 2 | >256 | 1 | >256 | ≤0.06 |
ATM, aztreonam; BIA, biapenem; FEP, cefepime; CAZ, ceftazidime; TOL, 395 ceftolozane; MEM, meropenem.
At a concentration of 8 mg/liter.
Pharmacokinetics.
The pharmacokinetic parameters for aztreonam, biapenem, ceftazidime, meropenem, and QPX7728 are shown in Table 2. The pharmacokinetic parameters used for both the mouse and human pharmacokinetic treatment regimen simulations are provided in Table 3.
TABLE 2.
Pharmacokinetic parameters following single doses of compounds administered by the intraperitoneal route
| Compound | Dose (mg/kg) | AUC0–∞a (mg*h/liter) | CL/Fb (liter/h/kg) | Cmaxc (mg/liter) | T1/2d (h) |
|---|---|---|---|---|---|
| Aztreonam | 150 | 131.86 | 1.13 | 249.47 | 0.29 |
| Biapenem | 100 | 104.52 | 0.96 | 222.70 | 0.22 |
| Ceftazidime | 300 | 150.75 | 1.99 | 233.20 | 0.28 |
| Meropenem | 300 | 145.79 | 2.06 | 326.81 | 0.19 |
| QPX7728 | 10 | 28.52 | 0.35 | 39.20 | 1.55 |
| QPX7728 | 30 | 70.01 | 0.43 | 95.30 | 1.43 |
| QPX7728 | 100 | 223.08 | 0.45 | 337.00 | 1.27 |
AUC0–∞, area under the concentration-time curve from 0 to infinity.
CL/F, oral clearance.
Cmax, maximum concentration of drug in serum.
T1/2, half-life.
TABLE 3.
Pharmacokinetic parameters used for the simulations
| Drug by organism | k01a (1/h) | k10b (1/h) | V/Fc (liter/kg) | Vd (liter) | Protein binding (%) | Reference(s) |
|---|---|---|---|---|---|---|
| Human | ||||||
| Aztreonam | 0.433 | 12.6 | 56 | 29 | ||
| Biapenem | 0.594 | 18.5 | 7 | Unpublished, 30 | ||
| Cefepime | 0.400 | 18.0 | 20 | 31 | ||
| Ceftazidime | 0.381 | 18.1 | 5 | 32 | ||
| Ceftolozane | 0.408 | 13.3 | 20 | 33 | ||
| Meropenem | 0.743 | 20.2 | 2 | 34 | ||
| Mouse | ||||||
| Aztreonam | 28.56 | 2.37 | 0.48 | 56.5 | This manuscript, 35 | |
| Biapenem | 16.38 | 3.16 | 0.30 | 2 | This manuscript, 30 | |
| Cefepime | 16.00 | 2.24 | 0.50 | 0 | 25–27 | |
| Ceftazidime | 9.51 | 4.37 | 0.40 | 0 | This manuscript, 36 | |
| Ceftolozane | 3.00 | 3.09 | 0.43 | 5 | 28 | |
| Meropenem | 13.73 | 3.61 | 0.57 | 10 | This manuscript, 37 |
k01, absorption rate constant.
k10, elimination rate constant.
V/F, volume of distribution/fraction absorbed.
V, volume of distribution.
Treatment regimens.
The comparison of the simulated percentage of the 24-h study duration that free-drug levels exceed the MIC (%24h ƒT>MIC) of each beta-lactam in mice and that of humans is shown in Table 4. Based on the simulations, the dosage regimens were expected to produce %24h ƒT>MIC that were similar to human dosage regimens for each beta-lactam.
TABLE 4.
Comparison of the 24-h free AUC and %24h ƒT>MIC of each beta-lactam in mice and in humans
| Drug | Species | Dosage regimena | %24h ƒT>MIC at MIC (mg/liter) of: |
24-h free AUC mg*h/liter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | ||||
| Aztreonam | Human | 2,000 mg TID by 1-h infusion | 0 | 0 | 22.5 | 45 | 66.9 | 88.6 | 100 | 100 | 100 | 100 | 484 |
| Mouse | 150 mg/kg q2h | 6 | 22 | 38 | 52.5 | 67 | 81.5 | 96.5 | 100 | 100 | 100 | 894 | |
| Biapenem | Human | 1,000 mg TID by 3-h infusion | 0 | 0 | 0 | 27.5 | 60 | 74.4 | 88.6 | 100 | 100 | 100 | 254 |
| Mouse | 100 mg/kg q2h | 16.5 | 29 | 40 | 50 | 62 | 73 | 84 | 95 | 100 | 100 | 1,229 | |
| Cefepime | Human | 2,000 mg TID by 2-h infusion | 0 | 0 | 34.1 | 61.8 | 88.6 | 100 | 100 | 100 | 100 | 100 | 667 |
| Mouse | 150 mg/kg q2h | 20 | 36.5 | 50.0 | 68.5 | 84 | 100 | 100 | 100 | 100 | 100 | 1,607 | |
| Ceftazidime | Human | 2,000 mg TID by 2-h infusion | 0 | 8.8 | 41.9 | 70.6 | 97.5 | 100 | 100 | 100 | 100 | 100 | 826 |
| Mouse | 300 mg/kg q2h | 25 | 41 | 55.5 | 69.5 | 83.5 | 98.0 | 100 | 100 | 100 | 100 | 1,809 | |
| Ceftolozane | Human | 2,000 mg TID by 1-h infusion | 0 | 17.5 | 44.3 | 68.2 | 88.8 | 100 | 100 | 100 | 100 | 100 | 884 |
| Mouse | 150 mg/kg q2h | 0 | 38 | 59 | 74.5 | 88.5 | 100 | 100 | 100 | 100 | 100 | 1,287 | |
| Meropenem | Human | 2,000 mg TID by 3-h infusion | 0 | 0 | 18.8 | 44.4 | 60.0 | 75.1 | 87.1 | 100 | 100 | 100 | 392 |
| Mouse | 300 mg/kg q2h | 20 | 30 | 41.5 | 51 | 60.5 | 70 | 79 | 89 | 100 | 100 | 1,575 | |
q2h, every 2 hours; TID, three times a day.
Thigh infection model.
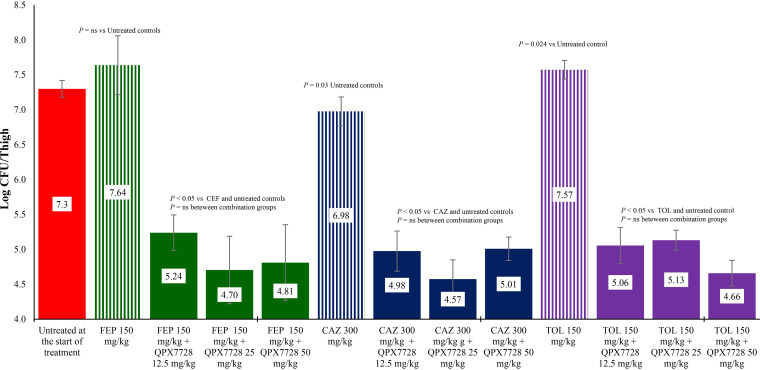
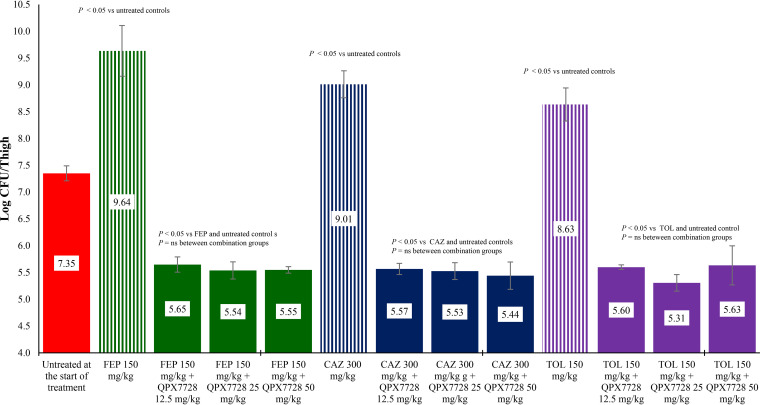
The activity of two carbapenems, three cephalosporins, and a monobactam alone or in combination with QPX7728 were evaluated against both strains of carbapenem-resistant K. pneumoniae. As shown in Fig. 1 and 2, treatment with ceftazidime, ceftolozane, or cefepime was ineffective at reducing bacterial counts for either strain compared with untreated control groups at the start of treatment. When the same exposures of these cephalosporins were combined with 12.5, 25, or 50 mg/kg of body weight of QPX7728, they produced about 2 log CFU reductions in bacterial counts for both strains compared with untreated controls at the start of treatment and all combinations with beta lactams alone (P < 0.05).
FIG 1.
Activity of cefepime (FEP), ceftazidime (CAZ), and ceftolozane (TOL) alone and in combination with QPX7728 against K. pneumoniae KP1096 in a neutropenic mouse thigh infection model. Treatments were administered every 2 hours for 24 hours by the intraperitoneal route.
FIG 2.
Activity of cefepime (FEP), ceftazidime (CAZ), and ceftolozane (TOL) alone and in combination with QPX7728 against K. pneumoniae KP1223 in a neutropenic mouse thigh infection model. Treatments were administered every 2 hours for 24 hours by the intraperitoneal route.
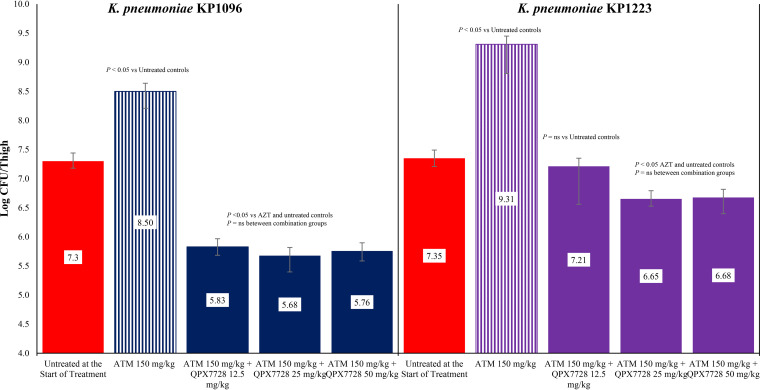
The activity of aztreonam alone and in combination with 12.5, 25, or 50 mg/kg of QPX7728 against both strains of carbapenem-resistant K. pneumoniae are shown in Fig. 3. The exposure of aztreonam allowed between 1 and 2 logs of bacterial growth in both K. pneumoniae strains. For K. pneumoniae KP1096, the combination of aztreonam with 12.5, 25, or 50 mg/kg of QPX7728 produced 1.47, 1.62, and 1.54 log CFU reductions in bacterial counts in the thigh, respectively. The reduction in bacterial counts with the combination therapy for all three dose regimens of QPX7728 was significantly greater than that observed for aztreonam alone (P < 0.05). For K. pneumoniae KP1223, the combination of aztreonam with 12.5, 25, or 50 mg/kg of QPX7728 produced 0.14, 0.70, and 0.67 log CFU reductions in bacterial counts in the thigh, respectively. The reduction in bacterial counts with aztreonam plus 12.5 mg/kg of QPX7728 was not significant compared with untreated controls at the start of treatment (P > 0.05). However, the bacterial counts with aztreonam in combination with 25 or 50 mg/kg of QPX7728 were significantly lower than that observed with aztreonam alone or untreated controls at the start of treatment (P < 0.05).
FIG 3.
Activity aztreonam (ATM) alone and in combination with QPX7728 against K. pneumoniae KP1096 and K. pneumoniae KP1223 in a neutropenic mouse thigh infection model. Treatments were administered every 2 hours for 24 hours by the intraperitoneal route.
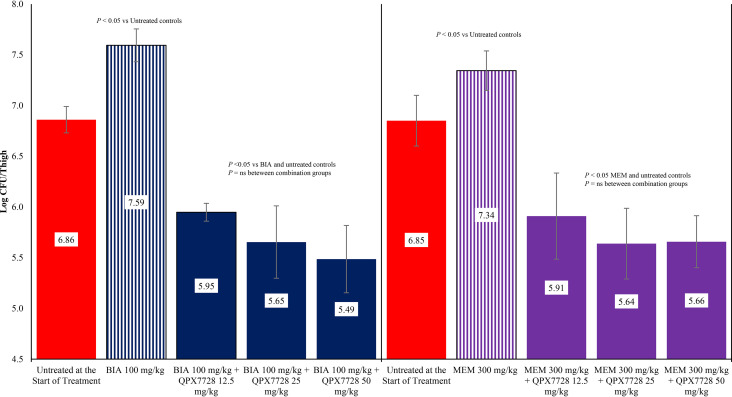
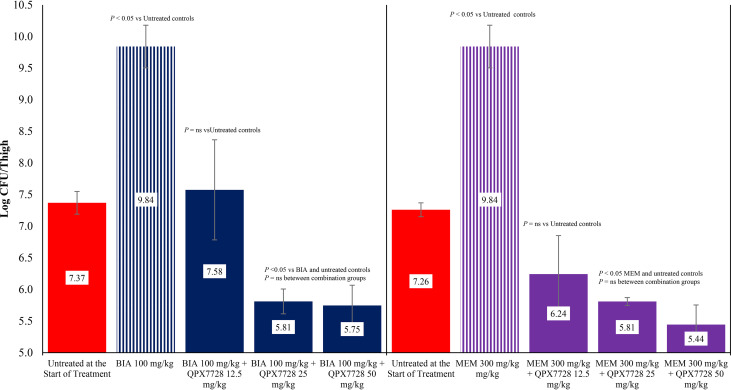
The activity of biapenem and meropenem alone and in combination with QPX7728 against K. pneumoniae strain KP1096 is shown in Fig. 4. Treatment with biapenem and meropenem allowed 0.73 and 0.49 log CFU, respectively, of bacterial growth compared with untreated controls at the start of treatment. The administration of biapenem in combination with 12.5, 25, or 50 mg/kg of QPX7728 produced 0.91, 1.21, and 1.37 log CFU reductions, respectively, in bacterial counts in the thigh compared with untreated controls at the start of treatment. Treatment with meropenem in combination with 12.5, 25, or 50 mg/kg of QPX7728 produced 0.94, 1.21, and 1.19 log CFU reductions, respectively, in bacterial counts in the thigh compared with untreated controls at the start of treatment, respectively. The reductions in bacterial counts observed with combination therapy for both carbapenems at all doses of QPX7728 were significantly greater than those observed with either biapenem or meropenem alone (P < 0.05).
FIG 4.
Activity of biapenem (BIA) and meropenem (MEM) alone and in combination with QPX7728 against K. pneumoniae KP1096 in a neutropenic mouse thigh infection model. Treatments were administered every 2 hours for 24 hours by the intraperitoneal route.
The activity of biapenem and meropenem alone and in combination with QPX7728 against K. pneumoniae strain KP1223 is shown in Fig. 5. Treatment with biapenem or meropenem allowed for, roughly, 2 log CFU of bacterial growth compared with untreated controls at the start of treatment. The administration of biapenem in combination with 12.5 mg/kg of QPX7728 did not produce a reduction in bacterial counts compared with untreated controls at the start of treatment (P > 0.05), but the bacterial counts were significantly lower with 12.5 mg/kg of QPX7728 than treatment with biapenem alone (P < 0.05). The administration of biapenem in combination of 25 or 50 mg/kg of QPX7728 produced 1.56 and 1.62 log CFU reductions, respectively, in bacterial counts in the thigh. These reductions in bacterial counts were significantly lower than that observed with biapenem alone and untreated controls at the start of treatment (P < 0.05). The administration of meropenem in combination with 12.5, 25, or 50 mg/kg of QPX7728 produced between 1.02, 1.45, or 1.82 log CFU reductions, respectively, in bacterial counts in the thigh. These reductions in bacterial counts were significantly lower than that observed with meropenem alone and untreated controls at the start of treatment (P < 0.05).
FIG 5.
Activity of biapenem (BIA) and meropenem (MEM) alone and in combination with QPX7728 against K. pneumoniae KP1223 in a neutropenic mouse thigh infection model. Treatments were administered every 2 hours for 24 hours by the intraperitoneal route.
QPX7728 alone did not have any antibacterial activity against K. pneumoniae KP1096. Treatment with QPX7728 at 50 mg/kg every 2 hours allowed for 1.14 log CFU/thigh of bacterial growth.
For all experiments, untreated control groups for both K. pneumoniae strains were sacrificed after 12 hours due to signs of distress. Over the 12-hour period, K. pneumoniae KP1096 grew by 0.7 log CFU/thigh and K. pneumoniae KP1223 grew by 2.0 log CFU/thigh.
DISCUSSION
The steady rise of bacterial resistance due to beta-lactamases in Enterobacteriaceae is threatening the utility of beta-lactams against these organisms (20–22).
In response, our laboratory has been investigating novel cyclic boronic acid beta-lactamase inhibitors for over a decade. Our first discovery and successful development candidate, vaborbactam, was specifically designed to inhibit serine carbapenemases, particularly the K. pneumoniae carbapenemase (KPC) enzyme. Vaborbactam is now approved in the United States and Europe in combination with meropenem as Vabomere (United States) or Vaborem (European Union). Since that discovery, our focus has been on improving the potency and spectrum of these inhibitors, with an emphasis on metallo-beta-lactamases. Through these investigations, we have discovered QPX7728, an ultrabroad-spectrum beta-lactamase inhibitor with activity against both serine (class A, C, and D) and metalloenzymes (class B) in Enterobacteriaceae. In addition, QPX7728 is less susceptible to non-beta-lactamase resistance mechanisms, such as efflux and porin mutations (16–19). Given this ultrabroad spectrum activity, QPX7728 was tested in vitro and in vivo in combination with multiple beta-lactams possessing various degrees of intrinsic stability to beta-lactamases against two strains of carbapenem-resistant K. pneumoniae. Both strains used in these studies produced SHV, TEM, and KPC beta-lactamases and had mutations in the outer membrane porins, OmpK 35 and OmpK 36 (23). The combination of resistance mechanisms rendered these strains highly resistant to each beta-lactam alone (all MICs were ≥64 mg/liter). The addition of QPX7728 at 8 mg/liter reduced the MICs of each beta-lactam at least 32-fold, with the highest MIC to the combination being 2 mg/liter. The dosage regimens in mice for each beta-lactam were selected to approximate the %24h ƒT>MIC of human regimens. The exposure simulations were based on single-dose pharmacokinetic (PK) studies from our laboratory with the exceptions of ceftolozane and cefepime. The PK profiles for both of these drugs were referenced from the literature and not replicated in our laboratory; as such, the simulated profiles reported may not reflect the exposures generated in the efficacy studies.
Based on the simulations, the selected dosage regimens for aztreonam, biapenem, cefepime, ceftazidime, ceftolozane, and meropenem alone resulted in bacterial stasis or bacterial growth compared with untreated controls at the start of treatment. In contrast, the combination of each beta-lactam with QPX7728 produced at least 1 log of bacterial killing against both strains of carbapenem-resistant K. pneumoniae, apart from aztreonam against K. pneumoniae strain KP1223.
Overall, these data demonstrate that QPX7728 restores the activity of multiple different beta-lactams, including cephalosporins, carbapenems, and monobactams, against highly resistant strains of K. pneumoniae. While these data were generated with only two strains and only cover a limited number of mechanisms that confer resistance to beta-lactams, the data suggest that QPX7728 administered in combination with multiple different beta-lactams could have clinical utility in the treatment of infections due to carbapenem-resistant Enterobacteriaceae.
MATERIALS AND METHODS
Mice.
Female Swiss mice (age, 5 to 6 weeks) were obtained from Envigo (Livermore, CA). All studies using animals were performed under protocols approved by an Institutional Animal Care and Use Committee (IACUC).
Antimicrobial agents.
Cefepime for injection (lot number 9D01006A41), ceftazidime for injection (lot number 108225C), and meropenem for injection (lot number DF-3297) were obtained from commercial sources. Cefepime, ceftazidime, and meropenem were prepared according to their package inserts. Aztreonam (lot number S07F022) powder was obtained from a commercial source and solubilized in 1% NaHCO3. Biapenem, ceftolozane, and QPX7728 were synthesized by Qpex Biopharma, Inc. (San Diego, CA) and solubilized in 0.9% saline.
Bacterial strain testing and MIC determination.
Two K. pneumoniae clinical isolates were used in these studies. The susceptibility of these isolates to aztreonam, biapenem, cefepime, ceftazidime, ceftolozane, and meropenem alone and in combination with 8 mg/liter of QPX7728 was determined by a broth microdilution assay according to CLSI reference methods (24). Assays were performed using a final volume of 100 μl. The bacterial suspensions were adjusted to yield a final cell density of ca. 5 × 105 CFU/ml. Each beta-lactam was prepared at a concentration equivalent to 2-fold the highest desired final concentration in culture medium and was then diluted directly into 96-well microtiter plates. For the determination of MICs in the presence of QPX7728, QPX7728 was added at a fixed concentration of 8 mg/liter. Microtiter plates were incubated for 16 to 18 h at 37°C and were read by using a microtiter plate reader (Molecular Devices, Sunnyvale, CA) at 600 nm, as well as by visual observation by using a microtiter plate reading mirror. The MIC was defined as the lowest concentration of antibiotic at which the visible growth of the organism was completely inhibited.
Neutropenic mouse thigh infection model.
Female mice (n = 2/group) were rendered neutropenic by an intraperitoneal (i.p.) injection of 150 mg/kg cyclophosphamide (Baxter, IL) on day 1 and day 4. On day 5, mice were infected by intramuscular injection of 0.1 ml of inoculum (107 CFU/ml) into both thigh muscles while under isoflurane anesthesia (5% isoflurane in oxygen running at 4 liters/min) (21). The infecting bacterial suspensions were prepared as follows: strains were grown in Mueller-Hinton broth (MHB) at 37°C under constant aeration (300 rpm) for 20 h. The infecting inoculum was prepared by removing an aliquot and diluting it into fresh MHB at 37°C, under constant aeration, for 3 h to reach an absorbance at 600 nm of 0.30 to 0.35. The bacterial suspensions were diluted in fresh MHB to make ∼107 CFU/ml by correlation of absorbance at 600 nm with predetermined plates.
Pharmacokinetics.
The pharmacokinetics of cefepime and ceftolozane in mice were referenced from the literature (25–28). The pharmacokinetics of aztreonam, biapenem, ceftazidime, meropenem, and QPX7728 were determined for this work in female Swiss mice. Mice were rendered neutropenic and infected in both thighs as described above. Two hours following infection, mice were administered a single dose by the i.p. route. At 0.08, 0.25, 0.5, 1, 2, 4, and 6 hours following administration, 3 mice/time point were euthanized and their blood collected by cardiac puncture and transferred to EDTA-containing tubes. Blood samples were centrifuged within 5 min of collection at 12,000 × g for 5 min to obtain plasma. An equal volume of morpholinopropanesulfonic acid (MOPS) buffer (pH 7) was added to plasma samples containing aztreonam and meropenem. All samples were stored at −80°C until analyzed.
Bioanalytical analysis.
Aztreonam standard curves were prepared in 50% 1 M MOPS/50% plasma at concentrations of 0.05 to 50.0 μg/ml. Twenty-five-microliter aliquots of sample were placed in 1.5-ml microcentrifuge tubes containing 100 μl of 4 μg/ml QPX7015 in 10%, 45%, 45% of water-methanol-acetonitrile (vol/vol/vol). Biapenem standard curves were prepared in mouse plasma at concentrations of 0.01 to 100 μg/ml. Fifty-microliter aliquots of sample were placed in 1.5-ml microcentrifuge tubes containing 200 μl of 2 μg/ml gatifloxacin and 2 μg/ml QPX7015 in 100 mM ammonium acetate in methanol. Ceftazidime standard curves were prepared in mouse plasma at concentrations of 0.01 to 100 μg/ml. Fifty-microliter aliquots of sample were placed in 1.5-ml microcentrifuge tubes containing 200 μl of 2 μg/ml gatifloxacin and 2 μg/ml QPX7015 in 100 mM ammonium acetate in methanol. Meropenem standard curves were prepared in 50% 1 M MOPS/50% plasma at concentrations of 0.04 to 50 μg/ml. Twenty-five-microliter aliquots of sample were placed in 1.5-ml microcentrifuge tubes containing 200 μl of 4.0 μg/ml doripenem in 10%, 45%, 45% of water-methanol-acetonitrile (vol/vol/vol). QPX7728 standard curves were prepared in 50-50 plasma-phosphate buffer at concentrations of 0.05 to 50.0 μg/ml. Thirty-microliter aliquots of sample were placed in 1.5-ml microcentrifuge tubes containing 30 μl of 2 μg/ml QPX7701 in 50-50 water-acetonitrile and 30 μl of 20 mM phosphate buffer (pH 7.4).
For all antibiotics, the samples were mixed using a vortex mixer and then centrifuged for 10 min at 15,000 × g using a tabletop centrifuge. The supernatant was removed and placed in a 96-well plate, and then 10 μl of each sample was injected onto a high-performance liquid chromatography-mass spectrometer (HPLC-MS) for quantification.
Plasma concentrations were fitted using a one-compartment model with first-order input and first-order elimination (Phoenix WinNonlin version 8.1; Certara USA, Inc., Princeton, NJ).
Treatment regimens.
Treatment regimens in mice were selected to approximate the %24h ƒT>MIC of each beta-lactam produced by their respective human dosage regimens (29–34). The total drug plasma profiles of each beta-lactam in humans were simulated using a one-compartment intravenous (i.v.) infusion model and first-order elimination (Phoenix WinNonlin version 8.1; Certara USA, Inc., Princeton, NJ) based on the literature. The free plasma profiles were calculated by correcting the total drug profiles for protein binding. For mice, the total drug plasma profiles of each beta-lactam were simulated using a one-compartment model with first-order input and first-order elimination (Phoenix WinNonlin version 8.1) based on single-dose studies from this work or from the literature. The plasma profiles were then corrected for protein binding and the dosage regimen selected that approximated the %24h ƒT>MIC of the human dosage regimen. Ceftazidime and meropenem were administered at 300 mg/kg every 2 hours. Aztreonam, cefepime, and ceftolozane were administered at 150 mg/kg every 2 hours. Biapenem was administered at 100 mg/kg every 2 hours. Each beta-lactam was administered alone and in combination with QPX7728 at doses of 12.5, 25, and 50 mg/kg starting 2 hours postinfection and were continued every 2 hours over 24 hours by the i.p. route.
CFU determination.
Untreated control animals were euthanized at the start of treatment to establish the baseline bacterial burden (2 hours postinfection). All treated and untreated control groups were euthanized by carbon dioxide asphyxiation. The thighs (n = 4/group) were removed aseptically and homogenized (Pro200 homogenizer; Pro Scientific, Monroe, CT) in ice-cold sterile saline. Serial 10-fold dilutions of the homogenized thighs were plated onto Mueller-Hinton agar, and the colonies were counted.
Statistical analysis.
Bacterial counts were analyzed using an unpaired t test (GraphPad Prism version 6.03). A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work, including the efforts of Mojgan Sabet, Ziad Tarazi, and David C. Griffith, was funded in part by the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA) under contract HHSO100201600026C.
REFERENCES
- 1.Adams DJ, Susi A, Nylund CM. 2020. Clinical characteristics, risk factors, and outcomes of patients hospitalized in the US military health system with carbapenem-resistant Enterobacteriaceae infection. Am J Infect Control 48:644–649. doi: 10.1016/j.ajic.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Thakuria B, Lahon K. 2013. The beta lactam antibiotics as an empirical therapy in a developing country: an update on their current status and recommendations to counter the resistance against them. J Clin Diagn Res 7:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. 2016. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis 85:459–465. doi: 10.1016/j.diagmicrobio.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Nicasio AM, Kuti JL, Nicolau DP. 2008. The current state of multidrug-resistant Gram-negative bacilli in North America. Pharmacotherapy 28:235–249. doi: 10.1592/phco.28.2.235. [DOI] [PubMed] [Google Scholar]
- 5.Ray S, Anand D, Purwar S, Samanta A, Upadhye KV, Gupta P, Dhar D. 2018. Association of high mortality with extended-spectrum beta-lactamase (ESBL) positive cultures in community acquired infections. J Crit Care 44:255–260. doi: 10.1016/j.jcrc.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Talbot GH, Jezek A, Murray BE, Jones RN, Ebright RH, Nau GJ, Rodvold KA, Newland JG, Boucher HW, Infectious Diseases Society of America. 2019. The Infectious Diseases Society of America's 10 x '20 initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration Approved by 2020): is 20 x '20 a possibility? Clin Infect Dis 69:1–11. doi: 10.1093/cid/ciz089. [DOI] [PubMed] [Google Scholar]
- 7.Bush K. 2018. Past and present perspectives on beta-lactamases. Antimicrob Agents Chemother 62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho JC, Zmarlicka MT, Shaeer KM, Pardo J. 2018. Meropenem/vaborbactam, the first carbapenem/beta-lactamase inhibitor combination. Ann Pharmacother 52:769–779. doi: 10.1177/1060028018763288. [DOI] [PubMed] [Google Scholar]
- 12.Zasowski EJ, Rybak JM, Rybak MJ. 2015. The beta-lactams strike back: ceftazidime-avibactam. Pharmacotherapy 35:755–770. doi: 10.1002/phar.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagace-Wiens PRS, Walkty A, Denisuik A, Golden A, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2018. Correction to: Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-beta-lactamase inhibitor combinations. Drugs 78:787. doi: 10.1007/s40265-018-0910-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagace-Wiens PRS, Walkty A, Denisuik A, Golden A, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2018. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-beta-lactamase inhibitor combinations. Drugs 78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 15.Vena A, Castaldo N, Bassetti M. 2019. The role of new beta-lactamase inhibitors in Gram-negative infections. Curr Opin Infect Dis 32:638–646. doi: 10.1097/QCO.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 16.Hecker SJ, Reddy KR, Lomovskaya O, Griffith DC, Rubio-Aparicio D, Nelson K, Tsivkovski R, Sun D, Sabet M, Tarazi Z, Parkinson J, Totrov M, Boyer SH, Glinka TW, Pemberton OA, Chen Y, Dudley MN. 2020. Discovery of cyclic boronic acid QPX7728, an ultrabroad-spectrum inhibitor of serine and metallo-beta-lactamases. J Med Chem 63:7491–7507. doi: 10.1021/acs.jmedchem.9b01976. [DOI] [PubMed] [Google Scholar]
- 17.Lomovskaya O, Nelson K, Rubio-Aparicio D, Tsivkovski R, Sun D, Dudley MN. 2020. Impact of intrinsic resistance mechanisms on potency of QPX7728, a new ultra-broad-spectrum beta-lactamase inhibitor of serine and metallo beta-lactamases in Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Antimicrob Agents Chemother 64:e00552-20. doi: 10.1128/AAC.00552-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomovskaya O, Tsivkovski R, Nelson K, Rubio-Aparicio D, Sun D, Totrov M, Dudley MN. 2020. Spectrum of beta-lactamase inhibition by the cyclic boronate QPX7728, an ultra-broad-spectrum beta-lactamase inhibitor of serine and metallo beta-lactamases: enhancement of activity of multiple antibiotics against isogenic strains expressing single β-lactamases. Antimicrob Agents Chemother 64:e00212-20. doi: 10.1128/AAC.00212-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castanheira M, Lindley J, Nelson K, Rubio-Aparicio D, Lomovskaya O. 2019. In vitro activity of the β-lactamase inhibitor QPX7728 combined with several β-lactams when tested against Acinetobacter baumannii and Pseudomonas aeruginosa. Abstract of Paper, AAR-711. ASM Microbe, San Francisco, CA. [Google Scholar]
- 20.Lynch JP, Clark NM, Zhanel GG. 2013. Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum β-lactamases and carbapenemases). Expert Opin Pharmacother 14:199–210. doi: 10.1517/14656566.2013.763030. [DOI] [PubMed] [Google Scholar]
- 21.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership Group. 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 23.Griffith DC, Sabet M, Tarazi Z, Lomovskaya O, Dudley MN. 2018. Pharmacokinetics/pharmacodynamics of vaborbactam, a novel beta-lactamase inhibitor, in combination with meropenem. Antimicrob Agents Chemother 63:e01659-18. doi: 10.1128/AAC.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 10th ed CLSI document M7-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Kaku N, Kosai K, Takeda K, Uno N, Morinaga Y, Hasegawa H, Miyazaki T, Izumikawa K, Mukae H, Yanagihara K. 2017. Efficacy and pharmacokinetics of the combination of OP0595 and cefepime in a mouse model of pneumonia caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 61:e00828-17. doi: 10.1128/AAC.00828-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Máthé A, Komka K, Forczig M, Szabó D, Anderlik P, Rozgonyi F. 2006. The effect of different doses of cisplatin on the pharmacokinetic parameters of cefepime in mice. Lab Anim 40:296–300. doi: 10.1258/002367706777611514. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki S, Okazaki K, Tsuji M, Yamaguchi K. 2004. Pharmacodynamics of S-3578, a novel cephem, in murine lung and systemic infection models. Antimicrob Agents Chemother 48:378–383. doi: 10.1128/aac.48.2.378-383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchers MJ, Mavridou E, Seyedmousavi S, van Mil AC, Lagarde C, Mouton JW. 2015. Plasma and epithelial lining fluid pharmacokinetics of ceftolozane and tazobactam alone and in combination in mice. Antimicrob Agents Chemother 59:3373–3376. doi: 10.1128/AAC.04402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.E.R. Squibb and Sons. Azactam (aztreonam) prescribing information. 2020. E.R. Squibb and Sons, East Brunswick, NJ. [Google Scholar]
- 30.Yamashita N, Kawashima K, Nomura K, Takeuchi H, Hikida M, Naruke T. 1994. Pharmacokinetics of biapenem in laboratory animals. Chemother 42:243–250. [Google Scholar]
- 31.Hospira Inc. Maxipime (cefepime) prescribing information. 2017. Hospira Inc, Lake Forest, IL. [Google Scholar]
- 32.Allergan, Inc. Avycaz (ceftazidime – avibactam) prescribing information. 2019. Allergan, Inc, Dublin, Ireland. [Google Scholar]
- 33.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubino CM, Bhavnani SM, Loutit JS, Morgan EE, White D, Dudley MN, Griffith DC. 2018. Phase 1 study of the safety, tolerability, and pharmacokinetics vaborbactam and meropenem alone and in combination following single and multiple doses in healthy adult subjects. Antimicrob Agents Chemother 62:e02228-17. doi: 10.1128/AAC.02228-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-beta-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Nichols WW, Mouton JW. 2015. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 59:2299–2304. doi: 10.1128/AAC.04627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabet M, Tarazi Z, Griffith DC. 2020. Pharmacodynamics of meropenem against Acinetobacter baumannii in a neutropenic mouse thigh infection model. Antimicrob Agents Chemother 64:e02388-19. doi: 10.1128/AAC.02388-19. [DOI] [PMC free article] [PubMed] [Google Scholar]