Activities of cefiderocol under simulated human plasma concentrations at the recommended dosing regimen of 2 g every 8 h with a 3-h infusion were evaluated using an in vitro chemostat model. Against a total of 6 meropenem-resistant Gram-negative strains with cefiderocol MICs of 0.5 to 4 μg/ml, including metallo-β-lactamase producers and carbapenem-resistant Acinetobacter baumannii, cefiderocol treatment showed a bactericidal effect within 8 h and sustained efficacy with no marked bacterial regrowth over 24 h.
KEYWORDS: cefiderocol, carbapenem resistant, Enterobacterales, Acinetobacter baumannii, chemostat model, nonfermenters
ABSTRACT
Activities of cefiderocol under simulated human plasma concentrations at the recommended dosing regimen of 2 g every 8 h with a 3-h infusion were evaluated using an in vitro chemostat model. Against a total of 6 meropenem-resistant Gram-negative strains with cefiderocol MICs of 0.5 to 4 μg/ml, including metallo-β-lactamase producers and carbapenem-resistant Acinetobacter baumannii, cefiderocol treatment showed a bactericidal effect within 8 h and sustained efficacy with no marked bacterial regrowth over 24 h.
TEXT
Cefiderocol, a novel parenteral siderophore cephalosporin, shows potent activities against a wide range of multidrug-resistant Gram-negative bacilli (1–3). Especially, cefiderocol is the only β-lactam antibiotic with activities against both Gram-negative pathogens harboring metallo-type β-lactamase (MBL) and carbapenem-resistant Acinetobacter species (4). The United States Food and Drug Administration (FDA) approved cefiderocol for the treatment of complicated urinary tract infections with susceptible breakpoints of 2 and 1 μg/ml against Enterobacterales and Pseudomonas aeruginosa, respectively (5). The susceptibility breakpoint was different from the provisional breakpoint of 4 μg/ml against Enterobacterales, P. aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia by the Clinical and Laboratory Standards Institute (6). In Europe, cefiderocol has been approved recently for the treatment of aerobic Gram-negative infections in adult patients with limited treatment options, and the European Committee on Antimicrobial Susceptibility Testing has set up breakpoints at 2 μg/ml for both Enterobacterales and P. aeruginosa. The purpose of this study is to evaluate the efficacy of cefiderocol under simulated human plasma concentrations over 24 h against clinical strains with a cefiderocol MIC around the breakpoint in a one-compartment in vitro chemostat model. MBL-producing strains were also included in this study because of the limited treatment options for such bacteria, and difficulties in evaluation by animal models have been reported due to the in vitro-in vivo discordance against MBL producers caused by the poor activity of MBL in animal models (7). Overall, this chemostat model is expected to provide useful information for treatment against isolates with cefiderocol MICs of 0.5 to 4 μg/ml, including MBL producers.
A total of 6 clinical isolates (2 P. aeruginosa, 1 A. baumannii, 1 Escherichia coli, and 2 Klebsiella pneumoniae) harboring either VIM, IMP, OXA-23, NDM, or KPC, with cefiderocol MICs of 0.5 to 4 μg/ml using iron-depleted cation-adjusted Mueller-Hinton broth (ID-CAMHB) (Table 1) (8), were evaluated by the in vitro chemostat model as reported previously (9). The isolates of Enterobacterales and P. aeruginosa were selected to have the MIC that was around the susceptible breakpoint by FDA, and one isolate of A. baumannii was selected to have a similar MIC to the test isolates of P. aeruginosa. Briefly, an exponentially growing bacterial suspension of 5.20 × 105 to 5.79 × 105 CFU/ml was prepared and then incubated at 37°C for 24 h with or without the simulated human plasma concentrations of each antimicrobial agent (see Text S1 in the supplemental material). The plasma concentration-time curves were recreated as follows: 2 g cefiderocol every 8 h (i.e., three times a day [t.i.d.]) as a 3-h infusion, 2.5 mg/kg colistin methanesulfonate every 12 h as a 0.5-h infusion, 5 mg/kg amikacin t.i.d. as a 0.5-h infusion, and 1 g meropenem t.i.d. as a 1-h infusion (Fig. 1). For cefiderocol and meropenem, free concentrations corrected by plasma protein binding ratio were used. For colistin and amikacin, total concentrations were used because either total or free concentrations have been shown to be important for pharmacokinetics/pharmacodynamics (PK/PD) by several different reports (10–13). One of the colistin-based combination therapies was used as a positive control because this is an important currently available option for combatting carbapenem-resistant Gram-negative pathogens, including MBL producers (14, 15). As the isolates used in this study were resistant to multiple classes of antibiotics, colistin-amikacin combination therapy was evaluated, although these isolates were resistant to amikacin. Meropenem was used as a negative control because all test isolates were resistant to carbapenem.
TABLE 1.
MICs of cefiderocol, colistin, amikacin, and meropenem against the test strains
| Strain | Acquired β-lactamase(s) | MIC (μg/ml) of: |
||||
|---|---|---|---|---|---|---|
| Cefiderocol | Colistin/amikacina | Colistin | Amikacin | Meropenem | ||
| P. aeruginosa | ||||||
| NUBL-7808 | VIM-2 | 0.5 | 0.25 | 0.5 | >32 | >32 |
| SR27001 | IMP-1 | 1 | 0.5 | 2 | >32 | >32 |
| A. baumannii SR08626 | OXA-23 | 0.5 | 0.125 | 0.5 | >32 | 32 |
| E. coli DU48916 | NDM-1, CTX-M-15, CMY-2, OXA-1 | 4 | 0.25 | 0.25 | >32 | 32 |
| K. pneumoniae | ||||||
| SR08667 | KPC-3, SHV | 2 | 2 | 1 | >32 | >32 |
| VA-384b | KPC-2, TEM-1, SHV-11, SHV-12 | 4 | 0.125 | 0.25 | 16 | >32 |
The MIC of colistin with 4 μg/ml amikacin was evaluated.
Information on K. pneumoniae strains harboring β-lactamases is provided in reference 19.
FIG 1.
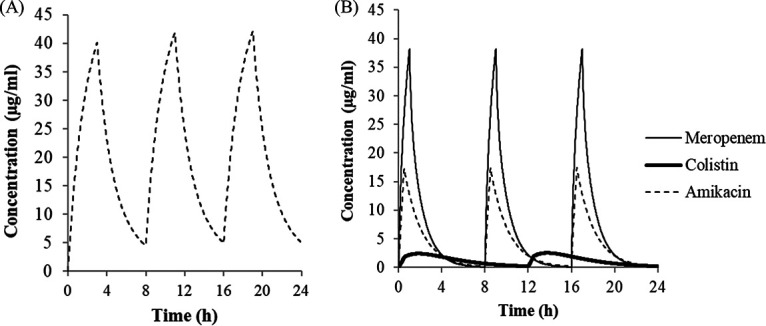
Concentration-time curves of cefiderocol (A) and colistin, amikacin, and meropenem (B) in a one-compartment in vitro chemostat model. For cefiderocol and meropenem, free concentrations corrected by plasma protein binding ratio were used.
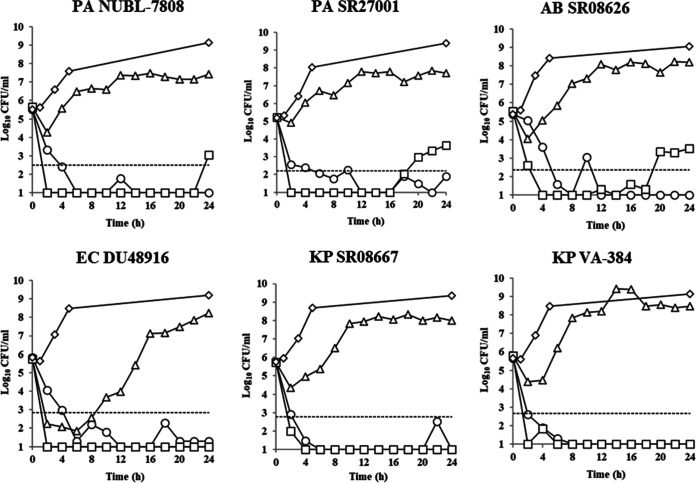
Against these carbapenem-resistant isolates, cefiderocol treatment showed potent activity that was comparable to that of colistin-based combination treatment (Fig. 2). Cefiderocol treatment showed more than a 3-log10 kill from the initial inoculum within 8 h posttreatment; thereafter, the 3-log10 kill was sustained until 24 h. The change of log10 CFU/ml from the initial inoculum after 24 h treatment was −3.30 to −4.77. The treatment by colistin plus amikacin caused more than a 3-log10 kill from the initial inoculum within 8 h posttreatment as well. However, the 3-log10 kill from the initial inoculum at 24 h posttreatment was not achieved in 3 nonfermenters as the number of the viable bacteria increased again after 20 h of treatment. The change of viable cells from the initial inoculum at 24 h posttreatment was −1.57 to −4.79. In contrast, meropenem treatment did not show the sustained bacterial killing during the treatment period and even the static effect after 24 h of treatment. The change of viable cells from the initial inoculum at 24 h posttreatment was 1.79 to 2.67.
FIG 2.
Number of viable cell-time curves exposed to the bacterial suspension under simulated human plasma concentrations of cefiderocol (open circles), colistin plus amikacin (squares), and meropenem (triangles) over 24 h. Each human dosing regimen was as follows: 2 g cefiderocol every 8 h as a 3-h infusion, 2.5 mg/kg colistin methanesulfonate every 12 h as a 0.5-h infusion, 5 mg/kg amikacin every 8 h as a 0.5-h infusion, and 1 g meropenem every 8 h as a 1-h infusion, respectively. Vehicle treatment is represented by diamonds. The 3-log10-CFU kill from the initial inoculum is represented by dotted horizontal lines. CFU, colony-forming units; PA, P. aeruginosa; AB, A. baumannii; EC, E. coli; KP, K. pneumoniae.
This is the first report on the evaluation of cefiderocol efficacy under simulated human PK using an in vitro chemostat model. The results of the in vitro chemostat model using iron-depleted cation-adjusted Mueller-Hinton broth (ID-CAMHB) were consistent with the observation from several in vivo animal studies, which have been used to evaluate the potential use of cefiderocol to treat the infections caused by carbapenem-resistant Gram-negative bacteria with a MIC of ≤4 μg/ml. The PK/PD studies using mouse thigh infection models caused by a variety of Gram-negative bacteria showed that a 1-log10 reduction in bacteria burden at 24 h was associated with 75% of the percentage of time that free cefiderocol concentrations are above the MIC (%fTMIC) on average (16). The Monte-Carlo simulation showed that a dose of 2 g every 8 h as a 3-h infusion provided >90% probability of target attainment with a pharmacodynamic target of 75 or 100% %fTMIC for a MIC of ≤4 μg/ml in nosocomial pneumonia patients (17). This potent efficacy was also confirmed by the efficacy studies using mouse thigh infection models under humanized PK (18). The data from this study further confirm efficacy against the isolates with a MIC of ≤4 μg/ml, which was consistent with these in vivo studies. It should be noted that this study provided the efficacy against NDM producers with a MIC of 4 μg/ml, which have never been appropriately evaluated due to the discrepancy between in vitro and in vivo studies. The good correlation of efficacy was observed between in vitro chemostat models using ID-CAMHB and in vivo studies, suggesting that these models could be used for further evaluation of efficacy and emergence of resistance during therapy against a variety of pathogens.
In summary, activities of the simulated human plasma concentrations of cefiderocol against strains with cefiderocol MICs of 0.5 to 4 μg/ml were evaluated in an in vitro chemostat model. The cefiderocol regimen showed bactericidal activities against carbapenemase-harboring carbapenem-resistant isolates of P. aeruginosa, A. baumannii, E. coli, and K. pneumoniae. These activities were comparable to that of a colistin-based regimen, suggesting cefiderocol as one of the therapeutic options for the treatment of infections caused by carbapenem-resistant Gram-negative pathogens, including MBL producers.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yoshichika Arakawa (Nagoya University) and GlaxoSmithKline Pharmaceuticals for providing the clinical isolates tested. We also thank Yutaka Jinushi and Keiichiro Hirooka at Shionogi TechnoAdvance Research Co., Ltd., for technical support.
All authors are employees of Shionogi & Co., Ltd., and have no conflicts of interest to report.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, Idowu T, Domalaon R, Schweizer F, Zhanel MA, Lagacé-Wiens PRS, Walkty AJ, Noreddin A, Lynch Iii JP, Karlowsky JA. 2019. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs 79:271–289. doi: 10.1007/s40265-019-1055-2. [DOI] [PubMed] [Google Scholar]
- 2.Yamano Y. 2019. In vitro activity of cefiderocol against a broad range of clinically important Gram-negative bacteria. Clin Infect Dis 69:S544–S551. doi: 10.1093/cid/ciz827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato T, Yamawaki K. 2019. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 69:S538–S543. doi: 10.1093/cid/ciz826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noval M, Banoub M, Claeys KC, Heil E. 2020. The battle is on: new beta-lactams for the treatment of multidrug-resistant Gram-negative organisms. Curr Infect Dis Rep 22:1. doi: 10.1007/s11908-020-0710-9. [DOI] [PubMed] [Google Scholar]
- 5.FDA. 2019. Cefiderocol injection. FDA, Silver Spring, MD: https://www.fda.gov/drugs/development-resources/cefiderocol-injection. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing, 29th ed CLSI document M100-ED29 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.Asempa TE, Abdelraouf K, Nicolau DP. 2020. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 75:997–1005. doi: 10.1093/jac/dkz532. [DOI] [PubMed] [Google Scholar]
- 8.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2019. Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn Microbiol Infect Dis 94:321–325. doi: 10.1016/j.diagmicrobio.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Homma T, Hori T, Ohshiro M, Maki H, Yamano Y, Shimada J, Kuwahara S. 2010. In vitro pharmacokinetic and pharmacodynamic evaluation of S-013420 against Haemophilus influenzae and Streptococcus pneumoniae. Antimicrob Agents Chemother 54:4300–4305. doi: 10.1128/AAC.00214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by Gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts JA, Lipman J. 2012. Editorial commentary: closing the loop—a colistin clinical study to confirm dosing recommendations from PK/PD modeling. Clin Infect Dis 54:1727–1729. doi: 10.1093/cid/cis311. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Hagihara M, Hirai J, Sakanashi D, Suematsu H, Nishiyama N, Koizumi Y, Yamagishi Y, Matsuura K, Mikamo H. 2017. Evaluation of amikacin pharmacokinetics and pharmacodynamics for optimal initial dosing regimen. Drugs R D 17:177–187. doi: 10.1007/s40268-016-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhadab AA, Ahmed MA, Brundage RC. 2018. Amikacin pharmacokinetic-pharmacodynamic analysis in pediatric cancer patients. Antimicrob Agents Chemother 62:e01781-17. doi: 10.1128/AAC.01781-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenhard JR, Bulman ZP, Tsuji BT, Kaye KS. 2019. Shifting gears: the future of polymyxin antibiotics. Antibiotics (Basel) 8:42. doi: 10.3390/antibiotics8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai B, Cai Y, Liew YX, Chua NG, Teo JQ, Lim TP, Kurup A, Ee PL, Tan TT, Lee W, Kwa AL. 2016. Clinical efficacy of polymyxin monotherapy versus nonvalidated polymyxin combination therapy versus validated polymyxin combination therapy in extensively drug-resistant Gram-negative bacillus infections. Antimicrob Agents Chemother 60:4013–4022. doi: 10.1128/AAC.03064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura R, Ito-Horiyama T, Takemura M, Toba S, Matsumoto S, Ikehara T, Tsuji M, Sato T, Yamano Y. 2019. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 63:e02031-18. doi: 10.1128/AAC.02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency. 2020. Assessment report. Fetcroja. European Medicines Agency, Amsterdam, The Netherlands. https://www.ema.europa.eu/en/documents/assessment-report/fetcroja-epar-public-assessment-report_en.pdf.
- 18.Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. 2017. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 61:e01022-17. doi: 10.1128/AAC.01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endimiani A, Hujer AM, Perez F, Bethel CR, Hujer KM, Kroeger J, Oethinger M, Paterson DL, Adams MD, Jacobs MR, Diekema DJ, Hall GS, Jenkins SG, Rice LB, Tenover FC, Bonomo RA. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the eastern USA. J Antimicrob Chemother 63:427–437. doi: 10.1093/jac/dkn547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.