Abstract
Purpose:
Intraductal papillary neoplasm of the bile duct (IPNB) is a precursor to invasive carcinoma and is a distinct pathologic diagnosis. The purpose of this study was to evaluate imaging features of IPNB on cross-sectional imaging studies with histopathologic correlation.
Materials and methods:
In this IRB approved, HIPAA compliant retrospective observational analysis of 23 pathology proven IPNB tumors 22 imaging studies were reviewed, 14 CT and 8 MRI scans. Features evaluated in consensus by two subspecialty-trained abdominal radiologists included: presence of specific lesion/mass within the bile duct, location within the biliary tree, size, morphology, enhancement characteristics, and bile duct caliber.
Results:
Majority of the subjects (16/18, 90%) had definite intraluminal mass, of which 7 (39%) had a polypoid mass with upstream diffuse biliary ductal dilation and 5 (28%) had a plaque-like mass with focal stricture and upstream biliary ductal dilatation. 6/18 (33%) subjects had low grade dysplasia, most commonly intestinal subtype, 7/18 (39%) subjects presented with invasive component, commonly pancreaticobiliary subtype, and 5/18 (28%) presented with high grade dysplasia.
Conclusion:
IPNB has increased predilection for extrahepatic bile ducts, commonly presenting as either an intraluminal polypoidal mass with associated upstream biliary ductal dilation or a focal plaque like mass with associated ductal stricture at the site of the tumor.
Keywords: Intraductal papillary neoplasm of bile duct, Premalignant, Extra hepatic polypoidal mass, Frond-like, Plaque-like stricture, Cholangiocarcinoma
1. Introduction
The World Health Organization described three pre-invasive biliary lesions in 2010: biliary intraepithelial neoplasia (BilIN), intraductal papillary neoplasm of the bile duct (IPNB) and mucinous cystic neoplasm [1]. IPNB accounts for 10–15% of bile duct tumors [2] and is further classified into two histologic grades: IPNB with low-grade intraepithelial neoplasia and IPNB with high-grade intraepithelial neoplasia with or without invasive cancer.
IPNB, a precursor to cholangiocarcinoma, is analogous to intraductal papillary mucinous neoplasm (IPMN) of the pancreas, although has a higher rate of malignant transformation compared to pancreatic IPMN [3]. IPNB with high-grade dysplasia/carcinoma in situ can take up to 1–2 years to transform into invasive carcinoma, and approximately 40–80% of IPNB can harbor an invasive component [3]. IPNB is more common in East Asian Countries compared to the West, accounting for 9.9–30% [4] and 7–11% of biliary ductal neoplasms [5,6], respectively. Risk factors include: hepatolithiasis, clonorchiasis infection, primary sclerosing cholangitis, and biliary malformations including choledochal cysts, and familial adenomatous polyposis or Gardner syndrome [7].
Ying et al. [8] has classified IPNB into seven different morphological types based on the imaging appearance. However, there is limited literature correlating the imaging appearances of IPNB on cross-sectional imaging with the pathologic features and histologic subtypes. The aim of this study was to identify the imaging appearances of IPNB on CT and MRI using histopathologic correlation of these pre-malignant lesions. In doing so, we also attempt to determine if there are any imaging or histologic features that are specific for establishing a recurrence-free interval.
2. Methods
2.1. Study cohort
The study was approved by the Institutional Review Board and informed consent was waived for this HIPAA compliant retrospective observational study. Inclusion criteria included patients 18 years or older with pathologic confirmation of IPNB between 1999 and 2018. Exclusion criteria included patients who did not have pre-operative cross-sectional imaging and/or those with synchronous biliary tract cancer. Post-operative imaging, if available was assessed to determine recurrence during surveillance.
2.2. Data collection
All subjects with diagnosis of IPNB were identified from the Department of Pathology database at our institution. Abdominal CT and MR imaging of all patients with this rare entity were reviewed on the McKesson Picture Archiving and Communication Systems by two board-certified fellowship trained abdominal radiologists (with 10 and 12 years’ experience) in consensus. The study cohort diagram is included in Fig. 1. Patient demographics and baseline characteristics including labs were collected.
Fig. 1.
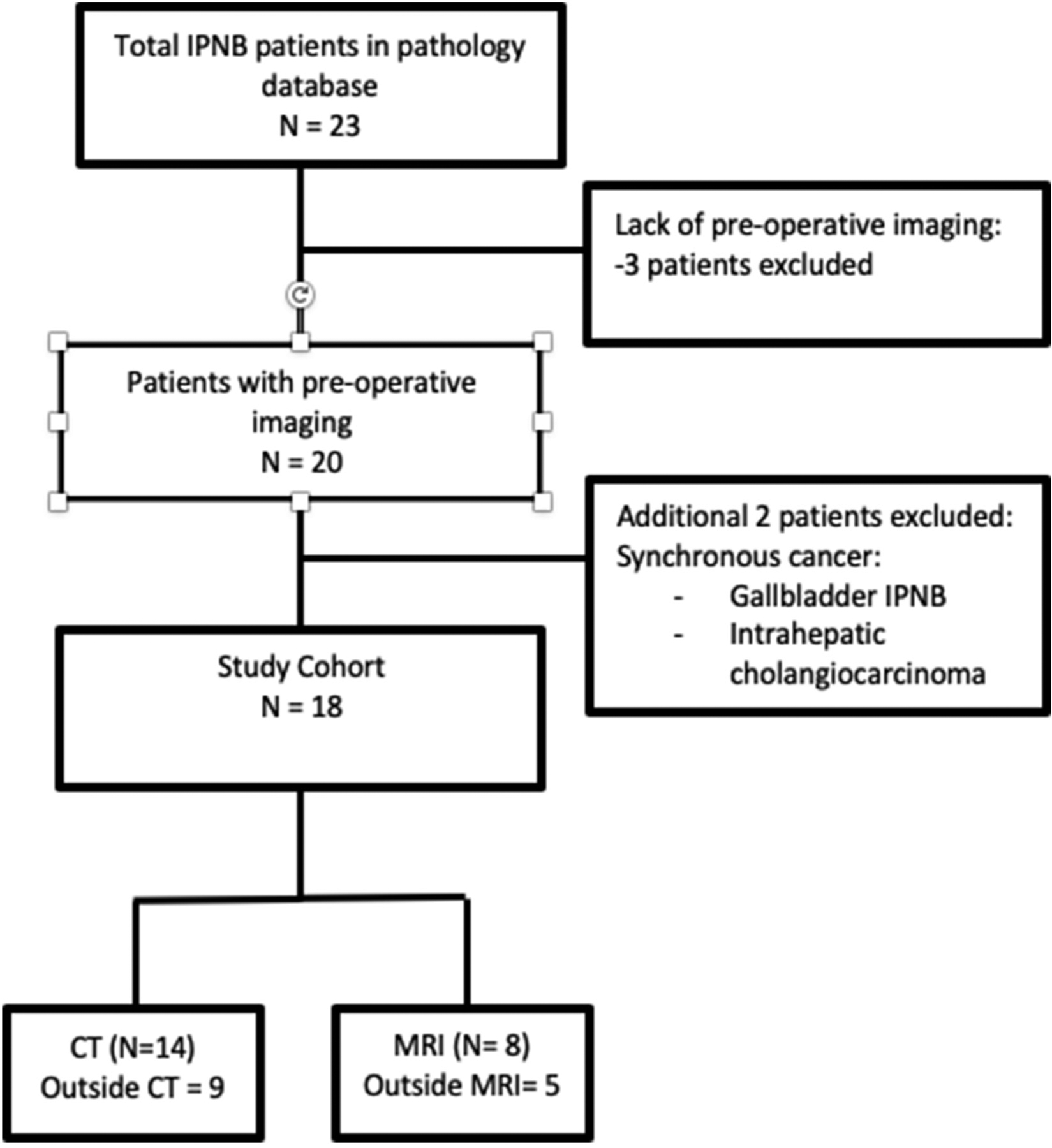
Study population flow diagram.
2.3. Imaging parameters
CT examinations were performed on a variety of 16- and 64- slice multi-detector CT scanners (Siemens Somatom, GE lightspeed, GE HD750). MRI examinations were performed on 1.5 T scanner (Phillips). Most (64% [14/22]) examinations were performed at an outside institution and transferred to University of Michigan for re-interpretation and further management. The CT examinations at our institution were performed following intravenous administration of 100–125 mL of iodinated contrast material (300 mg/mL) acquired in either a single portal venous phase (60 s delay) or acquired in both arterial (30 s delay) and portal venous phases. All CT examinations were reconstructed with a slice thickness of 2.5–5 mm and were acquired with the following settings: 100–120 kVp, variable mA. MRI examinations were acquired utilizing routine liver or cholangiocarcinoma protocol (coronal and axial T2 weighted imaging), dual-echo in and opposed phase, coronal and axial 3D MRCP with 3-D reformats, axial fat saturated T1Weighted gradient echo pre and dynamic post contrast imaging (0.1 mmol/kg of Gadolinium based contrast agent), delayed (15 min) fat-saturation post contrast T1WI.
2.4. Imaging features with tumor classification
Imaging appearances were recorded with parameters including: anatomic location of tumor in the biliary tree broadly classified as distal, perihilar, or intrahepatic IPNB, presence or absence of intraluminal mass, duct wall morphology, appearance of upstream biliary tree, morphology of intraluminal mass, associated periductal inflammation/infiltration, and post contrast appearance. The mass, if present, was morphologically categorized as intraluminal polypoidal, plaque-like, infiltrative, or intraluminal frond like mass with mural nodules (Fig. 2). Frequencies were reported as percentages.
Fig. 2.
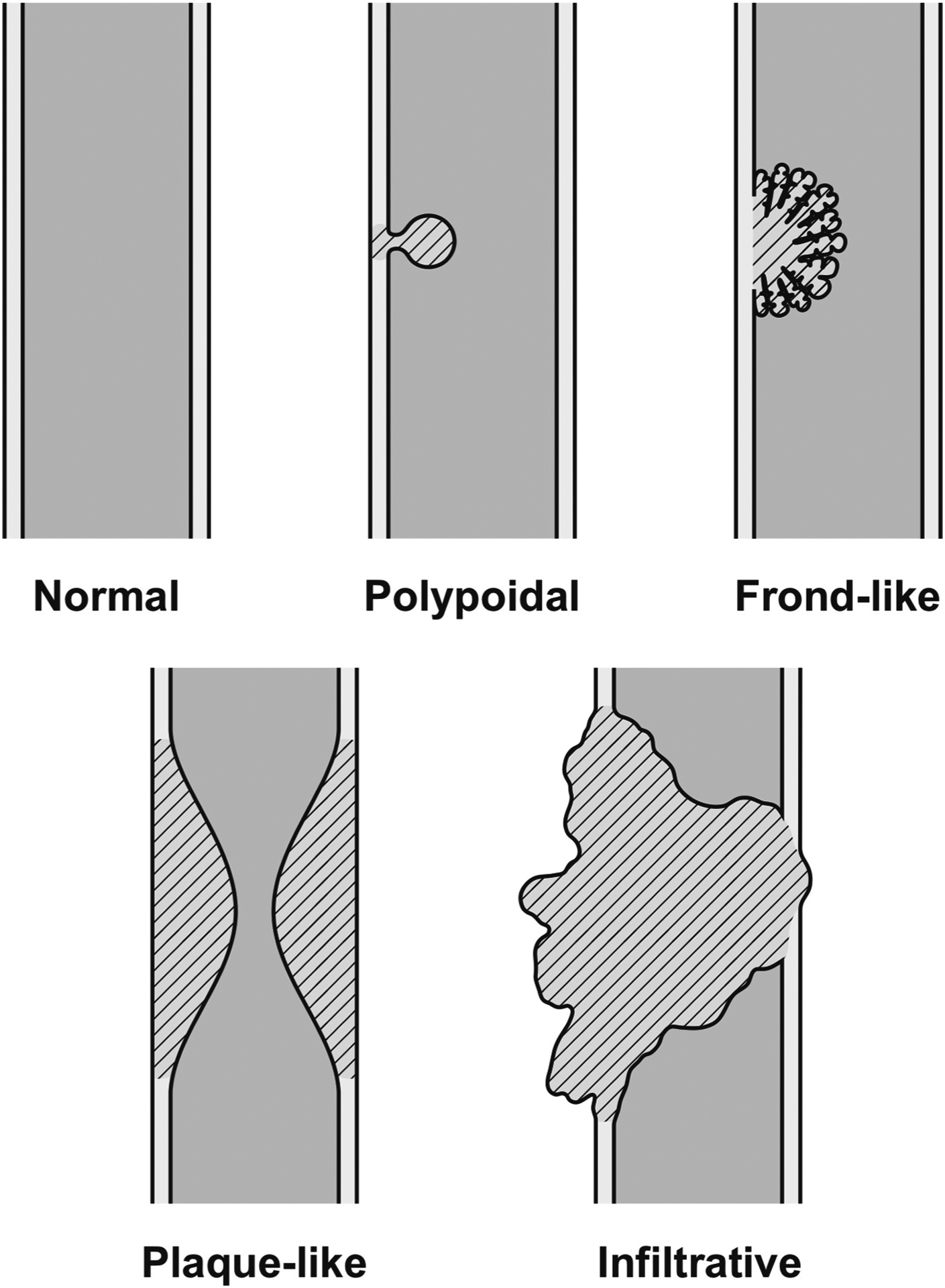
Characterization of intraluminal mass.
Histopathologic correlation was subcategorized into type of dysplasia (low grade, high grade and high grade with invasive cancer) and epithelial cell type (intestinal, pancreaticobiliary, gastric or oncocytic). Imaging features were correlated with histopathologic analysis to determine if phenotype had a greater predilection for a specific imaging feature. Other ancillary features include: presence or absence of loco-regional lymph nodes, portal vein thrombus, gallbladder morphology and/or presence of gallstones. Post-operative imaging reports if available, were also analyzed to determine recurrence.
3. Results
A total of 23 patients with pathologic diagnosis of IPNB were identified from the pathology database, of which three were excluded due to lack of pre-operative imaging. An additional two were excluded due to presence of concomitant intrahepatic cholangiocarcinoma and gallbladder IPNB. 18 subjects met inclusion criteria, out of which 10 subjects underwent CT, 4 MRI and 4 had both studies leading to a total of 22 imaging studies. The study cohort had equal male (n = 9) and female (n = 9) subjects. By ethnicity, there were 13 (72%) Caucasian, 1 (5.6%) Hispanic, 1 (5.6%) African American, and 1 (5.6%) Asian. Two (11%) subjects refused to reveal their race/ethnicity. Baseline demographics and characteristics are provided in Table 1.
Table 1.
Patient characteristics.
| Patients characteristics | N (%) |
|---|---|
| Study cohort | 18 |
| Age, median | 68 years |
| Gender | |
| Males | 9(50) |
| Females | 9(50) |
| Laboratory data (mean ± SD) | |
| White blood cell count (103/Vmm3) | 9 ± 3 |
| Total bilirubin (mg/dL) | 4 ± 7 |
| Alkaline phosphatase (IU/L) | 257 ± 221 |
| Aspartate aminotransferase (IU/L) | 76 ± 54 |
| Alanine aminotransferase (IU/L) | 88 ± 100 |
| Cancer antigen 19–9 (U/mL) | 1535 ± 2948 |
13/18 (72%) subjects had extrahepatic IPNB, of which 10 (55.6%) involved the distal common bile duct (CBD) either proximal to or at the ampulla and 3 (16.5%) involved the mid CBD. 4/18 (22%) had intrahepatic IPNB, without concomitant involvement of extrahepatic ducts. 1/18 (6%) subjects had perihilar IPNB (involvement of the common hepatic and intrahepatic ducts). (Table 2).
Table 2.
Anatomical distribution of intraductal papillary neoplasm of bile duct.
| Types based on anatomical distribution | N = 18 (%)a |
|---|---|
| Extrahepatic | 13 (72) |
| Distal CBD | 10 (56) |
| Mid CBD | 3 (17) |
| Intrahepatic | 4 (22) |
| Perihilar | 1 (6) |
Percentage are number of subjects.
16/18 (89%) subjects presented with an intraluminal ductal mass on imaging, of which 7 (39%) were polypoidal and had either upstream biliary ductal dilatation (n = 6) or a normal caliber duct (n = 1). 6/18 (33%) subjects presented as a plaque-like mass and stricture out of which 5 had upstream biliary ductal dilatation, and 1 had normal caliber upstream duct. One subject (5.5%) had dilated upstream duct with plaque like mass without stricture. There was one subject (5.5%) who had an infiltrative mass involving the mid CBD, spanning 5.1 cm in length, with associated upstream biliary ductal dilatation. Lastly, a subject (5.5%) presented as a papillary frond like mass with associated mural nodules within the left hepatic duct and associated localized upstream ductal dilatation. 2/18 (11%) subjects presented without intraluminal mass, out of which one had a focal stricture and one had normal caliber duct. There were no cases which presented as only ductal dilatation without an associated mass or stricture (Table 3).
Table 3.
A and B. Imaging appearance of IPNB.
| Biliary duct appearance | N = 18 (%)a |
|---|---|
| A) Presence of intraluminal mass | 16 (89) |
| • Focal polypoid mass | 7 (39) |
| - Upstream duct dilatation | 6 |
| - Normal caliber duct | 1 |
| • Plaque-like mass with stricture | 6 (33) |
| - dilated upstream duct | 5 |
| - normal caliber upstream duct | 1 |
| • Infiltrative mass (upstream duct dilated) | 1 (5.5) |
| • Frond like mass, mural nodules (localized duct dilatation) | 1 (5.5) |
| • Plaque-like mass without stricture and dilated upstream duct | 1 (5.5) |
| B) Absence of intraluminal mass | N = 2 (%) |
| • Normal caliber bile duct | 1 (5.5) |
| • Diffusely dilated bile duct | 0 (0) |
| • Bile duct stricture | 1 (5.5) |
Percentage are number of subjects.
The mean caliber of the dilated upstream CBD in those lesions which presented as a focal polypoid mass versus plaque-like mass with stricture was 2 cm (range 0.8–3.9 cm) and 1.3 cm (range 0.5–1.6 cm), respectively. The mean length of stricture was 1.2 cm (range 0.4–2.3 cm).
12/18 (67%) lesions had bile duct wall thickening at the level of the tumor, with a radiographic appearance of abnormal biliary ductal wall enhancement spanning a radial thickness of 0.3–0.7 cm. Of the remainder, an indwelling biliary stent (n = 1) precluded evaluation of the ductal wall morphology and 5/18 (28%) had normal duct wall morphology. There were variable imaging characteristics on CT and MRI, with most tumors iso to hypo-intense on pre-contrast T1 weighted image, mildly hyperintense on T2 weighted image, hyper-enhancing on arterial and portal venous phase imaging. Of the patients who underwent CT, all lesions were hyperdense on arterial phase and isodense on portal venous phase. 6/18 (33%) lesions also had periductal edema and inflammation which extended into the adjacent hepatic parenchyma.
Additional features recorded included the presence of prior cholecystectomy (n = 8, 55%). In patients with a gallbladder (n = 10), the lumen was distended to a mean of 8.7 cm craniocaudally (range 4.4–16.6 cm), of which 2 had gallstones. Portal vein was patent in all patients except for one case of bland thrombosis, of uncertain etiology. There were no cases of tumor thrombus. 9/18 (50%) subjects had mildly enlarged loco-regional lymph nodes involving the periportal region with a mean short axis diameter of 1.2 cm (range 0.8–3.4 cm), of which none were positive for malignancy on pathology.
3.1. Histopathologic correlation
On histopathology, 6/18 (33%) lesions had low grade dysplasia (Fig. 3), 5/18 (28%) had high grade dysplasia (Fig. 4), and 7/18 (39%) had high grade dysplasia with associated invasive cancer (Fig. 5). In 4 subjects, epithelial cell type could not be determined. Of the remaining 14 lesions, 9 presented with one epithelial subtype (pancreaticobiliary, gastric or intestinal) and 5 presented with more than one epithelial subtype (intestinal and pancreaticobiliary type (n = 3), and pancreaticobiliary and gastric cell type (n = 2)). Description of the imaging appearance associated with each of the different epithelial subtypes in this cohort are illustrated in Fig. 6A and B.
Fig. 3.
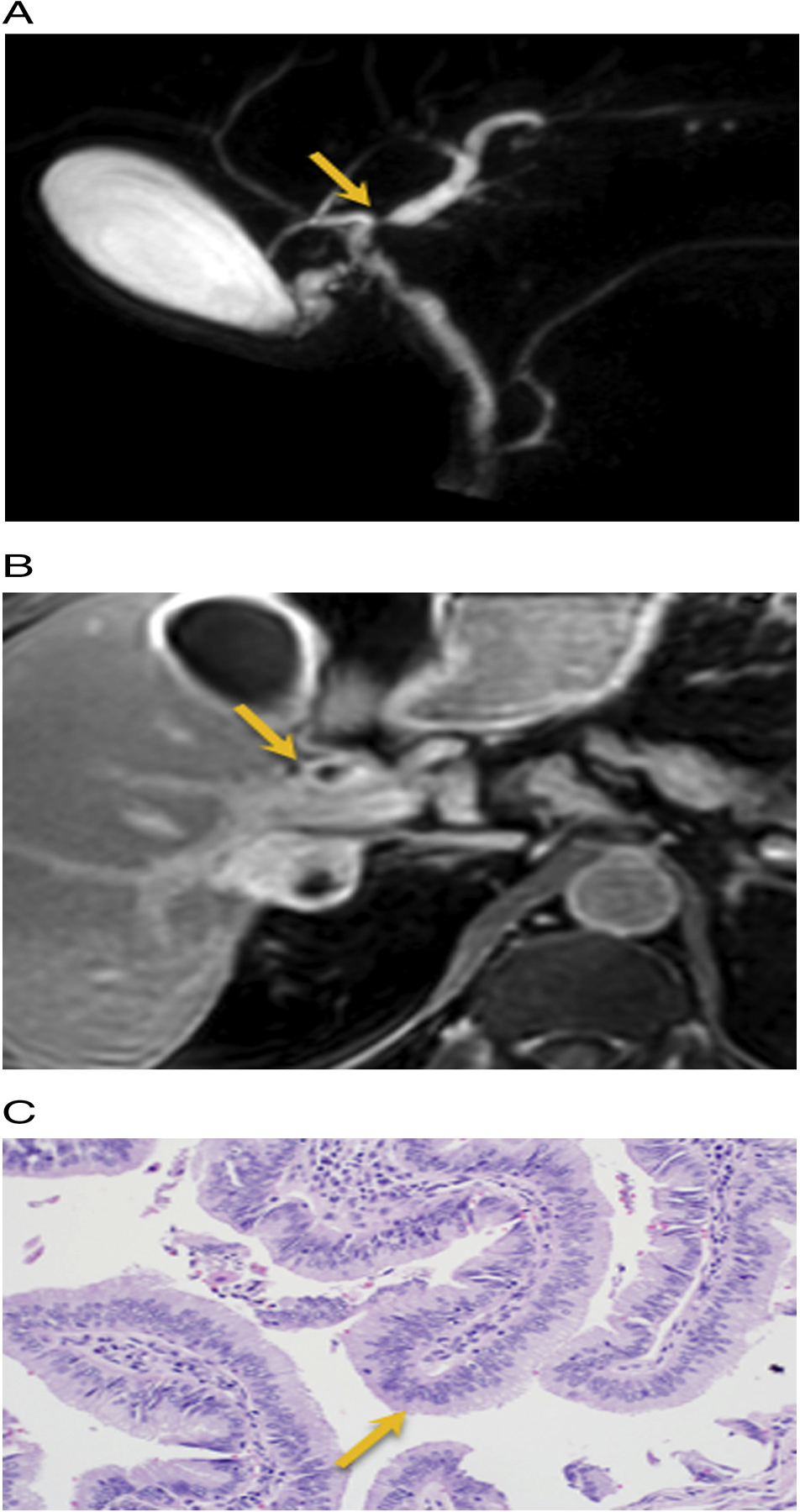
Stricture with plaque- like thickening. 59 year old male with painless jaundice. A) 3D MRCP images show a dominant stricture at the junction of right and left duct (arrow). B) Axial T1W post contrast delayed phase imaging at 15 min demonstrates ill-defined soft tissue thickening and enhancement at the confluence of right and left hepatic duct (arrow). C) H&E Histology: Biliary papillomatosis. IPNB with low grade intestinal dysplasia (arrow).
Fig. 4.
IPNB with intraluminal mass. 35-year old female with history of pineal and thyroid cancer presenting with pruritis and jaundice. A) Contrast enhanced CT demonstrates moderate to severe intrahepatic biliary ductal dilatation secondary to an obstructing polypoid intraluminal enhancing soft tissue mass (arrow). B) Histopathology: Intraluminal papillary growth, pancreaticobiliary type, with high-grade dysplasia (arrow).
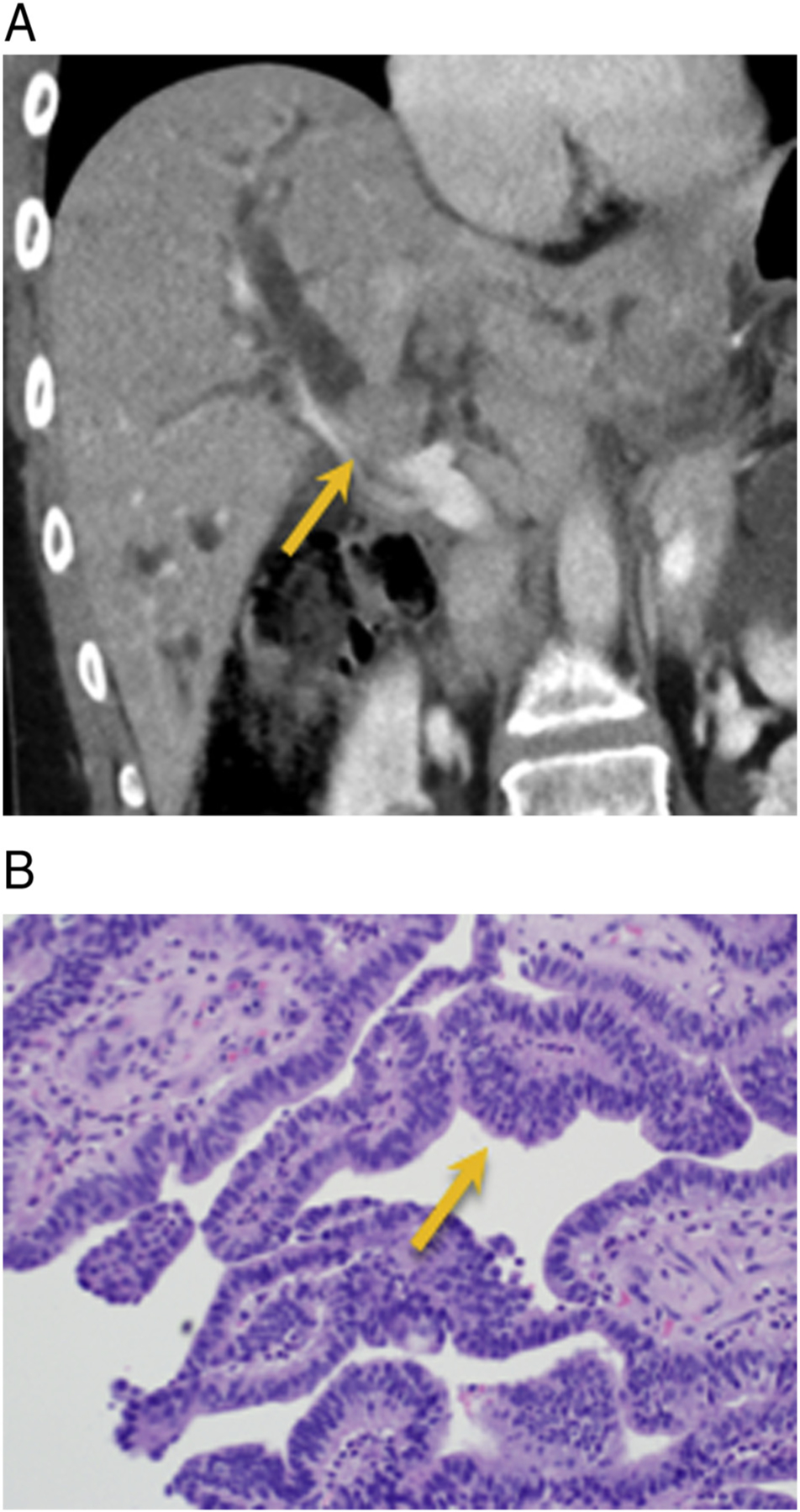
Fig. 5.
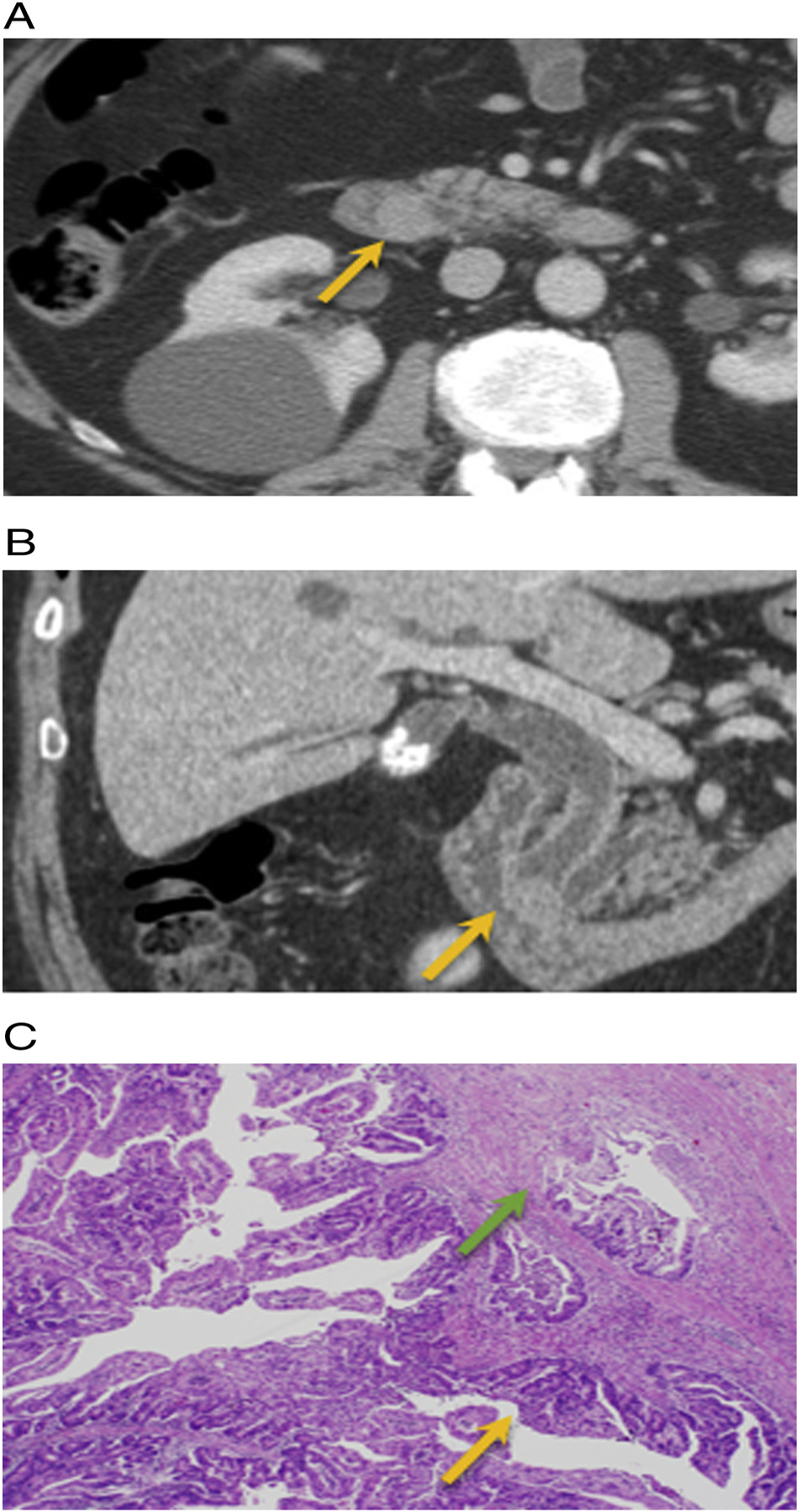
IPNB with invasive adenocarcinoma. 62-year old male with no significant past medical history presents with itching and orange colored urine. A and B) Axial and coronal post-contrast CT demonstrates a 1.5 cm enhancing distal CBD mass near the ampulla with upstream extrahepatic and intrahepatic biliary ductal dilation (arrow). Post whipple H&E histology: intraluminal papillary growth (orange) with foci of invasive adenocarcinoma (green arrow).
Fig. 6.
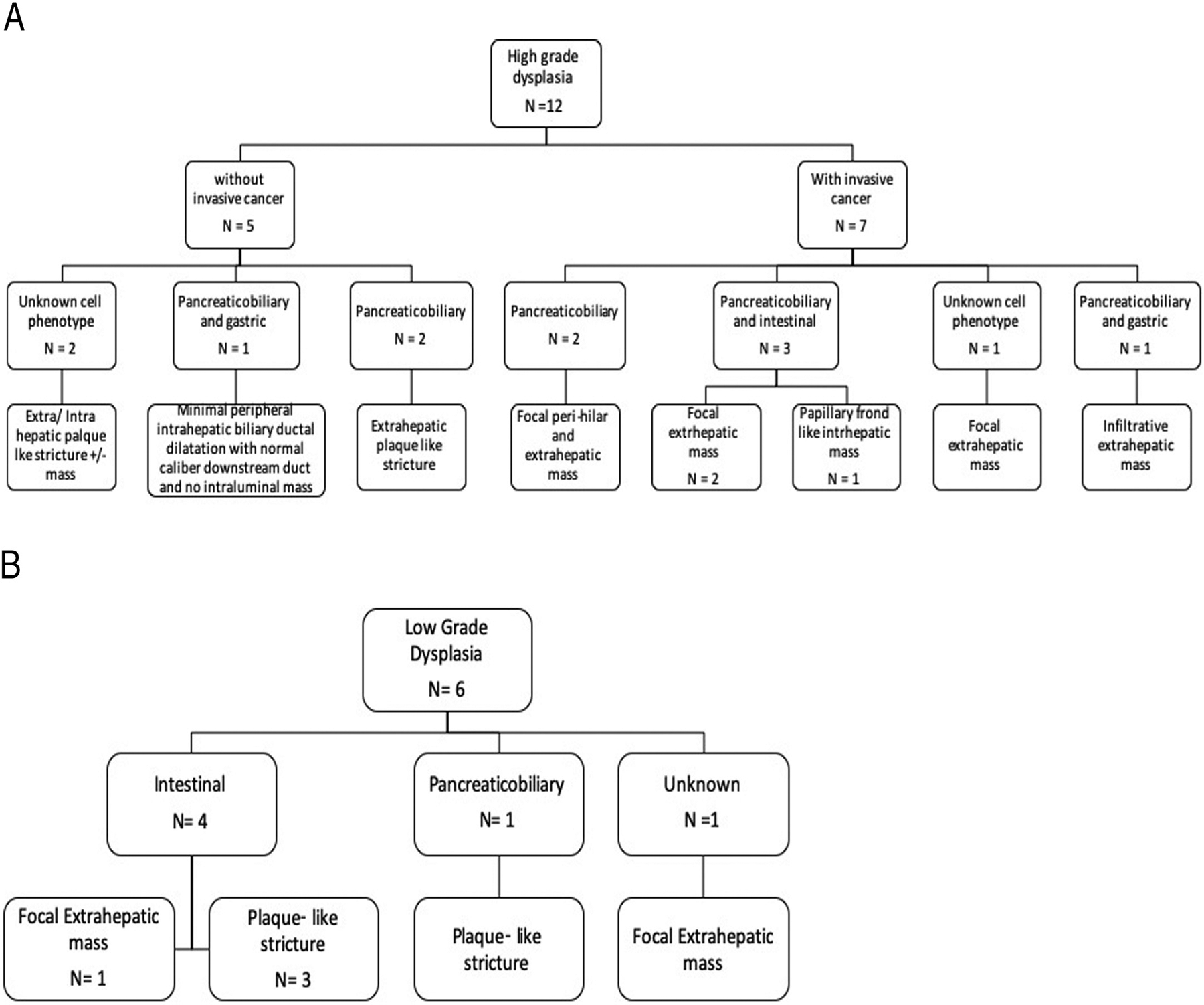
A. Histopathologic and Imaging correlation of High grade dysplasia with and without invasive transformation.
B. Histopathologic and Imaging correlation of low grade dysplasia.
Out of 18 subjects, most patients (n = 11, 61%) did not have follow up imaging. The remaining 7 subjects were followed for a mean of 3 years (range 0.2–5.2 years), with only one case of local recurrence, which was seen within the remnant common bile duct and was detected on routine surveillance imaging 1.5 year post resection. This recurrence radiographically presented as an intraluminal extrahepatic mass. No patients died from their IPNB diagnosis.
4. Discussion
Our study describes varied imaging appearances of IPNB on CT and MRI, often presenting either in an intrahepatic or extrahepatic location as an intraluminal focal polypoid mass, plaque-like mass with stricture, infiltrative mass of frond like mass with mural nodules. These imaging appearances are correlated with histopathological diagnosis.
A significant proportion of IPNB lesions identified presented with extrahepatic biliary duct involvement, either as a focal polypoid mass (n = 7) or plaque like mass with a stricture (n = 6). Of those patients with high grade dysplasia and invasive carcinoma on histopathology, a significant proportion 7/12 (58%) presented with extrahepatic distal biliary ductal involvement, either as a focal intraluminal mass or as a plaque-like mass with stricture, and the majority had upstream biliary ductal dilatation. Ogawa et al. described similar results with multi-detector computed tomography imaging modality in 36/37 consecutive IPNB patients, illustrating a higher rate of high grade dysplasia with invasive carcinoma in IPNB lesions which presented as an extrahepatic intraductal mass with associated biliary ductal dilatation [9]. There was only one case (6%) of pathologically proven intrahepatic IPNB without corresponding biliary ductal abnormality on imaging. This patient was diagnosed based on abnormal laboratory analysis.
Microscopically, IPNB is characterized by intraductal papillary proliferation of neoplastic cells with a distinct fibrovascular core. The cell lining can produce mucin of varying degrees, which, if produced in large quantities, can cause biliary ductal dilation upstream from the intraluminal mass as a result of obstruction and downstream of the mass from excess mucin production. This is an important imaging feature that should be taken into consideration when suggesting the potential diagnosis of IPNB. There are four distinct epithelial cell phenotypes described in the literature: pancreaticobiliary, intestinal, gastric, and oncocytic [2]. Most cases present with one of these four distinct phenotypes, although occasionally two phenotypes can co-exist in the same patient [10], as seen in our study. None of our patients had oncocytic cell type. Pancreaticobiliary phenotypes are most common and tend to have high grade dysplasia with an invasive component [10], a finding also seen in our study. Another important feature of these tumors described on pathology is frequent metaplasia over time with transformation from low grade to high-grade and eventually invasive adenocarcinoma [11]. Hence as a result, the histology can be variable throughout the lesion, with biopsy and or cell brushing underreporting the presence of malignancy.
In our study, those lesions with a histology of low grade dysplasia presented predominately with a plaque like structuring mass involving either the extra or intrahepatic ducts 4/6 (67%), as compared to the lesions with high grade dysplasia and invasive cancer, which had a higher frequency of presenting as a focal extrahepatic polypoid intraluminal mass with upstream biliary ductal dilatation 4/7 (57%).
IPNB has been described as the biliary counterpart to IPMN of the pancreas [12]. It is a slow growing tumor with a relatively indolent clinical course, although with a higher rate of malignant transformation to invasive cancer as compared to IPMN [13]. Only approximately 1/3rd of patients with IPNB produce mucin, whereas the majority of cases of IPMN produce macroscopic mucin secondary to predominance of intestinal phenotype [14]. In our study, intestinal phenotype was mainly present in IPNBs located within the extrahepatic bile duct. Two studies by Nakanuma et al. grouped IPNB into different types: type1 is analogous to pancreatic IPMN, occurring in intrahepatic and perihilar bile ducts with most cases presenting with low to intermediate grade dysplasia, and Type II occurring in peri-hilar, distal bile ducts and presenting with high grade dysplasia [15,16].
The invasive component of IPNB is histologically similar to cholangiocarcinoma, however, the malignant transformation that occurs in IPNB carries a better prognosis as compared to ductal cholangiocarcinoma [15]. The exact reason is unknown, although studies have hypothesized that it may be related to the indolent behavior of IPNB as compared to cholangiocarcinoma, as well as the underlying genetic make- up. Similarly, IPMN has a better prognosis than pancreatic adenocarcinoma, as described by Wasif et al., who evaluated overall survival in patients with invasive IPMN versus sporadic pancreatic adenocarcinoma, with improved overall survival in the former median survival of 21 months versus 14 months, p < 0.01, [17].
Despite the pre-invasive nature of these tumors, there were no positive lymph nodes on histopathology, a finding which is reportedly much more common in cholangiocarcinoma [18]. Regardless of the pathologic distinction between IPNB (a pre-invasive lesion) and cholangiocarcinoma (invasive cancer) which have differing prognosis and survival, imaging appearances of these tumors overlap, as described above [19]. Although, a recently published case report by Ritchie et al. describes IPNB as a precursor lesion to cholangiocarcinoma [20]. Since IPNB is a relatively newer diagnosis, further studies must be done to better understand its’ pathology and histology, as well as comparison with its possible counterparts, including cholangiocarcinoma, IPMN and pancreatic adenocarcinoma. Although the underlying histo-pathology and prognosis are different for IPNB and cholangiocarcinoma, given the strong overlap in imaging features, and the varying histology within an IPNB lesion, in terms of grades of dysplasia and epithelial subtype, treatment for each of these is similar, with similar post-operative follow up [3]. Given the limited number of reported IPNB cases in literature thus far, more prospective longitudinal observational studies are required to compare the recurrence rates of these lesions compared to their invasive counterpart cholangiocarcinoma in order to better predict outcomes and alter management such as extent of surgical resection and post-operative surveillance.
Of those patients with post-operative surveillance, there was only one case of recurrence found on routine post-operative surveillance imaging following hepaticojejunostomy in the remnant intrapancreatic segment of the common bile duct, approximately 1.5 years after initial resection (Fig. 7). Original imaging of this IPNB was an infiltrative extrahepatic mass, and pathology revealed high grade dysplasia with invasive cancer, combined pancreaticobiliary and gastric subtype. This patient subsequently underwent Whipple’s procedure. Matsumoto et al. [21] recently studied 306 patients with resected bile duct tumors, of which 21 were pathologically proven IPNB. Their study revealed higher likelihood of invasive component and worse prognosis with reduced recurrence free survival in extrahepatic IPNB compared to the intrahepatic type. Our study demonstrates similar findings, with a higher incidence of high grade dysplasia with an invasive component in extrahepatic IPNB as compared to intrahepatic IPNB, as seen in 71% (n = 5/7) versus 14% (1/7) of our cases, respectively.
Fig. 7.
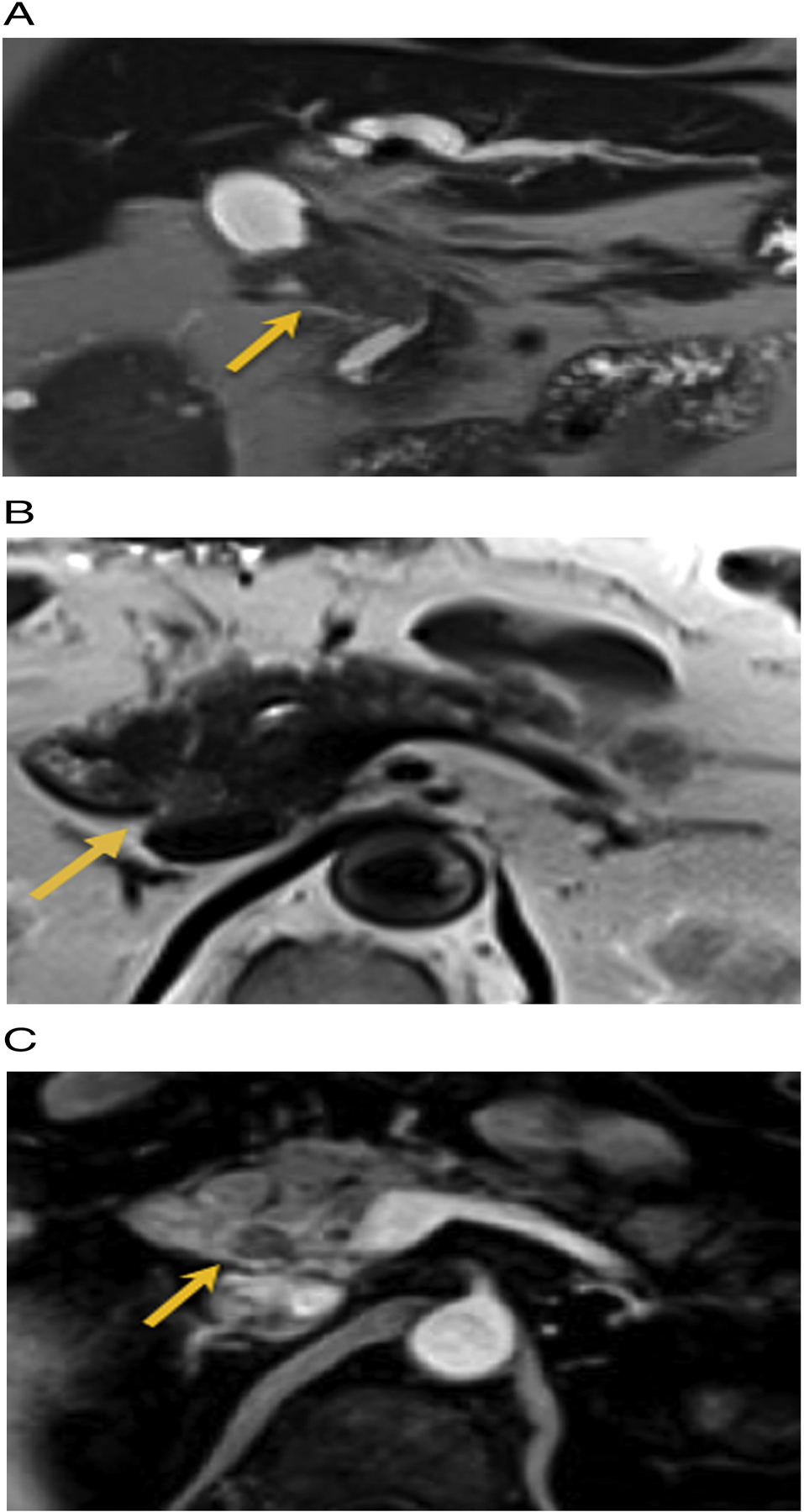
59 year old male with right upper quadrant pain. A) Pre-treatment T2 weighted MRI reveals a hypointense mass in the mid CBD (arrow). Treatment: extrahepatic bile duct excision and hepaticojejunostomy. Pathology: IPNB with high grade dysplasia and invasive carcinoma. B and C) Post-surgical MRI 1.5 years later: new expansile mass in the intrapancreatic portion of the remnant common bile duct which is mildly T2 hyperintense and hypo-enhancing on the post-contrast images (arrow). Patient subsequently underwent Whipple resection. Pathology: IPNB with high grade dysplasia.
There are a few limitations of this study. First, the study is limited by the retrospective observational design and single center data base. Secondly, the imaging appearances from a small sample of 18 subjects limits generalizing the appearances of IPNB with the different histologic subtypes. There were very few subjects who actually had postoperative follow up imaging, in our system, thus limiting the data on recurrence and further management options. Lastly, we did not compare the exact imaging appearance of cholangiocarcinoma and IPMN to IPNB at our institution to establish an internal validity on similarities or differences that would differentiate one lesion from the other.
In conclusion, while definitive imaging diagnosis of IPNB remains challenging, our study shows increased predilection of this cancer for extrahepatic bile ducts, most commonly presenting as either an intraluminal polypoid mass with associated upstream biliary ductal dilation or a focal plaque like mass with associated ductal stricture at the site of the tumor. While the presence of these described imaging findings should raise the suspicion for IPNB, given its strong overlap with cholangiocarcinoma on imaging and predilection for malignant transformation, treatment and surveillance requires a multidisciplinary approach, as treatment usually requires a Whipple procedure. Further multicenter longitudinal prospective trials to validate the results of our study is required.
Acknowledgments
Jiaqi Shi received partial funding from National Cancer Institute of the National Institutes of Health under Award Number K08CA234222.
Footnotes
Institutional review board approval was obtained and informed consent waived for this Health Insurance.
Portability and Accountability Act (HIPAA)-compliant retrospective cohort study.
Declaration of competing interest
Author declares that she has no conflict of interest.
References
- [1].Nagtegaal ID, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76(2):182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chatterjee A, et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: spectrum of imaging appearances. Radiographics 2019;39(2):388–412. [DOI] [PubMed] [Google Scholar]
- [3].Wan XS, et al. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol 2013;19(46):8595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ohtsuka M Similarity and differences between intraductal tumors of the bile duct. Am J Surg 2011;35(4):512–21. [DOI] [PubMed] [Google Scholar]
- [5].Rocha FG, Lee H, Katabi N, et al. Intraductal neoplasm of bile duct: A biliary equivalent of intraductal papillary neoplasm of pancreas. Hepatology 2012;56(4):1352–60. [DOI] [PubMed] [Google Scholar]
- [6].Park HJ, et al. Intraductal papillary neoplasm of the bile duct: clinical, imaging, and pathologic features. AJR Am J Roentgenol 2018;211(1):67–75. [DOI] [PubMed] [Google Scholar]
- [7].Lee SS, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer 2004;100(4):783–93. [DOI] [PubMed] [Google Scholar]
- [8].Ying S, et al. Morphological classification of intraductal papillary neoplasm of the bile duct. Eur Radiol 2018;28(4):1568–78. [DOI] [PubMed] [Google Scholar]
- [9].Ogawa H, et al. CT findings of intraductal papillary neoplasm of the bile duct: assessment with multiphase contrast-enhanced examination using multi-detector CT. Clin Radiol 2012;67(3):224–31. [DOI] [PubMed] [Google Scholar]
- [10].Nakanuma Y, et al. Tumorigenesis and phenotypic characteristics of mucin-producing bile duct tumors: an immunohistochemical approach. J Hepatobiliary Pancreat Sci 2010;17(3):211–22. [DOI] [PubMed] [Google Scholar]
- [11].Kunovsky L, et al. Mucinous cystic neoplasm of the liver or intraductal papillary mucinous neoplasm of the bile duct? A case report and a review of literature. Ann Hepatol 2018;17(3):519–24. [DOI] [PubMed] [Google Scholar]
- [12].Bennett S, et al. Clinical and pathological features of intraductal papillary neoplasm of the biliary tract and gallbladder. HPB (Oxford) 2015;17(9):811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Budzynska A, et al. Simultaneous liver mucinous cystic and intraductal papillary mucinous neoplasms of the bile duct: a case report. World J Gastroenterol 2014;20(14):4102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abdelkader A, et al. Cystic lesions of the pancreas: differential diagnosis and cytologic-histologic correlation. Arch Pathol Lab Med 2019;144(1):47–61. [DOI] [PubMed] [Google Scholar]
- [15].Nakanuma Y, et al. Clinicopathological characterization of so-called “cholangio-carcinoma with intraductal papillary growth” with respect to “intraductal papillary neoplasm of bile duct (IPNB)”. Int J Clin Exp Pathol 2014;7(6):3112–22. [PMC free article] [PubMed] [Google Scholar]
- [16].Nakanuma Y, Kakuda Y, Uesaka K. Characterization of intraductal papillary neoplasm of the bile duct with respect to the histopathologic similarities to pancreatic intraductal papillary mucinous neoplasm. Gut Liver 2019;13(6):617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wasif N, et al. Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer 2010;116(14):3369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee WJ, et al. Radiologic spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics 2001(21):S97–116. Spec No. [DOI] [PubMed] [Google Scholar]
- [19].Kim SA, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern–correlation with clinicopathologic findings. Radiology 2011;260(1):148–57. [DOI] [PubMed] [Google Scholar]
- [20].Ritchie DJ, Okamoto K, White SL. Intraductal papillary mucinous neoplasm of the biliary tract: a precursor lesion to cholangiocarcinoma. Radiol Case Rep 2019;14(4):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matsumoto T, et al. Impact of tumor location on postoperative outcome of intraductal papillary neoplasm of the bile duct. World J Surg 2019;43(5):1313–22. [DOI] [PubMed] [Google Scholar]


