Abstract
Background:
Socially determined vulnerabilities (SDV) to health disparities often cluster within the same individual. SDV are separately associated with increased risk of heart failure (HF). The objective of this study was to determine the cumulative effect of SDV to health disparities on incident HF hospitalization.
Methods and results:
Using the REasons for Geographic and Racial differences in (REGARDS) cohort study, we studied 25,790 participants without known HF and followed them 10+ years. Our primary outcome was an incident HF hospitalization through 12/31/2016. Guided by the Healthy People 2020 framework for social determinants of health, we examined 10 potential SDVs. We retained SDVs associated with incident HF hospitalization (p<0.10) and created a SDV count (0, 1, 2, 3+). Using the count, we estimated Cox proportional hazard models to examine associations with incident HF hospitalization, adjusting for potential confounders. Models were stratified by age (45–64, 65–74, and 75+ years) because past reports suggest greater disparities in HF incidence at younger ages. Participants were followed for a median of 10.1 years (IQR 6.5, 11.9). Black race, low educational attainment, low annual household income, zip code poverty, poor public health infrastructure, and lack of health insurance were associated with incident HF hospitalization. In adjusted models, among those 45–64 years, compared to having no SDV, having 1 SDV (HR 1.85; 95% CI 1.12–3.05), 2 SDV (HR 2.12; 95% CI 1.28–3.50) and 3+ SDVs (HR 2.45; 95% CI 1.48–4.04) were significantly associated with incident HF hospitalization (p for trend 0.001). We observed no significant associations for older individuals.
Conclusions:
A greater number of SDVs significantly increased risk of incident HF hospitalization among adults <65 years, which persisted after adjustment for cardiovascular risk factors. Using a simple SDV count that could be obtained from a social history during clinical assessment may identify younger individuals at increased risk.
Heart failure (HF) is a common chronic disease among older Americans.1 As there is no cure for HF, preventing its onset is of public health interest. Studies have identified predictors of incident HF including older age, male gender, cardiovascular disease (CVD) risk factors (e.g., diabetes, hypertension, smoking), and obesity.2–7 Recent studies investigated the effects of socio-demographic factors on incident HF and found that Black race, low education, low income, and neighborhood deprivation predict HF incidence.2, 6, 8–12 Racial disparities in HF are well-established; the prevalence of HF is greater among Blacks compared to Non-Hispanic Whites.9 Blacks develop HF at younger ages and have a 50% higher HF incidence at earlier ages than Whites.8, 10 Racial difference in incident HF are partially attributed to a greater burden of CVD risk factors among Blacks compared to Whites.10, 11 However, additional social determinants of health disparities may play an important role.2 Low educational attainment, low annual income, living in an area with relatively few healthcare services, and lacking health insurance put individuals at risk for incident HF.13–17
Despite our understanding of how individual social determinants affect incident HF hospitalization, we know little about their cumulative effect within the same individual.18 Socially determined vulnerabilities (SDV) to health disparities often cluster together within an individual.19 Someone with low educational attainment who experiences social isolation might be at higher risk for adverse outcomes. A recent REasons for Geographic and Racial Differences in Stroke (REGARDS) study assessed associations between SDVs and fatal coronary heart disease (CHD) and found that a greater number of SDVs was significantly associated with increased risk of fatal CHD. Adults with 2 SDVs had a 40% increased risk of fatal CHD compared to participants without SDVs. Understanding the joint impact of SDVs on the incidence of HF hospitalization is critical for preventing the onset of this debilitating and costly disease.
Given its rich self-reported and physiologic data and rigorously adjudicated CVD endpoints, the REGARDS study is well-suited to examine the cumulative effect of SDV to health disparities on incident HF hospitalization. We hypothesized that as a person’s number of SDV rose, the risk of incident HF hospitalization would rise. Because of prior observations of disparities for HF being greatest at younger ages, we hypothesized that the effect of a rising burden of SDV on risk of incident HF hospitalization would be greatest among individuals <65 years.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Monika Safford, MD (mms9024@med.cornell.edu) at Weill Cornell Medicine.
REGARDS Study:
REGARDS is a national, prospective, longitudinal cohort study evaluating racial and geographic disparities in stroke mortality.20 REGARDS recruited 30,239 community-dwelling, English-speaking individuals ≥45 years of age from 2003–2007 and is following participants for 10+ years.20
At enrollment, REGARDS participants completed a baseline computer assisted telephone interview, which ascertained sociodemographic information and medical history. Participants also received an in-home physical exam during which blood and urine samples were obtained, an electrocardiogram was performed, and a medication inventory was done through pill bottle review. This study was approved by the University of Alabama at Birmingham and Weill Cornell Medical College’s Institutional Review Board. All participants provided written informed consent.
Study cohort:
To assemble a cohort at risk for HF, we used HF-related medications to exclude individuals with suspected HF at baseline. The approach to determining suspected HF using medications was internally validated among a subgroup of REGARDS participants for whom Medicare claims were available. HF-related medications21 included: digoxin in the absence of atrial fibrillation, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker plus beta-blocker in the absence of hypertension; carvedilol; spironolactone; loop diuretics including furosemide, bumetanide, or torsemide; and/or a combination of hydralazine and nitrates. We excluded participants with: 1) missing data on self-reported atrial fibrillation, 2) on baseline medication use; and 3) participants with HF hospitalizations between the baseline CATI and in-home visit. Compared to Medicare claims, the negative predictive value was >95%. HF hospitalization rates was 27 per 1,000 person-years (PYs) among individuals with suspected HF versus 4 per 1,000 PYs among those without suspected HF.
Incident HF Hospitalizations:
REGARDS participants were contacted by phone to ascertain CVD outcomes every six-months.20 CVD events including incident HF hospitalizations were adjudicated by experts using a structured form,22 based on signs and symptoms of HF collected from chart-level data obtained from the hospital.21 We included incident HF hospitalizations through December 31, 2016. During follow-up calls, interviewers asked participants if they were hospitalized since the last call and, if so, why. Participant-reported reasons for hospitalizations were recorded and reviewed by a nurse-investigator. Medical records for CVD-related conditions were retrieved. Adjudication was based on medical records: 1) clinical presentation (symptoms of dyspnea on exertion, paroxysmal nocturnal dyspnea, night cough; and signs including peripheral edema, jugular venous distention, pulmonary rales, hepatomegaly, abnormal central venous pressure, tachycardia), 2) laboratory evaluation (b-type natriuretic peptide), 3) imaging (chest radiogram with cardiomegaly, pulmonary vascular congestion, or pleural effusion; or cardiac imaging such as echocardiography), and 4) medical treatments (weight loss of ≥4.5 kg in 5 days with diuresis).
SDV to health disparities:
The Healthy People 2020 framework of social determinants of health guided this work.23 We evaluated SDVs from 5 domains of the framework: 1) education (<high school); 2) economic stability (<$35,000 annual household income); 3) neighborhood/built environment (living in a zip code with >25% of residents living below the Federal poverty line, and living in a rural area as defined by rural urban commuting area codes 9 and 10); 4) health and healthcare (living in a Health Professional Shortage Area [HPSA], lacking health insurance, and living in a US state with poor public health infrastructure); and 5) social and community (Black race, social isolation). Social isolation was determined through two questions (not seeing friends or family members at least once a month; having nobody to care for you if you become seriously ill or disabled). States considered to have poor public health infrastructure were identified using longitudinal data from America’s Health Ranking (AHR)24 and represented states that were in the lowest decile in at least 80% of the decade prior to the REGARDS enrollment period (1993–2002).
Covariates:
To understand the mechanisms leading to associations between SDV and incident HF hospitalization, we sequentially adjusted for variables reflecting 1) socio-demographics, 2) medical conditions, 3) functional status, 4) health behaviors, and 5) physiologic variables. Socio-demographics included age at baseline, gender, and Southeastern region (stroke belt/buckle, defined as North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana and Arkansas; or non-stroke belt). Medical conditions included history of high blood pressure (self-report of hypertension diagnosis, use of antihypertensive medications, or blood pressure ≥140/90 mm Hg at the baseline in-home visit reflecting hypertension guidelines at the time of the observation period25), high cholesterol (self-reported diagnosis, total cholesterol >=240 or low density lipoprotein (LDL) cholesterol >160 mg/dL or high density lipoprotein (HDL) <40), diabetes (use of diabetes medications or insulin, or fasting blood glucose >126 mg/dL, or non-fasting glucose >200 mg/dL). Use of antihypertensive medications, statins, and insulin were included separately. Functional status was assessed with the Physical Component Summary (PCS) and the Mental Component Summary (MCS) scores. Health behaviors included smoking (currently vs. not), alcohol use (risky drinking based on sex-specific National Institute on Drug Abuse cut points vs. others), physical activity (enough activity to work up a sweat on most days of the week vs. others), and adherence to the Mediterranean diet using the Mediterranean diet score.26, 27 Physiologic variables included body mass index, systolic and diastolic blood pressure, total cholesterol, high density lipoprotein cholesterol, log transformed high sensitivity c-reactive protein, log transformed urinary albumin-to-creatinine ratio, and estimated glomerular filtration rate using the CKD-Epi equation.28
Statistical Analyses:
We examined bivariate associations between candidate SDV (described above in the SDV to health disparities section) and incident HF hospitalization, adjusting for age and gender. SDVs that demonstrated a statistically significant association (p<0.10) with incident HF were retained for further analysis. Using the retained SDVs (p<0.10), we created a SDV count (0, 1, 2, 3+) and described characteristics of our HF-free cohort within SDV count categories. We assessed multicollinearity among SDVs using variance inflation factors (VIF).19
To assess for effect modification by age, we tested interactions between SDV count and three age subgroups in an overall model: <65, 65–74, and 75+ years. Since the interaction term was significant (joint test p<0.0001), we present age-stratified results. Using Kaplan Meier plots, we depicted the cumulative risk of HF by SDV count by age group. Using the log-rank test, we assessed the equal incident HF hospitalization rates by SDV count for each age group.
We estimated Cox models to examine the effect of the SDV count on incident HF hospitalization, by age. First, a crude model examined the association between SDV count and incident HF. Second, a minimally-adjusted Cox model adjusted for age and gender. Finally, we added covariates in groups: 1) socio-demographics, 2) medical conditions and medications, 3) functional status, 4) health behaviors, and 5) physiologic variables. We calculated adjusted hazard ratios (aHR) and 95% confidence intervals (95% CI) for each estimate. To reduce the effect of missing data, we performed multiple imputation by chained equations on covariates that were missing.
As a sensitivity analysis, we conducted a competing risk survival analysis fitting Fine and Gray’s sub-distribution hazard model, where death from any cause was considered a competing event.29 Analyses were conducted in SAS version 9.4 and R 3.4.1.
Results:
Participant Characteristics:
We included 25,790 participants without suspected HF at baseline with exclusions in eFigure 1. The study sample included 13,487 (52%) participants aged 45–64 years; 8,214 (32%) aged 65–74 years; and 4,089 (16%) aged 75+ years. We observed missing data for some covariates, with the largest proportions of missing information were for Mediterranean diet scores (28%) and annual household income (12%). Missingness for the rest of the variables was <6%. The degree of missingness observed in our study was less than established thresholds of 50%.30 Among individuals with no missingness on SDVs who were 45–64 years, individuals with a greater number of SDVs were female, had hypertension and diabetes, had worse physical well-being, were smokers, and lived in the Southeast (Table 1). We observed similar characteristics among individuals with more SDVS in the two older age groups (eTables 1 and 2).
Table 1.
Participant Characteristics by Number of Socially Determined Vulnerabilities to Health Disparities and Incident Heart Failure, Age Group <65 years old.
| Groups by Number of Vulnerabilities | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 or more | p-value | |
| N | 2,971 | 3,801 | 2,549 | 2,820 | |
| Vulnerability Factors | |||||
| Black race, N (%) | 0 (0.0%) | 1396 (36.7%) | 1534 (60.2%) | 2373 (84.1%) | 0.001 |
| Low education (< High school), N (%) | 0 (0.0%) | 54 (1.4%) | 169 (6.6%) | 784 (27.8%) | 0.001 |
| Low annual household income (< $35,000), N (%) | 0 (0.0%) | 720 (18.9%) | 1411 (55.4%) | 2290 (81.2%) | <0.001 |
| Zip code level poverty (25% residents living below Federal poverty line), N (%) | 0 (0.0%) | 174 (4.6%) | 584 (22.9%) | 1599 (56.7%) | <0.001 |
| Residence in the worst ranked states for public health infrastructure, N (%)* | 0 (0.0%) | 1383 (36.4%) | 1110 (43.5%) | 1767 (62.7%) | <0.001 |
| No health insurance, N (%) | 0 (0.0%) | 74 (1.9%) | 290 (11.4%) | 1108 (39.3%) | <0.001 |
| Sociodemographic factors | |||||
| Age, Mean (SD) | 56.9 (4.9) | 57.2 (4.9) | 57.2 (4.9) | 57.3 (5.1) | 0.019 |
| Female, N (%) | 1415 (47.6%) | 2083 (54.8%) | 1547 (60.7%) | 1830 (64.9%) | <0.001 |
| Southeast region (Stroke Belt or Stroke Buckle), N (%)† | 1163 (39.1%) | 2231 (58.7%) | 1592 (62.5%) | 1968 (69.8%) | <0.001 |
| Medical Conditions | |||||
| Hypertension, N (%) | 1071 (36.0%) | 1828 (48.1%) | 1442 (56.6%) | 1854 (65.7%) | <0.001 |
| Dyslipidemia, N (%) | 1606 (54.1%) | 2036 (53.6%) | 1336 (52.4%) | 1510 (53.5%) | 0.73 |
| Diabetes, N (%) | 264 (8.9%) | 555 (14.6%) | 536 (21.0%) | 738 (26.2%) | <0.001 |
| Use of Medications | |||||
| Antihypertensive medications, N (%) | 1026 (34.5%) | 1670 (43.9%) | 1355 (53.2%) | 1631 (57.8%) | <0.001 |
| Statins, N (%) | 757 (25.5%) | 925 (24.3%) | 586 (23.0%) | 659 (23.4%) | 0.12 |
| Insulin, N (%) | 42 (1.4%) | 106 (2.8%) | 117 (4.6%) | 201 (7.1%) | <0.001 |
| Functional Status | |||||
| SF-12 physical component score, Median (IQR)‡ | 53.2 (47.78, 56.0) | 52.1 (45.1, 55.5) | 50.3 (40.1, 54.8) | 47.1 (36.0, 53.6) | <0.001 |
| SF-12 mental component score, Median (IQR)§ | 56.1 (52.8, 58.6) | 56.1 (51.7, 58.8) | 55.9 (50.1, 58.9) | 54.8 (45.3, 58.6) | <0.001 |
| Health Behaviors | |||||
| Current cigarette Smoking, N (%) | 362 (12.2%) | 601 (15.8%) | 533 (20.9%) | 824 (29.2%) | <0.001 |
| Risky alcohol consumption, N (%) | 183 (6.2%) | 177 (4.7%) | 95 (3.7%) | 91 (3.2%) | <0.001 |
| Physical activity, N (%) | 2163 (72.8%) | 2677 (70.4%) | 1686 (66.1%) | 1811 (64.2%) | <0.001 |
| High adherence to Mediterranean diet, N (%) | 629 (21.2%) | 781 (20.5%) | 399 (15.7%) | 341 (12.1%) | <0.001 |
| Physiological Factors | |||||
| Body mass index, Mean (SD)‖ | 28.6 (5.5) | 29.5 (6.0) | 30.6 (6.7) | 30.9 (7.1) | <0.001 |
| Systolic blood pressure, Mean (SD) | 121.1 (14.2) | 124.0 (15.4) | 126.2 (16.1) | 129.5 (17.1) | <0.001 |
| Diastolic blood pressure, Mean (SD) | 75.8 (9.0) | 77.2 (9.3) | 78.2 (9.4) | 79.70(10.5) | <0.001 |
| Total cholesterol, Mean (SD) | 195.6 (36.9) | 196.5 (39.2) | 197.3 (40.8) | 196.7 (42.2) | 0.47 |
| High density lipoprotein cholesterol, Median (IQR) | 49.0 (40.0, 61.0) | 50.0 (40.0, 61.0) | 49.0 (41.0, 60.0) | 50.0 (41.0, 61.0) | 0.20 |
| C-reactive protein≥3, N (%) | 819 (27.6%) | 1389 (36.5%) | 1123 (44.1%) | 1383 (49.0%) | <0.001 |
| Urinary Albumin/Creatinine ratio≥30 (mg/g), N (%) | 192 (6.5%) | 293 (7.7%) | 310 (12.2%) | 460 (16.3%) | <0.001 |
| Estimated GFR from the CKD-Epi equation, Median (IQR) | 94.2 (82.6, 99.7) | 95.1 (82.6, 103.1) | 96.6 (83.7, 108.3) | 99.5 (84.8, 112.2) | <0.001 |
Public Health Infrastructure is calculated based on the America’s Health Ranking data, and flags nine states (Louisiana, New Mexico, Mississippi, Nevada, South Carolina, Florida, Arkansas, Texas, Tennessee) that belonged to the states with the worst public health infrastructure for at least 8 years during the ten-year period prior REGARDS enrollment (1993- 2002).
REGARDS study oversampled residents from the stroke belt (Alabama, Arkansas, Louisiana, Mississippi, Tennessee, and the noncoastal regions in North Carolina, South Carolina, and Georgia) and the stroke buckle (the coastal regions within North Carolina, South Carolina, and Georgia).
Ranges from 0 to 100, and a higher score indicates better physical health.
Ranges from 0 to 100, and a higher score indicates better mental health.
Calculated as weight in kilograms divided by height in meters squared.
Note. p-values correspond with ANOVA for continuous variables that are normally distributed, Wilcoxon rank-sum (2 groups) or Kruskal-Wallis (>2 groups) for continuous variables that are skewed, and Pearson’s chi-squared or Fisher’s exact test for binary and categorical variables
SDV Factor Selection:
Black race, low educational attainment, low annual household income, zip code poverty, poor public health infrastructure, and lack of health insurance were all significantly associated with incident HF hospitalization at p<0.10 (Table 2). These SDVs were retained to create the SDV count (0, 1, 2, and 3+) that was used in Cox models described below. There was no evidence of multicollinearity between the six SDVS. Absolute values of correlation coefficients ranged from 0.04 to 0.29 (eFigure 2).
Table 2.
Minimally Adjusted Associations of Socially Determined Vulnerabilities and Incident Heart Failure
| Socially Determined Vulnerabilities | HR (95% CI)* | P |
|---|---|---|
| Black race | 1.23 (1.09, 1.40) | 0.001 |
| Low educational attainment (< High school) | 1.75 (1.50, 2.03) | <.0001 |
| Low annual household income (< $35,000) | 1.78 (1.55, 2.03) | <.0001 |
| Zip code with high poverty (> 25% residents living below Federal poverty line) | 1.21 (1.05, 1.40) | 0.009 |
| Residence in Health Professional Shortage Areas (HPSA) | 1.00 (0.89, 1.13) | 0.951 |
| Residence in the worst ranked states for public health infrastructure † | 1.20 (1.06, 1.35) | 0.003 |
| Lack of health insurance | 1.68 (1.29, 2.19) | 0.0001 |
| Rural residence | 1.02 (0.69, 1.50) | 0.926 |
| Social isolation (Not seeing friends or family members at least once a month) | 1.07 (0.82, 1.41) | 0.620 |
| Social isolation (Not having anyone to care for you if become seriously ill or disabled) | 1.05 (0.88, 1.26) | 0.584 |
Cox Proportional Hazard models results; associations of each vulnerability factor adjusted for age at baseline and gender;
Public Health Infrastructure is calculated based on the America’s Health Ranking data; nine states (Louisiana, New Mexico, Mississippi, Nevada, South Carolina, Florida, Arkansas, Texas, Tennessee) fell into the bottom 20% US states with the worst public health infrastructure for at least 8 years during the ten-year period prior REGARDS enrollment (1993–2002).
Incident HF hospitalization:
Over a median follow-up of 10.1 years (SD=3.3), we observed 1,109 incident HF hospitalizations. Of these, 276 hospitalizations (25%) occurred among participants aged 45–64 years, 441 (40%) occurred among 65–74 years, and 392 (35%) occurred among 75+ years. Median [IQR] time to HF hospitalization was 6.0 [3.0–9.2] for those aged 45–64 years, 6.0 [2.8–8.7] for 65–74 years, and 5.6 [3.2–9.2] for 75+ years. Kaplan Meier survival curves are shown in Figure 1. The log rank test p-value was <0.0001 for differences in the cumulative incidence of HF hospitalization among SDV groups for those aged 45–64 and 65–74, but not for those aged 75+ (p=0.59). Age-adjusted incidence rates of HF hospitalizations per 1,000 PYs by SDV groups and age are shown in eFigure 3. HF hospitalization incidence was lowest among those <65 years and highest among those 75+ years. In the <65 and 65–74 year old groups, HF hospitalization incidence increased with each additional SDV. For individuals with 3+ SDVs compared to no SDVs, HF hospitalization incidence was nearly 7 times higher in <65 years old stratum, and 2 times higher among those 65–74 years old. In the 75+ group, the highest HF hospitalization incidence was observed for individuals with 2 SDVs.
Figure 1.
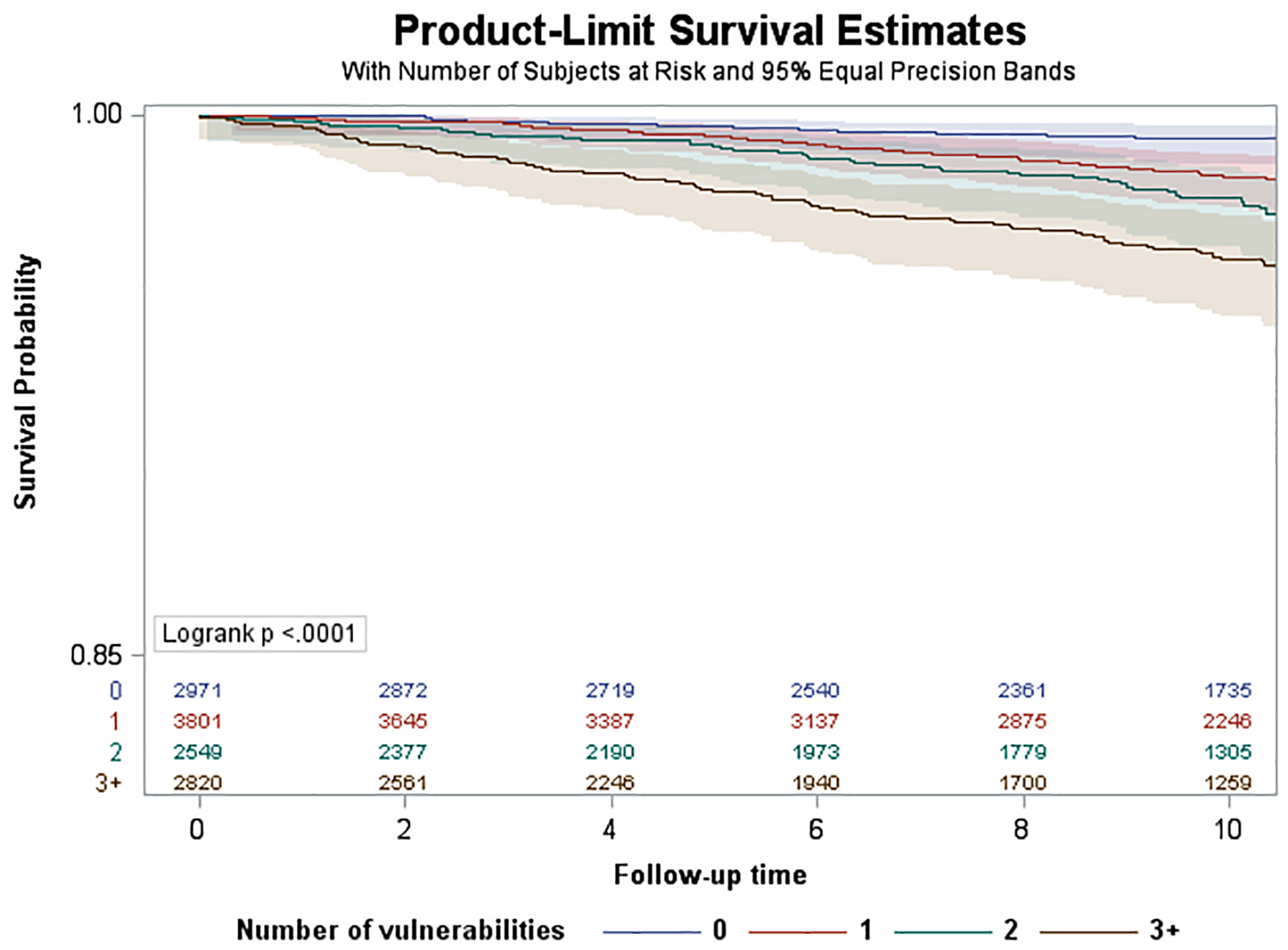
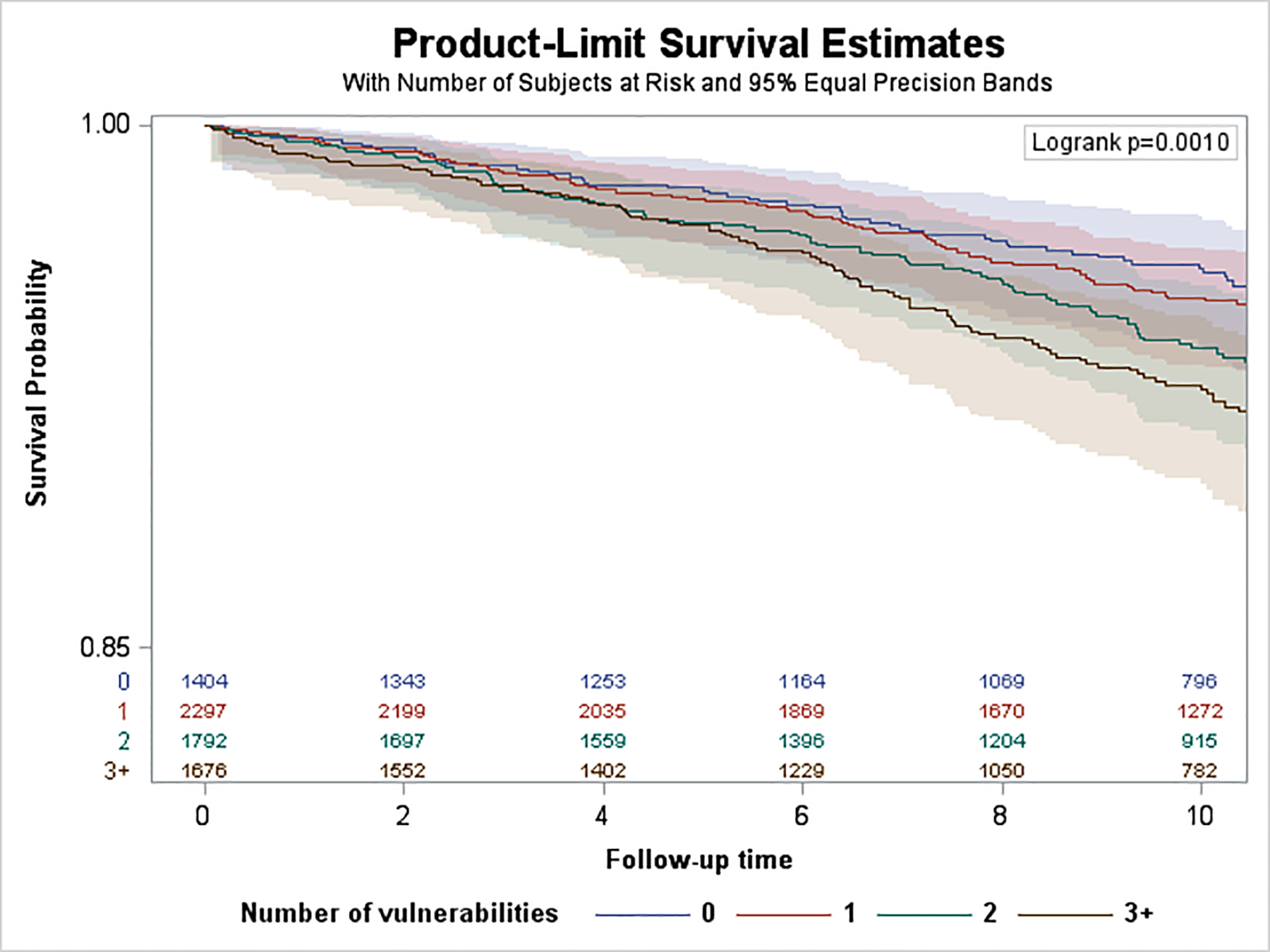
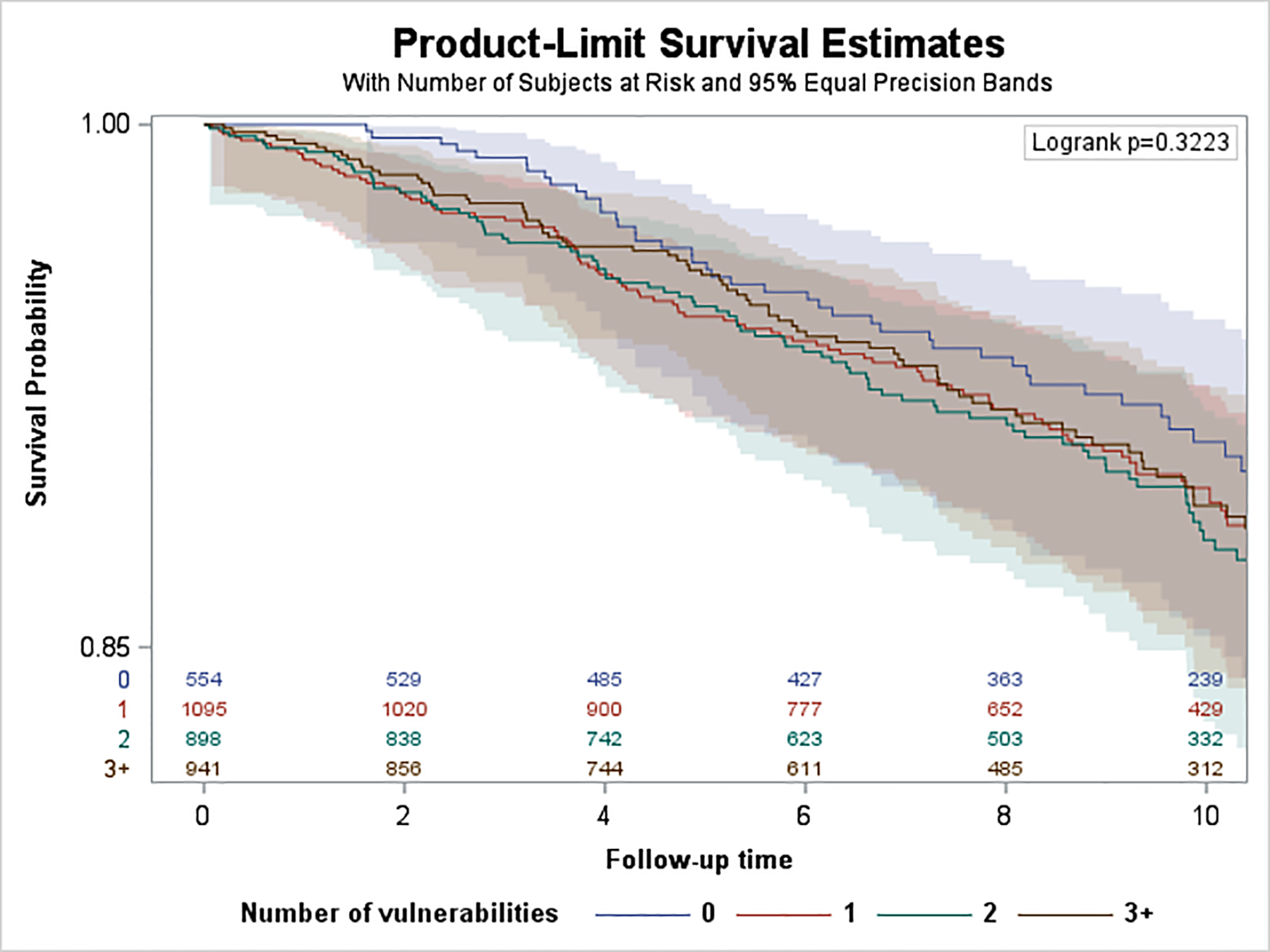
Kaplan-Meier Survival Curves by Age Strata. A. Age group <65 years. B, Age group 65–74 years. C, Age group 75+ years.
Adults 45–64 years:
In unadjusted models (eFigure 4), we observed significant associations between SDV count and incident HF hospitalization. HRs increased in a graded fashion with each additional SDV (1 SDV: 2.69; 95% CI 1.61–4.49; 2 SDVs: 4.13, 95% CI 2.47–6.91; 3+ SDVs: 7.16; 95% CI 4.39–11.67, p for trend <.0001). In models adjusting for age at baseline and gender (Figure 2), we continued to observe graded, statistically significant HRs for 1 SDV (2.72; 95% CI 1.63–4.54), 2 SDVs (4.25; 95% CI 2.54–7.13), and 3+ SDVs (7.41; 4.53–12.12) compared to 0 SDVs (p for trend <0.0001). In fully adjusted models (Figure 3), adjusted HRs for the association between SDV count and incident HF hospitalization were attenuated but remained significant. Compared to having 0 SDV, having 1 SDV had aHR 1.85 (95% CI 1.12–3.05), having 2 SDV (aHR 2.12; 95% CI 1.28–3.51) and 3+ SDVs (aHR 2.45; 95% CI 1.48–4.04) were significantly associated with incident HF hospitalization (p for trend <0.0001).
Figure 2.
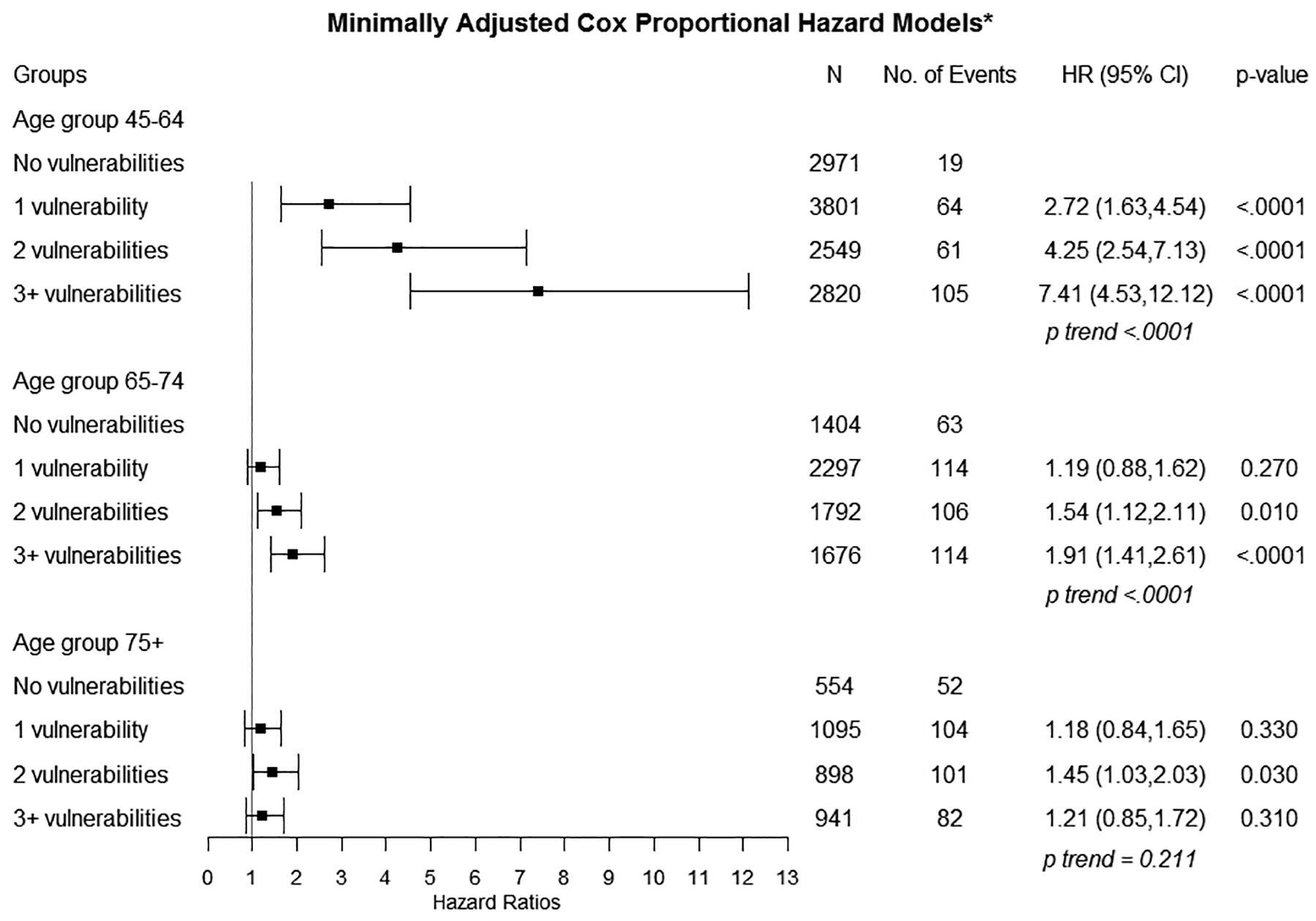
Minimally Adjusted Estimates for Associations Between Socially Determined Vulnerabilities and Incident Heart Failure
*Covariates include: age at baseline, gender; Complete case analysis
Figure 3.
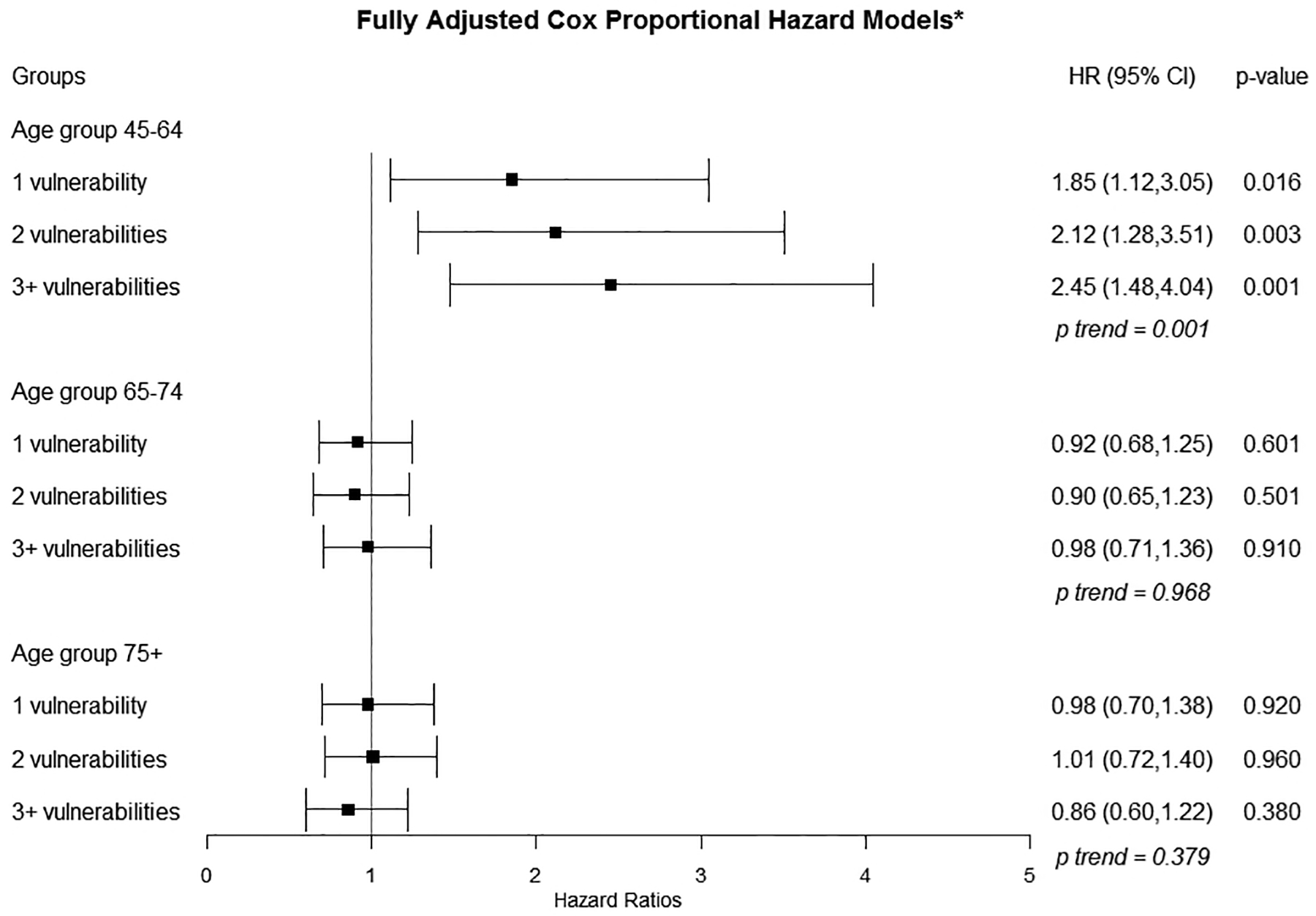
Fully Adjusted Estimates for Associations Between Socially Determined Vulnerabilities and Incident Heart Failure
*Covariates include: age at baseline, gender, region of residence, history of hypertension, history of hyperlipidemia, history of diabetes, use of hypertensive medications, statin use, insulin use, SF-12 physical and mental statuses, smoking, alcohol consumption, physical activity, Mediterranean diet score, body mass index, systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoproteins, log transformed c-reactive protein, log-transformed albumin-to-creatinine ratio, estimated glomerular filtration rate from the CKD-Epi equation; Missing data were imputed
Adults 65–74 years:
In unadjusted Cox models (eFigure 4), statistically significant associations were observed for individuals with 2 SDV (1.43; 1.04, 1.95) and 3+ SDV (1.72; 1.27–2.34) compared to 0 SDV, p for trend <.0001. In age and gender adjusted models (Figure 2), HRs remained significant for 2 SDVs (1.54; 95% CI 1.12–2.11) and 3+ SDVs (1.91; 95% CI 1.41–2.61) compared to 0 SDV (p for trend <0.0001). In fully adjusted Cox models (Figure 3), we did not observe significant associations between number of SDVs and incident HF hospitalization (p for trend=0.986).
Adults 75+ years:
Among the 554 adults with no SDV, we observed 39 incident HF hospitalizations. Among the 2,930 adults with 1 or more SDV, we observed 228 incident HF hospitalizations. None of the crude HRs for the 75+ year group were statistically significant, p for trend=0.602 (eFigure 4). In age and gender adjusted models (Figure 2), the only statistically significant minimally adjusted HR was for individuals with 2+ SDV (1.45; 95% CI 1.03–2.03) compared to 0 SDV, p for trend=0.211. We observed no significant associations between SDV count and incident HF hospitalization with fully-adjusted HRs near 1.0 (p for trend =0.379).
Sensitivity Analyses:
Examining death as a competing risk resulted in sub-distribution HR estimates that were nearly identical to the main analysis.
Discussion
As the number of SDVs increased, the risk of incident HF hospitalization increased among adults <65 years of age. An individual <65 years old with 2 SDVs had more than double increased risk of incident HF hospitalization, and an individual with 3+ SDVs had 2.5-fold increased risk of incident HF hospitalization. Findings persisted after adjusting for confounders, including demographics, functional status, medical history and medications, and physiological factors. Importantly, we found that associations between the cumulative burden of SDVs and incident HF hospitalization was present in crude associations but of lesser magnitude, which attenuated among those aged 65–74 years. We observed no associations among those aged 75+ years.
To our knowledge, this is the first study to examine the effect of an increasing number of SDVs within the same individual and incident HF hospitalization. We add to the literature by showing that the cumulative SDV burden has a large effect on incident HF hospitalization, specifically among individuals <65 years. This finding has important implications for population health management, indicating that efforts for preventing HF should focus on younger individuals with a high number of SDVs.
Our finding that the cumulative burden of SDV was associated with incident HF hospitalization after controlling for traditional CVD risk factors supports the notion that processes beyond traditional biology play a role in the pathogenesis of HF. Close links between CVD risk factors and SDV have been described.18, 31 It has been hypothesized that the link between CVD risk factors and SVD is in part mediated by allostatic load,32–35 where chronic stress and negative psychological states resulting from discrimination and oppression36 can lead to maladaptive rises in stress hormones and cytokines,31 with inflammatory state that negatively alters cardiac and vascular structure. Our findings, coupled with the suspected role of cytokines in the pathogenesis of HF,36 suggest that allostatic load may play a direct role in the development of HF beyond its influence on major HF risk factors like hypertension and coronary artery disease. This observation lends additional support for the need to develop strategies to address societal structures to reduce health disparities and improve morbidity and mortality related to CVD. Individuals with multiple SDVs may be undertreated19 with regard to traditional CVD risk factors, a process that could relate to implicit physician biases.37 We observed higher blood pressure and less statin use in those with 3+ SDVs. Whether or not individuals with high SDV burden represent a subpopulation for which more aggressive CVD risk reduction strategies than are currently delineated in guidelines (e.g., CVD risk of 5–7.5% according to the Pooled Cohort Risk Equations) could attenuate the effects of allostatic load remains unknown. Until additional research is conducted to guide clinical management, the six SDVs examined here - many collected during a standard medical encounter - could be added together by clinicians to identify individuals at increased risk, beyond traditional CVD risk factors.
Another possible explanation for our observations could relate to healthcare access. There are 5 key dimensions to access: approachability, acceptability, availability and accommodation, affordability, and appropriateness.38 Individuals with multiple SDV likely face challenges across multiple access dimensions.39 For example, an individual who lives in a region with poor health is less likely to have healthcare services that are physically available (availability and accommodation). An individual who lacks insurance may not be able to afford healthcare (affordability). Finally, an individual with low education may be less familiar with recommended screenings and less willing to follow medical direction (acceptability). Accordingly, an individual with 3 SDVs may experience challenges at each step, putting them at an incrementally increased disadvantage to receiving necessary care leading to less controlled CVD risk factors, and eventually HF. Our findings may help inform public health outreach efforts to support individuals with multiple SDVs.
It is notable that we only observed significant associations between cumulative SDV and incident HF hospitalization among younger adults. While this could have resulted from survival bias where those with higher SDV died before developing HF, we conducted a sensitivity analysis incorporating death as a competing risk and our findings remained unchanged. Prior literature demonstrated that social factors are less influential with advancing age40, an observation that could result from improved access to care via Medicare enrollment. Another possibility is that biologic factors like hypertension, diabetes, and coronary artery disease supersede the effect of social factors on incident HF hospitalization at older ages. Closer examination of individuals <65 years who do not develop incident HF in the setting of a high SDV burden is warranted to identify protective characteristics and factors that contribute to resilience.
Limitations:
An observational design limits our ability to draw causal inferences. Additionally, demographic and medical history variables were self-reported. Because participants were followed prospectively until they experienced an incident HF hospitalization event, SDVs were captured at the baseline survey so we were unable to examine the effects of time-varying SDVs (e.g., insurance status). We used incident HF hospitalization as a proxy for incident HF but recognize that some incident HF cases are diagnosed in the outpatient setting. Finally, the suspected HF-free cohort was internally validated with Medicare data, which is an imperfect gold standard for HF. We cannot corroborate the same operating characteristics would be observed in commercial claims data.41
Conclusions
Our study suggests that among individuals <65, the cumulative burden of SDV is an important risk factor for incident HF hospitalization that rises with each additional SDV to health disparities. This effect was not explained by CVD risk factors and confounders. Similar patterns were not observed for individuals 65+ years. While our findings should be confirmed in cohorts with larger samples of younger adults, the number of SDVs in individuals <65 years may be a simple and novel strategy to identify individuals at increased risk for incident HF hospitalization.
Supplementary Material
What is known:
Socially determined vulnerabilities to health disparities often cluster within the same individual
Separately, we know socially determined vulnerabilities are independently associated with an increased risk of heart failure.
What the study adds:
We determined the cumulative effect of socially determined vulnerabilities to health disparities on incident heart failure hospitalization.
This work found that a great number of socially determined vulnerabilities significantly increased the risk of incident heart failure hospitalization among adults younger than 65 years of age.
A simple count of socially determined vulnerabilities may be a quick and easy indicator of patients who are at increased risk of heart failure.
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/
Sources of Funding: This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. This work is also supported by R01 HL80477 and R01HL135199–02S1 from the National Heart Lung and Blood Institute (NHLBI), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, NIA or NHLBI. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data.
Disclosures: Dr. Safford receives salary support from Amgen for investigator initiated research. Dr. Levitan receives research support from Amgen, has served on Amgen advisory boards, and as a consultant for a research project funded by Novartis. My co-authors and I have no conflicts of interest or financial disclosures.
References
- 1. Kannel WB. Incidence and epidemiology of heart failure. Heart failure reviews. 2000;5:167–73. [DOI] [PubMed] [Google Scholar]
- 2. Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E and Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation Heart failure. 2012;5:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW and Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circulation Heart failure. 2008;1:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE and Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. Journal of the American College of Cardiology. 2000;35:1628–37. [DOI] [PubMed] [Google Scholar]
- 5. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C and Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Archives of internal medicine. 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 6. Ingelsson E, Lind L, Arnlov J and Sundstrom J. Socioeconomic factors as predictors of incident heart failure. Journal of cardiac failure. 2006;12:540–5. [DOI] [PubMed] [Google Scholar]
- 7. Roberts CB, Couper DJ, Chang PP, James SA, Rosamond WD and Heiss G. Influence of life-course socioeconomic position on incident heart failure in blacks and whites: the Atherosclerosis Risk in Communities Study. American journal of epidemiology. 2010;172:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD and Hulley SB. Racial differences in incident heart failure among young adults. The New England journal of medicine. 2009;360:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma A, Colvin-Adams M and Yancy CW. Heart failure in African Americans: disparities can be overcome. Cleveland Clinic journal of medicine. 2014;81:301–11. [DOI] [PubMed] [Google Scholar]
- 10. Yancy CW. Heart failure in African Americans: a cardiovascular engima. Journal of cardiac failure. 2000;6:183–6. [DOI] [PubMed] [Google Scholar]
- 11. Yancy CW. Heart failure in African Americans. The American journal of cardiology. 2005;96:3i–12i. [DOI] [PubMed] [Google Scholar]
- 12. Akwo EA, Kabagambe EK, Harrell FE Jr., Blot WJ, Bachmann JM, Wang TJ, Gupta DK and Lipworth L. Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States. Circulation Cardiovascular quality and outcomes. 2018;11:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. AHRQ. National Healthcare Disparities Report. 2003;2003. https://archive.ahrq.gov/qual/nhdr03/nhdr03.htm. Accessed February, 10, 2020.
- 14. AHRQ. 2015 National Healthcare Quality and Disparities Report and 5th Anniversary Update on the National Quality Strategy. 2016. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr15/index.html. Accessed February, 10, 2020.
- 15. CDC. CDC Health Disparities and Inequalities Report - United States, 2013. MMWR. 2013;62:1–184. [PubMed] [Google Scholar]
- 16. Institute of Medicine. Guidance for the National Healthcare Disparities Report. Washington, DC: National Academy Press; 2002. [PubMed] [Google Scholar]
- 17. Philbin EF, Dec GW, Jenkins PL and DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. The American journal of cardiology. 2001;87:1367–71. [DOI] [PubMed] [Google Scholar]
- 18. Erqou S, Echouffo-Tcheugui JB, Kip KE, Aiyer A and Reis SE. Association of cumulative social risk with mortality and adverse cardiovascular disease outcomes. BMC cardiovascular disorders. 2017;17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schroff P, Gamboa CM, Durant RW, Oikeh A, Richman JS and Safford MM. Vulnerabilities to Health Disparities and Statin Use in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Journal of the American Heart Association. 2017;6. doi: 10.1161/JAHA.116.005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS and Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 21. Goyal P, Mefford MT, Chen L, et al. Assembling and validating a heart failure-free cohort from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. BMC Med Res Methodol. 2020;20(1):53 Published 2020 Mar 4. doi: 10.1186/s12874-019-0890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH and Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. American journal of epidemiology. 2004;160:1152–8. [DOI] [PubMed] [Google Scholar]
- 23. HealhyPeople2020. Healthy People 2020: An Opportunity to Address the Societal Determinants of Health in the United States. 2010;2019. https://www.healthypeople.gov/sites/default/files/SocietalDeterminantsHealth.pdf. Accessed February 10, 2020.
- 24. United Health Foundation U. America’s Health Rankings. 2018;2018. https://www.americashealthrankings.org/learn/reports/2018-annual-report. Accessed February 10, 2020.
- 25. Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J and Forciea MA. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Annals of internal medicine. 2017;166:430–437. [DOI] [PubMed] [Google Scholar]
- 26. Tektonidis TG, Akesson A, Gigante B, Wolk A and Larsson SC. Adherence to a Mediterranean diet is associated with reduced risk of heart failure in men. European journal of heart failure. 2016;18:253–9. [DOI] [PubMed] [Google Scholar]
- 27. Wirth J, di Giuseppe R, Boeing H and Weikert C. A Mediterranean-style diet, its components and the risk of heart failure: a prospective population-based study in a non-Mediterranean country. European journal of clinical nutrition. 2016;70:1015–21. [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T and Coresh J. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fine JP and Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 30. Madley-Dowd P, Hughes R, Tilling K and Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. Journal of clinical epidemiology. 2019;110:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M and Yancy CW. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132:873–98. [DOI] [PubMed] [Google Scholar]
- 32. Chyu L and Upchurch DM. Racial and ethnic patterns of allostatic load among adult women in the United States: findings from the National Health and Nutrition Examination Survey 1999–2004. Journal of women’s health (2002). 2011;20:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duru OK, Harawa NT, Kermah D and Norris KC. Allostatic load burden and racial disparities in mortality. Journal of the National Medical Association. 2012;104:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B and Seeman TE. History of socioeconomic disadvantage and allostatic load in later life. Social science & medicine (1982). 2012;74:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McEwen BS and Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seta Y, Shan K, Bozkurt B, Oral H and Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. Journal of cardiac failure. 1996;2:243–9. [DOI] [PubMed] [Google Scholar]
- 37. Institute of Medicine Committee on U, Eliminating R and Ethnic Disparities in Health C. In: Smedley BD, Stith AY and Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care Washington (DC): National Academies Press (US) Copyright 2002 by the National Academy of Sciences; All rights reserved.; 2003. [PubMed] [Google Scholar]
- 38. Levesque JF, Harris MF and Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. International journal for equity in health. 2013;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calvillo-King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H and Halm EA. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28:269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rehkopf DH, Haughton LT, Chen JT, Waterman PD, Subramanian SV and Krieger N. Monitoring socioeconomic disparities in death: comparing individual-level education and area-based socioeconomic measures. American journal of public health. 2006;96:2135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kucharska-Newton AM, Heiss G, Ni H, Stearns SC, Puccinelli-Ortega N, Wruck LM and Chambless L. Identification of Heart Failure Events in Medicare Claims: The Atherosclerosis Risk in Communities (ARIC) Study. Journal of cardiac failure. 2016;22:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


