Abstract
The research described here presents data on the effect of galactans of red algae, carrageenans (λ/μ/ν-, κ-, κ/β-, and ι/κ-types), and agar on complement system activation in normal human serum. The experiments were based on well surfaces coated with triggering agents for binding initiating complement components —C3 and C4. The sulfated galactans inhibited C3 binding to lipopolysaccharide with direct dependence on the sulfation degree of polysaccharides. Sulfation degree was also important in carrageenans’ capacity to reduce C4 binding to mannan. However, C4 binding to antibodies was considerably activated by carrageenans, especially with 3,6-anhydrogalactose. The gelling carrageenans were able to block antigen binding centers of total serum IgM and with more intensity than non-gelling. No structural characteristics mattered in ameliorating C5 cleavage by plasmin in extrinsic protease complement activation, but λ/μ/ν- and κ/β-carrageenans almost completely inhibited C5 cleavage. Thus, galactans participated in cell surface biology by imitating surface glycans in inhibition of C3 binding and mannose binding lectin, but as to the tthe heclassical pathway these substances stimulated complement, probably due to their structure based on carrabiose.
Keywords: Carrageenan, Agar, Heparin, Complement, Lipopolysaccharide, Plasmin
1. Introduction
Red algae contain considerable amounts of sulfated galactans, and two groups of these polysaccharides, known as agars and carrageenans, find wide practical application in gelling and stabilizing food compounds. These galactans usually have an unbranched backbone built of alternating 3-linked β-d-galactopyranose and 4-linked α-galactopyranose residues. The latter has the l-configuration in the agar group of polysaccharides and d-configuration in carrageenans. Additionally, 4-linked residues may be present as 3,6-anhydro derivatives (Usov, 1998).
Carrageenans are composed of repeating units of [→3)-β-d-Galp-(1→4)-α-d-Galp-(1→] (‘diads’ or ‘carrabiose’ disaccharides), mainly substituted by sulfate groups (Stortz & Cerezo, 2002) and rarely with other substituents (Chiovitti et al., 1998; Estevez, Ciancia, & Cerezo, 2004). Carrageenans are classified into families by the location of the sulfate groups in the β-galactose moiety. Then, a particular name is given to each structural disaccharide unit based on sulfate group locations and presence or absence of the 3,6-anhydro sugar in the α-galactose moiety. Carrageenans found in nature usually contain more than one carrabiose unit, forming hybrid structures, and the number and structure of diads varies with algal species and life stage (Cosenza, Navarro, Ponce, & Stortz, 2017; Craigie, 1990). Some physico-chemical characteristics of carrageenans with predominant λ-, κ-, or ι-diad contents enable their use as gelling and stabilizing agents, which are properties carrageenans share with agars (Lahaye, 2001; Usov, 1998). Carrageenans and agars also exhibit a wide spectrum of biological activities regarding human health (Koutsaviti, Ioannou, & Roussis, 2018; Pereira & Critchley, 2020; Pereira, 2018). Sulfated galactans from red algae have been observed to interact with the serine protease system—the complement (Baker, Nicklin, & Miller, 1986; Davies, 1965) and coagulation/fibrinolysis cascades (dos Santos-Fidencio, Gonçalves, Noseda, Duarte, & Ducatti, 2019; Opoku, Qiu, & Doctor, 2006).
Complement is the fluid-phase part of innate immunity contributing to infectious and non-infectious diseases and is composed of cascading proteases that assemble with almost immediate reactivity at abnormal landscapes of foreign and altered host cell surfaces (Fig. 1 ) (Lubbers, Van Essen, Van Kooten, & Trouw, 2017; Ricklin, Mastellos, Reis, & Lambris, 2018). Almost immediate reactivity is achieved by a pivotal component of the complement system—C3. C3 has an ability to cleave spontaneously into C3a and C3b fragments and amplifies its own production by a positive feedback loop. The activity of C3 loop on cell surfaces depends on whether it encounters surfaces with complement stimulating factors (e.g. antibodies, bacterial carbohydrates) or surfaces with absent receptors against the C3/C3b attack (Harrison, 2018; Lachmann, 2018).
Fig. 1.
A simple scheme of complement activation and major steps for further proliferation of the complement cascade in tissues with all complement components. The main cleavage fragments of complement are responsible for many of the host defense-mediated functions of complement, such as chemoattraction, phagocytosis, and cell lysis.
The C3 stimulating factors on surfaces are C4 and C2 converted to C3 convertase by pattern recognition receptor (PRR)-associated serine proteases. Depending on PRRs, complement activation is divided into ‘lectin’ and ‘classical’ activation pathways. For the ‘lectin pathway,’ the triggering PRRs are mannan-binding lectin (MBL), ficolins, and collectins detecting pathogen-associated molecular sugar patterns or altered glycosylation patterns on abnormal host cells. In the ‘classical pathway’, the PRR is C1q, activated upon recognition of the Fc portion of target cell bound immunoglobulins or pentraxins (Lubbers et al., 2017). C3/C3b is capable of covalently binding to the surface on its own, in the absence of activity of other complement pathways. Such is the case when C3b/C3(H2O) takes advantage of the surfaces lacking polyanions necessary for the stabilization of Factor H (‘protected surface’) in the ‘alternative pathway’ of complement activation. Factor H is a soluble PRR of lectin nature, accelerating C3 convertase decay. Cells coated with bacterial endotoxin (smooth lipopolysaccharides, LPS) may be the most important in vivo activator by this mechanism (Blaum, 2017; Lachmann, 2018).
Complement components can be directly cleaved by coagulation/ fibrinolytic factors, resulting in ‘extrinsic protease pathway’ (Amara et al., 2010; Barnum, 2017). This non-canonical complement activation pathway opens a possible link to why many complement disorders feature pathologic thrombosis as a hallmark clinical manifestation (Baines & Brodsky, 2017).
Since the earliest works on carrageenan and complement, our understanding of complement organization and methods in the field have drastically evolved. Initially, carrageenans’ action on complement was limited only to classical and alternative pathways and was assayed with the model based on the phenomenon of immune hemolysis (Baker et al., 1986; Davies, 1965). This article describes the ability of red algal polysaccharides to affect the human complement system in tissue containing all complement cascade proteins-serum by analyzing C3 binding to well plate surfaces coated with Escherichia coli LPS, C4 binding to wells coated with IgG or mannan molecules, and, finally, changes in C5a concentration in human serum activated with plasmin.
2. materials and methods
Chemical compounds studied in this article: ι-carrageenan (PubChemCID: 101231952); κ-carrageenan (PubChemCID: 11966249); β-carrageenan (PubChemCID: 102199626); λ-carrageenan (PubChemCID: 101231953); LPS (PubChemCID: 11970143); heparin (PubChemCID: 772); mannan (PubChemCID: 25147451).
2.1. Reagents
Commercial unfractionated heparin as sodium salt (cat no. 101931, lot no. 2024H, St. Louis, Sigma, USA) and commercial LPS from the bacterium E. coli 055:B5 (cat no. L2880, lot no. 025M4040 V, Sigma, St. Louis, MO, USA) were purchased from Sigma, as was mannan from Saccharomyces cerevisiae, prepared by alkaline extraction (cat no. M7504, lot no. SLCC2157). Normal human IgG was manufactured by Statens Serum Institute (007740, SSI, Denmark). Human plasmin was from RENAM (cat no. FA-3, lot no. 0818, Moscow, Russia). Specific enzyme-linked immunosorbent assay (ELISA) kit, used to measure C5a concentrations, was purchased from Cytokine, Saint-Petersburg, Russia. Human complement C4c was purchased from LeeBiosolutions (cat no. 194-41, lot no. 08D1609). Anti-human-C3 and C4 monoclonal antibody (mAb) conjugated with horseradish peroxidase (HRP) were purchased from Cytokine, Saint-Petersburg, Russia. Food agar of the first class, brand 700 from Ahnfeltia tobuchiensis (Primorsky Krai, Russia) and agarose (cat no. A9539, Sigma) were used for comparison in experiments of C3 binding to E. coli LPS and catalytic cleavage of C5 by plasmin.
2.2. Isolation and characterization of carrageenans
Red seaweeds Chondrus armatus (Gigartinaceae), Tichocarpus crinitus (Tichocarpaceae), and Ahnfeltiopsis flabelliformis (Phyllophoraceae) were collected along the Russian coast of the Japanese Sea in 2016–2017. Morphological and anatomic characteristics of the seaweeds were determined according to Perestenko (1994) and identified by light microscopy by Prof. E. Titlynov and Dr. Oksana Belous from the A.V. Zhirmunsky National Scientific Center of Marine Biology, Far East Branch of the Russian Academy of Sciences FEB RAS. According to the identification, C. armatus was represented by male gametophyte and T. crinitus and A. flabelliformis by female gametophytes with cystocarps. The polysaccharides were extracted from dried algae (5 g) with hot water (300 mL) at 80 °C for 3 h, a total of three times, according to the protocol (Yermak, Kim, Titlynov, Isakov, & Solov’eva, 1999). The suspensions were centrifuged (4000 rpm), residues recovered, and supernatants were filtered through a Vivaflow 200 membrane (Sartorius, Göttingen, Germany) with a 100 kDa pore size to remove low molecular weight compounds. The polysaccharides were precipitated from solutions with a triple volume of 96 % ethanol. The precipitate was separated, washed with ethanol, suspended in hot water, and fractionated into gelling and non-gelling fractions by 4 % KCl for C. armatus, 1 % KCl for T. crinitus, and 4 % CaCl2 for A. flabelliformis total polysaccharides, respectively. The structures of the obtained fractions were established according to published protocols (Barabanova et al., 2005; Kravchenko et al., 2016; Yermak et al., 1999).
To determine the content of 3,6-anhydrogalactose, total reductive hydrolysis of the carrageenans and agar in 2 M Trifluoroacetic acid (TFA) (100 °C, 4 h) with 4-methylmorpholinborane was carried out, and then, aldononitrile acetates were obtained (Usov & Elashvili, 1991). Other monosaccharides (galactose, glucose, xylose) were determined as alditol acetates according to a previously published protocol (Kravchenko et al., 2020). The sulfate ester content of the polysaccharides was determined by turbidimetry (Dodgson & Price, 1962). The protein content of carrageenans and agar was determined according to the Lowry method (Lowry, Rosebrough, Farr, & Randall, 1951).
To determine the configuration of 4-linked 3,6-anhydrogalactose in food agar and soluble fraction of C. armatus, the polysaccharide samples were subjected to partial acid hydrolysis as described by Kravchenko et al. (2020). Agarose (Sigma-Aldrich, USA) and kappa-carrageenan from Kappaphycus alvarezii (Sigma-Aldrich, USA) were used as standards for the production of aldononitrile acetates of agarobiose and carrabiose.
Carrageenan viscosimetric molecular weights were calculated using the Mark-Houwink equation: [η] = KMα, where [η] is the intrinsic viscosity, and K and α are empirical constants for carrageenans, being 3 × 10−3 and 0.95 at 25 °C in 0.1 M NaCl, respectively (Rochas, Rinaudo, & Landry, 1990). The viscosity of polysaccharide solution (1–2 mg mL-1 in 0.1 M NaCl) was measured with a modified Ubbelohde viscometer (Design Bureau Puschino, Russia), and the intrinsic viscosity of the polysaccharide sample was calculated by extrapolation of the dependence ln (η)rel/C to infinite dilution using the least squares method.
Infrared spectroscopy (IR) spectra of the polysaccharides (as films) were recorded on a Vector 22 Fourier transform spectrophotometer Equinox 55 (Bruker, USA) taking 120 scans with 4 cm–1 resolution. Spectral regions of 1900–700 cm−1 were scanned, and the baseline was corrected for scattering. The spectra were normalized by monosaccharide ring skeleton absorption at 1074 cm–1 (A1074 ≈ 1.0).
The polysaccharides (3 mg) were deuterium-exchanged twice with heavy water (D2O, 0.6 mL) by freeze-drying prior to examination in a solution of 99.95 % D2O, and the 1H and 13C Nuclear magnetic resonance (NMR) spectra were recorded using a DRX-500 (125.75 MHz) spectrometer (Bruker, Hamburg, Germany) operating at 50 °C. Chemical shifts were described relative to the internal standard, acetone (δC 31.45, δH 2.25). The NMR data were acquired and processed using XWIN-NMR 1.2 software (Bruker).
2.3. Human serum
The study protocol was approved by the medical ethical committee of the local hospital (Vladivostok, Russia). Informed consent was obtained from all donors. To obtain human serum, blood was drawn in Clot Activator Tubes (product code: 613060202, Improvacuter®, China). Serum samples from 25 apparently healthy adult donors were pooled and double centrifuged for 10 min, first at 3000 and then at 14,000 g. The serum was subsequently aliquoted and frozen at 80 °C for future study, as recommended by Lachmann (2010).
2.4. Assessment of C3 binding to LPS-coated plates (alternative pathway)
Functional activity of the alternative pathway (AP) was assessed by an ELISA-based assay with immobilized E. coli LPS as a ligand according to a previous protocol with slight modifications (Damgaard et al., 2017). To coat Nunc Maxisorb plates (Denmark) with LPS, LPS was dissolved in phosphate buffered saline (PBS) at a concentration of 10 μg mL−1 and incubated for 16 h at room temperature. Residual binding sites were blocked by 200 μL of 1 % bovine serum albumin (BSA) in PBS for 1 h at 37 °C. The investigated polysaccharide samples were added to the LPS-coated plate (20 μL, C = 0.1, 1.0, 5.0, and 10.0 mg mL−1). Serum samples were diluted in Tris-buffered saline (TBS) with 0.05 % Tween-20, 9.5 mM ethylene glycol tetraacetic acid (EGTA), and 5 mM Mg2+ (pH 7.5) to inhibit activity of the lectin and classical pathways (1:3 v/v) and added to the plate (80 μL per well), followed by incubation for 1 h at 37 °C. Wells receiving only buffer were used as negative controls and heparin as positive controls. Complement binding was assessed by commercially available products (Cytokine, Saint-Petersburg, Russia)—anti-human-C3 mAb conjugated with HRP, followed by the detection with tetramethylbenzidine (TMB), according to the manufacturer’s instructions. The absorbance was read at 450 nm on a microtiter plate reader.
2.5. Complement deposition by classical and lectin pathway activity
The method was based on a protocol described elsewhere by Petersen, Thiel, Jensen, Steffensen, & Jensenius (2001). Microtiter wells (Maxisorb, Nunc, Kamstrup, Denmark) were coated with 100 μL of 0.1 μg mL−1 normal human IgG or 0.1 μg mL−1 mannan from S. cerevisiae in 100 mM Na2CO3/NaHCO3, pH 9.6. After incubation overnight at room temperature, residual protein-binding sites were blocked by the addition of 200 μL of buffer containing 1 mg mL−1 BSA, 10 mM Tris-Cl, and 145 mM NaCl (pH 7.4) for 1 h at 37 °C. After each step, plates were washed three times with 200 μL of TBS with 0.05 % (v/v) Tween 20 and 5 mM CaCl2 (TBS/tw/Ca2+). After a final wash, the investigated polysaccharide samples were added to the IgG- or mannan-coated plates (20 μL, C = 0.01, 0.1, 1.0, and 10.0 mg mL-1) and 80 μL of 1:200 diluted serum in 20 mM Tris-HCl buffer with 10 mM CaCl2, 1 M NaCl, 0.05 % v/v Triton X-100, and 0.1 % w/v BSA, pH 7.4. Wells receiving only buffer were used as negative controls and heparin as positive controls. All dilutions were added in duplicate. Following incubation overnight at 4 °C and a wash using TBS/tw/Ca2+, C4b-depositing capacity was assessed by adding 0.5 μg C4 in 100 μL of TBS/tw/Ca2+. After incubation for 2 h at 37 °C and a wash as described above, deposited C4b was detected by anti-human-C4 mAb conjugated with HRP, followed by the detection with TMB, according to the manufacturer’s instructions. The absorbance was read at 450 nm on a microtiter plate reader. The tests were carried out in triplicate in two independent experiments.
2.6. Determination of galactans ability to bind serum antibodies
A commercial diagnostic ELISA kit “Immunoscreen-G,M,A-ELISA-BEST” (ZAO Vector-Best, Russia) for the simultaneous determination of the concentrations of total immunoglobulins of classes G, M, A in human blood serum was used. The kit included three types of strips, which differed in the specificity of antibodies immobilized on the inner surface of the wells to heavy chains of IgG, IgM or IgA. At the first stage of immunonalysis, 20 μL of 1:1500 serum diluted in PBS/Tween 20, 80 μL of PBS/Tween 20, and 20 μL of polysaccharide (C = 2 mg mL−1) were incubated in the wells of all 3 strip types. The wells with control instead of polysaccharide samples contained 20 μL of vehicle. Then the plate was washed, treated with a conjugate of mAb to light chains of immunoglobulins (kappa and lambda chains) with horseradish peroxidase. The formed immune complexes were detected by the enzymatic reaction of peroxidase with hydrogen peroxide in the presence of a chromogen (TMB). The optical density of solutions in the wells after termination of the reaction was measured at the main wavelength of 450 nm. The intensity of staining is proportional to the concentrations of IgG, IgM, IgA.
2.7. Effect of algal polysaccharides on complement in serum activated by plasmin
The ability of the investigated polysaccharides to affect complement activation induced by plasmin in human serum was investigated by changes in the concentration of C5a anaphylatoxin. The generation of C5a was assessed by ELISA (Cytokine, Saint-Petersburg, Russia) according to the manufacturer’s instructions. The only modification to the protocol was on the step of 60 min incubation with first antibodies by addition of plasmin (0.5 U mL−1, final value) and the investigated polysaccharides or heparin with varying concentrations (10, 100, and 1000 μg mL−1, final value). Two controls were used, one with serum only and a second with serum and plasmin. Concentration of generated C5a was expressed in ng mL−1 from triplicates of two independent experiments.
2.8. Statistical analysis
All data are expressed as the means ± standard deviations. Statistical analysis was performed using one-way repeated measures analysis of variance (ANOVA) with Tukey post-hoc test. In tests with multiple sample concentrations pairwise comparisons were calculated for the highest concentration value. A probability value (P) less than 0.05 was considered significant.
3. Results
Polysaccharides were extracted from red seaweed C. armatus, T. crinitus, and A. flabelliformis and fractionated by KCl or CaCl2 into insoluble and soluble fractions, as described in the methods. In our work, mainly insoluble or gelling fractions of polysaccharides and one non-gelling or soluble fraction of C. armatus were used. Table 1 contains structural characteristics and disaccharide repeating units of the carrageenans, food agar from A. tobuchiensis and agarose (Sigma) used in the current study. The molecular weights of these polysaccharides were higher than 200 kDa. According to chemical analysis data, these polysaccharides varied in the degree of sulfation and the amount of 3,6-anhydrogalactose (Table 1). The non-gelling fraction of C. armatus is characterized by the highest degree of sulfation and very low content of 3,6-anhydro derivative. The protein contents in polysaccharides did not exceed 5 %. Agar and agarose differ from carrageenans by the lowest degree of sulfation. The resulting sequence of sulfation degree of the samples is λ/μ/ν > ι/κ > κ > κ/β > agar > agarose.
Table 1.
The major disaccharide repeating unit structures of carrageenans from algae of the families Gigartinaceae, Tichocarpaceae, and Phyllophoraceae, commercial agar and agarose.
| Algal species/fraction | Sample | Disaccharide repeating unit structure |
Composition, % of sample weight |
Molar ratio Gal:AnGal: SO3Na | Polysaccharide molecular weight, kDa | |||
|---|---|---|---|---|---|---|---|---|
| 3-linked | 4-linked | Gal | AnGal | SO3Na | ||||
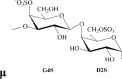
|
C. armatus soluble |
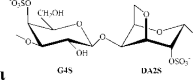
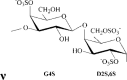
λ/μ/ν-carrageenan | G2S | D2S,6S | 26.8 | 0.5 | 31.0 | 1:0.02:1.8 | 200.0 |
|
C. armatus insoluble |
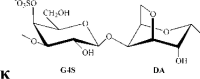
κ-carrageenan | G4S | DA | 32.8 | 22.0 | 23.8 | 1.0:0.8:1.1 | 560.0 |
|
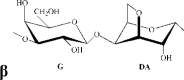
T. crinitus insoluble |
κ/β-carrageenan | G4S/G | DA/DA | 39.5 | 27.5 | 18.7 | 1.0:0.8:0.7 | 328.0 |
|
A. flabelliformis insoluble |
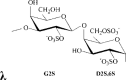
ι/κ-carrageenan | G4S/G4S | DA2S/DA | 31.6 | 15.6 | 30.2 | 1.0:0.6:1.5 | 330.0 |
| A. tobuchiensis | agar | G | LA | 43.7 | 33.5 | 14.3 | 1.0:0.9:0.5 | |
| commercial | agarose | G | LA | 54.5 | 52.4 | 1.0 | 1.0:1.0:0.02 | |
 |
 |
 |
||||||
 |
 |
 |
||||||
Remarks: G: 3-linked β-d-galactopyranose; G2S: 3-linked β-d-galactopyranose 2-sulfate; G4S: 3-linked β-d-galactopyranose 4-sulfate; D2S,6S: 4-linked α-d-galactopyranose 2,6-disulfate; DA: 4-linked 3,6-anhydro-α-d-galactopyranose; DA2S: 4-linked 3,6-anhydro-α-d-galactopyranose 2-sulfate, with letter code nomenclature by Knutsen, Myslabodski, Larsen, and Usov (1994).
The structures of the obtained fractions were studied by Fourier transform infrared (FTIR) and NMR spectroscopies, and the obtained spectra were compared with spectra of polysaccharides isolated by us from these species of algae, as detailed previously (Barabanova et al., 2005; Kalitnik et al., 2015; Kravchenko et al., 2016; Yermak et al., 1999). Absorption bands in the IR spectra and chemical shifts in the NMR spectra were assigned via comparison to signals of known carrageenan and agar structures (Kolender & Matulewicz, 2004; Miller & Blunt, 2000; Pereira, Amado, Critchley, Van de Velde, & Ribeiro-Claro, 2009; Pereira, Gheda, & Ribeiro-Claro, 2013; Van de Velde, Knutsen, Usov, Rollema, & Cerezo, 2002).
In this work, we present the IR spectra of the studied polysaccharides and the 1H and 13C NMR spectra of the carrageenans. An intense absorption band in the region of 1250 cm−1 in the IR spectra of all studied carrageenans (Fig. 2 A–D) indicated the presence of a significant number of sulfate groups (–S = O asymmetric vibration) (Pereira et al., 2009), in agreement with results of chemical analysis (Table 1). Absorption bands at 932 and 849 cm−1 in IR spectra of insoluble fractions were characteristic of 3,6-anhydrogalactose (C—O vibration) and the secondary axial sulfate group at C-4 of the 3-linked β-d-galactose residue, respectively (Fig. 2A–C). This made it possible to assign the polysaccharides to κ-type carrageenans. The IR spectrum of the insoluble fraction of A. flabelliformis also had a pronounced absorption band at 805 cm-1 (Fig. 2C), belonging to the secondary axial sulfate group at C-2 of a 4-linked 3,6-anhydro-α-d-galactose of ι-disaccharide unit (Pereira et al., 2009). The absorption band at 890 cm-1 in the IR spectrum of the insoluble fraction of T. crinitus (Fig. 2B) evidenced the presence of non-sulfated β-d-galactose residues, typical for β-carrageenan (Renn et al., 1993). There was no absorption band corresponding to 3,6-anhydrogalactose in the IR spectrum of the soluble fraction of C. armatus (Fig. 2D), consistent with chemical analysis (Table 1). On the contrary, there was a wide absorption band at 815–830 cm−1 corresponding to the primary equatorial sulfate group at C-6 and the secondary equatorial sulfate group at C-2 of 4-linked α-d-galactose, which were characteristic of λ-carrageenan (Pereira et al., 2009). It should be noted that there was an absorption band in this range in the IR spectra of ν- and μ-carrageenans (the biosynthetic precursor of ι- and κ-carrageenan, respectively). So, the FTIR spectroscopy data indicated that soluble fraction from C. armatus was likely represented by mixture of λ-, ν- and μ-carrageenan types. According to partial reductive hydrolysis, soluble fraction of C. armatus consisted of only [→3)-β-d-Galp-(1→4)-α-d-Galp-(1→] disaccharide units (carrabiose).
Fig. 2.
IR spectra of κ- (A), κ/β- (B), ι/κ- (C), and λ/μ/ν- (D) carrageenans and agar (E).
Thus, FTIR spectroscopy data suggest that KCl-insoluble polysaccharides from C. armatus were represented by κ-carrageenan (Yermak et al., 1999), whereas KCl-insoluble polysaccharides fractions from T. crinitus and A. flabelliformis had hybrid structures and were identified as κ/β-carrageenan (Barabanova et al., 2005) and ι/κ-carrageenan respectively (Kravchenko et al., 2016).
In contrast to the IR spectra of carrageenans, the IR spectrum of agar contained a weak absorption band at 1250 cm−1 (Fig. 2E), which indicated a lower content of sulfate esters in this polysaccharide compared to carrageenans that was consistent with chemical analysis (Table 1). As in the case of gelling carrageenans (Fig. 2A–C), the IR spectrum of agar (Fig. 2E) contained an absorption band at 932 cm−1, typical for 3,6-anhydrogalactose, as well as an intense absorption band at 890 cm−1, belonging to unsulfated 3-linked β-d-galactose (Pereira et al., 2013). The partial reductive hydrolysis of food agar showed that the polysaccharide contained only [→3)-β-d-Galp-(1→4)-α-l-AnGalp-(1→] disaccharide units (agarobiose) without any [→3)-β-d-Galp-(1→4)-α-d-AnGalp-(1→] disaccharide units (carrabiose). This distinction made classification as agar possible.
FTIR spectroscopy data were confirmed by NMR spectroscopy analysis, as the carrageenans were subjected to both 1H and 13C NMR analyses. The spectra are presented as Supplementary materials. The two signals at 103.1 ppm and 96.2 ppm in the anomeric carbon resonance area of the both spectra of insoluble fractions (C. armatus and A. flabelliformis) were assigned to C-1 of the 3-linked β-d-galactose residue (G4S) and C-1 of the 4-linked 3,6-anhydro-α-d-galactose (DA) of κ-carrageenan, respectively (Supplementary 1). An intense signal at 92.9 ppm and less intense signal at 95.8 ppm, among the six signals observed in the anomeric carbon resonance region of the 13C NMR spectrum of the insoluble fraction from A. flabelliformis, were characteristic of C-1 of the 4-linked 3,6-anhydro-α-d-galactose-2-sulfate (DA2S) of ι-carrageenan and C-1 of the 4-linked 3,6-anhydro-α-d-galactose (DA') of β-carrageenan, respectively (Supplementary 1B). There were poorly resolved signals at 102.9, 103.1, and 103.2 ppm in the 13C NMR spectrum, resulting from overlapping C-1 signals of the 3-linked β-d-galactose 4-sulfate of the ι- (G4S’) and κ-carrageenans (G4S) and the 3-linked β-d-galactose (G) of β-carrageenan, respectively (Usov & Shashkov, 1985). The NMR spectroscopy data indicate that the content of the ι-type disaccharide units in the polymer chain of ι/κ-carrageenan was predominant. The ratio of ι- and κ-units was 2:1, and β-carrageenan was present in minor quantities.
Well-resolved 1H and 13C NMR spectra of soluble fraction from C. armatus could not be recorded, even at high temperature, because of high polysaccharide viscosity and, probably, disordered macromolecular organization. However, we were able to identify some of the main signals by comparing our spectra with literature data (Van de Velde et al., 2002). There were four signals in the anomeric carbon resonance area of the 13C NMR spectrum (Supplementary 2). Signals at 103.3 and 91.6 ppm were attributed to C-1 of 3-linked β-d-galactose 2-sulfate (G2S-1) and 4-linked α-d-galactose 2,6-disulfate (D2S,6S-1), respectively, of λ-carrageenan (Van de Velde et al., 2002). The broad signal at 105.3 ppm was likely related to 3-linked β-d-galactose 4-sulfate of μ- (G4S- 1) and ν- (G4S'-1) carrageenans (biosynthetic precursors of κ- and ι-carrageenans, respectively). At the same time, a wide signal at 98.6 ppm was attributed to 4-linked α-d-galactose 6-sulfate (D6S-1) and α-d-galactose 2,6-disulfate of μ- (D6S-1) and ν- (D2S,6S'-1) carrageenans, respectively (Van de Velde et al., 2002). In addition, the intense signal at 61.6 ppm in the upfield region of the 13C NMR spectrum was characteristic of the C-6 of 3-linked β-d-galactose of λ- (G2S-6), μ- (G4S-6), and ν- (G4S'-6) carrageenans. A wide, poorly resolved signal at 69.3 ppm corresponded to 4-linked α-galactose sulfated at C-6 (D2S,6S, D6S, D2S,6S'). At the same time, weak signal at 64 ppm can be attributed to C-4 of 3-linked β-d-galactose 2-sulfate (G2S-4) of λ-carrageenan. The 13C NMR data were consistent with the 1H NMR (not shown). There was a broad signal at 5.52–5.59 ppm in the α-anomeric proton resonance area, which was attributed to H-1 of the 4-linked α-d-galactose 2,6-disulfate of λ- (5.59 ppm) and ν- (5.52 ppm) carrageenans. In addition, a weak signal at 5.26 ppm in the spectrum suggested the presence of μ-carrageenan (H-1 of 4-linked α-d-galactose 6-sulfate). Thus, the non-gelling polysaccharide from C. armatus was a mixture of λ- μ- and ν-carrageenans.
The 1H NMR spectrum of κ/β-carrageenan (Supplementary 3) contained four signals in the anomeric proton resonance area. The signals at 5.09 and 5.11 ppm were characteristic of the H-1 of 4-linked 3,6-anhydro-α-d-galactose of β- (DA’) and κ-carrageenans (DA), respectively. The signals at 4.62 and 4.64 ppm were assigned to the H-1 of 3-linked β-d-galactose (G) and 3-linked β-d-galactose 4-sulfate (G4S) of the β- and κ-carrageenans, respectively (Kolender & Matulewicz, 2004; Van de Velde et al., 2002).
3.1. The influence of red algal galactans on total functional complement activation
The influence of the investigated galactans on binding C3 complement component to plate wells coated with LPS was studied by an ELISA-based method. Results displayed in Fig. 3 A revealed that, in general, the investigated polysaccharides inhibited C3 binding to plate wells coated with LPS. This capacity was dependent on the polysaccharide sample and concentration. Heparin was the most potent inhibiting agent in this assay, almost independent of concentration in the range of values in the experiment, and the decrease by its action reached 59–68%, relative to the negative control. Among the galactans, their effect decreased, as follows: λ/μ/ν > κ/β > κ > ι/κ > agar. More precisely, at the highest concentration (2 mg mL−1), all carrageenans, on average, reduced C3 binding by 70 %, just like heparin, and agar and agarose by 40 and 20 %, respectively. By lowering concentrations, the investigated samples, unlike heparin, gradually lost their inhibiting potential.
Fig. 3.
Binding of C3 and C4 complement components to well surfaces coated with E. coli LPS (A), human IgG (B), or S. cerevisiae mannan (C) in the presence of carrageenan (λ/μ/ν-, κ-, κ/β-, and ι/κ-types) and agar (agar, agarose) groups in varying concentrations. All concentrations are expressed in final values, as % change in C3 or C4 concentration on the well surface relative to the vehicle control (100%) in three replicates from two independent experiments. The asterisk (*) indicates significant differences <0.05 by one-way ANOVA followed by Tukey post hoc comparisons for the highest sample concentration value.
Regarding C4 binding to the mannan-coated surface (Fig. 3B), the investigated samples were affected less efficiently. Heparin, again, reduced C4 binding to the mannan-coated surface, depending on concentration (35 % decrease at the highest concentration of 2 mg mL−1). The most active samples were λ/μ/ν- and κ-carrageenans, inhibiting C4 binding to mannan, on average, by 30 % within the concentration range used in this test. The hybrid carrageenan structures of κ/β and ι/κ were almost inactive. The wells containing agar and agarose gellified in C4 binding to mannan- and antibody-coated surfaces.
Another tendency was observed when we studied C4 binding to antibody-coated surfaces (Fig. 3C). Heparin illustrated inhibiting potential at the two highest concentrations (0.2 and 2 mg mL−1) by about 25–40 % and was inert at lower concentrations. Carrageenans stimulated C4 binding, especially at high concentrations. Of the polysaccharides, κ/β- and κ-carrageenans’ actions at the highest concentrations resulted in the most pronounced activity—a four-fold increase in C4 binding to antibody-coated surfaces. λ/μ/ν-Carrageenan was the least active one (two-fold increase at the highest concentration), and ι/κ-type, independent of concentration, showed a two-fold increase relative to the negative control (100 %).
3.2. Binding of red algal polysaccharides to serum immunoglobulins
The ability of the investigated samples to affect concentrations of the total IgG, IgA, IgM of human serum was analyzed. Table 2 contains data on total IgM measured in serum in the presence of the investigated samples. The results revealed that the galactans were able to affect total serum IgM and insignificantly other types of serum Igs. The strongest binding towards total serum IgM was observed for gelling carrageenans.
Table 2.
Measured concentration of total serum IgM in the presence of polysaccharides.
| Sample | % |
|---|---|
| control | 100.0 ± 2.8 |
| heparin | 101.4 ± 3.9 |
| λ/μ/ν-carrageenan | 91.2 ± 2.1* |
| κ-carrageenan | 82.4 ± 2.8* |
| κ/β-carrageenan | 81.0 ± 1.8* |
| ι/κ-carrageenan | 76.8 ± 1.6* |
The asterisk (*) indicates significant differences <0.05 relative to vehicle control (100 %).
3.3. Influence of red algal galactans on the extrinsic protease pathway of complement activation induced by plasmin
The effect of red algal polysaccharides on the extrinsic protease pathway of complement by activating human serum with a component of a coagulation system (plasmin) was studied (Fig. 4 ). The measure of serum activation was determined by the concentration of a cleaved C5 component—C5a—in fluid phase by means of an ELISA method. Fig. 4 contains the control- and control + for human serum with and without plasmin, showing activation by 50 % (from 43 to 62 ng mL−1). Heparin was inactive in this test, while the investigated samples illustrated some degree of inhibition at the highest concentration, with λ/μ/ν- and κ/β-carrageenans being the most impressive (almost to the level of control-).
Fig. 4.
C5a concentration in serum activated with plasmin (0.5 U mL−1, final value) in the presence of red algal galactans: carrageenan (λ/μ/ν-, κ-, κ/β-, and ι/κ-types) and agar (agar, agarose) groups in varying concentrations. All concentrations are expressed as final values. Control- is non-activated serum, and control + is serum activated with plasmin. The results are expressed C5a concentration (ng mL-1) from three replicates of two independent experiments. The asterisk (*) indicates significant differences <0.05 by one-way ANOVA followed by Tukey post hoc comparisons for the highest sample concentration value.
4. Discussion
As a dietary fiber, carrageenans encounter in human organisms only the gastrointestinal tract (EFSA Panel on Food Additives & Nutrient Sources added to Food (ANS) et al., 2018). To dampen the immune response elicited by the presence of luminal antigens appears to be one the main functions of the mucosal immunity (Brownlee, Dettmar, Strugala, & Pearson, 2006; Cummings et al., 2004). As a result the complement role there is dictated by location and is heavily inclined to opsonization but not lysis of invading bacteria. In other words, the complement composition is limited to C4, C3, factor B, and C1q, with notably low or absent complement C5–C9 proteins composing membrane attack complex for cell lysis (Sina, Kemper, & Derer, 2018). The experimental design of complement’s functional activity in the current article was focused on the enzyme immunoassay method of C3 or C4 tethering to a suitable solid phase (Harboe, Thorgersen, & Mollnes, 2011). Heparin was used as a reference here because of its capacity to inhibit complement (Weiler, Edens, Linhardt, & Kapelanski, 1992) and because carrageenan's ability to act in a similar manner to heparin, gives a promising direction in the glycomimetic drug field (Buck et al., 2006; Groult et al., 2019; Poupard et al., 2017).
All cell surfaces are coated with a layer of glycocalyx composed from glycans in many different molecular forms (Ernst & Magnani, 2009). Differences in cell surface glycans can serve as markers of a cell’s identity (e.g. developmental state, tissue type, self versus non-self discrimination. The major leading factor in reading cell surface as self is Factor H which fixates on surface polyanions (glycoproteins containing sialic acid residues, heparan sulfate, and other glycosaminoglycans) and moves the ongoing balance of complement activation-inactivation on cells towards inactivation (Collins & Troeberg, 2019; Langford-Smith, Day, Bishop, & Clark, 2015; Pangburn et al., 2009). Our results demonstrated that, for C3 binding to LPS-coated surfaces, i.e. without polyanions necessary for Factor H, the galactans inhibited this process, although with less efficacy than heparin (Fig. 3A). Influence of the sulfated galactans in C3 binding and visible dependence on the sulfation degree allows us to assume they function as surface polyanions. Some degree of C3 binding inhibition to LPS-coated surfaces by the non-sulfated galactan agarose might be explained with agarose’s ability to directly bind C3 but not stabilize Factor H on the surface (Hetland & Eskhland, 1986). Thus, sulfated red algal galactans should be capable of decreasing the inflammatory reaction by strengthening surface readings as less non-self in the alternative pathway and amplification loop because of their polyanion nature.
Factor H is not significant in the case of mannan-driven complement attack, however, our data illustrated that carrageenans still can provide cell surface protection but with far less efficacy than for C3 binding (Fig. 3B). The only exception was observed for the most sulfated non-gelling λ/μ/ν-type carrageenan sample, which had a comparable to heparin effect. The C4 deposition on wells used in the assay reflected the activity of serine protease circulating in complex with MBL (MBL-associated serine protease-2, MASP-2) (Petersen et al., 2001). Hence, carrageenans probably inhibit MBL and/or MASP-2, up-regulating the lectin pathway, and facilitate Factor H, down-regulating the alternative pathway and amplification loop. The lectin pathway has an extensive scope of therapeutic potential, especially in models of myocardial or gastrointestinal ischemia-reperfusion injury. However, it has only been actively studied for the last 10 years (Ricklin et al., 2018), so hypothesizing possible applications of algal sulfated polysaccharides at this moment is difficult.
When wells are coated with antibodies, the classical pathway becomes a leading force, allowing recognition of immune complexes by C1q cleaving upon recognition into the homologous to MASP proteases (C1r and C1s; Petersen et al., 2001). Our results revealed that, in general, carrageenans, contrary to heparin, augmented this pathway of complement activation (Fig. 3C), which corresponds to the hemolytic complement studies (Baker et al., 1986). In our experiment without cells, the increasing C4 deposition onto well plates in the presence of carrageenans must be connected with the increase in amount of antibody during the incubation step with serum and samples. Blood serum contains substantial amounts of an interesting variety of antibodies, called natural/spontaneous antibodies (NA). The most prominent functions of NAs are homeostatic (broadly reactive against self-antigens, tumor-specific patterns, cell-surface-exposed structures of necrotic cells, or plasma proteins leaking destroyed cells, etc.) and protective against infections spreading hematologically. However, for protection, they act as recognition proteins, like MBL and C-reactive protein; evoke strong complement-mediated inflammatory response; and are capable of recognizing evolutionarily fixed epitopes in foreign antigens (Holodick, Rodríguez-Zhurbenko, & Hernández, 2017; Lutz, Binder, & Kaveri, 2009; Ochsenbein & Zinkernagel, 2000). The most abundant NA in humans (∼1 % of the total serum immunoglobulins with major reactive type being of IgG and especially IgM variety; McMorrow, Comrack, Sachs, & DerSimonian, 1997) is directed against ‘α-gal epitope’ with the structure α-Galp-(1→3)-β-Galp-(1→4)-GlcpNAc-R (2018, Galili, 2013, 2020). The investigated polysaccharides could bind NA of human serum (EFSA Panel on Food Additives & Nutrient Sources added to Food (ANS) et al., 2018) because the →4)-α-Galp-(1→3)-β-Galp-(1→ portion of the xenoantigen is a disaccharide repeating unit of a carrageenan chain. Structural features of the galactans in our study also matter because polysaccharides containing 3,6-anhydrogalactose (κ, κ/β, ι/κ) were more potent activators compared to the non-gelling type. This property of carrageenans to bind NA has been tested in our study (Table 2). The data suggested an ability of carrageenans to connect with antigen binding parts of total IgM of human serum leading as a result to a decrease in number of IgM reacting with mAb against light chains of immunoglobulins. The gelling types more actively bound IgM, corroborating the more substantial C4 binding to antibodies-coated surface in the presence of carrageenans. Drawing conclusions about the degree of influence by structural characteristics, like varying sulfate positions, was difficult but could be connected with NAs’ property of polyreactivity, accompanied with a degree of specificity (Bovin et al., 2012). The former is for homeostatic functions and the latter mostly for protective functions. The mucous layer of the gastrointestinal tract contains homeostatic polyreactive NAs, mostly of the IgA variety, with an innate role to coat and contain the resident commensal microorganisms and provide protection against detrimental ones (Bunker et al., 2017; Wells et al., 2017). No reports of allergic reaction to carrageenan as a food ingredient have been registered in humans (EFSA Panel on Food Additives & Nutrient Sources added to Food (ANS) et al., 2018). However, this complement activation in the presence of anti-Gal NAs has been successfully explored in the accelerated wound healing model by application of α-gal nanoparticles (Galili, 2013). Carrageenans, in turn, have a long history of topical administration in tissue engineering and wound healing (Ditta et al., 2020) for a variety of bioengineering applications, and antiviral microbicides hydrogels (Yegappan, Selvaprithiviraj, Amirthalingam, & Jayakumar, 2018) or other compounds. One of the mechanisms of the antiviral action of carrageenans is due to their negative charge which bind virus positively charged glycoproteins responsible for attachment to a host cell (Damonte, Matulewicz, & Cerezo, 2004). At the same time, anti-Gal-mediated neutralization and complement-mediated lysis of the viruses after incubation of the viruses expressing α-gal epitopes in human serum or, with purified anti-Gal antibody had been shown, but no such effects for viruses lacking α-gal epitopes (Galili, 2018).
With topical administration of red algal polysaccharides, one might also consider useful knowledge of their influence on complement through other homeostatic cascades by the ‘extrinsic protease pathway,’ encompassing complement interaction with the coagulation cascade and fibrinolytic proteins. This interaction unlike canonical complement activation is believed to take place on several host cell types with normal surface landscapes, like platelets and endothelial cells, activated by complement fragments (e.g. C4a protein released from C4 during activation of the classical and lectin pathways) (Ricklin, 2018). Our very simple experiment, without cells and surfaces imitating them, allowed us to extricate onlygalactans’ effect on the reaction of complement activation in solution by a fibrinolytic protein, plasmin (Fig. 4), the strongest activator of C5 (Amara et al., 2010). Previously, heparin was determined to be inert to plasmin (Andrade-Gordon & Strickland, 1986); our data showed that heparin is also inert to plasmin-induced complement activation in serum (Fig. 4). However, red algal polysaccharides slightly retarded this process with little dependence on structural characteristics and sulfate content, but two carrageenans with and without κ-units at the highest concentration almost abolished C5 activation.
5. Conclusion
In summary, the red algal sulfated polysaccharides affected the complement system and its interplay with fibrinolytic components. These substances have the potential to participate in cell surface biology by inhibiting C3 binding to the surface in a similar fashion as cell regulators of the glycosaminoglycan family, depending on sulfation degree. Sulfation degree was also important in carrageenans’ capacity to reduce C4 binding in lectin complement activation. However, C4 binding in the classical complement was considerably activated in the presence of carrageenans with 3,6-anhydrogalactose. No structural characteristics apparently mattered in ameliorating C5 cleavage by plasmin occurring in extrinsic protease complement activation.
CRediT authorship contribution statement
E.V. Sokolova: Conceptualization, Methodology (Biological), Funding acquisition, Writing - original draft, Investigation. A.O. Kravchenko: Methodology (Chemical), Writing - review & editing (Chemical part). N.V. Sergeeva: Resources. A.I. Kalinovsky: Methodology (NMR spectroscopy data). V.P. Glazunov: Methodology (IR-spectroscopy data). L.N. Bogdanovich: Resources. I.M. Yermak: Writing - original draft (Chemical part), Writing - review & editing.
Acknowledgements
This study was supported by the Russian Science Foundation (RSF) Grant 20-74-00006. The study was carried out on equipment from the Collective Facilities Center, “The Far Eastern Center for Structural Molecular Research (NMR/MS) PIBOC FEB RAS.” Ekaterina Sokolova would like to express to PJL, an amazing person and no less amazing scientist, her deepest respect and admiration.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.carbpol.2020.117251.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Amara U., Flierl M.A., Rittirsch D., Klos A., Chen H., Acker B., et al. Molecular intercommunication between the complement and coagulation systems. The Journal of Immunology. 2010;185(9):5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Gordon P., Strickland S. Interaction of heparin with plasminogen activators and plasminogen: Effects on the activation of plasminogen. Biochemistry. 1986;25(14):4033–4040. doi: 10.1021/bi00362a007. [DOI] [PubMed] [Google Scholar]
- Baines A.C., Brodsky R.A. Complementopathies. Blood Reviews. 2017;31(4):213–223. doi: 10.1016/j.blre.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K.C., Nicklin S., Miller K. The role of carrageenan in complement activation. Food and Chemical Toxicology. 1986;24(9):891–895. doi: 10.1016/0278-6915(86)90315-7. [DOI] [PubMed] [Google Scholar]
- Barabanova A.O., Yermak I.M., Glazunov V.P., Isakov V.V., Titlyanov E.A., Solov’eva T.F. Comparative study of carrageenans from reproductive and sterile forms of Tichocarpus crinitus (Gmel.) Rupr (Rhodophyta, Tichocarpaceae) Biochemistry (Moscow) 2005;70(3):350–356. doi: 10.1007/s10541-005-0121-4. [DOI] [PubMed] [Google Scholar]
- Barnum S.R. Complement: A primer for the coming therapeutic revolution. Pharmacology & Therapeutics. 2017;172:63–72. doi: 10.1016/j.pharmthera.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Blaum B.S. The lectin self of complement factor H. Current Opinion in Structural Biology. 2017;44:111–118. doi: 10.1016/j.sbi.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Bovin N., Obukhova P., Shilova N., Rapoport E., Popova I., Navakouski M., et al. Repertoire of human natural anti-glycan immunoglobulins. Do we have auto-antibodies? Biochimica et Biophysica Acta (BBA)-General Subjects. 2012;1820(9):1373–1382. doi: 10.1016/j.bbagen.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Brownlee I.A., Dettmar P.W., Strugala V., Pearson J.P. The interaction of dietary fibres with the colon. Current Nutrition and Food Science. 2006;2(3):243–264. [Google Scholar]
- Buck C.B., Thompson C.D., Roberts J.N., Müller M., Lowy D.R., Schiller J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathogens. 2006;2(7) doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker J.J., Erickson S.A., Flynn T.M., Henry C., Koval J.C., Meisel M., et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017;358(6361) doi: 10.1126/science.aan6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiovitti A., Bacic A., Craik D.J., Kraft G.T., Liao M.L., Falshaw R., et al. A pyruvated carrageenan from Australian specimens of the red alga Sarconema filiforme. Carbohydrate Research. 1998;310(1-2):77–83. [Google Scholar]
- Collins L.E., Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. Journal of Leukocyte Biology. 2019;105(1):81–92. doi: 10.1002/JLB.3RU0618-246R. [DOI] [PubMed] [Google Scholar]
- Cosenza V.A., Navarro D.A., Ponce N.M., Stortz C.A. In: Industrial applications of renewable biomass products. Goyanes S.N., D’Accorso N.B., editors. Springer; Cham: 2017. Seaweed polysaccharides: Structure and applications; pp. 75–116. [Google Scholar]
- Craigie J.S. In: Biology of red algae. Cole K.M., Sheath R.G., editors. Cambridge University Press; New York: 1990. Cell wall; pp. 221–258. [Google Scholar]
- Cummings J.H., Antoine J.M., Azpiroz F., Bourdet-Sicard R., Brandtzaeg P., Calder P.C., et al. PASSCLAIM 1—Gut health and immunity. European Journal of Nutrition. 2004;43(2):ii118–ii173. doi: 10.1007/s00394-004-1205-4. [DOI] [PubMed] [Google Scholar]
- Damgaard C., Reinholdt J., Palarasah Y., Enevold C., Nielsen C., Brimnes M.K., et al. In vitro complement activation, adherence to red blood cells and induction of mononuclear cell cytokine production by four strains of Aggregatibacter actinomycetemcomitans with different fimbriation and expression of leukotoxin. Journal of Periodontal Research. 2017;52(3):485–496. doi: 10.1111/jre.12414. [DOI] [PubMed] [Google Scholar]
- Damonte E.B., Matulewicz M.C., Cerezo A.S. Sulfated seaweed polysaccharides as antiviral agents. Current Medicinal Chemistry. 2004;11(18):2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- Davies G.E. Inhibition of complement by carrageenin: Mode of action, effect on allergic reactions and on complement of various species. Immunology. 1965;8(3):291. [PMC free article] [PubMed] [Google Scholar]
- Ditta L.A., Rao E., Provenzano F., Sánchez J.L., Santonocito R., Passantino R., et al. Agarose/κ-carrageenan-based hydrogel film enriched with natural plant extracts for the treatment of cutaneous wounds. International Journal of Biological Macromolecules. 2020;164:2818–2830. doi: 10.1016/j.ijbiomac.2020.08.170. In press. [DOI] [PubMed] [Google Scholar]
- Dodgson K.S., Price R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Journal of Biochemistry. 1962;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos-Fidencio G.C., Gonçalves A.G., Noseda M.D., Duarte M.E.R., Ducatti D.R. Effects of carboxyl group on the anticoagulant activity of oxidized carrageenans. Carbohydrate Polymers. 2019;214:286–293. doi: 10.1016/j.carbpol.2019.03.057. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Younes M., Aggett P., Aguilar F., Crebelli R., Filipič M.…Kuhnle G.G. Re‐evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA Journal. 2018;16(4) doi: 10.2903/j.efsa.2018.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B., Magnani J.L. From carbohydrate leads to glycomimetic drugs. Nature Reviews Drug Discovery. 2009;8(8):661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez J.M., Ciancia M., Cerezo A.S. The system of galactans of the red seaweed, Kappaphycus alvarezii, with emphasis on its minor constituents. Carbohydrate Research. 2004;339(15):2575–2592. doi: 10.1016/j.carres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Galili U. Anti‐Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140(1):1–11. doi: 10.1111/imm.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U. Why do we produce anti-gal: Evolutionary appearance of anti-gal in old world primates. The Natural Anti-Gal Antibody As Foe Turned Friend In Medicine. 2018;23 doi: 10.1016/B978-0-12-813362-0.00002-6. [DOI] [Google Scholar]
- Galili U. Human natural antibodies to mammalian carbohydrate antigens as unsung heroes protecting against past, present, and future viral infections. Antibodies. 2020;9(2):25. doi: 10.3390/antib9020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groult H., Cousin R., Chot-Plassot C., Maura M., Bridiau N., Piot J.M., et al. Λ-carrageenan oligosaccharides of distinct anti-heparanase and anticoagulant activities inhibit MDA-MB-231 breast cancer cell migration. Marine Drugs. 2019;17(3):140. doi: 10.3390/md17030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Thorgersen E.B., Mollnes T.E. Advances in assay of complement function and activation. Advanced Drug Delivery Reviews. 2011;63(12):976–987. doi: 10.1016/j.addr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Harrison R.A. Vol. 40. Springer; Berlin Heidelberg: 2018. The properdin pathway: An “alternative activation pathway” or a “critical amplification loop” for C3 and C5 activation? pp. 15–35. (Seminars in Immunopathology). January. No. 1. [DOI] [PubMed] [Google Scholar]
- Hetland G., Eskhland T. Formation of the functional alternative pathway of complement by human monocytes in vitro as demonstrated by phagocytosis of agarose beads. Scandinavian Journal of Immunology. 1986;23(3):301–308. doi: 10.1111/j.1365-3083.1986.tb01971.x. [DOI] [PubMed] [Google Scholar]
- Holodick N.E., Rodríguez-Zhurbenko N., Hernández A.M. Defining natural antibodies. Frontiers in Immunology. 2017;8:872. doi: 10.3389/fimmu.2017.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalitnik A.A., Marcov P.A., Anastyuk S.D., Byankina Barabanova A.O., Glazunov V.P., Popov S.V., Ovodov Y.S., Yermak I.M. Gelling polysaccharide from Chondrus armatus and its oligosaccharides: The structural peculiarities and anti-inflammatory activity. Carbohydrate Polymers. 2015;115:768–775. doi: 10.1016/j.carbpol.2014.04.070. [DOI] [PubMed] [Google Scholar]
- Knutsen S.H., Myslabodski D.E., Larsen B., Usov A.I. A modified system of nomenclature for red algal galactans. Botanica Marina. 1994;37(2):163–170. [Google Scholar]
- Kolender A.A., Matulewicz M.C. Desulfation of sulfated galactans with chlorotrimethylsilane. Characterization of β-carrageenan by 1H NMR spectroscopy. Carbohydrate Research. 2004;339:1619–1629. doi: 10.1016/j.carres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Koutsaviti A., Ioannou E., Roussis V. Bioactive seaweeds for food applications. Academic Press; 2018. Bioactive seaweed substances; pp. 25–52. [Google Scholar]
- Kravchenko A.O., Anastyuk S.D., Sokolova E.V., Isakov V.V., Glazunov V.P., Helbert W., et al. Structural analysis and cytokine-induced activity of gelling sulfated polysaccharide from the cystocarpic plants of Ahnfeltiopsis flabelliformis. Carbohydrate Polymers. 2016;151:523–534. doi: 10.1016/j.carbpol.2016.05.086. [DOI] [PubMed] [Google Scholar]
- Kravchenko A.O., Anastyuk S.D., Glazunov V.P., Sokolova E.V., Isakov V.V., Yermak I.M. Structural characteristics of carrageenans of red alga Mastocarpus pacificus from Sea of Japan. Carbohydrate Polymers. 2020;229 doi: 10.1016/j.carbpol.2019.115518. [DOI] [PubMed] [Google Scholar]
- Lachmann P.J. Preparing serum for functional complement assays. Journal of Immunological Methods. 2010;352(1–2):195–197. doi: 10.1016/j.jim.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lachmann P.J. Looking back on the alternative complement pathway. Immunobiology. 2018;223(8-9):519–523. doi: 10.1016/j.imbio.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Lahaye M. Developments on gelling algal galactans, their structure and physico-chemistry. Journal of Applied Phycology. 2001;13(2):173–184. [Google Scholar]
- Langford-Smith A., Day A.J., Bishop P.N., Clark S.J. Complementing the sugar code: Role of GAGs and sialic acid in complement regulation. Frontiers in Immunology. 2015;6:25. doi: 10.3389/fimmu.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Lubbers R., Van Essen M.F., Van Kooten C., Trouw L.A. Production of complement components by cells of the immune system. Clinical and Experimental Immunology. 2017;188(2):183–194. doi: 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H.U., Binder C.J., Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends in Immunology. 2009;30(1):43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- McMorrow I.M., Comrack C.A., Sachs D.H., DerSimonian H. Heterogeneity of human anti-pig natural antibodies cross-reactive with the gal (α1,3) galactose epitope. Transplantation. 1997;64(3):501–510. doi: 10.1097/00007890-199708150-00021. [DOI] [PubMed] [Google Scholar]
- Miller I.J., Blunt J.W. New 13C NMR methods for determining the structure of algal polysaccharides, Part 1. The effect of substitution on the chemical shifts of simple Diad galactans. Botanica Marina. 2000;43:239–250. [Google Scholar]
- Ochsenbein A.F., Zinkernagel R.M. Natural antibodies and complement link innate and acquired immunity. Immunology Today. 2000;21(12):624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- Opoku G., Qiu X., Doctor V. Effect of oversulfation on the chemical and biological properties of kappa carrageenan. Carbohydrate Polymers. 2006;65(2):134–138. [Google Scholar]
- Pangburn M.K., Rawal N., Cortes C., Alam M.N., Ferreira V.P., Atkinson M.A. Polyanion-induced self-association of complement factor H. The Journal of Immunology. 2009;182(2):1061–1068. doi: 10.4049/jimmunol.182.2.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. Biological and therapeutic properties of the seaweed polysaccharides. International Biology Review. 2018;2(2):1–50. [Google Scholar]
- Pereira L., Amado A.M., Critchley A.T., Van de Velde F., Ribeiro-Claro P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and RT-Raman) Food Hydrocolloids. 2009;30:1–7. [Google Scholar]
- Pereira L., Critchley A.T. The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? Journal of Applied Phycology. 2020;32:1875–1877. doi: 10.1007/s10811-020-02143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Gheda S.F., Ribeiro-Claro P.J.A. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. International Journal of Carbohydrate Chemistry. 2013;22:7. [Google Scholar]
- Perestenko L.P. Nauka [In Russian]; St. Petersburg: 1994. The red algae of the far eastern seas of Russia. [Google Scholar]
- Petersen S.V., Thiel S., Jensen L., Steffensen R., Jensenius J.C. An assay for the Mannan-binding lectin pathway of complement activation. Journal of Immunological Methods. 2001;257(1-2):107–116. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- Poupard N., Groult H., Bodin J., Bridiau N., Bordenave-Juchereau S., Sannier F., et al. Production of heparin and λ-carrageenan anti-heparanase derivatives using a combination of physicochemical depolymerization and glycol splitting. Carbohydrate Polymers. 2017;166:156–165. doi: 10.1016/j.carbpol.2017.02.040. [DOI] [PubMed] [Google Scholar]
- Renn D.W., Santos G.A., Dumont L.E., Parent C.A., Stanley N.F., Stancioff D.J., et al. β-Carrageenan: Isolation and characterization. Carbohydrate Polymers. 1993;22:247–252. [Google Scholar]
- Ricklin D., Mastellos D.C., Reis E.S., Lambris J.D. The Renaissance of complement therapeutics. Nature Reviews Nephrology. 2018;14(1):26. doi: 10.1038/nrneph.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochas C., Rinaudo M., Landry S. Role of the molecular weight on the mechanical properties of kappa–carrageenan gels. Carbohydrate Polymers. 1990;12:255–266. [Google Scholar]
- Sina C., Kemper C., Derer S. Vol. 37. Academic Press; 2018. The intestinal complement system in inflammatory bowel disease: Shaping intestinal barrier function; pp. 66–73. (Seminars in Immunology). [DOI] [PubMed] [Google Scholar]
- Stortz C.A., Cerezo A.S. Novel findings in carrageenans, agaroids and “hybrid” red seaweed galactans. Current Topics in Phytochemistry. 2002;4:121–134. [Google Scholar]
- Usov A.I. Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocolloids. 1998;12(3):301–308. [Google Scholar]
- Usov A.I., Elashvili M.Y. Quantitative-determination of 3,6-anhydrogalactose derivatives and specific fragmentation of the red algal galactans under reductive hydrolysis conditions. Bioorganicheskaya Khimiya. 1991;17(6):839–848. [Google Scholar]
- Usov A.I., Shashkov A.S. Polysaccharides of algae XXXIV: Detection of iota-carrageenan in Phyllophora brodittei (Tum.) J Ag. (Rhodophyta) using 13C-NMR spectroscopy. Botanica Marina. 1985;28:367–373. [Google Scholar]
- Van de Velde F., Knutsen S.H., Usov A.I., Rollema H.S., Cerezo A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends in Food Science & Technology. 2002;13(3):73–92. [Google Scholar]
- Weiler J.M., Edens R.E., Linhardt R.J., Kapelanski D.P. Heparin and modified heparin inhibit complement activation in vivo. The Journal of Immunology. 1992;148(10):3210–3215. [PubMed] [Google Scholar]
- Wells J.M., Brummer R.J., Derrien M., MacDonald T.T., Troost F., Cani P.D., et al. Homeostasis of the gut barrier and potential biomarkers. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2017;312(3):G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegappan R., Selvaprithiviraj V., Amirthalingam S., Jayakumar R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydrate Polymers. 2018;198:385–400. doi: 10.1016/j.carbpol.2018.06.086. [DOI] [PubMed] [Google Scholar]
- Yermak I.M., Kim Y.H., Titlynov E.A., Isakov V.V., Solov’eva T.F. In: Sixteenth International seaweed symposium. Kain J.M., Brown M.T., Lahaye M., editors. Springer; Dordrecht: 1999. Chemical structure and gel properties of carrageenans from algae belonging to the Gigartinaceae and Tichocarpaceae, collected from the Russian pacific coast; pp. 555–562. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






