Summary
Bleomycin electrochemotherapy (ECT) has emerged as a treatment modality for locally advanced metastatic melanoma over the past decade. The phenomenon of reversible electroporation enhances cell permeability when a pulsed electrical current is applied to tissues. This facilitates enhanced cytotoxicity of bleomycin with minimal systemic side effects.
We present two case analyses of patients with advanced metastatic melanoma of lower limb which did not respond to alternative therapies, including immunotherapy and isolated limb perfusion, but had a positive clinical response to bleomycin ECT. Locoregional control of the tumour was gained along with positive functional outcomes for the patients including increased mobility and reduced malodour.
Bleomycin ECT is an exciting new therapeutic modality in the armamentarium of the plastic surgeon. Operating parameters have been developed and refined which facilitate its safe use along with incorporation into international melanoma guidelines. Evidence in the literature supports its use in select cases, however, it is vital that we share our experiences in its use so that its role can be better defined. Particularly in the era of rapidly developing systemic treatments which are decreasing mortality and thereby increasing the number of patients requiring locoregional disease managment.
Keywords: Melanoma, Electrochemotherapy, Bleomycin, Cutaneous metastases
Introduction
Melanoma Bleomycin electrochemotherapy (ECT) has emerged as a treatment modality for locally advanced metastatic melanoma over the past decade. The phenomenon of reversible electroporation enhances cell permeability when a pulsed electrical current is applied to tissues.1,2 This facilitates greater local cytotoxicity of chemotherapeutic agents such as Bleomycin, whilst reducing systemic side effects due to the lower systemic dose required. Indeed, the cytotoxicity of bleomycin is enhanced up to 700-fold in combination with reversible electroporation technology.2 Bleomycin ECT is predominantly indicated for accessible neoplastic lesions including palliative management of locally advanced metastatic melanoma.1,3 Despite this, many melanoma surgeons are still unfamiliar with this treatment modality and it is likely underutilised.
In this report we discuss our bleomycin ECT treatment regime and care pathway in the context of this new era of targeted therapy (TT) and immuno-therapy (IT). We present two case analyses of patients with advanced metastatic melanoma of lower limb where options for standard therapies, including isolated limb perfusion, had been exhausted but in which a dramatic clinical response to bleomycin ECT was observed.
Case report
The device used for both cases was the Cliniporator™ (IGEA Clinical Biophysics, Italy), with a centred hexagonal needle electrode, and was used in concordance with published guidelines.4
Case 1
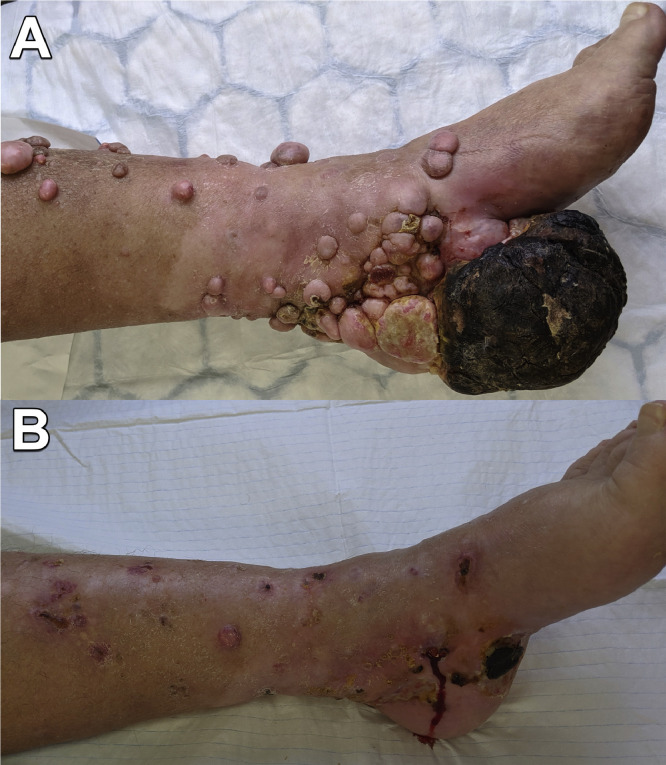
A 70-year-old woman with no other significant medical co-morbidities or regular medications was diagnosed with a 15 mm Breslow thickness left foot acral malignant melanoma (BRAF wild type). She had wide excision and a sentinel lymph node biopsy (SLNB) was positive. She declined groin dissection (preceded adjuvant therapy availability). A year later recurrent disease was identified on positron emission tomography (PET) in the popliteal fossa lymph nodes and adjacent to the graft site on the sole of the foot. She had six cycles of pembrolizumab followed by T-VEC (Talimogene laherparepvec oncolytic virus) and isolated limb perfusion (ILP) over the following year. In-transit metastases and lesions on her leg significantly impacted her quality of life with pain, bleeding and resultant reduced mobility. Whilst ILP did reduce the size of some of the lesions her symptoms did not improve significantly, and she was referred for consideration of ECT. She had debulking and bleomycin ECT to her extensive lesions with 171 sequences completed in total (see Table 1). The treatment resulted in considerable reduction in tumour mass with less bleeding and less painful mobilization with partial weight bearing on crutches (Figure 1). She had increased pain and wound care requirements for a period of several months following bleomycin ECT. She had a further round of bleomycin ECT five months later with good effect and remained independent in activities of daily living. Post-treatment pain recurred following the second round of bleomycin ECT and was managed with oral analgesia alone.
Table 1.
Bleomycin electrochemotherapy delivery.
| Probe Configuration | Frequency (kilohertz, kHz) | Sequences (n) | Duration (min) | |
|---|---|---|---|---|
| Case 1 | Hexagonal | 5 kHz | Total: 171 | 39 min |
| Case 2 | Hexagonal | 5 kHz | Total: 95 | 12 min |
Figure 1.
Case 1. Left lower leg and foot, medial aspect. (A) Pre-operative. (B) 6-months post-operative.
Case 2
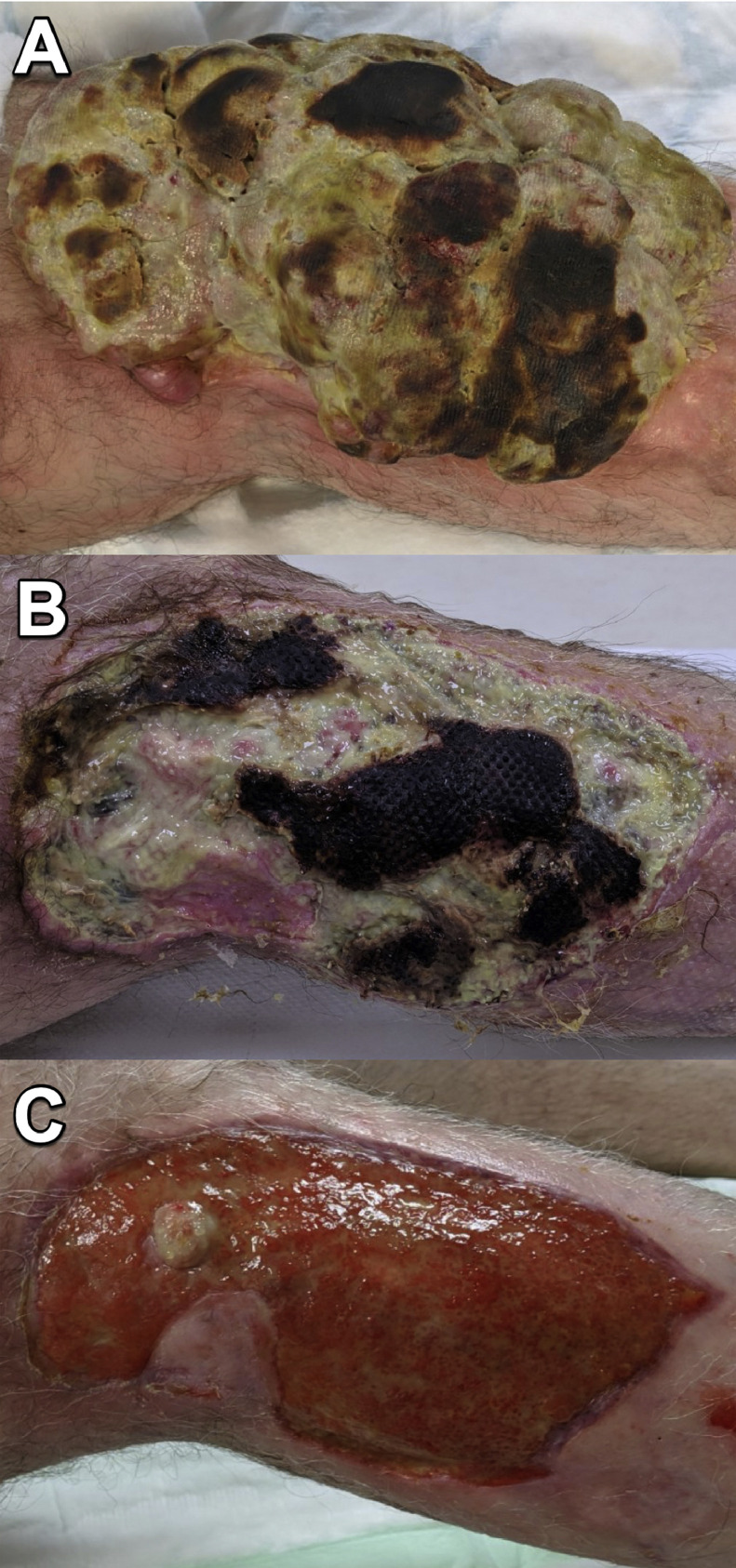
A 51-year-old gentleman with a background of coeliac disease was diagnosed with malignant melanoma (BRAF wild type) of the right calf. Wide excision was carried out. Six years later the disease recurred with regional lymph node involvement and local disease recurrence. He received immunotherapy (7 doses of nivolumab) and, following a groin dissection, this was changed to ipilimumab. A single cycle induced auto-immune meningitis which necessitated cessation of treatment and a protracted course of prednisolone. Cutaneous and subcutaneous recurrences were initially surgically excised. T-VEC therapy had a limited effect on local disease and he was referred for ECT. His main complaint was the size and growth of a single metastasis lateral to his knee. The malodour was disabling, its size prevented wearing trousers and he was experiencing significant pain requiring opioid analgesics (dihydrocodeine). He had debulking and bleomycin ECT, completing 95 sequences in total (see Table 1). The bleomycin ECT treatment was successful in reducing and maintaining the reduction in tumour bulk (Figure 2). Following bleomycin ECT the patient was able to wear trousers and the malodorous smell had resolved. There was increased pain following bleomycin ECT, however, at four months this significantly reduced. Of note, the patient developed common peroneal nerve palsy, 3/5 power (NRC scale), improving to 4/5 power which may have been due to bleomycin ECT or tumour invasion.
Figure 2.
Case 2. Left knee, medial aspect. (A) Pre-operative. (B) 1-month post-operative. (C) 3-months post-operative (proximal tumour recurrence subsequently treated with excision).
Discussion
Management of locally advanced metastatic melanoma is challenging with a plethora of available treatment modalities including excisional and ablative surgery, systemic therapies, ILP, radiotherapy and ECT.3 Systemic therapies (TT and IT) are rapidly evolving and increasingly available, they significantly prolong the course of the disease, are potentially curative and increase the number of patients who may benefit from local disease management.5
Bleomycin ECT is now recognised as a safe treatment for cutaneous tumours and skin metastases with established standard operating procedures.4 This is evidenced by its incorporation into clinical guidelines internationally.3,6,7 The evidence base is growing and a recently published cohort study of by the InspECT research group including 394 melanoma lesions found that 78% (306) had a complete or partial response, and 58% (229) had a complete response to bleomycin ECT.8 Additionally, there is emerging evidence that bleomycin ECT may be particularly effective in combination with immunotherapies, acting synergistically and producing enhanced systemic anti-tumour effects (the ‘abscopal effect’).9,10 This is of particular importance to patients, such as those in this report, with a ‘wild-type’ BRAF gene precluding the use of TT to the BRAF and MEK pathway. These patients cannot benefit from the highly effective TT combination of BRAF and MEK inhibitors.5 Post-treatment pain is a recognised complication of bleomycin ECT, has a reported incidence of 39% and is manageable with oral analgesia in the majority of cases.8
Clinicians determine on a case-by-case basis, or regional availability of services, which local peripheral treatments are used. National and international guidelines do not currently specify an algorithmic approach to determine which modality should be considered first-line.3,7 This approach appropriately reflects the paucity of comparative studies evaluating peripheral treatment modalities for locally advanced metastatic melanoma.10 We have presented two cases with a dramatic response to electrochemotherapy resulting in improved symptoms and quality of life. In both cases bleomycin ECT was considered after other modalities were utilised based on local dogmatic practice in which ILP is generally first-line.
Conclusions
These cases highlight the clinical efficacy of bleomycin ECT in managing locally advanced metastatic melanoma in the context of a new era of systemic therapies and improved survival. More research, in particular comparative studies, are required to establish the relative efficacy of bleomycin ECT versus other modalities. As the evidence base evolves guidelines must be regularly updated to ensure that clinicians are aware of the options available for their patients and their efficacy. Additionally, if modalities are clinically non-inferior cost-effectiveness analysis is warranted.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
None.
Funding
None.
References
- 1.Probst U., Fuhrmann I., Beyer L., Wiggermann P. Electrochemotherapy as a new modality in interventional oncology: a review. Technol Cancer Res Treat. 2018;17 doi: 10.1177/1533033818785329. 1533033818785329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mir L.M., Orlowski S., Belehradek J., Jr., Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27:68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence Melanoma: assessment and management https://www.nice.org.uk/guidance/ng14/chapter/1-Recommendations#managing-stage-iv-melanoma (Accessed 01/03/2020).
- 4.Gehl J., Sersa G., Matthiessen L.W. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57:874–882. doi: 10.1080/0284186X.2018.1454602. [DOI] [PubMed] [Google Scholar]
- 5.Pasquali S., Hadjinicolaou A.V., Chiarion Sileni V., Rossi C.R., Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2 doi: 10.1002/14651858.CD011123.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence Electrochemotherapy for metastases in the skin from tumours of non-skin origin and melanoma https://www.nice.org.uk/guidance/IPG446 (Accessed 01/05/2020).
- 7.Garbe C., Amaral T., Peris K. European consensus-based interdisciplinary guideline for melanoma. Part 2: treatment – update 2019. Eur J Cancer. 2020;126:159–177. doi: 10.1016/j.ejca.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Kunte C., Letule V., Gehl J. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176:1475–1485. doi: 10.1111/bjd.15340. [DOI] [PubMed] [Google Scholar]
- 9.Goggins C.A., Khachemoune A. The use of electrochemotherapy in combination with immunotherapy in the treatment of metastatic melanoma: a focused review. Int J Dermatol. 2019;58:865–870. doi: 10.1111/ijd.14314. [DOI] [PubMed] [Google Scholar]
- 10.Read R.L., Thompson J.F. Managing in-transit melanoma metastases in the new era of effective systemic therapies for melanoma. Expert Rev Clin Pharmacol. 2019;12:1107–1119. doi: 10.1080/17512433.2019.1689121. [DOI] [PubMed] [Google Scholar]