Abstract
Lipopolysaccharide (LPS), an endotoxin, is known to induce inflammatory response and oxidative stress in rodents. We evaluated the protective role of Euglena tuba extract (ETME) against LPS induced inflammatory response and oxidative stress in male Balb/c mice. Male Balb/c mice were divided into 4 groups. Group 1 (control) were intraperitoneally administered 0.5 mL PBS. Group 2, 3 and 4 were treated with a single dose of LPS (i.p. 40 mg/kg body weight). Prior 1 h, Group 3 and 4 received orally 100 mg/kg body weight and 200 mg/kg body weight ETME respectively. Biomarkers of oxidative stress including TBARS, SOD, Catalase, Liver marker enzyme (SGPT and SGOT), Nitric Oxide, and inflammatory cytokines including IL-6 and TNF-α, were estimated in serum. Oxidative stress and inflammatory markers were significantly increased in the LPS treated group, whereas ETME treated group at different concentrations protected mice from pro inflammatory cytokines and oxidative stress. Our results indicate that 70% methanolic extract of Euglena tuba can efficiently counteract free radical generation and increased level of inflammatory cytokine in an LPS induced mice model.
Keywords: Mice, Lipopolysaccharide, Inflammatory cytokines, Oxidative stress, Euglena tuba
Introduction
Euglena tuba is an alga that is predominantly found in freshwater ponds, puddles, lakes, and river banks, forming seasonal algal bloom prominently at warm temperatures during conditions of lower dissolved oxygen and acidic environment. Blooms fully cover the pond forming a deep red to greenish coloured continuous layer on the surface of the water (Deb 2015). Various bioactive compounds like phenolics, flavonoids, alkaloids, tannins, terpenoids, saponins, carbohydrates, and ascorbic acid are present in Euglena tuba which provide many documented medicinal properties (Chaudhuri et al. 2014). Studies have shown that Euglena tuba has antioxidant, hepatoprotective, iron-chelator, antitumor activities and can induce apoptosis through ROS- mediated MAPK regulation (Panja et al. 2014, 2016).
Lipopolysaccharide (LPS), an endotoxin, is the key component of the outer membrane of Gram-negative bacteria. The administration of LPS to mice is a widely used strategy to induce an acute systemic inflammatory response. Incorporation of LPS to the body activates an intracellular signaling pathway and causes the production of inflammatory cytokines which are responsible for activating innate immune response by regulating inflammatory mediators such as TNF-α, IL-6, and NO (Buras et al. 2005; Schulte et al. 2013). In response to these cytokines, reactive oxygen species are generated from neutrophils and other phagocytic cells which triggers oxidative stress (Sugino et al. 1987). Uncontrolled inflammation and excessive oxidative stress may be the leading symptoms of sepsis culminating in multiple organ failure and death (Steven et al. 2017). The symptoms of COVID 19 also show an abnormally high inflammatory response (Zhang et al. 2020).
Based on already available reports that Euglena tuba has excellent anti-inflammatory and antioxidant property, we focus our study to investigate the ameliorative anti-inflammatory and antioxidant effect of 70% methanolic extract of Euglena tuba (ETME) on LPS induced inflammatory response and resultant severe oxidative stress in mice.
Method and material
Sample collection and characterization
The algal sample was collected in the month of October from a pond in district Kangra of the state of Himachal Pradesh, India, situated at 28° 22′ to 33° 12´ N, 75° 47′ to 79° 04′E and 31° 40′–32° 25′ East longitudes and 70° 35′–77° 5′ North latitudes. Samples were preserved in 4% formalin for identification and observed under the light microscope. Morphological features were taken in account and characterized (Kumar et al. 2016).
Extract preparation
The collected samples were thoroughly cleaned under the sterile condition with distilled water 3–4 times to remove dirt and then centrifuged at 1000 x g to wash out contaminating bacteria. The collected pellet of the biomass of Euglena tuba was dried in sunlight for seven days and then finely powdered. In 100 mL solvent (methanol: water 7:3) the powder (10 g) was soaked and stirred for 17 h, then centrifuged at 3000 g. The process was repeated again with the obtained pellet by using a 100 mL fresh solvent. The total obtained supernatant was concentrated under reduced pressure in a rotary evaporator. The concentrated solution was lyophilized and the 70% methanolic extract of Euglena tuba (ETME) was obtained which was kept at −20 °C for future use.
Animals and experimental design
6–8 weeks old male Balb/c mice were purchased from the Indian Institute of Toxicological Research, Lucknow, India. The animals were grouped and housed in polyacrylic cages with 6 animals per cage and maintained under standard laboratory conditions. Animals had free access to standard diet purchased from Paramount Techno Company and water ad libitum. Animals were randomly divided into four groups, each comprising six animals. Group 1 the controls were intraperitoneally administered 0.5 mL PBS. Group 2,3 and 4 were treated with a single dose of LPS (O55: B5 E.coli purchased from Sigma Aldrich, Mumbai) (i.p. 40 mg/kg body weight) (Lee et al. 2013). Prior 1 h Group 3 and 4 received orally 100 mg/kg body weight and 200 mg/kg body weight ETME respectively. Blood samples were drawn 3 h after LPS treatment from orbital sinus and plasma was separated. The survival of the mice was monitored for 5 days and sacrificed by cervical dislocation. All experiments using mice were done in accordance with the guidelines of the Institutional Ethical Committee (839/GO/Re/04/CPCSEA).
Nitric oxide (NO)
Nitric oxide levels was measured in the plasma by Griess reaction (Yamamoto et al. 1998). The samples were incubated with Griess reagent (0.1% naphthalene diamine HCl; 1% sulfanilamide in 5% phosphoric acid mixed as 1:1) and the pink-colored product thus formed was measured at 540 nm on a spectrophotometer. Production of Nitric oxide was calculated by comparing standard sodium nitrite conc. and the results are expressed as μmol/L NO.
Total protein content was estimated in plasma samples by using the method of Lowry et al. (1951).
Estimation of cytokine level (IL-6 and TNF – α) in the serum of mice
Cytokine levels were estimated following the directions given as per the manufacturer’s manual (Krishgen Bio framework, India) as described previously in detail (Kumar et al. 2020). The result is reported in Pg/ mL.
Thiobarbituric acid reactive substances (TBARS)
Malondialdehyde (MDA) in plasma was determined by reaction with thiobarbituric acid (TBA) (Buege and Aust 1978). Briefly; 0.1 mL Tris –HCL buffer, 0.1 mL Ferrous sulphate and 0.1 mL Ascorbic acid. 0.05 mL sample was added and the volume was made up to 1.0 mL with DDW. 1.0 mL TCA and 2 mL TBA were added after incubation at 37 °C for 15 min. Tubes were plugged and incubated for 15 min. in a boiling water bath. After incubation, tubes were centrifuged at 3000 rpm for 10 min. and the supernatant was read at 532 nm. The concentration of MDA was calculated using the extinction coefficient of 1.56 × 105 M−1 cm−1. Results are expressed as nmol MDA/mg protein.
Superoxide dismutase (SOD)
The activity of the enzyme superoxide dismutase was determined according to the method of Kono (1978). Auto oxidation of hydroxylamine HCL generates superoxide anions. These anions bring about the reduction of NBT to blue formazone. SOD inhibits the reduction of NBT induced by hydroxylamine HCL. The activity of the enzyme was expressed as units/mg protein, where one unit of enzyme is defined as the amount of enzyme inhibiting the rate of reaction by 50%.
Catalase
Catalase activity was assayed by the method of Luck (1971). Catalase is an enzyme that catalyzes the decomposition of hydrogen peroxide to oxygen. The rate of decomposition of hydrogen peroxide is assessed spectrophotometrically at 240 nm. The activity of the enzyme is expressed as μmoles of hydrogen peroxide decomposed per min per mg protein, using the molar extinction coefficient of hydrogen peroxide (71 M−1 cm-1).
Measurement of liver marker enzyme
Determination of serum SGPT, and SGOT level was performed using reagent kits from Span diagnostic and ERBA diagnostics and measurements were made on an Erba Mannheim Chem.-7 analyzer.
Statistical analysis
All data are expressed as mean ± SD of six measurements and statistical analysis was performed using GraphPad Prism 7.04 software. Data were analyzed by one-way ANOVA. A probability of P < 0.05 was considered significant.
Results
Nitric oxide
Figure 1 shows levels of NO in plasma of all groups. The NO levels were found to be significantly (p < 0.05) higher in the LPS group with respect to control group while significant decreases (p < 0.05) were seen in the ETME group as compared to the LPS group.
Fig. 1.
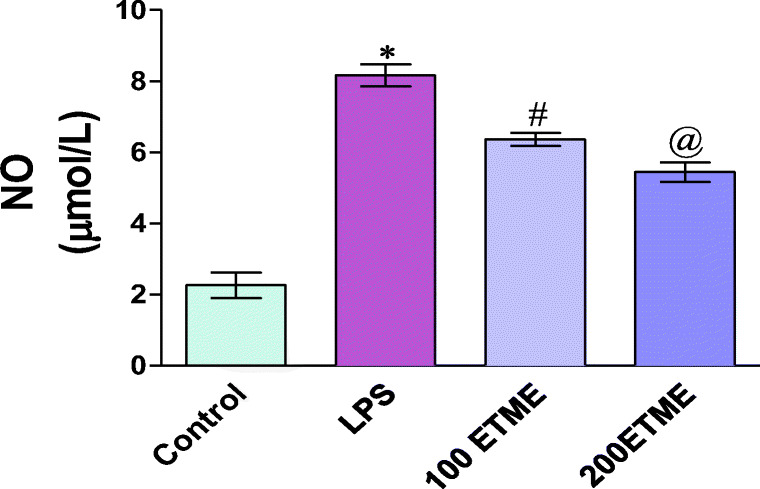
Effect of different doses of ETME on the level of NO in LPS induced oxidative stress.*p < 0.05 significant changes occurs between 100 ETME to the LPS group, @ represent significant p < 0.05 change occurs between 200 ETME compared to the LPS group
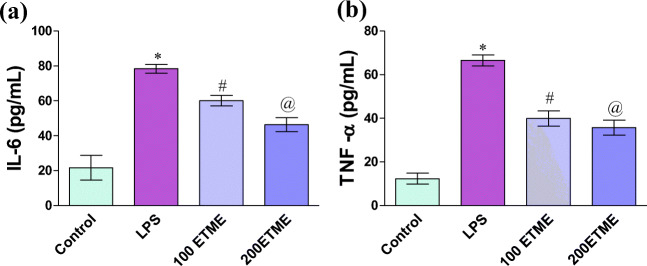
Cytokine level (IL-6 and TNF – α) in the serum of mice
Cytokine levels are represented in Fig. 2a and b. The activity of both cytokines is significantly (p < 0.05) increased in LPS treated groups of mice with respect to control. A significant reduction is seen in both ETME groups of mice when compared with LPS group.
Fig. 2.
a and b represents the cytokine level in mice serum.*, # and @ represent the significant increase (p < 0.05) in the value of cytokines with respect to their control LPS and different concentration of ETME supplementation
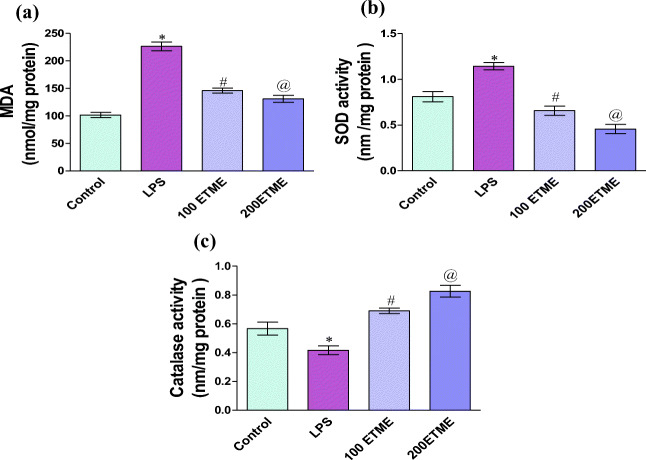
Thiobarbituric acid reactive substances
Lipid peroxidation is one of the important oxidative stress markers measured in the form of MDA and value is reported in the form of nmol/mg protein. According to variously reported studies lipid peroxidation increases with oxidative stress. Our result also confirms increase in the level of lipid peroxidation in the LPS group as compared to control whereas both ETME groups show significant (p < 0.05) decrease in the MDA level with respect to LPS group Fig. 3a.
Fig. 3.
a Effect of different doses of ETME on the level of MDA in LPS induced oxidative stress.*p < 0.05 compared to the control group, # p < 0.05 significant changes occurs between 100 ETME to the LPS group, @ represent significant p < 0.05 change occurs between 200 ETME compared to the LPS group. b Effect of different doses of ETME on the SOD enzyme in LPS induced oxidative stress.*p < 0.05 compared to the control group, # p < 0.05 significant changes occurs between 100 ETME to the LPS group, @ represent significant p < 0.05 change occurs between 200 ETME compared to the LPS group. c Effect of different doses of ETME on the catalase enzyme in LPS induced oxidative stress.*p < 0.05 significant changes occurs between 100 ETME to the LPS group, @ represent significant p < 0.05 change occurs between 200 ETME compared to the LPS group
Superoxide dismutase
Figure 3b, shows the SOD activity. Significantly increased (p < 0.05) value of SOD are found in the LPS group as compared to control, whereas both ETME groups show significant (p < 0.05) decrease in the SOD level with respect to LPS group.
Catalase
Catalase is one of the important antioxidant biomarkers markers. Figure 3c, represents the level of catalase activity. In the LPS group of mice, there was (p < 0.05) significantly decrease in the level of enzyme activity with respect to the control group of mice while significantly increased (p < 0.05) in the enzyme activity are found in 100 & 200 ETME groups when compared to the LPS group. 200 ETME supplementation provides a better result with respect to 100 ETME.
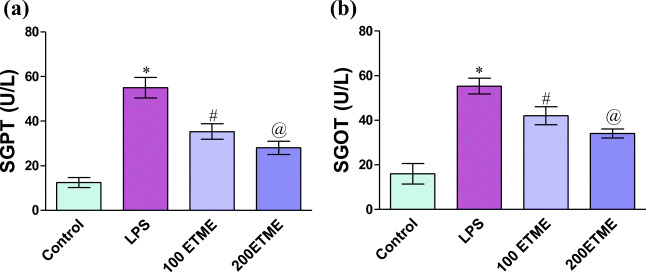
Serum SGPT and SGOT level
SGOT and SGPT level are shown in Fig. 4a and b. The activity of both markers is significantly (p < 0.05) increased in LPS treated groups of mice with respect to control. A significant reduction is seen in both ETME groups of mice when compared with LPS group.
Fig. 4.
a and b The serum SGPT and SGOT are represented are in Fig. 2.*, # and @ represent the significant increase (p < 0.05) between control, LPS and different concentration of ETME supplementation
Discussion
Microalgae are the best antioxidants used in different pathologies related to the generation of free radicals associated with several diseases (Sathasivam et al. 2019). Antioxidant properties of frequently consumed food, vegetables, and other herbs have been confirmed as a good source of potent antioxidant due to their phytochemicals (Proteggente et al. 2002). Polyphenols and flavonoids are strong antioxidants that can act as reducing agent (Karaman et al. 2010), free radical scavengers (Kähkönen et al. 1999), inhibitors of lipid peroxidation (Williams et al. 2004) and thereby preventing oxidative damage (Ross and Kasum 2002). HPLC analysis of Euglena tuba extract corroborate the presence of bioactive compounds including tannic acid,reserpine, methyl gallate, catechin, ascorbic acid and rutin, the presence of these compounds validate the strong antioxidant property of ETME (Chaudhuri et al. 2014). Moreover GC-MS analysis of ETME also showed the presence of compounds such as isovanillin, 3-hydroxy-5-methoxy benzaldehyde, α-D-glucopyranoside, methyl-2,6-dihydroxy-4-methyl benzoate, methyl palmitate, 14,17-octadecadienoic acid, and viminalol which may contribute to antioxidant, antiperoxidative, anti-inflammatory and anticancer activities (Panja et al. 2014).
Overproduction of free radicals increases the level of oxidants which is the underlying mechanism of oxidative damage. Excessive oxidative stress is a distinguishing aspect of LPS. Therapies targeting redox abnormalities could be useful for improving the management of sepsis. Studies have revealed that prolonged vascular inflammation and oxidative stress in response to infection or endotoxin (LPS) induction is a distinct trait related to sepsis (Steven et al. 2017). The antioxidant defense system of the body involves a range of antioxidants and enzymes such as SOD, CAT, GST, and GSH. Our results provide evidence that the administration of LPS alters the level of antioxidant enzymes and that oral administration of the Euglena tuba extract can provide protection against oxidative stress. ETME shows in vitro hydroxyl radical scavenging activity that can eliminate hydroxyl radical and significantly decrease the level of lipid peroxidation, suggesting that may ETME function as a good antioxidant that can effectively reduce the cellular toxicity of LPS (Chaudhuri et al. 2014).
A high level of SOD and low level of Catalase is an adaptive response to increased oxidative damage created due to the administration of endotoxin LPS (Portolés et al. 1996). Our result suggests that ETME shows superoxide radical scavenging activity that might be due to the presence of flavonoids which effectively scavenge superoxide anions (Sunil et al. 2011). However, the level of Catalase significantly increases by the action of phenolics linked to enzymatic and non-enzymatic reactions of antioxidants (Nirmal et al. 2008).
The development of reactive oxygen and nitrogen species is an important part of the innate immune response. High level of NO is generated by i-NOS after induction by any pathogen, cytokines, growth factors and endotoxin which plays a significant role in the pathology of several diseases such as arthritis, diabetes, atherosclerosis, and sepsis (Nathan 1992; Nussler and Billiar 1993). The overproduction of NO is the characteristic feature of endotoxin. LPS induction enhances the expression of i-NOS that consequently activates the nuclear factor (NF-κB) and increases the production of NO (Virdis et al. 2005). However, ETME reduces the toxicity of NO due to the presence of anti-inflammatory compounds which might inhibit nitrite formation by directly competing with oxygen in the reaction with nitric oxide (Chaudhuri et al. 2014).
The liver is the main organ of oxidative and detoxifying action. During stress conditions increase in the amount of ROS and inflammation plays an important role in altering the liver function markers SGOT and SGPT. Our results show increase in the level of both marker enzymes in the LPS treated group of mice whereas the different concentration of ETME reversed this effect, higher conc. of ETME provides better results, so our finding support that the ETME supplement reduced ROS and inflammation and also work as a hepatoprotective compound.
Our findings assume great significance in present times when science is trying to find ways to limit the mortality caused by COVID 19. The major cause of multiple organ failure in patients of COVID 19 is due to the production of very high levels of pro-inflammatory cytokines which is referred to as ‘cytokine storm’ (Sallard et al. 2020; Stebbing et al. 2020). Our results show that ETME may reduce the level of LPS induced cytokines thus providing an intervention strategy for the management of COVID 19.
Conclusion
Our finding demonstrates that 70% methanolic extract of Euglena tuba can efficiently counteract free radical generation and increased level of pro-inflammatory cytokine in an LPS induced mice model. The outcome of this study strongly suggests that the extract of Euglena tuba can be used as a therapeutic agent to reduce the risk of systemic inflammation and oxidative stress in chronic diseases. The results may also provide a line of defense for COVID 19.
Acknowledgments
We would like to thank Dr. Rakesh Kumar for collection of Euglena samples.
Abbreviations
- LPS
Lipopolysaccharide
- ETME
Methanolic extract of Euglena tuba
- TBARS
Thiobarbituric acid reactive substances
- SOD
Superoxide Dismutase
- NBT
Nitroblue tetrazolium
- SGPT
Serum Glutamic Pyruvic Transaminase
- SGOT
Serum Glutamic Oxaloacetic Transaminase
- NO
Nitric oxide
- i NOS
Inducible nitric oxide synthase
Financial support
This work was supported by a research grant from SERB-DST, Govt. of India (EMR/2016/006470).
Data availability
The information that helps the finding of this study is accessible from the corresponding author upon reasonable request.
Compliance with ethical standards
Ethics approval
All experiments using mice were done in accordance with the guidelines of the Institutional Ethical Committee (839/GO/Re/04/CPCSEA).
Conflict of interest
The authors of this manuscript have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In Methods in enzymology 52:302–310. Elsevier. 10.1016/S0076-6879(78)52032-6 [DOI] [PubMed]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Chaudhuri D, Ghate N, Deb S, Panja S, Sarkar R, Rout J, Mandal N. Assessment of the phytochemical constituents and antioxidant activity of a bloom forming microalgae Euglena tuba. Biol Res. 2014;47(1):24. doi: 10.1186/0717-6287-47-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S. Morphology and biochemical study of a microalga Euglena tuba reported from the aquatic ecosystem of cachar. Res Rev J Pharmacog Phytochem. 2015;3:1–10. [Google Scholar]
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47(10):3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Karaman Ş, Tütem E, Başkan KS, Apak R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay. Food Chem. 2010;120(4):1201–1209. doi: 10.1016/j.foodchem.2009.11.065. [DOI] [Google Scholar]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186(1):189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- Kumar R, Toppo K, Mandotra SK, Suseela MR, Seth MK, Minhas U, Kesherwani R, Gupta SP. Quantitative analysis and first report of Euglena tuba from Himachal Pradesh, India. IJSR. 2016;5(12):1336–1339. [Google Scholar]
- Kumar R, Akhtar F, Rizvi SI. Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidaemic model of rat. Clin Exp Pharmacol Physiol. 2020;47(4):571–582. doi: 10.1111/1440-1681.13221. [DOI] [PubMed] [Google Scholar]
- Lee MH, Kang H, Lee K, Yang G, Ham I, Bu Y, Kim H, Choi HY. The aerial part of Taraxacum coreanum extract has an anti-inflammatory effect on peritoneal macrophages in vitro and increases survival in a mouse model of septic shock. J Ethnopharmacol. 2013;146(1):1–8. doi: 10.1016/j.jep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Luck H. (1971) Catalase. In: Hu, B, Ed, Methods of Enzymatic Analysis 3:279
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6(12):3051–3064. doi: 10.1096/fasebj.6.12.1381691. [DOI] [PubMed] [Google Scholar]
- Nirmal J, Babu CS, Harisudhan T, Ramanathan M. Evaluation of behavioural and antioxidant activity of Cytisus scoparius link in rats exposed to chronic unpredictable mild stress. BMC Complement Altern Med. 2008;8(1):15. doi: 10.1186/1472-6882-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54(2):171–178. doi: 10.1002/jlb.54.2.171. [DOI] [PubMed] [Google Scholar]
- Panja S, Chaudhuri D, Ghate NB, Mandal N. Phytochemical profile of a microalgae Euglena tuba and its hepatoprotective effect against iron-induced liver damage in Swiss albino mice. J Appl Microbiol. 2014;117(6):1773–1786. doi: 10.1111/jam.12643. [DOI] [PubMed] [Google Scholar]
- Panja S, Ghate NB, Mandal N. A microalga, Euglena tuba induces apoptosis and suppresses metastasis in human lung and breast carcinoma cells through ROS-mediated regulation of MAPKs. Cancer Cell Int. 2016;16(1):13. doi: 10.1186/s12935-016-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portolés MT, Catalá M, Antón A, Pagani R. Hepatic response to the oxidative stress induced by E. coli endotoxin: glutathione as an index of the acute phase during the endotoxic shock. Mol Cell Biochem. 1996;159(2):115–121. doi: 10.1007/BF00420913. [DOI] [PubMed] [Google Scholar]
- Proteggente AR, Pannala AS, Paganga G, Buren Lv, Wagner E, Wiseman S, van de PF, Dacombe C, Rice-Evans CA. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic Res. 2002;36(2):217–233. doi: 10.1080/10715760290006484. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22(1):19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N, Florence A. Type 1 interferons as a potential treatment against COVID-19. Antivir Res. 2020;178:4. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allah EF. Microalgae metabolites: a rich source for food and medicine. Saudi Journal of Biological Sciences. 2019;26(4):709–722. doi: 10.1016/j.sjbs.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediat Inflamm. 2013;2013:16. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven S, Dib M, Roohani S, Kashani F, Münzel T, Daiber A. Time response of oxidative/nitrosative stress and inflammation in LPS-induced endotoxaemia—a comparative study of mice and rats. Int J Mol Sci. 2017;18(10):12. doi: 10.3390/ijms18102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Dohi K, Yamada K, Kawasaki T. The role of lipid peroxidation in endotoxin-induced hepatic damage and the protective effect of antioxidants. Surgery. 1987;101(6):746–752. doi: 10.5555/uri:pii:0039606087900894. [DOI] [PubMed] [Google Scholar]
- Sunil AG, Kesavanarayanan KS, Kalaivani P, Sathiya S, Ranju V, Priya RJ, Pramila B, Paul FS, Venkhatesh J, Babu CS. Total oligomeric flavonoids of Cyperus rotundus ameliorates neurological deficits, excitotoxicity and behavioral alterations induced by cerebral ischemic–reperfusion injury in rats. Brain Res Bull. 2011;84(6):394–405. doi: 10.1016/j.brainresbull.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Virdis A, Colucci R, Fornai M, Blandizzi C, Duranti E, Pinto S, Bernardini N, Segnani C, Antonioli L, Taddei S. Cyclooxygenase-2 inhibition improves vascular endothelial dysfunction in a rat model of endotoxic shock: role of inducible nitric-oxide synthase and oxidative stress. J Pharmacol Exp Ther. 2005;312(3):945–953. doi: 10.1161/01.HYP.0000253085.56217.11. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Akbar SKMDF, Masumoto O. Increased nitric oxide (NO) production by antigen-presenting dendritic cells is responsible for low allogeneic mixed leucocyte reaction (MLR) in primary biliary cirrhosis (PBC) Clin Exp Immunol. 1998;114(1):94–101. doi: 10.1046/j.1365-2249.1998.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, Reiter RJ. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:6. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The information that helps the finding of this study is accessible from the corresponding author upon reasonable request.