Abstract
Background and aims
Acute onset diabetes and diabetic ketoacidosis (DKA) can be precipitated by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in individuals with no history of diabetes. However, data regarding the follow-up of these individuals are scarce.
Methods
Three patients (data of two patients already published) with acute onset diabetes and DKA, precipitated by coronavirus disease 2019 (COVID-19), were followed for 14 weeks to assess the behavior of the diabetes. Detailed history, anthropometry, laboratory investigations, imaging studies, clinical course and outcomes were documented.
Results
Three individuals developed symptoms suggestive of SARS CoV-2 infection. After a few days, they were detected to have COVID-19 pneumonia, based on reverse transcription-polymerase chain reaction (RT-PCR) assay and chest imaging. In the meantime, they also developed acute onset diabetes and DKA, which were precipitated by COVID-19. They responded well to treatment, including intravenous fluids and insulin. After around one week, they were transitioned to multiple shots of subcutaneous insulin. After about 4–6 weeks, their insulin requirement diminished and oral antihyperglycemic drugs were initiated. At the last follow-up (14 months), they had controlled glycemia with oral antihyperglycemic medicines.
Conclusions
COVID-19 can induce acute onset diabetes and DKA in some individuals with no history of diabetes. These features resemble type 1 diabetes. However, after 4–6 weeks, their requirement for exogenous insulin diminishes and respond to oral antihyperglycemic medications. Long term follow up is required to further understand the type of diabetes induced by SARS CoV-2 infection in these individuals.
Keywords: SARS CoV-2, COVID-19, Diabetic ketoacidosis, DKA, Coronavirus
Highlights
-
•
In some individuals, COVID-19 induces diabetes resembling type 1 diabetes, characterized by acute onset and DKA.
-
•
However, their requirement for insulin diminishes after around 4–6 weeks.
-
•
Subsequently their diabetes can be controlled with oral antihyperglycemic medicines.
1. Introduction
COVID-19 can precipitate acute hyperglycemic crises-DKA and hyperosmolar hyperglycemic state (HHS) in individuals with either new onset diabetes or previously undiagnosed diabetes [[1], [2], [3], [4], [5], [6], [7]]. Acute onset diabetes and DKA as presenting feature are characteristic of type 1 diabetes. There is a recent report that indicated that SARS CoV-2 infection induced autoantibody-negative insulin dependent diabetes in a young Caucasian boy. However, there is no follow up available to assess the behavior of the diabetes [8]. In this report, we tried to assess the behavior of the diabetes in three patients with no history of diabetes, who had developed acute onset diabetes and DKA after SARS CoV-2 infection. We followed our patients till 14 weeks to assess the clinical course and behavior of the diabetes.
2. Methods
Demographic details, medical history, physical examination, laboratory investigations including glutamic acid decarboxylase-65 (GAD-65) antibodies, computed tomography (CT) imaging studies, treatment given, clinical course till 14 weeks and management outcomes were documented prospectively. Informed consent was obtained from all patients for the study. DKA was defined as plasma glucose >250 mg/dL, a positive test for urine or serum ketones, and arterial pH < 7.35 and/or serum HCO3 <18 mmol/L.
3. Results
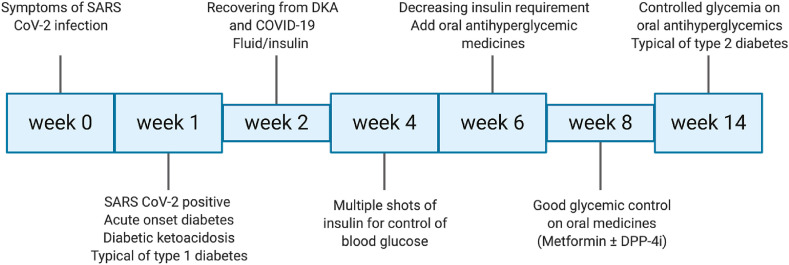
Our cases had no history of diabetes prior to the current illness. All the patients developed symptoms and signs suggestive of SARS CoV-2 infection. Investigations revealed COVID-19, based on RT PCR assay and CT chest imaging (Table 1 ). In the meantime, they developed acute onset diabetes and DKA, similar to patients with typical type 1 diabetes (Fig. 1 ). They were managed in a tertiary care facility, in the intensive care unit, with intravenous fluids, antibiotics and insulin. They responded well to the treatment. They were subsequently transitioned to three shots of rapid acting and one shot of long acting insulin. They achieved good glucose control. They were discharged from the hospital in the 3rd week, but multiple shots of insulin were continued as treatment for diabetes. During follow-up in the outpatient clinic, their requirement for exogenous insulin diminished and oral antihyperglycemic medicines were added (metformin 1000 mg, twice daily in one patient, and a combination of metformin 500 and sitagliptin 50 mg, twice daily in two patients). They continued to maintain good glycemic control on oral medicines, despite stoppage of insulin. At the last follow-up (around 14 weeks), all patients had controlled glycemia (fastings and post-prandials) as well as glycated hemoglobin (HbA1c) (Table 1). This easy control of diabetes with metformin or a combination with DPP-4 inhibitor is a characteristic feature of type 2 diabetes. Thus our patients’ diabetes began like type 1 diabetes and further clinical course resembled type 2 diabetes (Fig. 1).
Table 1.
Patient characteristics, laboratory variables and clinical course of our cases.
| Characteristics at admission | Case 1 | Case 2 | Case 3 | REFERENCE RANGE |
|---|---|---|---|---|
| Age (years) | 30 | 60 | 34 | – |
| Gender | M | M | M | – |
| BMI (kg/m2) | 28.6 | 26.2 | 27.3 | <23.0 |
| Duration of diabetes | Diagnosed at admission | Diagnosed at admission | Diagnosed at admission | – |
| HbA1c (%) | 9.6 | 12.6 | 12.0 | <5.7 |
| Plasma glucose (mg/dL) | 555 | 582 | 940 | <100 |
| pH | 7.07 | 7.30 | 7.21 | 7.25–7.35 |
| HCO3 (mmol/L) | 6.1 | 13 | 14 | 22–28 |
| Anion gap (mmol/L) | 11.9 | 16.2 | 17.2 | 12–18 |
| Lactate (mmol/L) | 1.22 | 1.13 | 0.82 | 0.5–1.5 |
| Urinary ketones | 4+ (>100 mg/dL) | 3+ (61–100 mg/dL) | 3+ (61–100 mg/dL) | Negative (<10 mg/dL) |
| CRP (mg/L) | 156.6 | 13.7 | 26.6 | 0–10 |
| IL-6 (pg/mL) | 60 | 12 | – | <6 |
| Ferritin (ng/mL) | 817.0 | 135.0 | 619.0 | 17.9–464.0 |
| D-dimer (mg/L) | 1.86 | 0.88 | 0.40 | 0.00–0.50 |
| Procalcitonin (ng/mL) | 1.04 | 0.04 | – | 0.00–0.05 |
| SARS CoV-2 RT-PCR assay | Positive | positive | positive | – |
| COVID-19 severity | Severe | Moderate | Mild | – |
| GAD 65, IgG, serum (IU/mL) | <5.00 | – | <5.0 | <10.0 |
| Anti-diabetic medicines at 14 weeks’ follow up | Metformin 500 mg plus sitagliptin 50 mg combination, twice daily | Metformin SR 500 BID, Sitagliptin 100 mg OD | Metformin 1000 mg BID | – |
| HbA1c at last follow-up (14 weeks) | 6.2 | 7.4 | 6.9 | <7.0 |
BMI, body mass index; HbA1c, glycated hemoglobin; CRP, C-reactive protein; GAD65, Glutamic Acid Decarboxylae-65.
Fig. 1.
Follow-up of patients with acute onset diabetes and diabetic ketoacidosis precipitated by SARS CoV-2 infection. At the onset, diabetes resembles type 1 diabetes, characterized by acute onset and DKA as presenting feature. At around 4–6 weeks, exogenous insulin requirement diminishes and glycemia can be controlled with oral-antihyperglycemic medicines (metformin ± dipeptidyl peptidase-4 inhibitor).
4. Discussion
We published a report of two cases who had developed DKA following SARS-CoV-2 infection [9]. Subsequently, we managed another patient who presented with DKA and had no history of diabetes. There is the possibility of COVID-19 either inducing new-onset diabetes or unmasking previously undiagnosed diabetes. Another issue is regarding the type of diabetes (type 1 vs. type 2 diabetes). Our patients probably had undiagnosed diabetes which was unmasked by COVID-19. This was indicated by elevated HbA1c at the time of admission in all the three patients. The details are given in Table 1.
In this report, we want to discuss the follow-up of these three patients, as it further clarified the picture of these patients as far as their type of diabetes is concerned. Initially, we thought that COVID-19 has induced type 1 diabetes in these patients, as acute onset and presentation with DKA are characteristic features of type 1 diabetes. However, when we followed these patients over time. We noticed that their requirement for exogenous insulin diminished significantly over 4–6 weeks. We were able to initiate oral antihyperglycemic medicines (metformin monotherapy or in combination with a dipeptidyl peptidase-4 inhibitor). All the three patients maintained good glycemic control with oral antihyperglycemic medicines till the last follow up (around 14 weeks). Maintenance of good glycemic control on metformin or a combination with DDP-4 inhibitor is a characteristic feature of typical new onset type 2 diabetes.
SARS-CoV-2 enters human cells via angiotensin-converting enzyme 2 (ACE2), which is also found in human pancreatic β-cells, suggesting that SARS-CoV-2 might alter pancreatic β-cell function and impair insulin secretion [[10], [11], [12]]. Furthermore, proinflammatory cytokines increase ACE2 expression in β-cells, thereby enhancing β-cell sensitivity to SARS-CoV-2 during inflammatory conditions [12]. In several recently published studies, patients were reported with DKA associated with COVID-19 [[1], [2], [3], [4], [5], [6], [7]]. In these studies, COVID-19 precipitated DKA in patients who were not previously known to have diabetes. We hypothesize that SARS CoV-2 causes direct cytotoxic injury to pancreatic β-cells, thereby leading to acute deficiency of insulin. This leads to acute onset of diabetes and precipitates DKA in these patients with otherwise type 2 diabetes. When the SARS CoV-2 infection abates, pancreatic β-cells recover over weeks and months, and exogenous insulin requirement goes down. The patients maintain glycemic control on oral antihyperglycemic medicines, as is norm in all patients with new onset or newly diagnosed type 2 diabetes. One report also described autoantibody-negative insulin dependent diabetes in a 19-year male following SARS-CoV-2 infection [8]. This was not an autoimmune-mediated type 1 diabetes, as all the five autoantibodies, usually seen in individuals with type 1 diabetes, were absent. Although authors tried to indicate that their patient had type 1 diabetes caused by direct cytotoxic injury to pancreatic β-cells by SARS-CoV-2 infection, however, only long-term follow up would clear the picture regarding type of diabetes [8].
5. Conclusions
COVID-19 can induce acute onset diabetes and precipitate DKA in some individuals with no prior history of diabetes. Acute onset of diabetes and DKA as presenting features in adults with previously no history of diabetes would indicate type 1 diabetes. However, these patients could be managed with oral antihyperglycemic medicines once DKA subsides and patients recuperate. Long term follow-up of these individuals is needed to further understand the type of diabetes.
Authors contribution
MSK had the idea, wrote the manuscript, revised and approved the manuscript. PKR was involved in patient care, collected data and approved the manuscript. SG was involved in patient care, collected data and approved the manuscript. AM was involved in patient care, collected data, approved the manuscript. SKM was involved in patient management, revised and approved the manuscript.
Declaration of competing interest
There are no conflicts of interest relevant to this article.
References
- 1.Goldman N., Fink D., Cai J., Lee Y.N., Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020;166:108291. doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaney A.I., Griffin G.D., Simon E.L. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med. 2020;S0735–6757(20) doi: 10.1016/j.ajem.2020.05.114. 30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metabol. 2020 doi: 10.1111/dom.14057. 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palermo N.E., Sadhu A.R., McDonnell M.E. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab. 2020;105(8):dgaa360. doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim N.Y., Ha E., Moon J.S., Lee Y.H., Choi E.Y. Acute hyperglycemic crises with coronavirus disease-19: case reports. Diabetes Metab J. 2020;44(2):349e53. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollstein T., Schulte D.M., Schulz J. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. 2020 doi: 10.1038/s42255-020-00281-8. [DOI] [PubMed] [Google Scholar]
- 9.Reddy P.K., Kuchay M.S., Mehta Y., Mishra S.K. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020;14(5):1459–1462. doi: 10.1016/j.dsx.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fignani D., Licata G., Brusco N. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 is expressed in human pancreatic islet β-cells and is upregulated by inflammatory stress. 2020. Preprint at bioRxiv. [DOI] [PMC free article] [PubMed]