Abstract
Background
Integrase strand-transfer inhibitors (INSTIs) have been associated with weight gain among women living with HIV (WLWH). We aimed to investigate the association between INSTIs and change in cardiometabolic risk indicators.
Setting
Retrospective cohort
Methods
Data from 2006–2017 were analyzed from WLWH enrolled in the longitudinal Women’s Interagency HIV Study who were virally-controlled on antiretroviral therapy (ART) for ≥5 consecutive semiannual visits. Women who switched/added an INSTI to ART (INSTI group) were compared to women who remained on non-INSTI ART (non-INSTI group). Outcomes included changes in fasting lipids and glucose; hemoglobin A1c (HbA1c); blood pressure (BP); and incident diabetes, hypertension, and insulin resistance. Outcomes were measured 6–12 months before and 6–18 months after INSTI switch/add in the INSTI group with comparable visits in the non-INSTI group. Longitudinal linear regression models compared change over time in each outcome by study group.
Results
1118 participants (234 INSTI, 884 non-INSTI) were followed for a median 2.0 (Q1 1.9, Q3 2.0) years. Participants were median age 49 years, 61% Black, and 73% overweight or obese (BMI ≥25kg/m2). Compared to non-INSTI, the INSTI group experienced greater increases in HbA1c (+0.05 vs. −0.06 mg/dL, p=0.0318), systolic BP (+3.84 vs. +0.84 mmHg, p=0.0191), and diastolic BP (+1.62 vs. −0.14 mmHg, p=0.0121), with greatest change in HbA1c among women on INSTIs with ≥5% weight gain.
Conclusions
INSTI use was associated with unfavorable changes in HbA1c and systolic and diastolic BP during short-term follow up. Further research is needed to understand long-term cardiometabolic effects of INSTI use.
Keywords: Human Immunodeficiency Virus, HIV; Integrase strand transfer inhibitors, INSTI; Antiretroviral therapy, ART; Diabetes Mellitus, DM; blood pressure, BP; hypertension, HTN
INTRODUCTION
Integrase strand-transfer inhibitors (INSTIs) are a highly effective class of ART that demonstrate a favorable profile in clinical trials1 and have revolutionized antiretroviral therapy (ART). High tolerability coupled with potency have led to guidelines recommending INSTIs as first-line agents for treatment-naïve persons living with HIV (PLWH).2,3 Unfortunately, recent studies have shown weight gain with INSTI use in both treatment-naïve4–6 and in treatment-experienced individuals,7–11 with some studies suggesting a greater effect in women.9,11
Despite increasing recognition of weight gain associated with INSTI use, most studies evaluating weight gain after INSTI use did not concurrently report cardiometabolic risk indices that may worsen with weight gain, such as glycemic control and lipid profiles. Previous INSTI clinical trial data have generally suggested more favorable lipid profiles with INSTI-containing regimens than other ART.12,13 However, recent case reports have raised concern about possible glycemic side effects associated with INSTI-based regimens.14–16 Unfortunately, much of these data about metabolic consequences with INSTI use are from clinical trials in treatment-naïve individuals or case reports. There are limited studies of the metabolic consequences of INSTI use among virally controlled PLWH who are switched to INSTI-containing ART, and in particular among women living with HIV (WLWH) who appear to have higher risk of INSTI-associated weight gain but are underrepresented in clinical studies.9,17,18
Our group recently demonstrated increased weight gain and body circumference measurements associated with INSTI use among virally-controlled WLWH enrolled in the Women’s Interagency HIV Study (WIHS).11 We therefore evaluated metabolic outcomes associated with INSTI use among participants from the WIHS by comparing changes in hemoglobin A1c (HbA1c), blood pressure (BP), fasting glucose (FG) and lipid profiles, and incident insulin resistance, hypertension, and diabetes among virally-controlled women who had switched to or added an INSTI to their ART regimen (INSTI group) to women remaining on ART without addition of an INSTI (non-INSTI group).
METHODS
Study Population
We utilized data from WLWH enrolled in the WIHS cohort,19,20 including participant visits occurring from 2006 to 2017 as recently described.11 In summary, WIHS is the largest longitudinal cohort study of WLWH and at-risk HIV-uninfected women in the United States. WIHS participants undergo study visits every 6 months with clinical and medication history assessed via standardized, interviewer-administered survey instruments, physical examinations, and specimen collection. WIHS participants were followed at 10 sites - Atlanta, GA; Birmingham, AL/Jackson, MS combined site; Bronx, NY; Brooklyn, NY; Chapel Hill, NC; Chicago, IL; Los Angeles, CA; Miami, FL; San Francisco, CA; and Washington, DC.
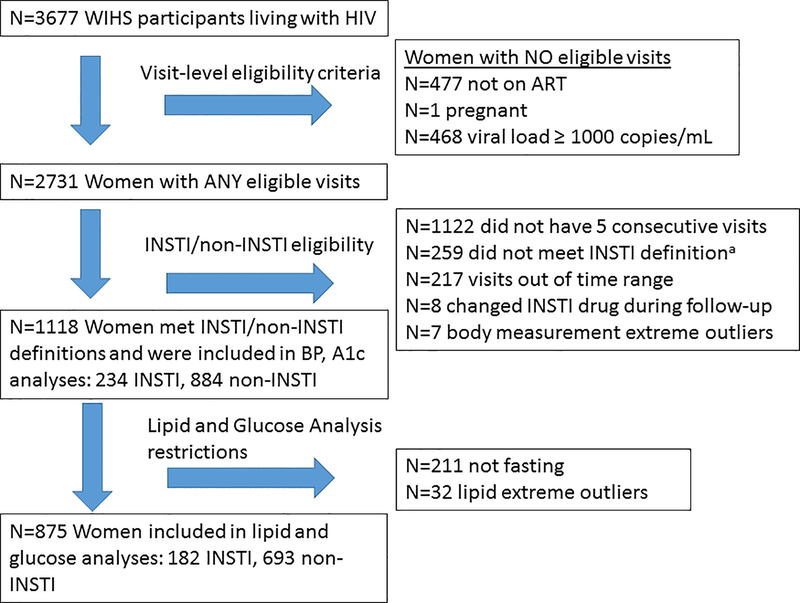
All WLWH who were enrolled in WIHS in 2006 were assessed for eligibility in this analysis (Figure 1). To avoid the known metabolic changes associated with ART initiation21,22 and achieving viral suppression,5 we elected to include only participants with an HIV RNA below 1000 copies/mL for the duration of follow-up; this allowed us to identify women who were likely to be adherent to ART while allowing for the occurrence of transient low-level viremia,23,24 similar to a previous clinical cohort analysis.10 Criteria for the INSTI group included: (1) history of switching from a non-INSTI ART regimen which could include a nucleoside reverse transcriptase inhibitor (NRTI) backbone plus either a non-NRTI (NNRTI), protease inhibitor (PI), or entry inhibitor (EI) to an INSTI, or adding an INSTI to an ART regimen; (2) five consecutive WIHS visits where participants were both taking ART by self-report and HIV RNA <1000 copies/mL; and (3) remaining on the same INSTI drug for at least two study visits after switching or adding an INSTI. The study visit in which INSTI use was first reported was considered the “switch/add” visit. The five consecutive study visits selected for the INSTI group were the “switch/add” visit, two visits preceding the “switch/add” visit (pre-visits, study visits 1 and 2), and two visits following the “switch/add” visit (post-visits, study visits 4 and 5). Criteria for the comparison (non-INSTI) group included: (1) five consecutive WIHS visits among participants who were both taking ART by self-report and had HIV RNA <1000 copies/mL; and (2) remained on an ART regimen that did not contain an INSTI for those five consecutive visits. Comparable visits for the non-INSTI group were anchored at a similar midpoint by calendar year as the INSTI group. Women who were ART-naïve were excluded from both groups. Additionally, visits from WLWH when pregnant were excluded from both groups due to the fluctuations in outcome variables associated with pregnancy. For lipid and blood glucose analyses, women were required to have a fasting blood draw at least one pre-visit and one post-visit. Lastly, women with one or more extreme post-visit values pertaining to the outcomes were removed from analysis (fasting glucose ≥350, HDL >150, triglycerides ≥500, pre-visit HbA1c ≥9) as such extreme values were rare, felt to be less indicative of ART effects, and resulted in poor model fit.
Figure 1. Study schema. The Women’s Interagency HIV Study (WIHS).
a INSTI definition: (1) history of switching from a non-INSTI ART regimen to an INSTI, or adding an INSTI to an ART regimen; (2) five consecutive WIHS visits where participants were both taking ART by self-report and HIV RNA was less than 1000 copies/mL; and (3) remain on the same INSTI drug for two study visits after switching or adding an INSTI
Body measurement data from seven participants were excluded because extreme changes in outcome variables suggested inaccurate data. Lipid data from 32 participants were excluded for extreme values if any of the following criteria were met: fasting glucose ≥350, HDL >150, triglycerides ≥500, pre-visit HbA1c ≥9.
The WIHS study protocol has been approved by Institutional Review Boards at all participating sites, and all study participants provided written informed consent for use of their data. This secondary data analysis was reviewed and approved by the WIHS Executive Committee, and de-identified data were used.
Data Collection
BP measurements were collected using the automated Dinamap monitor (Dinamap Procare Series, GE Medical Systems). Participants sat quietly for five minutes without talking before measurement. Three seated BP measurements from the participant’s right arm, with feet flat on the floor and back supported, were obtained at each visit and averaged. Weight (kilogram, kg) and BMI (calculated as weight/height2) were measured in a standard method by trained staff.11
Blood samples were collected at pre-specified visits (approximately annually) and were sent to Quest Diagnostics (Baltimore, MD) for analysis. Participants were instructed to be fasting for a minimum of 8 hours prior to the morning of their visit; fasting was determined by self-report. If the participant was fasting, total cholesterol, HDL, calculated LDL, TG, glucose, and insulin levels were obtained; HbA1c levels were obtained regardless of fasting status (but were not available from April 1, 2006-September 30, 2010). If values were available from more than one pre-visit or post-visit (~5% of women), the visit closest to the switch/add visit was used.
Statistical Analysis
Baseline demographic and clinical characteristics as well as baseline and incident metabolic outcomes for INSTI and non-INSTI were compared using chi-square or Fisher’s exact tests for categorical variables and two-sample t-tests or Wilcoxon rank sum tests for continuous variables.
Primary outcomes included HbA1c, systolic and diastolic BP, FG, total cholesterol, LDL, HDL, triglycerides. In order to incorporate the longitudinal nature of the data, separate longitudinal linear regression models assessed change over time for each outcome by study group (INSTI vs. non-INSTI). Models contained study visit (1,2,4,5), study group (INSTI, non-INSTI), a study visit*study group interaction term and included an AR(1) covariance structure to account for with-in person correlation in outcomes over time (study visit). In addition, models were adjusted for age, race, income, education, current smoking status, use of NNRTI (vs. other anchor ART drug) during any pre-visit, use of tenofovir disoproxyl fumarate (TDF, vs. other NRTI), and use of cholesterol-lowering medication. Pre-specified contrasts with α=0.05 compared change (defined as the average of the post-visits (4 and 5) minus the average of the pre-visits (1 and 2) by study group. Model-based estimates of mean change and confidence intervals by study group are reported. Model fit was assessed through residual plots. Hemoglobin A1c values excluded 4 INSTI and 10 non-INSTI participants with absolute mean change ≥ 2.5% due to poor model fit; as such, these extreme changes were descriptively summarized (Supplemental Digital Content Table 1). Primary outcomes were then evaluated using models stratified by clinically significant change in weight from baseline (<5% vs. ≥5%, as previously described).
Secondary outcomes were incident cases of diabetes mellitus, hypertension, and insulin resistance occurring after the “switch/add” visit. Those with these metabolic outcomes during a pre-visit were excluded from analysis. Diabetes mellitus was defined as a) self-reported anti-diabetic medication use or b) either (1) FG greater than 125 mg/dL or (2) HbA1c greater than 6.4%. Hypertension was defined as self-reported antihypertensive medication use or systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg. Insulin resistance was defined using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) method,25 with values ≥2 mg/dL indicating insulin resistance.26 Analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Demographic and Clinical Characteristics
There were 1118 WIHS participants included in this analysis, with 884 in the non-INSTI and 234 in the INSTI groups with a median 2.0 years (Q1 1.9, Q3 2.0) of follow-up. Baseline demographic and clinical characteristics are shown in Table 1. Women were median age 49 years (Q1 43, Q3 55), 61% were Black, 50% reported an annual income below $12,000, and 66% reported a high school education or less. Baseline HIV viral load was undetectable in 81%, detectable but below 200 copies/mL in 14%, and between 200 to 1,000 copies/mL in 5% of participants. Approximately 73% of participants had a baseline BMI ≥25 kg/m2 with a median body weight of 76 kg (Q1 64, Q3 92). Cholesterol-lowering medications were used by 21% of participants, 22% had diabetes mellitus, and 46% had hypertension at baseline.
Table 1.
Baseline demographics and clinical characteristics of study participants. The Women’s Interagency HIV Study (WIHS).
| Demographic | Overall (N=1118) | INSTI (N=234) | Non-INSTI (N=884) | p-value |
|---|---|---|---|---|
| Age, y, median (Q1, Q3) | 49 (43,55) | 50 (45,56) | 49 (43,55) | 0.0598 |
| Race, N (%) | 0.0863 | |||
| African-American | 680 (60.8) | 140 (59.8) | 540 (61.1) | |
| Hispanic | 266 (23.8) | 48 (20.5) | 218 (24.7) | |
| Other | 172 (15.4) | 46 (19.7) | 126 (14.3) | |
| Annual Income, N (%) | 0.4778 | |||
| ≤$12,000 | 555 (49.6) | 114 (48.7) | 441 (49.9) | |
| $12,001–24,000 | 254 (22.7) | 61 (26.1) | 193 (21.8) | |
| >$24,000 | 276 (24.7) | 56 (23.9) | 220 (24.9) | |
| Insurance Coverage, N (%) | 1079 (96.5) | 228 (97.4) | 851 (96.3) | 0.4058 |
| Education, N (%) | 0.1477 | |||
| High school or less | 741 (66.3) | 146 (62.4) | 595 (67.3) | |
| Beyond high school | 371 (33.2) | 87 (37.2) | 284 (32.1) | |
| Smoker, N (%) | 382 (34.2) | 68 (29.1) | 314 (35.85) | 0.0658 |
| Injection Drug Use, N (%) | 0.3995 | |||
| Never use | 888 (79.4) | 188 (80.3) | 700 (79.2) | |
| Current use | 2 (0.2) | 1 (0.4) | 1 (0.1) | |
| Former use | 220 (19.7) | 43 (18.4) | 177 (20.0) | |
| Non-Injection Drug Use, N (%) | 0.1045 | |||
| Never use | 333 (29.8) | 58 (24.8) | 275 (31.1) | |
| Current use | 205 (18.3) | 41 (17.5) | 164 (18.6) | |
| Former use | 571 (51.1) | 133 (56.8) | 438 (49.6) | |
| Baseline ART, N (%) | ||||
| NNRTI | 555 (49.6) | 83 (35.5) | 472 (53.4) | <0.0001 |
| EFV use | 345 (30.9) | 43 (18.4) | 302 (34.2) | <0.0001 |
| PI | 572 (51.2) | 161 (68.8) | 411 (46.5) | <0.0001 |
| NRTIa | ||||
| AZT | 104 (9.3) | 32 (13.7) | 72 (8.1) | 0.0096 |
| ABC | 215 (19.2) | 64 (27.4) | 151 (17.1) | 0.0004 |
| TDF | 864 (77.3) | 152 (65.0) | 712 (80.5) | <0.0001 |
| INSTI added, N (%) | ||||
| Raltegravir | 85 (7.6) | 85 (36.3) | ||
| Elvitegravir | 52 (4.7) | 52 (22.2) | ||
| Dolutegravir | 97 (8.7) | 97 (41.5) | ||
| CD4 count ≤200 cells/μL, N (%) | 40 (3.6) | 8 (3.4) | 32 (3.6) | 0.8806 |
| Viral load, N (%) | 0.0171 | |||
| Undetectableb | 910 (81.4) | 176 (75.2) | 734 (83.0) | |
| Detectable but <200 copies/mL | 158 (14.1) | 42 (18) | 116 (13.1) | |
| 200–1,000 copies/mL | 50 (4.5) | 16 (6.8) | 34 (3.8) | |
| Weight, kg, median (Q1, Q3) | 76 (64,92) | 74 (63,90) | 77 (64,93) | 0.3151 |
| BMI, kg/m2, median (Q1, Q3) | 29 (25,35) | 29 (25,35) | 29 (25,35) | 0.3407 |
| <18.5 kg/m2, N (%) | 20 (1.8) | 2 (0.9) | 18 (2.0) | 0.3490 |
| 18.5–24.9 kg/m2, N (%) | 258 (23.1) | 59 (25.2) | 199 (22.5) | |
| ≥25 kg/m2, N (%) | 813 (72.7) | 167 (71.4) | 646 (73.1) | |
| Diabetes Mellitus, N (%)c | 248 (22.2) | 57 (24.4) | 191 (21.6) | 0.3675 |
| Hypertension, N (%) | 511 (45.7) | 102 (43.6) | 409 (46.3) | 0.4648 |
| Cholesterol medication use, N (%) | 229 (20.5) | 68 (29.1) | 161 (18.2) | 0.0003 |
Abbreviations: INSTI, integrase strand transfer inhibitor; ART, antiretroviral therapy; y, years; NNRTI, non-nucleotide reverse transcriptase inhibitor; EFV, efavirenz; PI, protease inhibitor; NRTI, nucleotide reverse transcriptase inhibitor; AZT, zidovudine; ABC, abacavir; TDF, tenofovir disoproxil fumarate; BMI, body mass index.
Not mutually exclusive. Column percentages may not total 100 due to rounding.
Limit of detection for viral load assay was ≤ 80 copies/mL for 4% of visits, ≤48 for 14% of visits, and ≤20 for 83% of visits.
Diabetes mellitus was defined as self-reported anti-diabetic medication use or either (1) two FG greater than 125 mg/dL or (2) hemoglobin A1c greater than 6.4% and FG>125 mg/dL
At baseline, there were no significant differences between INSTI and non-INSTI groups regarding age, race, income, insurance status, education, smoking status, and drug use. Compared to the non-INSTI group at baseline, women in the INSTI group were more likely to have a detectable viral load and have reported cholesterol medication use. The baseline lipid profiles, HbA1C, and blood pressure measurements were similar between groups, though fasting glucose was slightly higher in the non-INSTI group (Table 2). Baseline diabetes and hypertension prevalence did not differ by group. When compared to the non-INSTI group at baseline, women in the INSTI group were more likely to be on a PI and abacavir and less likely to be on an NNRTI and TDF. Participants without fasting pre- and post-visits were excluded from analyses of fasting outcomes; non-fasting participants (N=52 and N=191 non-INSTI) were similar to fasting participants (N=182 INSTI and N=693 non-INSTI) in most baseline demographic and clinical characteristics, except non-fasting women were more likely to be Black, have education beyond high school, report former injection drug use, have diabetes at baseline, have a BMI < 25 kg/m2, and have a detectable (but <200 copies/mL) viral load (all p<0.05, data not shown).
Table 2.
Baseline and model-adjusted change over time in glycemic control and blood pressure in WLWH who switched to INSTI or remained on non-INSTI antiretroviral therapy.
| Metabolic Outcome | Baseline Mean (SD) INSTIa | Baseline Mean (SD) Non-INSTIa | Mean Change (95% CI) INSTIb | Mean Change (95% CI) Non-INSTIb | Difference Between Means, INSTI-Non-INSTI (95% CI)b | p-value b |
|---|---|---|---|---|---|---|
| Outcomes from full study population (N=234 INSTI, N=884 Non-INSTI) | ||||||
| Hemoglobin A1c, mg/dLc | 5.86 (0.86) | 5.89 (1.07) | +0.05 (−0.04, 0.13) | −0.06 (−0.10, −0.01) | +0.10 (0.01, 0.20) | 0.0318 |
| Systolic blood pressure, mmHg | 119.06 (14.22) | 120.84 (16.21) | +3.84 (1.61, 6.08) | +0.84 (−0.32, 2.00) | +3.00 (0.49, 5.51) | 0.0191 |
| Diastolic blood pressure, mmHg | 73.20 (8.19) | 74.19 (9.03) | +1.62 (0.39, 2.85) | −0.14 (−0.78, 0.49) | +1.76 (0.39, 3.14) | 0.0121 |
| Outcomes from fasting subset population (N=182 INSTI, N=693 Non-INSTI) d | ||||||
| Glucose, mg/dL | 90.61(17.21) | 94.10(22.79) | +4.44 (1.29, 7.59) | +2.71 (1.08, 4.33) | +1.73 (−1.79, 5.26) | 0.3349 |
| Total cholesterol, mg/dL | 183.03(36.13) | 181.38(36.53) | −2.64 (−7.32, 2.04) | +2.51 (0.11, 4.91) | −5.13 (−10.37, 0.06) | 0.0530 |
| LDL, mg/dL | 101.14(32.31) | 99.19(30.19) | −0.17 (−4.22, 3.89) | +2.19 (0.11, 4.27) | −2.36 (−6.88, 2.17) | 0.3072 |
| HDL, mg/dL | 55.41(17.71) | 57.19(19.33) | −2.71 (−4.71, −0.71) | +0.14 (−0.90, 1.17) | −2.85 (−5.08, −0.62) | 0.0125 |
| TG, mg/dL | 132.47(61.72) | 125.27(68.58) | −0.73 (−9.34, 7.88) | +1.21 (−3.19, 5.62) | −1.95 (−11.56, 7.66) | 0.6913 |
Abbreviations: WLWH, women living with HIV; INSTI, integrase strand transfer inhibitor; SD, standard deviation; CI, confidence interval; LDL, Low density lipoprotein; HDL, High density lipoprotein; TG, triglycerides
Baseline means are unadjusted using the first available value from pre visits included in the analysis
From longitudinal linear regression models for each outcome adjusting for study visit, study group, study visit*study group, age, race, site, income, education, current smoking status, use of non-nucleotide reverse transcriptase inhibitor (NNRTI) use during any pre-visit, use of tenofovir disoproxyl fumarate (TDF), and use of cholesterol medication, and incorporating within person correlation of the outcomes over time (study visit). Pre-specified contrasts compared change (defined as the average of the post-visits minus the average of the pre-visits in each outcome by study group.
Hemoglobin A1C values exclude 4 INSTI and 10 non-INSTI participants with mean change ≤ −2.5% (2 INSTI, 2 non-INSTI) and ≥+2.5% (2 INSTI, 8 non-INSTI) due to poor model fit with inclusion of extreme values.
Only participants with documented blood drawn after at least 8 hours of fasting are included.
Cardiometabolic Outcomes
The INSTI group experienced significantly greater increases in HbA1c (+0.05% vs. −0.06%, p=0.0318), systolic BP (+3.84 vs. +0.84 mmHg, p=0.0191), and diastolic BP (+1.62 vs. −0.14 mmHg, p=0.0121) compared to the non-INSTI group. Among those who were fasting, the INSTI group had greater decreases in HDL (−2.71 vs. +0.14 mg/dL, p=0.0125) during follow-up, without significant differences in TG, total cholesterol, glucose, or LDL (Table 2).
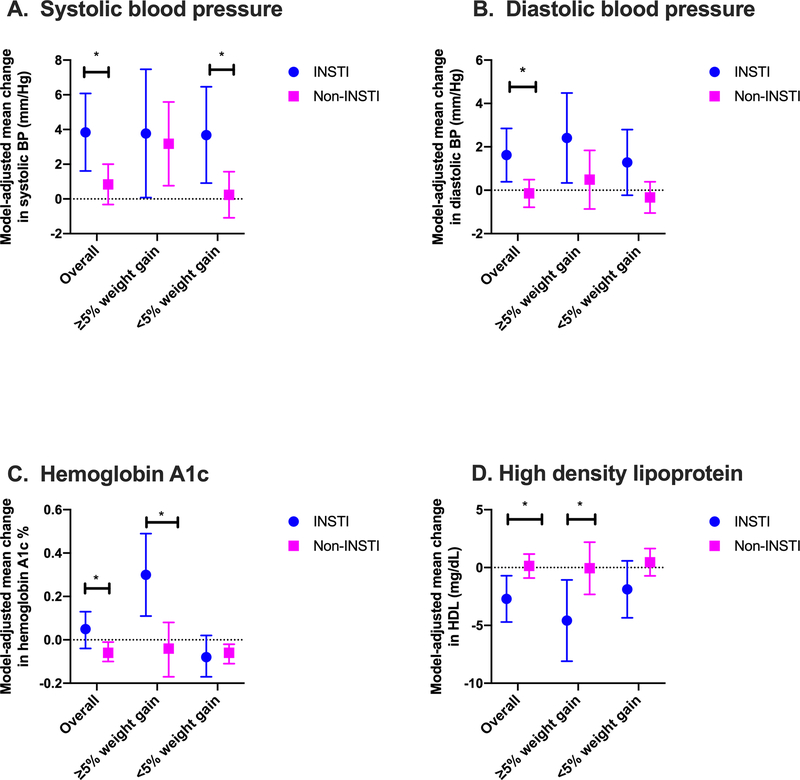
Overall, 77.1% (852) of non-fasting participants experienced <5% body weight gain over time, while 22.9% (253) participants experienced ≥5% body weight gain. When stratified by study group, there were 31.9% (74) non-fasting participants in the INSTI group with ≥5% weight gain and 20.5% (179) in the non-INSTI group. When stratified by percent change in body weight, participants with ≥5% body weight gain in the INSTI group experienced greater increases in HbA1c compared with the non-INSTI group (+0.30% vs. −0.04%, p=0.0034) (Figure 2). However, HbA1c did not differ significantly in participants with <5% body weight gain between INSTI and non-INSTI groups. Among participants with <5% body weight gain, the INSTI group had a greater increase in systolic BP compared to the non-INSTI group (+3.68 mmHg vs. +0.24 mmHg, p=0.0276). Participants with <5% body weight gain in the INSTI group had a greater increase in diastolic BP compared to the non-INSTI group (+1.28 mmHg vs. −0.33 mmHg, p=0.0582). Additionally, participants with ≥5% body weight gain the INSTI group experienced greater reductions in HDL compared to the non-INSTI group, (−4.58 vs. −0.06 mg/dL, p=0.0324) (Supplemental Digital Content Table 2).
Figure 2: Change in cardiometabolic indicators stratified by degree of weight gain.
INSTI use was associated with unfavorable changes in blood pressure (2A and 2B), hemoglobin A1c (2C), and high density lipoprotein (2D). For all except systolic BP, change was greater in women with ≥5% body weight gain. Statistical tests refer to difference between means (INSTI minus non-INSTI); * denotes p<0.05
Development of incident diabetes (5% (8 participants) in the INSTI group and 2% (15 participants) in the non-INSTI group) and hypertension (24% (32 participants) in the INSTI group and 20% (97 participants) in the non-INSTI group) did not differ between groups. However, among women who were fasting in the INSTI group (17%, 11 participants) had a lower observed incidence of insulin resistance (defined as HOMA score ≥2 among women with baseline HOMA score <2), than women in the non-INSTI group (31%, 86 participants), p=0.0514.
DISCUSSION
In this longitudinal study of virally-controlled WLWH, INSTI use was associated with a number of modest but statistically significant changes in cardiometabolic risk indicators over a relatively short time following addition or switch to INSTI. Greater increases in HbA1c and systolic and diastolic blood pressures were observed within a median follow-up of only 2 years, with HbA1c changes most notable among women who also experienced clinically significant (≥5%) body weight gain. In the context of the increasing concern about weight gain associated with INSTI use,4,7–10 these small but early signals of worsening cardiometabolic risk indicators indicate a critical need for long-term studies of the effects of INSTIs on these measures.
There is growing concern not only about weight gain associated with INSTI use, but also hyperglycemia and diabetes.14,16,27 In clinical trials, dolutegravir use was previously associated with grade 2 hyperglycemia (ranging from 126 to 250 mg/dL) in 6–9% of individuals.28 Our observations add to these findings by showing a small but significant increase in HbA1c among WLWH who switch to INSTIs over short-term follow-up. While insulin resistance often precedes the onset of diabetes,29 any instance of metabolic syndrome without insulin resistance has been found to increase risk for diabetes by three-fold.30 Although we did not observe a difference in incident diabetes mellitus between the two groups, this study adds to the growing body of literature that raises concern for glycemic complications associated with INSTI use, particularly among WLWH, and emphasizes the need for long-term follow-up. Additionally, the finding of greater increases in HbA1c with INSTI use but a lower incidence of insulin resistance observed in our analysis was unexpected, and the reason is not clear. Although it may be an artifact of sample size, this finding could suggest an alternative mechanism for hyperglycemia associated with INSTI use and warrants further investigation. Standardized analyses for not only weight gain but also BP, blood lipids, fasting glucose and insulin, and HbA1c should be included as part of future studies of INSTIs.31
A significant increase in both systolic and diastolic pressure was observed, though we did not observe significant differences in incident hypertension. Recent studies have emphasized the importance of targeting lower blood pressure goals to avoid cardiovascular disease events.32,33 Although the absolute increase in systolic and diastolic BP in our study was modest, there is evidence that even slight increases in systolic and diastolic BP can increase the risk for coronary heart disease,34 supporting the need for longer follow up of these measures. Evaluation of blood pressure, incident hypertension, and cardiovascular events should continue to be evaluated in future research studies.
The observed favorable lipid profiles seen with INSTI use in this analysis also supports previous data. Multiple previous reports have shown elevations in total cholesterol, LDL, and triglycerides with PIs,35–37 which were the predominant ART regimen used in the non-INSTI group. Additionally, the NEAT022 study group found improvement in blood lipid profiles, apart from HDL, among a predominantly male group (11% women) switching from a PI-based regimen to dolutegravir.38 Similar to our findings, the NEAT022 investigators found a decrease in HDL among individuals switching to a dolutegravir regimen. Interestingly, ADVANCE, a recent study of predominantly WLWH comparing dolutegravir and efavirenz, found no significant differences in lipid profiles, including HDL.18 Our work expands on these findings by showing consistent results in WLWH with viral suppression, even when controlling for cholesterol medication use.
This work has several limitations. Although we did not find an increased risk for incident diabetes or hypertension and the absolute changes in HbA1c and BP were small, this may be related to the relatively short follow-up time. A minimum follow-up time of 6–12 months after INSTI initiation was specifically chosen to isolate the effect of the INSTI and avoid the introduction of other confounding factors.39 However, longer exposure to INSTIs may be necessary for the development of incident outcomes, thus necessitating studies with extended follow-up. A recent study identified an association between weight gain and concurrent use of tenofovir alafenamide (TAF) with dolutegravir.18 Unfortunately, there were no women in our study on TAF, thus limiting our ability to comment on the possible relationship between weight gain, TAF, and INSTIs. Additionally, our analyses evaluated metabolic side effects related to use of INSTIs only among women. While recent reports have indicated that WLWH may be more vulnerable to these side effects,5 further research assessing sex differences is warranted. Finally, despite controlling for unbalanced variables in our multivariable regression, as a retrospective cohort study, our work is limited by the inability to control for unmeasured confounders and cannot establish causality.
Conclusion
In summary, virally-controlled WLWH who switched to or added an INSTI to their ART regimen were found to have modest unfavorable changes in HbA1c and blood pressure when compared to WLWH remaining on non-INSTI ART, during relatively short follow up. In addition, more pronounced HbA1c changes were observed in women with ≥5% body weight gain. As PLWH, particularly women, already have a higher risk of cardiovascular disease,40,41 additional studies with longer follow up are warranted to evaluate metabolic complications related to long-term INSTI use. Investigation of cardiometabolic outcomes in future studies of new INSTIs used for treatment and prevention is necessary.
Supplementary Material
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241-01; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201-01; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204-01; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202-01; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193-01; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245-01; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240-01; Connie Wofsy Women’s HIV Study, Northern California CRS (Bradley Aouizerat and Phyllis Tien), U01-HL146242-01; Los Angeles CRS (Roger Detels), U01-HL146333-01; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205-01; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203-01; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208-01; UAB-MS CRS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-HL146192-01; UNC CRS (Adaora Adimora), U01-HL146194-01. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (grant numbers UL1 TR002378, TL1 TR002382) to N.A.S; National Institute of Allergy and Infectious Diseases (K23 AI124913) to C.D.L.
Footnotes
Potential Conflicts of Interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Dow DE, Bartlett JA. Dolutegravir, the Second-Generation of Integrase Strand Transfer Inhibitors (INSTIs) for the Treatment of HIV. Infectious diseases and therapy. 2014;3(2):83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 17 December 2018. [Google Scholar]
- 3.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. Jama. 2018;320(4):379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgi K, Jenkins C, Rebeiro PF, et al. Greater Weight Gain Among Treatment-Naive Persons Starting Integrase Inhibitors. Presented at CROI: Conference on Retroviruses and Opportunistic Infections; 2019 Mar 4–7; Seattle, WA, USA. [Google Scholar]
- 5.Bakal DR, Coelho LE, Luz PM, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother. 2018;73(8):2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squires K, Kityo C, Hodder S, et al. Integrase inhibitor versus protease inhibitor based regimen for HIV-1 infected women (WAVES): a randomised, controlled, double-blind, phase 3 study. Lancet HIV. 2016;3(9):e410–e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palella FJ, Rayeed N, Li J, et al. Weight Gain Among Virally Suppressed Persons who Switch to InSTI-Based ART. Presented at CROI: Conference on Retroviruses and Opportunistic Infections; 2019 Mar 4–7; Seattle, WA, USA. [Google Scholar]
- 8.Lake JE, Wu K, Erlandson KM, et al. Risk Factors for Excess Weight Gain Following Switch to Integrase Inhibitor-Based ART. Presented at CROI: Conference on Retroviruses and Opportunistic Infections; 2019 Mar 4–7; Seattle, WA, USA. [Google Scholar]
- 9.Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS (London, England). 2017;31(10):1499–1500. [DOI] [PubMed] [Google Scholar]
- 10.Norwood J, Turner M, Bofill C, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerchberger AM, Sheth AN, Angert CD, et al. Weight Gain Associated with Integrase Stand Transfer Inhibitor Use in Women. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231. [DOI] [PubMed] [Google Scholar]
- 13.Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253–264. [DOI] [PubMed] [Google Scholar]
- 14.Fong PS, Flynn DM, Evans CD, Korthuis PT. Integrase strand transfer inhibitor-associated diabetes mellitus: A case report. Int J STD AIDS. 2017;28(6):626–628. [DOI] [PubMed] [Google Scholar]
- 15.Horikawa M, Toyoda M, Saito N, et al. Raltegravir-associated Diabetic Ketoacidosis in a Patient with HIV Infection: A Case Report. Tokai J Exp Clin Med. 2018;43(1):19–23. [PubMed] [Google Scholar]
- 16.McLaughlin M, Walsh S, Galvin S. Dolutegravir-induced hyperglycaemia in a patient living with HIV. J Antimicrob Chemother. 2018;73(1):258–260. [DOI] [PubMed] [Google Scholar]
- 17.Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, et al. Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N Engl J Med. 2019;381(9):816–826. [DOI] [PubMed] [Google Scholar]
- 18.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019;381(9):803–815. [DOI] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 20.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). International journal of epidemiology. 2018;47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrin M, Tate JP, Akgun KM, et al. Weight Gain and Incident Diabetes Among HIV-Infected Veterans Initiating Antiretroviral Therapy Compared With Uninfected Individuals. J Acquir Immune Defic Syndr. 2016;73(2):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(12):1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mira JA, Macias J, Nogales C, et al. Transient rebounds of low-level viraemia among HIV-infected patients under HAART are not associated with virological or immunological failure. Antivir Ther. 2002;7(4):251–256. [DOI] [PubMed] [Google Scholar]
- 24.Gallant JE. Making sense of blips. J Infect Dis. 2007;196(12):1729–1731. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 26.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC endocrine disorders. 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulgan T Factors Associated With Insulin Resistance in Adults With HIV Receiving Contemporary Antiretroviral Therapy: a Brief Update. Curr HIV/AIDS Rep. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolutegravir (Tivicay) [Package Insert]. Research Triangle Park, NC, USA: ViiV Healthcare, 2017. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tivicay/pdf/TIVICAY-PI-PIL.PDF. Accessed 4/2019. [Google Scholar]
- 29.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340(8825):925–929. [DOI] [PubMed] [Google Scholar]
- 30.Meigs JB, Rutter MK, Sullivan LM, Fox CS, D’Agostino RB Sr., Wilson PW. Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes care. 2007;30(5):1219–1225. [DOI] [PubMed] [Google Scholar]
- 31.Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? Journal of virus eradication. 2019;5(1):41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright JT Jr., Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano Y, Reis JP, Colangelo LA, et al. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline With Cardiovascular Events Later in Life. Jama. 2018;320(17):1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Archives of internal medicine. 1993;153(5):598–615. [DOI] [PubMed] [Google Scholar]
- 35.Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS (London, England). 1999;13(10):F63–70. [DOI] [PubMed] [Google Scholar]
- 36.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS (London, England). 1998;12(7):F51–58. [DOI] [PubMed] [Google Scholar]
- 37.Rhew DC, Bernal M, Aguilar D, Iloeje U, Goetz MB. Association between protease inhibitor use and increased cardiovascular risk in patients infected with human immunodeficiency virus: a systematic review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;37(7):959–972. [DOI] [PubMed] [Google Scholar]
- 38.Gatell JM, Assoumou L, Moyle G, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS (London, England). 2017;31(18):2503–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koethe JR, Jenkins CA, Lau B, et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS research and human retroviruses. 2016;32(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanna DB, Ramaswamy C, Kaplan RC, et al. Sex- and Poverty-Specific Patterns in Hiv-Associated Cardiovascular Disease Mortality in New York City, 2007–2017. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao SG, Galaviz KI, Gay HC, et al. Factors Associated With Excess Myocardial Infarction Risk in HIV-Infected Adults: A Systematic Review and Meta-analysis. J Acquir Immune Defic Syndr. 2019;81(2):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.