Abstract
We evaluated simple laboratory variables to discriminate COVID-19 from bacterial pneumonia or influenza and for the prospective grading of COVID-19. Multivariate logistic regression and receiver operating characteristic curve were used to estimate the diagnostic performance of the significant discriminating variables. A comparative analysis was performed with different severity. The leukocytosis (P = 0.017) and eosinopenia (P = 0.001) were discriminating variables between COVID-19 and bacterial pneumonia with area under the curve (AUC) of 0.778 and 0.825. Monocytosis (P = 0.003), the decreased lymphocyte-to-monocyte ratio (P < 0.001), and the increased neutrophil-to-lymphocyte ratio (NLR) (P = 0.028) were predictive of influenza with AUC of 0.723, 0.895, and 0.783, respectively. Serum amyloid protein, lactate dehydrogenase, CD3+ cells, and the fibrinogen degradation products had a good correlation with the severity of COVID-19 graded by age (≥50) and NLR (≥3.13). Simple laboratory variables are helpful for rapid diagnosis on admission and hierarchical management of COVID-19 patients.
Keywords: Coronavirus disease 2019 (COVID-19), Bacterial pneumonia, Influenza, Differential diagnosis, Prospective grading
Highlights
-
•
Laboratory features help differential diagnosis and the grade of COVID-19.
-
•
Leukocytosis and eosinopenia discriminate COVID-19 from bacterial pneumonia.
-
•
Monocytosis, NLR, and LMR distinguish COVID-19 from influenza.
-
•
Age, NLR, SAA, LDH, CD3+ cells, and FDP assist the stratification of COVID-19.
1. Introduction
As of May 17, 4,534,731 cases and 307,537 deaths have been reported in up to 200 countries, areas, or territories (WHO, 2020). SARS-CoV-2 is extremely contagious (Chen, 2020; Wu et al., 2020), but the time from the initial infection to the viral discharge is still not clear. However, the transmission may occur early in the course of SARS-CoV-2 infection before the onset of the illness (Rothe et al., 2020; Zou et al., 2020). The symptom-based screening, the radiography, and even the nucleic acid detection were insufficient to rule out COVID-19 (Winichakoon et al., 2020; Zou et al., 2020). Moreover, the nonspecific clinical manifestation in patients with COVID-19 has posed a differential diagnosis dilemma, particularly in the high-occurrence season of respiratory diseases (Bordi et al., 2020; Chen et al., 2020; Sevenhuysen et al., 2020). Misdiagnosing COVID-19 as other respiratory diseases may lead to serious public consequences, whereas misdiagnosing other respiratory diseases as COVID-19 may lead to unnecessary panic and infection control. Previously published studies have shown that certain hematological/biochemical changes (e.g., leukocytopenia, lymphopenia) may be helpful in the diagnosis of COVID-19, but the diagnostic performance of them in the differential diagnosis model was limited. Currently, the rapidly increasing number of confirmed cases made the medical system overloaded. An efficient model of triage is demanded to assist physicians or public health authorities to perform hierarchical management of the patients and improve the utilization of medical resources.
In this study, we aimed to identify commonly requested laboratory variables that either distinguish COVID-19 from bacterial pneumonia and influenza or assist the clinicians in the quick stratification of patients into different prognostic categories.
2. Methods
2.1. Patients and data collection
The laboratory data were collected at Renmin Hospital of Wuhan University, Wuhan, Hubei, China. We retrospectively reviewed the laboratory parameters of 48 patients with COVID-19 before they were confirmed by real-time polymerase chain reaction. To find out the hematological and biochemical characteristics of COVID-19 with different severity, the patients were divided into 4 grades according to previous studies with sufficient clinical data (Liu et al., 2020; Yang et al., 2020). Age < 50 years and neutrophil-to-lymphocyte ratio (NLR) < 3.13 were regarded as grade A (n = 26), and age < 50 years and NLR ≥ 3.13 were regarded as grade B (n = 6). Age ≥ 50 years and NLR < 3.13 were defined as grade C (n = 7), and age ≥ 50 years and NLR ≥ 3.13 were defined as D (n = 9). A total of 51 inpatients were included with the evidence of bacterial infection and pneumonia. We collected the data of 48 outpatients with influenza after they were identified by colloidal gold-based immunoassays for influenza virus A or B antibody. Fifty-one healthy individuals were from the routine check-up data. The details about the inclusion and exclusion criteria of the cases were in the supplementary material. The project was approved by the hospital's ethics committee (ZE2020-027-01) and was in accordance with the Helsinki Declaration as revised in 2013.
2.2. Statistical analysis
All the cutoff values were the upper limit or lower limit of the reference interval. For categorical data, we used Fisher's exact test or chi-square test to compare the proportions of patients with abnormal variables. We then performed a multivariate logistic regression analysis to determine the independent discriminating variables. Based on the logistic regression analysis results, the area under the curve (AUC) from the receiver operating characteristic curve (ROC) was calculated with MedCalc software to evaluate the diagnostic efficiency. For variable data in the comparative analysis, 1-way analysis of variance and the Kruskal–Wallis test were used to compare the average levels in different severity. The data are expressed as mean ± SD. The statistical analysis was performed using SPSS 19.0 with a 2-sided statistically significant P value < 0.05. Figures were drawn with GraphPad Prism 5.0 software.
3. Results
3.1. Demographics of patients with COVID-19, bacterial pneumonia, and influenza
The proportion of males and females was not significantly different in the 3 groups. COVID-19 and bacterial pneumonia tended to occur in patients aged 25–65 years old (P = 0.889), while influenza was prone to affect a younger population, particularly those <49 years old (Table 1 , P < 0.001).
Table 1.
The demographics of patients with COVID-19, bacterial pneumonia, or influenza.
| COVID-19 | Bacterial pneumonia | P valuea | Influenza | P valuea | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 23/48(47.92%) | 24/51(47.06%) | 0.932 | 29/48(60.42%) | 0.219 |
| Male | 25/48(52.08%) | 27/51(52.94%) | 19/48(39.58%) | ||
| Age, years | |||||
| Mean ± SD | 42.04 ± 15.43 | 44.88 ± 11.54 | 0.889 | 29.65 ± 11.67 | <0.001 |
| Age range | 4–75 | 20–75 | 13–64 | ||
| <25 | 2/48 (4.17%) | 1/51 (1.96%) | 20/48 (41.67%) | ||
| 25–49 | 30/48 (62.50%) | 30/51 (58.82%) | 24/48 (50.00%) | ||
| 50–65 | 11/48 (22.92%) | 18/51 (35.29%) | 4/48 (8.33%) | ||
| >65 | 5/48 (10.42%) | 2/51 (3.92%) | 0/48 (0.00%) |
Compared with COVID-19.
3.2. Hematological and biochemical features of patients with COVID-19, bacterial pneumonia, and influenza
The percentage of patients with abnormal laboratory parameters was shown in Table 2 . A markedly higher proportion of patients with influenza had lymphopenia, monocytosis, elevated NLR, and reduced lymphocyte-to-monocyte ratio (LMR). These significant changes in cell count in influenza patients were mainly caused by the changes in the percentage of the corresponding cells rather than the total leukocyte count. Patients with COVID-19 had leukopenia and eosinopenia, whereas patients with bacterial pneumonia had leukocytosis and neutrophilia. Intriguingly, the proportion of eosinopenia was more than 3 times in the 2 viral infection groups than in bacterial pneumonia (P < 0.001). We included 51 healthy individuals to obtain the normal ranges of NLR and LMR. The range of NLR and LMR in our study was 0.9–3.72 and 2.74–6.69, respectively. The proportion of increased NLR (>3.72) or decreased LMR (<6.69) in influenza was 72.9% or 100%, which was significantly higher than that in COVID-19 (both P < 0.001). The elevated serum amyloid protein (SAA) was more common in viral infection rather than bacterial infection.
Table 2.
Number of participants with abnormalities in the routine blood test and in the most used inflammatory markers, categorized by patients with COVID-19, bacterial pneumonia, or influenza.
| COVID-19 |
Bacterial pneumonia |
Influenza |
|||
|---|---|---|---|---|---|
| N/n (%) | N/n (%) | P valuea | N/n (%) | P valuea | |
| Leukocytosis (>9.5 × 109/L) |
3/48 (6.25%) |
12/46 (26.09%) |
0.011 | 3/48 (6.25%) |
1.000 |
| Leukopenia (<3.5 × 109/L) |
11/48 (22.92%) |
1/46 (2.17%) |
0.004 | 3/48 (6.25%) |
0.040 |
| Neutrophilia (>6.3 × 109/L) |
5/48 (10.42%) |
14/46 (30.43%) |
0.021 | 8/48 (16.67%) |
0.371 |
| Neutrophilia (%) (>75%) |
5/48 (10.42%) |
10/46 (21.74%) |
0.165 | 17/48 (35.42%) |
0.004 |
| Lymphopenia (<0.8 × 109/L) |
14/48 (29.17%) |
3/46 (6.52%) |
0.006 | 22/48 (45.83%) |
0.140 |
| Lymphopenia (%) (<20%) | 13/48 (27.08%) |
15/46 (32.61%) |
0.558 | 38/48 (79.17%) |
<0.001 |
| Monocytosis (>0.6 × 109/L) |
7/48 (14.58%) |
16/46 (34.78%) |
0.023 | 33/48 (68.75%) |
<0.001 |
| Monocytosis (%) (>10%) | 16/48 (33.33%) |
7/46 (15.23%) |
0.041 | 33/48 (68.75%) |
0.001 |
| Eosinopenia (<0.02 × 109/L) |
36/48 (75.00%) |
8/46 (17.39%) |
<0.001 | 29/48 (60.42%) |
0.127 |
| Eosinopenia (%) (<0.4%) |
36/48 (75.00%) |
9/46 (19.57%) |
<0.001 | 30/48 (62.50%) |
0.186 |
| NLR (>3.72) | 12/48 (25.00%) |
14/46 (30.43%) |
0.556 | 35/48 (72.92%) |
<0.001 |
| LMR (<2.74) | 20/48 (41.67%) |
16/46 (34.78%) |
0.530 | 48/48 (100%) |
<0.001 |
| CRP (>10 mg/L) |
26/48 (54.17%) | 27/42 (64.29%) |
0.330 | 12/15 (80.00%) | 0.129 |
| SAA (>10 mg/L) |
40/48 (83.33%) | 24/42 (57.14%) | 0.010 | 14/15 (93.33%) | 0.673 |
Compared with COVID-19.
3.3. Independent discriminating variables between COVID-19 and bacterial pneumonia or COVID-19 and influenza
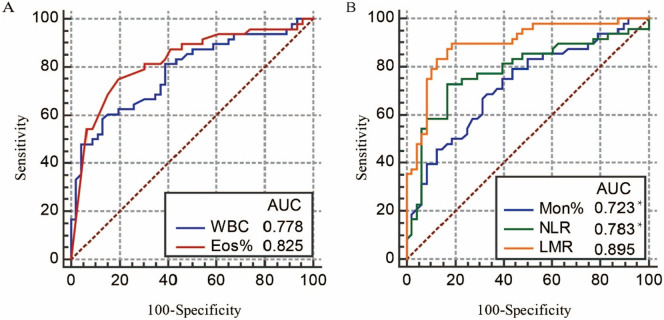
The significant laboratory variables above were included in the logistic regression analysis. To avoid multicollinearity or the effect of total leukocyte count, we included the percentages rather than the absolute counts of different white blood cells. For the comparison of bacterial pneumonia and COVID-19, leukocytes, monocytes, eosinophils, and SAA were entered into the logistic regression model (Table 3 ). Multivariate analysis showed that a leukocyte count >9.5 and eosinophil <0.4% were the only independent discriminating variables between bacterial pneumonia and COVID-19 with an AUC of 0.778 and 0.825, respectively (Fig. 1 ). For the differentiation of influenza and COVID-19, the 5 significant variables—leukocyte, neutrophil, monocyte, NLR, and LMR—were included. The lymphocyte was excluded after the collinearity analysis. Lastly, the independent discriminating variables between influenza and COVID-19 were monocyte >10%, NLR >2.72, and LMR <2.74 (Table 3). The odds ratio of LMR was 96.442. The LMR appeared to have the best discriminatory ability with an AUC of 0.895, which was significantly different from monocyte and NLR (P = 0.019 and 0.033, respectively) (Fig. 1). The performance of the significant variables was summarized in Table S1 (Supplementary material).
Table 3.
Multivariate predictors of bacterial pneumonia versus COVID-19 or influenza versus COVID-19.
| Characteristic | Odds ratio (95% CI) | P value | |
|---|---|---|---|
|
Bacterial pneumonia to COVID-19 |
WBC (×109/L) | ||
| 3.5–9.5 | Reference | ||
| <3.5 | 5.531 (0.569–53.756) | 0.140 | |
| >9.5 | 0.134 (0.026–0.699) | 0.017 | |
| Monocytes (%) | |||
| <10% | Reference | ||
| >10% | 1.838 (0.966–3.498) | 0.064 | |
| Eosinophils (%) | |||
| >0.4% | Reference | ||
| <0.4% | 6.654 (2.210–20.027) | 0.001 | |
| SAA (mg/L) | |||
| <10 | Reference | ||
| >10 | 2.798 (0.826–9.479) | 0.098 | |
|
Influenza to COVID-19 |
Age (years) | 2.255 (0.856–5.941) | 0.100 |
| WBC (×109/L) | |||
| 3.5–9.5 | Reference | ||
| <3.5 | 0.172 (0.010–3.099) | 0.233 | |
| >9.5 | 0.247 (0.008–7.260) | 0.418 | |
| Neutrophil (%) | |||
| <75% | Reference | ||
| >75% | 0.691 (0.207–2.311) | 0.549 | |
| Monocytes (%) | |||
| <10% | Reference | ||
| >10% | 0.147 (0.042–0.521) | 0.003 | |
| NLR | |||
| <3.72 | Reference | ||
| >3.72 | 0.141 (0.024–0.811) | 0.028 | |
| LMR | |||
| <2.74 | 96.442 (8.332–1116.248) | <0.001 | |
| >2.74 | Reference | ||
WBC = white blood cell; SAA = serum amyloid A; NLR = neutrophil-to-lymphocyte ratio; LMR = lymphocyte-to-monocyte ratio.
Fig. 1.
(A) The ROC curve and AUC of leukocytes and the percentage eosinophils in the differential diagnosis of bacterial pneumonia and COVID-19. (B) The ROC curve and AUC of the percentage of monocytes, NLR, and LMR in the differential diagnosis of influenza and COVID-19. WBC = white blood cell, leukocytes; Eos% = the percentage of eosinophils; Mon% = the percentage of monocytes; NLR = neutrophil-to-lymphocyte ratio; LMR = lymphocyte-to-monocyte ratio. Compared with LMR, * P < 0.05.
3.4. Laboratory characteristics of COVID-19 in different disease severity
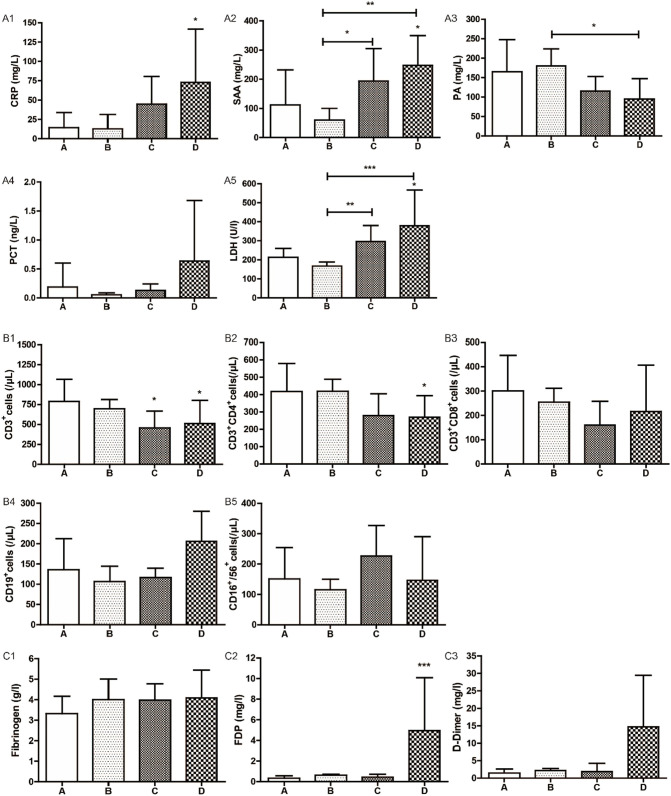
To find out the hematological and biochemical characteristics of COVID-19 with different severity, we divided the patients with COVID-19 into 4 grades, from mild to severe as grade A to grade D. The level of SAA, lactate dehydrogenase (LDH), and the absolute count of CD3+cells had a strong correlation with the disease severity (Fig. 2 ). Fibrinogen degradation products (FDPs) were significantly increased only in grade D. Although the upward trend of D-dimer was the same as FDPs, the difference was not significant (Fig. 2).
Fig. 2.
The average levels of various hematological and biochemical indicators in different severity of COVID-19. The inflammatory markers (A1–A5), the lymphocyte subsets (B1–B5), and the markers of fibrinolysis (C1–C3) in different grades of COVID-19. A, grade A, age < 50 years, NLR < 3.13; B, grade B, age < 50 years, NLR ≥ 3.13; C, grade C, age ≥ 50 years, NLR < 3.13; D, grade D, age ≥ 50 years, NLR ≥ 3.13. The average levels were displayed by mean and SD. *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
The COVID-19 has not been effectively contained so far. It is impracticable to quarantine all patients with fever of unknown etiology. Currently, the focus is on how to improve the suspicion for COVID-19 and rule out other infections with respiratory symptoms or undifferentiated fever. This study has identified several laboratory features on admission that clearly discriminate COVID-19 from bacterial pneumonia and influenza.
Multivariate analysis showed that leukocytosis combined with eosinopenia could simply discriminate COVID-19 from bacterial pneumonia. However, leukopenia and lymphopenia, though prevalent in COVID-19 patients in previous studies (Chen et al., 2020; Huang et al., 2020), have a lower incidence in our study and have little discriminatory abilities. Interestingly, three-quarters of the patients with COVID-19 on admission had eosinopenia. At present, however, there has been little discussion about the relationship between eosinopenia and COVID-19. Several studies displayed a significant correlation between eosinopenia and duration of hospitalization and mortality in patients with acute exacerbation of chronic obstructive pulmonary disease (Holland et al., 2010; Rahimi-Rad et al., 2015). The eosinopenia in COVID-19 may be due to the change of its distribution in acute stress as well as the direct destruction or the suppression of formation in bone marrow by SARS-CoV-2 (Spreng, 2000; Kim et al., 2013; Rahimi-Rad et al., 2015). It is indicated that the acute stress response is more severe in patients with SARS-CoV-2 infection.
Influenza posed a diagnostic dilemma in countries with concurrent outbreaks of COVID-19 (Bordi et al., 2020; Sevenhuysen et al., 2020) due to the nonspecific symptoms and analogical shedding pattern (Tsang et al., 2015; Zou et al., 2020). Three simple hematologic parameters contributed to a rapid distinction between COVID-19 and influenza: monocytosis, elevated NLR, and significantly reduced LMR. Lymphopenia and/or monocytosis had been reported to be present in 90% of patients with influenza, and LMR < 2 was a screening indicator for influenza infection (Merekoulias et al., 2010). In our study, only 29.17% of the new COVID-19 patients and 45.83% of the outpatients with influenza had lymphopenia, both of which were much lower than the corresponding rates in patients with these 2 diseases (Merekoulias et al., 2010; Chen et al., 2020; Huang et al., 2020; Wang et al., 2020). Previous studies showed that the excess recruitment of monocytes to the lungs led to increased immunopathology and mortality (Shi and Pamer, 2011). NLR displayed good predictive power for pneumonia (Cataudella et al., 2017; Nam et al., 2018; Paliogiannis et al., 2018). As it was reported, NLR was significantly higher in the noneosinophilic group (eosinophils<2%) in comparison to the eosinophilic group (eosinophils>2%) (Karakonstantis et al., 2018). However, the diagnostic performance of eosinophils in COVID-19 is still controversial (Li et al., 2020; Zhang et al., 2020). Therefore, elevated NLR, eosinopenia, and monocytosis may explain why patients with COVID-19-induced acute respiratory distress syndrome (ARDS) had lower severity of illness than H1N1-induced ARDS (Tang et al., 2020c).
The early risk stratification of COVID-19 is helpful for the hierarchical management of the patients. Recently, some studies showed that NLR was an independent risk factor for mortality in hospitalized patients with COVID-19 (Liu et al., 2020; Yang et al., 2020). According to the previous study (Liu et al., 2020), we divided the patients with COVID-19 into 4 grades based on age (≥50 years) and NLR (≥3.13) to seek for more laboratory parameters to assist the stratification. SAA, LDH, CD3+ T cells, and FDP levels were strongly associated with the severity of the disease, consistent with other reports (Chen et al., 2020; Huang et al., 2020; Tang et al., 2020a; Tang et al., 2020b). However, no significant differences were found between grade A and grade B. This result indicated that age played a more important role than NLR in the stratification of COVID-19.
There are several limitations to this study. The most important limitation is the possibility of misclassification bias (e.g., viral pneumonia misclassified as bacterial pneumonia) because of the low specificity of these simple laboratory parameters. Differentiating viral from bacterial pneumonia is difficult even with a variety of methods. Furthermore, the discriminative ability of these simple laboratory variables in mixed infection requires further evaluation. Finally, only outpatients with influenza were considered. Therefore, the conclusions may not be applicable to patients with more severe influenza disease.
5. Conclusions
This study provides a constellation of simple hematological and biochemical laboratory parameters for differential diagnosis and prospective grading of COVID-19. This simple, efficient model of triage may assist physicians or public health authorities with the hierarchical management of the patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Lin Song: Conceptualization, Resources, Investigation. En-Yu Liang: Methodology, Writing - original draft. Hong-Mei Wang: Validation, Writing - review & editing. Yan Shen: Formal analysis, Visualization. Chun-Min Kang: Writing - review & editing. Yu-Juan Xiong: Writing - review & editing. Min He: Validation, Writing - review & editing. Wen-Jin Fu: Writing - review & editing. Pei-Feng Ke: Supervision. Xian-Zhang Huang: Conceptualization, Supervision, Project administration.
CRediT authorship contribution statement
Lin Song: Conceptualization, Resources, Investigation. En-Yu Liang: Methodology, Writing - original draft. Hong-Mei Wang: Validation, Writing - review & editing. Yan Shen: Formal analysis, Visualization. Chun-Min Kang: Writing - review & editing. Yu-Juan Xiong: Writing - review & editing. Min He: Validation, Writing - review & editing. Wen-Jin Fu: Writing - review & editing. Pei-Feng Ke: Supervision. Xian-Zhang Huang: Conceptualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diagmicrobio.2020.115169.
Appendix A. Supplementary data
Supplementary material
References
- Bordi L., Nicastri E., Scorzolini L., Di Caro A., Capobianchi M.R., Castilletti C. Differential diagnosis of illness in patients under investigation for the novel coronavirus (SARS-CoV-2), Italy, February 2020. Euro Surveill. 2020;25(8) doi: 10.2807/1560-7917.ES.2020.25.8.2000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataudella E., Giraffa C.M., Di Marca S., Pulvirenti A., Alaimo S., Pisano M. Neutrophil-to-lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J Am Geriatr Soc. 2017;65(8):1796–1801. doi: 10.1111/jgs.14894. [DOI] [PubMed] [Google Scholar]
- Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M., Alkhalil M., Chandromouli S., Janjua A., Babores M. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology. 2010;15(1):165–167. doi: 10.1111/j.1440-1843.2009.01651.x. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakonstantis S., Kalemaki D., Tzagkarakis E., Lydakis C. Pitfalls in studies of eosinopenia and neutrophil-to-lymphocyte count ratio. Infect Dis (Lond) 2018;50(3):163–174. doi: 10.1080/23744235.2017.1388537. [DOI] [PubMed] [Google Scholar]
- Kim Y.H., Park H.B., Kim M.J., Kim H.S., Lee H.S., Han Y.K. Prognostic usefulness of eosinopenia in the pediatric intensive care unit. J Korean Med Sci. 2013;28(1):114–119. doi: 10.3346/jkms.2013.28.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ding X., Xia G., Chen H.G., Chen F., Geng Z. Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: a retrospective case–control study. EClinicalMedicine. 2020;100375 doi: 10.1016/j.eclinm.2020.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merekoulias G., Alexopoulos E.C., Belezos T., Panagiotopoulou E., Jelastopulu D.M. Lymphocyte to monocyte ratio as a screening tool for influenza. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K.W., Kim T.J., Lee J.S., Kwon H.M., Lee Y.S., Ko S.B. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49(8):1886–1892. doi: 10.1161/STROKEAHA.118.021228. [DOI] [PubMed] [Google Scholar]
- Paliogiannis P., Fois A.G., Sotgia S., Mangoni A.A., Zinellu E., Pirina P. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27(147) doi: 10.1183/16000617.0113-2017. pii: 170113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi-Rad M.H., Asgari B., Hosseinzadeh N., Eishi A. Eosinopenia as a marker of outcome in acute exacerbations of chronic obstructive pulmonary disease. Maedica (Buchar) 2015;10(1):10–13. [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenhuysen C., Lee L., Nwosu A., Smith T., Whitmore L., Bastien N. National influenza mid-season report, 2019–2020. Can Commun Dis Rep. 2020;46(5):25–28. doi: 10.14745/ccdr.v46i01a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng M. Possible health effects of noise induced cortisol increase. Noise Health. 2000;2(7):59–64. [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Du R., Wang R., Cao T., Guan L., Yang C. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. pii: S0012–3692(0020)30558–30554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T.K., Cowling B.J., Fang V.J., Chan K.H., Ip D.K., Leung G.M. Influenza A virus shedding and infectivity in households. J Infect Dis. 2015;212(9):1420–1428. doi: 10.1093/infdis/jiv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. pii: jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winichakoon P., Chaiwarith R., Liwsrisakun C., Salee P., Goonna A., Limsukon A. Negative nasopharyngeal and oropharyngeal swab does not rule out COVID-19. J Clin Microbiol. 2020;58(5) doi: 10.1128/JCM.00297-20. e00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Coronavirus (COVID-19) 2020. https://covid19.who.int/
- Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2020:1–7. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material