To the Editor
Dimethyl fumarate (DMF) is a commonly used therapy for relapsing remitting multiple sclerosis (RRMS). DMF primarily affects the adaptive immune system - T cells (CD8+ > CD4+ and memory > naïve) and B cells (memory > naïve) [1]–[3]. A recent study identified effects of DMF on the NK cell compartment noting an increased proportion of CD56bright NK cells [4]. CD56bright NK cells play an immunoregulatory role through cytokine production, as well as cytolysis of autoreactive T cells [5]. Multiple MS therapies modulate NK cells, and altering the NK cell compartment is thought to be a key mechanism of action of daclizumab [6],[7]. Whether DMF has direct effects on NK cell function is not known.
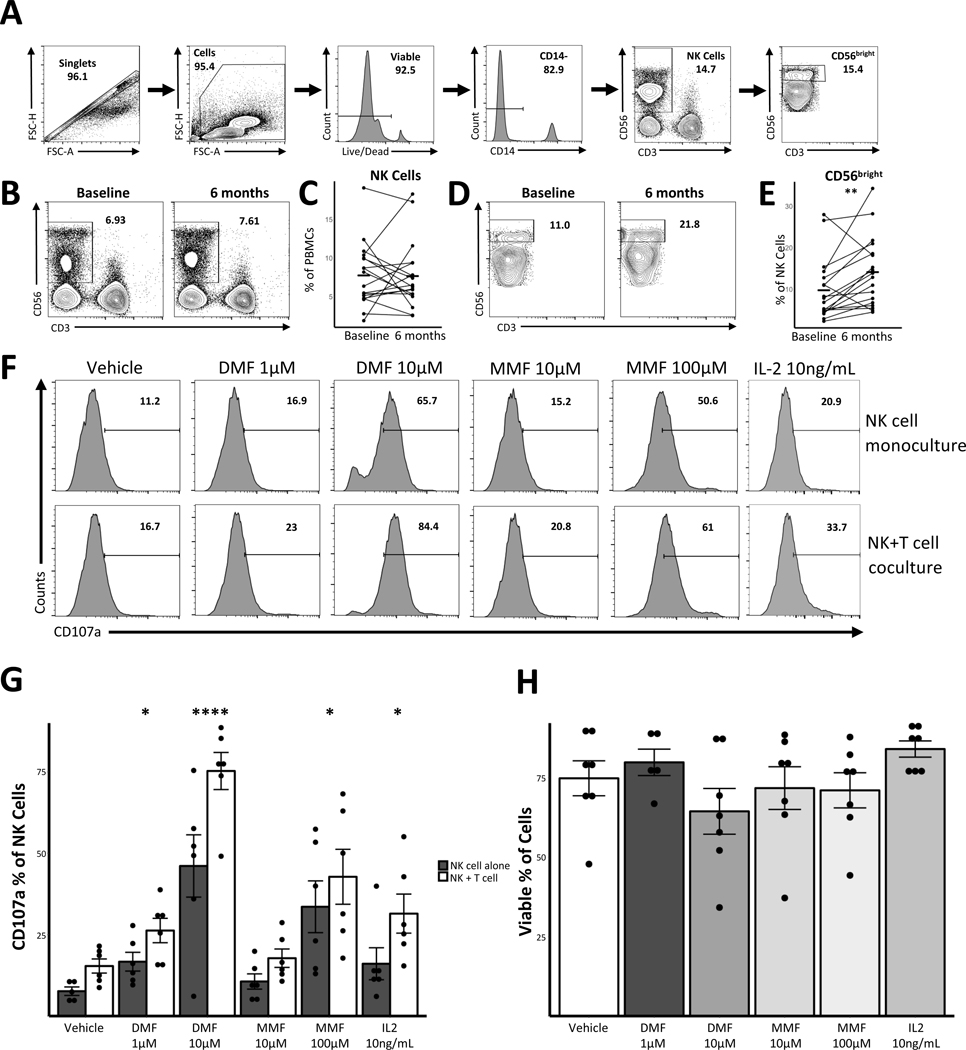
We recruited 18 RRMS patients initiating DMF and obtained peripheral blood mononuclear cells (PBMCs) at baseline and 6-months post-initiation. Cohort characteristics have been previously published [2]. We performed multiparametric flow-cytometry to identified immune cell subsets and noted no change in the NK cell population (Figure 1B, C) consistent with previous studies [1],[4]. However, DMF treatment led to a 44.5 % relative increase in the proportion of CD56bright NK cells (9.79 % vs 14.15 %, p=0.008; Figure 1D, E) confirming the findings of Medina et al. CD56bright NK cells can lyse autoreactive T cells by releasing perforin, granzyme-B and granzyme-K and potentially limit autoimmunity [5]. They are more likely to cross the blood brain barrier and are enriched in the CSF [8], highlighting the potential importance of changes noted with DMF treatment.
Figure 1. Dimethyl fumarate treatment leads to an increase in CD56bright NK cells and enhances NK cell function.
Cryopreserved PBMCs from 18 RRMS patients collected at baseline and 6-month time points were thawed and stained for viability and surface markers. NK cells were gated as shown in Panel A. NK cells as a percentage of viable PBMCs were compared between baseline and end of study (B, C). The CD56bright sub-set of NK cells as a percentage of NK cells was also quantified and compared between baseline and end of study (D, E). Statistical significance determined by Wilcoxon signed-rank test. We treated negatively selected NK cells from healthy controls (NK cell isolation kit, Miltenyi Biotec) with indicated doses of DMF, MMF, or IL-2 for 12 hours. NK cells were then washed and co-cultured for 24 hours at 1:5 E:T ratio with or without autologous labeled T cells. T cells were negatively isolated (pan-T cell isolation kit, Miltenyi Biotec) and activated for 3–5 days with anti CD3/CD28 beads per manufacturer’s instructions (DynaBeads, Life Technologies). CD107a antibody was present for entire duration of co-culture. Cells were collected and stained for viability (Live/Dead, Life Technologies) and surface markers (CD14 [BioLegend], CD56 & CD107a [Life Technologies], CD3 [Miltenyi Biotec]). (F) is representative plot showing change in CD107a expression on NK cells in mono or co-culture and in presence of DMF, MMF, or IL-2. (G) shows quantification of 7 individual donors from 3 independent co-culture experiments. Error bars show standard error of the mean. Reference group for comparisons in (G) was vehicle. (H) depicts quantification of viability data from various NK cell monoculture conditions (n=7). * p<0.05; ** p<0.01; **** p<0.0001.
We then examined the effect of both DMF and MMF on NK cell function. NK cells were isolated and treated with either DMF or MMF for 24 hours. Using CD107a expression to measure NK cell degranulation, we noted an increase in the proportion of degranulated NK cells following treatment with a range of DMF and MMF concentrations (Figure 1F, G). These changes occurred in the absence of dramatic effects on NK cell viability (Figure 1H). We next assessed whether DMF or MMF treatment led to increased NK cell cytotoxicity in NK-T cell co-culture [8]. We cultured NK cells (pre-treated with either DMF or MMF) with autologous activated T cells and noted increased NK cell degranulation with DMF and MMF treatment compared to controls (Figure 1F, G). There has been some controversy about the relative importance of DMF and its metabolite MMF in mediating biological effects of this medication, however we noted significant effects with both DMF and MMF at biologically relevant concentrations [9].
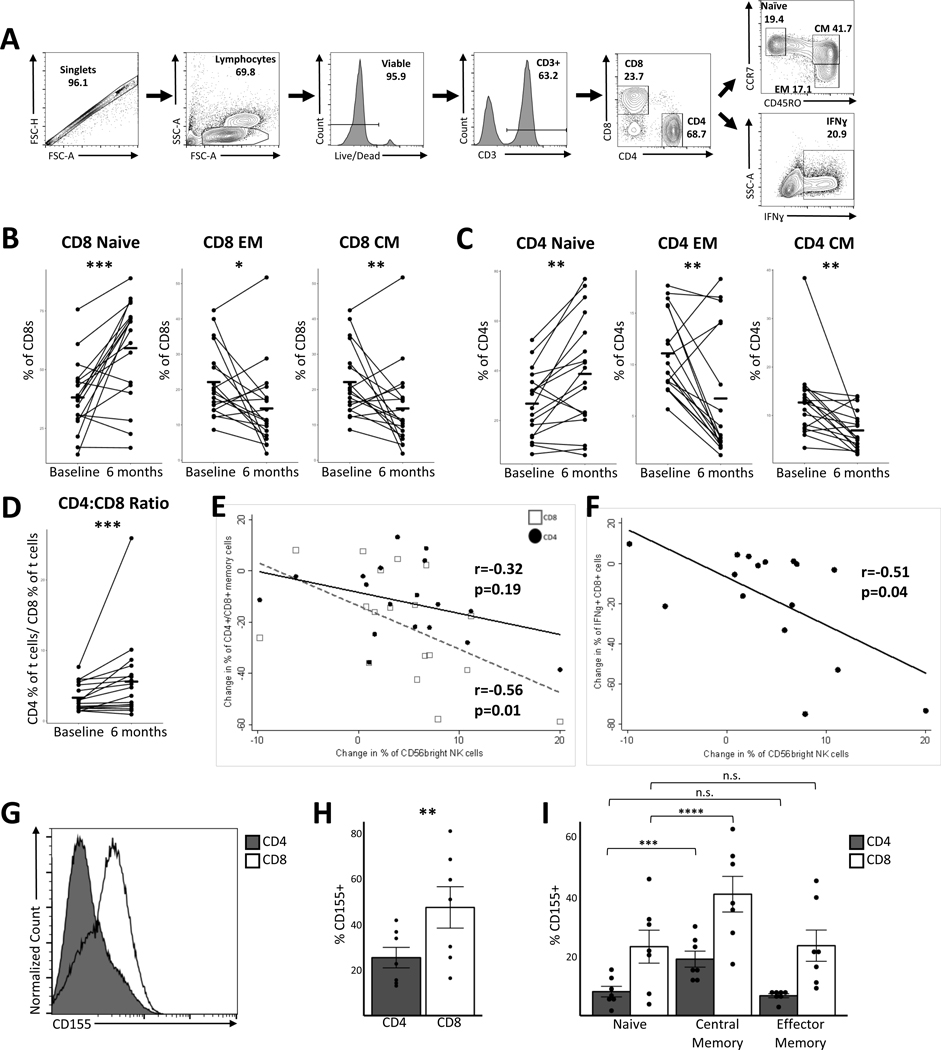
To assess whether the observed changes in the NK cell compartment might be related to previously described immunological effects of DMF, we examined relationships between the change in CD56bright NK cells and various T cell populations. As noted in prior studies, we identified preferential depletion of CD8+ T cells (increased CD4:CD8 ratio) and reductions in the central memory and effector memory subsets in both CD8+ and CD4+ T cells [1],[10] (Figure 2 B-D). We noted a significant correlation between the change in CD56bright NK cells and the reduction in CD8+ memory cells (r= −0.56, p =0.01; Figure 2E). There was a weaker correlation with the change in CD4+ memory cells (r=−0.32, p=0.19; Figure 2E). We also noted significant correlations between the reduction in proportions of CD8+ and CD4+ T cells producing pro-inflammatory cytokines - interferon-gamma (IFNγ) and tumor necrosis factor-alpha (TNFα) and the change in CD56bright NK cells (CD8+ IFNγ vs CD56bright: r=−0.51, p=0.04, Figure 2F; CD8+ TNFα vs CD56bright: r=−0.54, p=0.03; CD4+ IFNγ vs CD56bright: r=−0.48, p=0.06; CD4+ IFNγ vs CD56bright : r=−0.56, p=0.02). These data provide additional support for the hypothesis that increases in CD56bright NK cell population mediate some of the immunological effects of DMF.
Figure 2. Changes in the NK cell compartment correlate with changes in T cell subsets and higher CD155 expression is noted in T cell subsets preferentially affected by DMF.
PBMCs from both study timepoints were thawed and analyzed for T cell surface. We also activated thawed PBMCs for 5 days with anti CD3/CD28 beads (DynaBeads, Life Technologies) followed by stimulation with PMA/Ionomycin (10nM/1.3mM) in presence of Brefeldin-A/Monensin (10μM/2 μM) for 4 hours and performed intracellular staining for cytokine production. Gating strategy is depicted in (A). (B) and (C) show shifts in memory populations of CD8+ and CD4+ T cell before and after DMF treatment. (D) shows change in CD4+:CD8+ ratio before and after DMF treatment. (E) depicts correlation between change in CD4+ or CD8+ memory populations (CD45RO+) and change in CD56bright NK cells with DMF treatment. (F) depicts correlation between change in IFNγ producing CD8+ T cells and change in CD56bright NK cells with DMF treatment. Negatively selected T cells from healthy controls were activated for 3–4 days with anti CD3/CD28 beads (DynaBeads, Life Technologies) and staining was performed for CD4, CD8, CD45RO, CCR7, and CD155. (G) is a representative plot showing expression of CD155 on activated CD8+ T cells compared to CD4+. (H) is quantification of CD155 expression on CD4+ and CD8+ T cells [n = 7]. (I) shows CD155 expression on various memory subsets of both CD4+ and CD8+ T cells [n = 7]. Comparisons in (B), (C), and (D) are between baseline and end of study. In (H) comparison is between CD4+ and CD8+ T cells. In (I) comparison is between naive and central/effector memory for CD4+ and CD8+ sub-sets. Statistical significance measured using two-tailed paired student t-test. Correlations depicted in (E) and (F) were performed using a Spearman’s correlation coefficient [r]. Flow antibodies are from BioLegend (CD4, CD8, CCR7, IFNγ), eBioscience (CD45RO), and Miltenyi Biotec (CD3). * p<0.05; ** p <0.01; *** p<0.001.
The interaction between T cells and NK cells involves multiple surface molecules on both cells. To further understand the preferential targeting of CD8+ and memory T cells by DMF, we profiled the expression of CD155 (Polio virus receptor) on activated T cells. CD155 interacts with CD226 (DNAM-1) on NK cells and leads to increased cytolysis of T cells [3]. We found a greater proportion of CD155+ cells among CD8+ T cells compared to CD4+ T cells (Figure 2G, H). We also noted increased CD155+ expression in central memory T cells compared to naive cells in both CD4+ and CD8+ T cells (Figure 2I). A recent study demonstrated that CD155 expression on activated T cells in MS is important for cytolysis by NK cells[8]. Thus, the observed pattern of expression of CD155 in the T cell compartment is consistent with the preferential targeting of CD8+ T cells and memory cells noted with DMF treatment.
In summary, we demonstrate immunological effects of DMF treatment in MS patients on the NK cell compartment. DMF led to increased proportion of immunoregulatory CD56bright NK cells and this change was correlated with the reductions in CD8+ memory T cells and pro-inflammatory cytokine producing T cells. We also demonstrate that DMF and its metabolite MMF lead to increased NK cell degranulation and this change in NK cell phenotype and function may be part of the immunological mechanism of action of DMF in patients with MS.
ACKNOWLEDGMENTS
The authors would like to acknowledge Cindy Darius and Julie Fiol for assistance with phlebotomy, Jinyan Bo and David Buchanan for technical assistance. This study was funded by an Investigator-initiated trial grant from Biogen (US-BGT-15-10858) to PAC. PB was supported by a Career Transition Award from the NMSS, the John F. Kurtzke Clinician scientist development award from the AAN and a Junior Faculty Award from the Race to Erase MS
CONFLICT OF INTEREST
Mr. Smith has nothing to disclose. Dr. Bhargava has nothing to disclose. Dr. Calabresi reports grants from Medimmune, grants from Biogen, grants from Teva, grants from Novartis, personal fees from Vertex, personal fees from Biogen, outside the submitted work.
REFERENCES
- 1.Longbrake EE, et al. Mult Scler.2016;22:1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith MD, et al. Ann Clin Transl Neurol.2017;4:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross CC, et al. Neurol Neuroimmunol Neuroinflammation.2016;3:e183. [Google Scholar]
- 4.Medina S, et al. Mult Scler.2017:135245851771708. [Google Scholar]
- 5.Jiang W, et al. J Immunol.2011;187:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielekova B, et al. Proc Natl Acad Sci USA.2006;103:5941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saraste M, et al. Neurol Sci.2007;28:121–126. [DOI] [PubMed] [Google Scholar]
- 8.Gross CC, et al. Proc Natl Acad Sci USA.2016;113:E2973–82. [Google Scholar]
- 9.Litjens NH, et al. BMC Pharmacol.2004;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer CM, et al. Neurol Neuroimmunol Neuroinflammation.2015;2:e76. [Google Scholar]