Abstract
The coronavirus disease 2019 (COVID-19) pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has killed more than one million people as of October 1, 2020. Consequently, a search is on for a treatment that can bring the pandemic to an end. However, treatments (vaccine, antiviral, plasma) that are directed at specific viral proteins (RNA polymerase, spike proteins) may not work well against all strains of the virus. Therefore, it is hypothesized that a therapy based on multiple treatments is needed for COVID-19 patients and to bring the pandemic to an end. Here, it is proposed that a combination of cool air therapy (CAT) and purified air technology (PAT) in an oxygen species cool air respirator (OSCAR) could be used to reduce viral (SARS-CoV-2) load and severity of illness in COVID-19 patients through the individual dose–response relationship. In addition, the proposed therapy (CAT + PAT in OSCAR), which works by a more general physical and chemical mechanism, should work well with other treatments (vaccine, antiviral, plasma) that target specific viral proteins (RNA polymerase, spike proteins) to provide a safe and effective multiple therapy approach for ending the COVID-19 pandemic caused by SARS-CoV-2.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, Cool air therapy, Purified air technology, Oxygen species cool air respirator, Dose–response, Pandemic
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has killed over one million people as of October 1, 2020 [1], [2]. This virus is very contagious and can spread through the air, by close contact, in aerosol droplets, by the fecal-oral route, and by contact with contaminated surfaces [1], [3].
Although most cases of COVID-19 are asymptomatic or mild, a good proportion are severe resulting in hospitalization, intensive care (oxygen therapy on a ventilator), and death; especially, among those with comorbidities [2], [4]. Consequently, a search is on for a treatment that can protect the public from this highly contagious and deadly virus [5]. However, treatments (vaccine, antiviral, plasma) directed at specific viral proteins (RNA polymerase, spike proteins) may not be effective against all strains of SARS-CoV-2 [6], [7], [8], [9].
The hypothesis
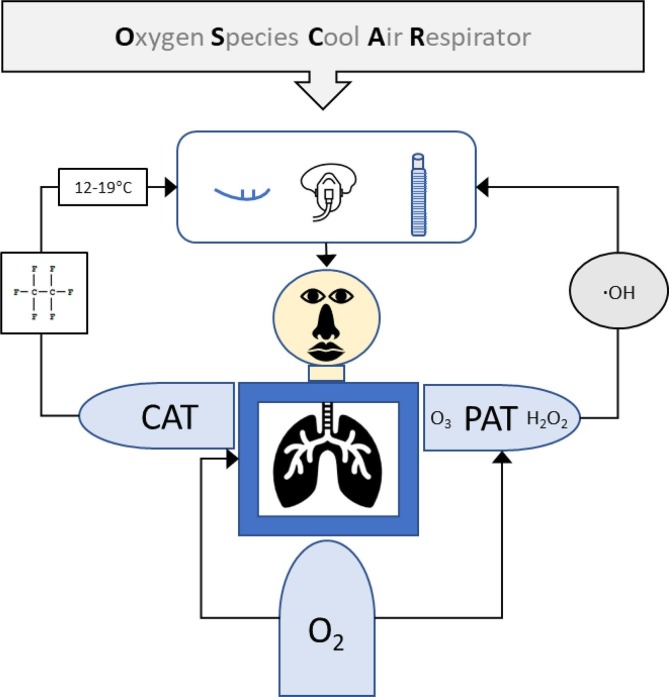
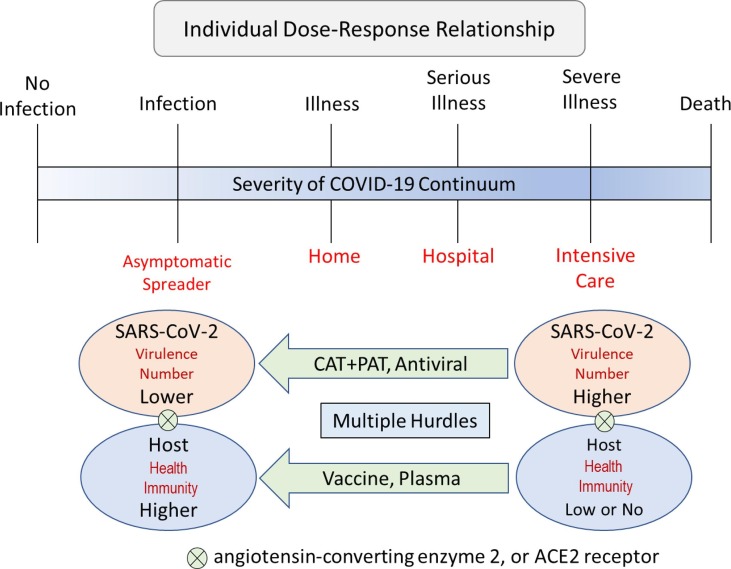
Therefore, it is hypothesized that a therapy based on multiple treatments is needed to end the COVID-19 pandemic caused by SARS-CoV-2. In the present study, it is proposed that a therapy based on a combination of cool air therapy (CAT) and purified air technology (PAT) in an oxygen species cool air respirator (OSCAR) (Fig. 1 ) is needed to reduce viral (SARS-CoV-2) load and severity of illness in COVID-19 patients through the individual dose–response relationship (Fig. 2 ).
Fig. 1.
Flow diagram of the proposed Oxygen Species Cool Air Respirator (OSCAR) for safe and effective treatment of COVID-19 patients using a combination (multiple therapy) of cool air therapy (CAT) and purified air technology (PAT) to reduce viral load and severity of illness caused by SARS-CoV-2. Abbreviations: COVID-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; C2F6 = perfluorocarbon coolant; O3 = ozone; H2O2 = hydrogen peroxide; .OH = hydroxyl radical; and O2 = oxygen.
Fig. 2.
A multiple therapy (hurdle) approach for reducing viral load and severity of COVID-19 illness caused by SARS-CoV-2 through the individual dose–response relationship. Abbreviations: COVID-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; CAT = cool air therapy; and PAT = purified air technology.
Cool Air Therapy (CAT)
Current evidence indicates that coronaviruses like SARS-CoV-2 bind to cellular receptors like angiotensin-converting enzyme 2 (ACE2) receptors [9], [10] and enter target cells by endocytosis [1], [5], [11], [12]. Interestingly, cool temperatures (12 to 19 °C) reduce membrane fluidity and block endocytosis of ligand-receptor complexes [13], [14] as well as virus-receptor complexes [15], [16], [17] by target cells. Thus, CAT may be an effective treatment for COVID-19 by blocking endocytosis and replication of SARS-CoV-2 by target cells (epithelial) in the respiratory tract; but is it safe?
In anesthetized rats on ventilators, lung function (resistance and elastance) is not different at respiratory tract temperatures of 13 °C (cool) and 37 °C (normal) indicating that CAT may be safe [18]. In fact, there is a commercial device (RhinoChill) that uses a nasal canula to deliver evaporative perfluorocarbon coolant to safely reduce respiratory tract and core body temperature of cardiac arrest and brain injury patients [19], [20], [21]. Thus, an existing CAT (RhinoChill) could be used to safely reduce surface temperature in the respiratory tract of COVID-19 patients to the extent needed to block endocytosis and replication of SARS-CoV-2 by target cells. A possible additional benefit of CAT may be a reduction in the cytokine storm seen is severe cases of COVID-19, which is associated with uptake and replication of SARS-CoV-2 in respiratory epithelial cells [9].
Purified Air Technology (PAT)
Over a decade ago, research was proposed (https://www.sbir.gov/sbirsearch/detail/82681 ) to evaluate efficacy and safety of a commercial PAT device (AirOcare) for reducing contagions (mold, bacteria, virus) in air and on contact surfaces in a hospital environment. The abstract for this proposed research is quoted here because it describes well the potential of this existing PAT (AirOcare) for treating Covid-19 patients and preventing community spread of SARS-CoV-2:
“By utilizing air purification methods used internationally in fruit preservation and food storage facilities, we propose to test AirOcare’s proprietary units to sterilize surgical and dental instruments; and, to sterilize entire rooms which could include operating rooms in the field during combat situations. As air circulates through the chamber, part of the oxygen is converted into ozone and to reactive oxygen species, including superoxide and hydrogen peroxide. The simultaneous production of superoxide anion and hydrogen peroxide is likely to produce hydroxyl radicals, one of the most powerful oxidants in nature. We hypothesize that oxidants produced in the chamber of the AirOcare system should irreversibly oxidize important biomolecules in spores, bacteria and virus particles with no effect to the environment because they have a relatively short half-life. In Phase I of this SBIR proposal we propose to design the chamber to the proper dimensions and design the system to maximize ozone and hydrogen peroxide formation. We will evaluate the potential of this new chamber for sterilization by exposing bacterial and viral particles for various periods of time and at various steady state concentrations of ozone and hydrogen peroxide. The sterilizing potential will be evaluated by determining inhibition in replication rate. BENEFITS: Validation of innovative, effective method for sterilizing surgical and dental instruments, as well as operating rooms.”
In a subsequent study [22], this PAT was found to reduce airborne bacteria in a meat processing room. Thus, an existing PAT (AirOcare) could be used to safely deliver reactive oxygen species to the inspired and expired air of COVID-19 patients to kill the virus (SARS-CoV-2) in vivo and ex vivo. It may also be an effective treatment in confined spaces (elevators, classrooms, buses) to reduce viral load and spread of SARS-CoV-2.
Mechanism of the hypothesis
Thus, it is hypothesized that CAT and PAT could be combined in an oxygen species cool air respirator (OSCAR) to deliver a multiple therapy treatment to block endocytosis and replication of SARS-CoV-2 in target cells of the respiratory tract, and kill SARS-CoV-2 in the inspired and expired air of COVID-19 patients (Fig. 1) resulting in reduced viral load and severity of illness through the individual dose–response relationship (Fig. 2), which is explained next.
Individual dose-response relationship
When a person is exposed to a contagion (bacteria, virus, mold), they either become ill or they do not (binary event) [23], [24], [25]. They become ill if the dose consumed is equal to or greater than the illness dose, which is the minimum dose of contagion needed to cause an illness (infection with symptoms of the disease) for that particular interaction between the contagion and the host. More specifically, the peak response of the person (host) to contagion exposure falls on a continuum from no infection to infection to illness to serious illness to severe illness to death (Fig. 2) [26], [27]. Where an individual peak response falls on this continuum depends on the final contagion load, which in turn depends on the post-exposure interaction between the contagion (number and virulence) and host (health ↔ immunity) [23], [28]. An additional and important consideration for SARS-CoV-2 is the complement of ACE2 receptors present on target cells of the host [9], [10]. Thus, even if the proposed therapy (Fig. 1) is only partly effective in reducing viral (SARS-CoV-2) load, it should have a clinical benefit by reducing severity of illness in COVID-19 patients through the individual dose–response relationship.
Testing the hypothesis
The hypothesis can be tested in an animal or human ex vivo lung perfusion model [29], [30] followed by validation in animal and human clinical trials. The ex vivo studies would identify safe and effective levels of CAT and PAT in OSCAR and how well they work with other treatments (vaccine, antiviral, plasma) before confirmation in animal and human clinical trials.
Basis of the hypothesis
The idea for blocking endocytosis of SARS-CoV-2 by target cells in the respiratory tract is based on the author’s prior research experience in endocrinology. Specifically, the author used cool temperatures (12 or 16℃) to block endocytosis of hormone (insulin or glucagon)-receptor complexes by target cells (rat or chicken adipocytes) so that he could determine the number and affinity of hormone receptors on the cell surface by 125I-hormone binding assays [13], [14].
The idea for killing SARS-CoV-2 by reactive oxygen species originated from the author’s prior knowledge of a research project that evaluated an existing PAT device (AirOcare) for its ability to reduce airborne bacterial contamination in a meat processing room [22].
The origin for the individual dose–response relationship component of the present hypothesis is the author’s prior and current research experience in quantitative microbial risk assessment and modeling of dose–response using human outbreak [31], [32] and of human feeding trial data [26], [27], [33], [34] for Salmonella [23], [35], [36], [37].
The multiple therapy part of the current hypothesis is based on a common practice in the author’s current field of research (food safety) where multiple antimicrobial agents (organic acids, oxidants) and inactivation methods (heat, irradiation) are used in sequence or together to reduce or eliminate pathogens in food [38], [39], [40], [41]. By using multiple hurdles, lower levels of each treatment can be used to achieve the same efficacy while reducing risk of unwanted side effects, such as decreases in food quality.
The same principle (multiple hurdle) should work with COVID-19. In fact, there is no vaccine or single treatment that works against viruses that cause SARS, MERS, Hepatitis C, AIDS, and the common cold. Thus, it seems prudent to try a multiple therapy approach against the virus (SARS-CoV-2) that causes COVID-19.
Conclusions
There is emerging evidence of strain variation for SARS-CoV-2 [6], [7], [8], which could complicate development, effectiveness, and longevity of treatments (vaccine, antiviral, plasma) for COVID-19 that are aimed at specific viral proteins (RNA polymerase, spike proteins) [9]. However, strain variation will have less effect on efficacy of the proposed treatment (CAT + PAT in OSCAR) because it is a physical and chemical method with a more general mechanism of action. In addition, the proposed treatment (CAT + PAT in OSCAR) should work well with the other treatments (vaccine, antiviral, plasma) under development resulting in a safe and effective multiple therapy approach for treatment of COVID-19 illness caused by SARS-CoV-2.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Mention of trade names or commercial products is solely for providing specific information and does not imply recommendation or endorsement by the U. S. Department of Agriculture (USDA), which is an equal opportunity provider and employer. The ideas expressed in this paper are those of the author and do not represent an official position of USDA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110353.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Vella F., Senia P., Ceccarelli M. Transmission mode associated with coronavirus disease 2019: a review. Eur Rev Med Pharmacol Sci. 2020;24:7889–7904. doi: 10.26355/eurrev_202007_22296. [DOI] [PubMed] [Google Scholar]
- 2.Abduljalil J.M., Abduljalil B.M. Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbes New Infect. 2020;35:100672. doi: 10.1016/j.nmni.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaper R. Transmission of SARS-CoV-2 through the air. Curr Med Res Pract. 2020;10:196–197. doi: 10.1016/j.cmrp.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang N., Shen H.M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachetti M., Marini B., Benedetti F. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18:179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C., Liu Z., Chen Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol. 2020;92:667–674. doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81 doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B, Huang S, Yin L, The cytokine storm and COVID-19, J Med Virol doi (2020), p. 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed]
- 10.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunes-Correia I., Ramalho-Santos J., Nir S., Pedroso de Lima M.C. Interactions of influenza virus with cultured cells: detailed kinetic modeling of binding and endocytosis. Biochem. 1999;38:1095–1101. doi: 10.1021/bi9812524. [DOI] [PubMed] [Google Scholar]
- 12.Owczarek K, Szczepanski A, Milewska A, et al. Early events during human coronavirus OC43 entry to the cell, Sci Rep 8 (2018), p. 7124. [DOI] [PMC free article] [PubMed]
- 13.Oscar T.P. Glucagon binding to receptors on the surface of chicken adipocytes. J Anim Sci. 1995;73:728–737. doi: 10.2527/1995.733728x. [DOI] [PubMed] [Google Scholar]
- 14.Oscar T.P. Down-Regulation of Glucagon Receptors on the Surface of Broiler Adipocytes. Poult Sci. 1996;75(8):1027–1034. doi: 10.3382/ps.0751027. [DOI] [PubMed] [Google Scholar]
- 15.Grove J., Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunes-Correia I., Nir S., Pedroso de Lima M.C. Kinetics of influenza virus fusion with the endosomal and plasma membranes of cultured cells. Effect of temperature. J Membr Biol. 2003;195:21–26. doi: 10.1007/s00232-003-2040-x. [DOI] [PubMed] [Google Scholar]
- 17.Wang G., Hernandez R., Weninger K., Brown D.T. Infection of cells by Sindbis virus at low temperature. Virol. 2007;362:461–467. doi: 10.1016/j.virol.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Arantes-Costa F.M., Zoriki S., Santos M.H., Kobata C.H., Vieira J.E., Martins M.A. Effects of ventilation, humidity and temperature on airway responsiveness to methacholine in rats. Eur Respir J. 2002;19:1008–1014. doi: 10.1183/09031936.02.00232402. [DOI] [PubMed] [Google Scholar]
- 19.Abou-Chebl A., Sung G., Barbut D., Torbey M. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke. 2011;42:2164–2169. doi: 10.1161/STROKEAHA.110.613000. [DOI] [PubMed] [Google Scholar]
- 20.Grave M.S., Sterz F., Nurnberger A. Safety and feasibility of the RhinoChill immediate transnasal evaporative cooling device during out-of-hospital cardiopulmonary resuscitation: a single-center, observational study. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busch H.J., Eichwede F., Fodisch M. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation. 2010;81:943–949. doi: 10.1016/j.resuscitation.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Patel J.R. Evaluation of reactive oxygen species generating AirOcare system for reducing airborne microbial populations in a meat processing plant. Sens. Instrumen. Food Qual. 2009;3(1):57–61. [Google Scholar]
- 23.T.P. Oscar, Dose-response model for 13 strains of Salmonella, Risk Anal 24 (2004), pp. 41-49. [DOI] [PubMed]
- 24.Oscar T.P. The development of a risk assessment model for use in the poultry industry. J Food Safety. 1998;18(4):371–381. [Google Scholar]
- 25.Oscar T.P. A quantitative risk assessment model for Salmonella and whole chickens. Int J Food Microbiol. 2004;93(2):231–247. doi: 10.1016/j.ijfoodmicro.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 26.McCullough N.B., Eisele C.W. Experimental human salmonellosis. I. Pathogenicity of strains of Salmonella meleagridis and Salmonella anatum obtained from spray-dried whole egg. J Infect Dis. 1951;88:278–289. doi: 10.1093/infdis/88.3.278. [DOI] [PubMed] [Google Scholar]
- 27.McCullough N.B., Eisele C.W. Experimental human salmonellosis. III. Pathogenicity of strains of Salmonella newport, Salmonella derby, and Salmonella bareilly obtained from spray-dried whole egg. J Infect Dis. 1951;89:209–213. doi: 10.1093/infdis/89.3.209. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T., Bartrand T.A., Weir M.H., Omura T., Haas C.N. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010;30:1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spratt J.R., Mattison L.M., Iaizzo P.A. Lung transplant after prolonged ex vivo lung perfusion: predictors of allograft function in swine. Transpl Int. 2018;31:1405–1417. doi: 10.1111/tri.13315. [DOI] [PubMed] [Google Scholar]
- 30.Loor G. EVLP: ready for prime time? Semin Thorac Cardiovasc Surg. 2019;31(1):1–6. doi: 10.1053/j.semtcvs.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Teunis P.F., Kasuga F., Fazil A., Ogden I.D., Rotariu O., Strachan N.J. Dose-response modeling of Salmonella using outbreak data. Int J Food Microbiol. 2010;144:243–249. doi: 10.1016/j.ijfoodmicro.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Bollaerts K., Aerts M., Faes C., Grijspeerdt K., Dewulf J., Mintiens K. Human salmonellosis: estimation of dose-illness from outbreak data. Risk Anal. 2008;28:427–440. doi: 10.1111/j.1539-6924.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 33.McCullough N.B., Eisele C.W. Experimental human salmonellosis: II. Immunity studies following experimental illness with Salmonella meleagridis and Salmonella anatum. J Immunol. 1951;66:595–608. [PubMed] [Google Scholar]
- 34.McCullough N.B., Eisele C.W. Experimental human salmonellosis. IV. Pathogenicity of strains of Salmonella pullorum obtained from spray-dried whole egg. J Infect Dis. 1951;89:259–265. doi: 10.1093/infdis/89.3.259. [DOI] [PubMed] [Google Scholar]
- 35.Oscar TP, Salmonella prevalence alone is not a good indicator of poultry food safety, Risk Anal, doi (2020), p. 10.1111/risa.13563. [DOI] [PubMed]
- 36.Oscar TP, Process risk model for Salmonella and ground chicken, J Appl Microbiol, 127 (2019), pp. 1236-1245. [DOI] [PubMed]
- 37.Oscar TP, Risk of salmonellosis from chicken parts prepared from whole chickens sold in flow pack wrappers and subjected to temperature abuse, J Food Prot, 80 (2017), pp. 1496-1505. [DOI] [PubMed]
- 38.McMeekin T.A. Quantifying the hurdle concept by modeling the bacterial growth/no growth interface. Int J Food Microbiol. 2000;55:93–98. doi: 10.1016/s0168-1605(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 39.Nyhan L., Begley M., Mutel A., Qu Y., Johnson N., Callanan M. Predicting the combinatorial effects of water activity, pH and organic acids on Listeria growth in media and complex food matrices. Food Microbiol. 2018;74:75–85. doi: 10.1016/j.fm.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Bucher O., Farrar A.M., Totton S.C. A systematic review-meta-analysis of chilling interventions and a meta-regression of various processing interventions for Salmonella contamination of chicken. Prev Vet Med. 2012;103:1–15. doi: 10.1016/j.prevetmed.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Thames HT, Sukumaran AT, A review of Salmonella and Campylobacter in broiler meat: emerging challenges and food safety measures Foods 9 (2020), p. doi: 10.3390/foods9060776. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.