Highlights
-
•
The root causes of the SARS-CoV-2 pandemic are disputed.
-
•
Some authors claim consumption of bushmeat was responsible.
-
•
Others argue that environmental encroachment and degradation were important.
-
•
These differences stem in part from individualist versus ecological conceptual frameworks.
Keywords: COVID-19, SARS-CoV-2, Zoonoses, Ecological, Eco-social, Epistemology
Abstract
An accurate understanding of why zoonoses such as SARS-CoV-2 are emerging at an increased rate, is vital to prevent future pandemics from the approximately 700,000 viruses with zoonotic potential. Certain authors have argued that the consumption of wildlife, or human contact with bats was responsible for the emergence of SARS-CoV-2. Others argue that a range of anthropogenic environmental degradations have played a vital role in the emergence of SARS-CoV-2 and other zoonoses. In this opinion piece, I argue that these divergent viewpoints stem, in part, from different foundational conceptual frameworks – biomedical individualist and eco-social frameworks, respectively. Based on the fact that the eco-social framework provides a more complete account of the different types of causal factors underpinning the emergence of zoonoses, I propose that the COVID-19 pandemic provides an additional reason for the health sciences to ground its theory of health and disease in an eco-social conceptual framework.
1. Introduction
The rate of emergence of zoonoses, such as SARS-CoV-2, has been increasing over the past 40 years (Jones et al., 2008; Everard et al., 2020; Jones et al., 2013; Smith et al., 2014). What was responsible for this increase in general and the emergence of SARS-CoV-2 in particular? It is crucial that the health sector answers this question correctly. In this opinion piece, I contrast two answers to this question and suggest that our underlying conceptual frameworks of disease influence the answer we choose. One answer is that specific circumstances explain each outbreak. The emergence of SARS-CoV-2, for example, was due to the consumption of wildlife, or the placement of multiple species in close species in proximity (such as the Wuhan wet-market) was responsible (Rothan and Byrareddy, 2020; Guo et al., 2020; Lai et al., 2020; Shereen et al., 2020; Singhal, 2020). This view is logically consistent with a biomedical individualist conception of the determinants of health and disease, since this framework focuses on 'downstream’ determinants of disease. An alternative answer is that a range of anthropogenic environmental degradations played a vital role in the emergence of zoonoses such as SARS-CoV-2 (Everard et al., 2020; Volpato et al., 2020; UNEP, 2020; Johnson et al., 2015). This explanation is more likely to be favored by those who operate within an ecological conceptual framework. Because this ecological explanation provides a more complete account of the different types of causal factors underpinning the emergence of zoonoses such as SARS-CoV-2, I will argue that the COVID-19 pandemic provides another motivation to more explicitly ground our theory of health and disease in an ecological (and social) conceptual framework.
2. The need for an explicitly eco-social conceptual framework of health
Theories are vital to structure our ideas so as to bring facts together in a way that they form a coherent whole and explain the causal connections between the facts (McMichael, 1999; Krieger, 1994). An optimal theory of the determinants of health and disease should provide an accurate portrayal of all the important determinants in a way that illustrates the interrelationships, the relative importance of the various determinants and facilitates proportionate and effective responses to threats to health (Krieger, 1994). If the theory conceals certain determinants or privileges certain causes over others, then it should be replaced by a theory which does not do this (Krieger, 1994; Susser and Susser, 1996).
An example of a necessary transition in theory in the health sciences, was the switch from miasmatic theories to the germ theory in the late 19th century (Krieger, 1994). Microbes were identified as being responsible for specific diseases and soon thought by many to be the major cause of disease (McMichael, 1999). In the ensuing decades further evidence would emerge to back up this theory of specific diseases being caused by specific agents: specific micronutrient deficiencies and particular toxin or occupational exposures were shown to be responsible for specific diseases and later on the link was made between specific genetic changes and individual diseases (Krieger, 1994). These insights led to the biomedical individualist conception of health and disease which claimed that specific environmental, microbiological, immunological and nutritional agents, considered almost solely at the level of individuals, could explain the majority of disease (Susser and Susser, 1996). This has been dominant theory for the health sciences over the past century (McMichael, 1999; McMichael et al., 2015; Jones et al., 2017).
This theory has been critiqued as placing too much focus on individual, cellular and subcellular levels at the expense of considering what was happening at population levels (McMichael, 1999; Krieger, 1994; Susser and Susser, 1996; McMichael et al., 2015). As an alternative, various ecological or ecosocial theories have been proposed (McMichael, 1999; Krieger, 1994; Susser and Susser, 1996; McMichael et al., 2015; Jones et al., 2017; Benach, 2020; Parkes et al., 2020; Shafiei et al., 2017; Ortiz and Levins, 2017; Hancock, 2015; Yang et al., 2015). Ecology is the formal study of the interdepencies between groups of organisms, populations, and species and their surroundings (Susser and Susser, 1996). Ecological and ecosocial frameworks seek to integrate social and biological interdependencies in a dynamic ecological perspective to develop multilevel conceptual frameworks for understanding the origins and distribution of disease (Krieger, 1994, 1994; Susser and Susser, 1996; McMichael et al., 2015; Yang et al., 2015). This multilevel perspective is particularly useful for explaining the emergence of zoonoses (Yang et al., 2015).
3. What drives the emergence of Nipah virus, SARS-CoV-2 and other zoonoses?
The outbreaks of Nipah virus starting in 1999 offer an example of the contrasting way that the biomedical individualist and ecosocial frameworks portray the emergence of zoonoses. Certain authors limited their analysis of the emergence of this zoonosis to human interactions with domestic pigs and the host species (fruit bats) (Bellini et al., 2005). As a result, the strategies they recommend to prevent further outbreaks are limited largely to reducing contacts between these species or better personal protective equipment for humans (Bellini et al., 2005). This conceptualization fits well within a biomedical individualist approach that focuses on ‘downstream’ factors at the expense of considering broader ecological factors (Smith et al., 2014; McMichael, 1999; Susser and Susser, 1996). Other authors adopted an explicitly interdisciplinary, ecosocial approach that revealed the important role that an array of upstream factors (anthropogenic environmental changes and socioeconomic factors) played in the emergence of this virus (Daszak et al., 2013).
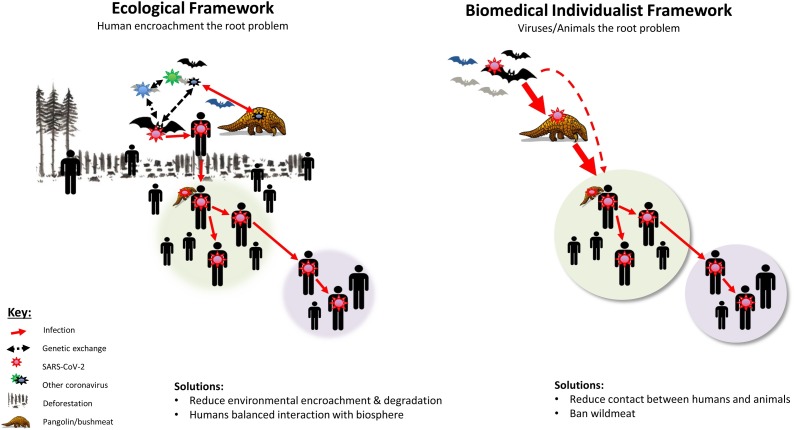
A similar contrast has been evident with SARS-CoV-2. Phylogenetic analyses reveal that SARS-CoV-2 was likely transmitted to humans from bats in the Yunnan province, China or from pangolins (Tang et al., 2020; World Health Organization, 2020; Zhang et al., 2019; Menachery et al., 2017; Jin et al., 2020). Whilst we cannot be sure of the route of transmission, bat zoonoses frequently result from human encroachment on wildlife areas (Menachery et al., 2017; Smith and Wang, 2013; Chomel et al., 2007; Wynne and Wang, 2013), whereas transmission from pangolins is likely linked to their trade and consumption (Zhang et al., 2019). Seen from a biomedical individualist framework, these transmission routes could be impeded by banning the trade and consumption of wild animals and the entry of humans to sites of bat habitation – a solution proposed in various forms in numerous papers (Fig. 1 ) (Rothan and Byrareddy, 2020; Guo et al., 2020; Shereen et al., 2020; Singhal, 2020; World Health Organization, 2020; Chomel et al., 2007; Wynne and Wang, 2013; Ahmad et al., 2020; Wolfe et al., 2005).
Fig. 1.
A schematic representation of the contrast between ecological (left) and biomedical individualist (right) conceptual frameworks for understanding the origins of SARS-CoV-2 and other zoonoses. According to the individualist framework, SARS-CoV-2 emerged following humans consuming wildlife (represented by a pangolin) or coming into close contact with wild animals such as bats. The ecological framework expands this explanation by incorporating insights such as the extensive genetic recombination between coronaviruses in different species which produce a wide array of novel viruses that can expand into new or expanding niches. The expanding human ecological footprint in conjunction with environmental encroachment and degradation increase the probability of novel pandemic zoonoses such as SARS-CoV-2.
Viewed from an ecological perspective, far more radical reforms would be necessary (Fig. 1). SARS-CoV-2 is just one of a number of emerging human infections, the majority of which are zoonoses (Jones et al., 2008, 2013; Wolfe et al., 2005). A number of studies have assessed why the number of infectious diseases outbreaks and rate of emergence of zoonoses has been increasing over the past 40 years (Jones et al., 2008; Everard et al., 2020; Jones et al., 2013; Smith et al., 2014). These studies have found that a key contributory factor has been widespread anthropogenic ecosystem degradation (Jones et al., 2008; Everard et al., 2020; Jones et al., 2013; Johnson et al., 2015; Daszak et al., 2000). For example, deforestation and other changes in land usage have been linked to the emergence of a number of infectious diseases such as Ebola, Zika and HIV (Jones et al., 2008; Everard et al., 2020; Jones et al., 2013; Dobson et al., 2020). Likewise human activities such as intensified agriculture and livestock production, climate change and human activities that reduce biodiversity have all been linked to increased risk of transmission of infectious diseases (UNEP, 2020; Keesing et al., 2010; Civitello et al., 2015). An additional factor, that has received less attention, is that humans, by virtue of our number, biomass and connectivity, represent a large and growing niche for emerging infections (McMichael et al., 2015). Humans and our livestock are now estimated to comprise 96 % of all mammalian biomass on earth (Bar-On et al., 2018). If one factors in human encroachment and how interconnected the global population of humans is, ecological/evolutionary theory would predict that humans will be affected by increasing numbers of infectious diseases, such as novel corona- and influenza-viral infections (Fig. 1) (Choi et al., 2015; Belshe, 2005; Guan et al., 2010). Coronaviruses, for example, circulate as a large number of species from four genera and are characterized by high rates of genetic recombination between species (Jin et al., 2020; Su et al., 2016; Hu et al., 2017; Chan et al., 2013). These are among the features that enable them to evolve fairly rapidly to fit new niches (Jin et al., 2020; Su et al., 2016; Hu et al., 2017). Genetic studies of SARS-CoV-1 and 2 have revealed that both emerged following serial episodes of genetic recombination in different hosts (Menachery et al., 2017; Jin et al., 2020; Su et al., 2016). Furthermore, analyses of coronaviruses from bats in China have found multiple novel SARS-CoV-type viruses that have arisen by recombination and are capable of infection of human cell lines. These viruses have not, thus far, been detected in humans (Hu et al., 2017).
Seen from an ecological framework, unless these underlying environmental pressures are addressed, the probability of zoonotic emergence of a new coronavirus or one of the estimated 700,000 other unidentified viruses with zoonotic potential (Carroll et al., 2018) would be expected to increase with time. To prevent this emergence, the ecological framework would promote far more radical reforms than banning wildlife consumption and wet-markets. Addressing the underlying determinants would require reducing human’s encroachment of natural habitats as well as their overall ecological footprint (Everard et al., 2020; UNEP, 2020).
4. SARS-CoV-2 is not the worst consequence of environmental degradation
The collective demands of contemporary human consumption are estimated to require approximately 1.7 planet earths (Global Footprint Network, 2020). This excess consumption has important consequences for human and planetary health beyond the emergence of zoonoses. These include climate change and mass extinctions of various life forms (Everard et al., 2020; McMichael et al., 2015). As an example, the total insect biomass has declined between 76 % and 60-fold over the past few decades in different world regions (Hallmann et al., 2017; Lister and Garcia, 2018). An estimated 40 % of all insect species are now threatened with extinction (Sánchez-Bayo and Wyckhuys, 2019). Other phyla have experienced comparable declines (Rosenberg et al., 2019) with overall extinction rates now being estimated to being 10 to 1000-fold higher than baseline rates (Lamkin and Miller, 2016; Wilson, 2016). The consequences are large for both planetary- and human-health. Bees, for example, play a key role in food security through their role as pollinators (Sánchez-Bayo and Wyckhuys, 2019). They have however been dying in such numbers that in numerous regions bees now have to be artificially trucked in to pollinate fruit trees (Sánchez-Bayo and Wyckhuys, 2019). Various reviews have concluded that the key drivers of these population-declines and extinctions are habitat destruction, global warming and widespread pesticide usage (Sánchez-Bayo and Wyckhuys, 2019; Wilson, 2016; Ellis, 2019). Excess consumption and associated habitat destruction is thus driving not only the emergence of new zoonoses but also mass extinctions.
With its focus on individual level analyses, the biomedical individualist framework has been late to recognize and respond to these environmental threats. This provides an additional argument for the health sciences to adopt explicitly ecological frameworks (McMichael et al., 2015; Haines and Ebi, 2019).
5. The social component of eco-social
Ecological conceptual frameworks should explicitly include a social component for a number of reasons. Social relations play a critical role in human health and wellbeing (Krieger, 1994). An extensive array of research has, for example, established that social and economic equity is good for health. Equality has been associated with a healthier social-fabric that results in lower violent crime rates, better mental health and improved overall health including longer life expectancy (Pickett and Wilkinson, 2015). Cutting human consumption will thus need to be done in a way that promotes rather than diminishes equity (McMichael et al., 2015). Imposing bans on wildlife consumption for populations with few alternative food sources and without providing alternatives, would be deemed unethical from this perspective. Consumption of wildlife has been shown to be a critical source of nutrition for certain impoverished communities (Volpato et al., 2020; Borgerson et al., 2016).
6. Other health problems are also better understood from an ecological framework
Cogent arguments have been made that a range of other health problems are best understood from eco-social perspectives. This includes the One Health perspective on antimicrobial resistance (Lammie and Hughes, 2016), increases in non-zoonotic infectious diseases (Gebreyes et al., 2014), decreased diversity of the microbiota in westernized populations (Schnorr et al., 2014) and increased obesity and allergies in various populations around the world (McMichael et al., 2015; Lammie and Hughes, 2016; Haahtela et al., 2015).
An important limitation of this analysis is that it may have been too simplistic in classifying particular authors as operating either within a biomedical or an eco-social conceptual framework. My objective was not, however, to conduct detailed analyses of the theoretical underpinnings of individual papers. Rather it was to assess if the wildlife consumption/animal contact theory is more logically consistent with the individualist- as opposed to the ecological conceptual framework. A further limitation is that it could be argued that I have assumed that the biomedical individualist and eco-social perspectives are in opposition to one another. This was not intentional. Numerous proponents of eco-social conceptualizations of health have argued cogently that their conceptual framework includes space for all the evidence-based findings of biomedical individualism at the individual level. The key distinction is that eco-social frameworks also actively include thinking at higher levels of aggregation in explicitly multilevel frameworks (Krieger, 1994; Susser and Susser, 1996; McMichael et al., 2015; Yang et al., 2015).
7. Conclusion
An important lesson of the increasing rate at which zoonoses (including SARS-CoV-2) are emerging is that humans uncontrolled consumption is placing excessive pressure on natural ecosystems. Unless reversed, the anthropogenic destruction of habitats will lead to both the emergence of new zoonoses and accelerated extinction rates (Sánchez-Bayo and Wyckhuys, 2019; Wilson, 2016; Ellis, 2019). We might disagree on how much to reduce humanities footprint and how best to accomplish this, but the evidence that we need to do this appears incontrovertible (McMichael et al., 2015; Watts et al., 2018). Reaching this conclusion does however require thinking at an ecological level. For this reason, the prevention of future zoonotic pandemics and related crises would be assisted by the health sciences embracing an explicitly eco-social theoretical framework of health and disease (Jones et al., 2017; Parkes et al., 2020; Hancock, 2015). As part of this process, reasoning along multi-level ecological lines could be taught as part of training in the health sciences (McMichael et al., 2015). This will hopefully contribute to the health sciences rekindling the philosophy of Hippocrates who considered a healthy environment to be the key foundation for all health (Hippocrates, 1978).
CRediT authorship contribution statement
Chris Kenyon: Conceptualization, Visualization, Writing - review & editing.
Declaration of Competing Interest
The author declares that he has no conflict of interest
References
- Ahmad T., Khan M., Haroon Musa T.H., Nasir S., Hui J. COVID-19: zoonotic aspects. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101607. Epub 2020/03/01. PubMed PMID: 32112857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On Y.M., Phillips R., Milo R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. U. S. A. 2018;115(25):6506–6511. doi: 10.1073/pnas.1711842115. Epub 2018/05/23. PubMed PMID: 29784790; PubMed Central PMCID: PMCPMC6016768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W.J., Harcourt B.H., Bowden N., Rota P.A. Nipah virus: an emergent paramyxovirus causing severe encephalitis in humans. J. Neurovirol. 2005;11(5):481–487. doi: 10.1080/13550280500187435. [DOI] [PubMed] [Google Scholar]
- Belshe R.B. The origins of pandemic influenza--lessons from the 1918 virus. N. Engl. J. Med. 2005;353(21):2209–2211. doi: 10.1056/NEJMp058281. Epub 2005/11/25. PubMed PMID: 16306515. [DOI] [PubMed] [Google Scholar]
- Benach J. We must take advantage of this pandemic to make a radical social change: the coronavirus as a global health, inequality, and eco-social problem. Int. J. Health Serv. 2020 doi: 10.1177/0020731420946594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgerson C., McKean M.A., Sutherland M.R., Godfrey L.R. Who hunts lemurs and why they hunt them. Biol. Conserv. 2016;197:124–130. [Google Scholar]
- Carroll D., Daszak P., Wolfe N.D., Gao G.F., Morel C.M., Morzaria S. The global virome project. Science. 2018;359(6378):872–874. doi: 10.1126/science.aap7463. Epub 2018/02/24. PubMed PMID: 29472471. [DOI] [PubMed] [Google Scholar]
- Chan J.F., To K.K., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21(10):544–555. doi: 10.1016/j.tim.2013.05.005. Epub 2013/06/19. PubMed PMID: 23770275; PubMed Central PMCID: PMCPMC7126491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.J., Torremorell M., Bender J.B., Smith K., Boxrud D., Ertl J.R. Live animal markets in Minnesota: a potential source for emergence of novel influenza a viruses and interspecies transmission. Clin. Infect. Dis. 2015;61(9):1355–1362. doi: 10.1093/cid/civ618. Epub 2015/08/01. PubMed PMID: 26223994; PubMed Central PMCID: PMCPMC4599395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel B.B., Belotto A., Meslin F.-X. Wildlife, exotic pets, and emerging zoonoses. Emerging Infect. Dis. 2007;13(1):6. doi: 10.3201/eid1301.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello D.J., Cohen J., Fatima H., Halstead N.T., Liriano J., McMahon T.A. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl. Acad. Sci. 2015;112(28):8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science. 2000;287(5452):443–449. doi: 10.1126/science.287.5452.443. Epub 2000/01/22. PubMed PMID: 10642539. [DOI] [PubMed] [Google Scholar]
- Daszak P., Zambrana-Torrelio C., Bogich T.L., Fernandez M., Epstein J.H., Murray K.A. Interdisciplinary approaches to understanding disease emergence: the past, present, and future drivers of Nipah virus emergence. Proc Natl Acad Sci U S A. 2013;110(Suppl 1):3681–3688. doi: 10.1073/pnas.1201243109. Epub 2012/09/01. PubMed PMID: 22936052; PubMed Central PMCID: PMCPMC3586606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P., Pimm S.L., Hannah L., Kaufman L., Ahumada J.A., Ando A.W. Ecology and economics for pandemic prevention. Science. 2020;369(6502):379–381. doi: 10.1126/science.abc3189. Epub 2020/07/25. PubMed PMID: 32703868. [DOI] [PubMed] [Google Scholar]
- Ellis E.C. To conserve nature in the anthropocene, half earth is not nearly enough. One Earth. 2019;1(2):163–167. [Google Scholar]
- Everard M., Johnston P., Santillo D., Staddon C. The role of ecosystems in mitigation and management of Covid-19 and other zoonoses. Environ. Sci. Policy. 2020;111:7–17. doi: 10.1016/j.envsci.2020.05.017. Epub 2020/06/06. PubMed PMID: 32501392; PubMed Central PMCID: PMCPMC7247996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreyes W.A., Dupouy-Camet J., Newport M.J., Oliveira C.J., Schlesinger L.S., Saif Y.M. The global one health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl. Trop. Dis. 2014;8(11) doi: 10.1371/journal.pntd.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Footprint Network . Global Footprint Network; 2020. Living Planet Index 2016.http://wwwfootprintnetworkorg/en/indexphp/GFN/ [Google Scholar]
- Guan Y., Vijaykrishna D., Bahl J., Zhu H., Wang J., Smith G.J. The emergence of pandemic influenza viruses. Protein Cell. 2010;1(1):9–13. doi: 10.1007/s13238-010-0008-z. Epub 2011/01/05. PubMed PMID: 21203993; PubMed Central PMCID: PMCPMC4875113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. Epub 2020/03/15. PubMed PMID: 32169119; PubMed Central PMCID: PMCPMC7068984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahtela T., Laatikainen T., Alenius H., Auvinen P., Fyhrquist N., Hanski I. Hunt for the origin of allergy - comparing the Finnish and Russian Karelia. Clin. Exp. Allergy. 2015;45(5):891–901. doi: 10.1111/cea.12527. Epub 2015/03/17. PubMed PMID: 25772429. [DOI] [PubMed] [Google Scholar]
- Haines A., Ebi K. The imperative for climate action to protect health. N. Engl. J. Med. 2019;380(3):263–273. doi: 10.1056/NEJMra1807873. Epub 2019/01/17. PubMed PMID: 30650330. [DOI] [PubMed] [Google Scholar]
- Hallmann C.A., Sorg M., Jongejans E., Siepel H., Hofland N., Schwan H. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0185809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock T. Population health promotion 2.0: an eco-social approach to public health in the Anthropocene. Can. J. Public Health. 2015;106(4):e252–5. doi: 10.17269/cjph.106.5161. Epub 2015/08/19. PubMed PMID: 26285199; PubMed Central PMCID: PMCPMC6972308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippocrates . Airs, waters and places. An essay on the influence of climate, water supply and situation on health. In: GER e Lloyd., editor. Hippocratic Writings. Penguin; London, England: 1978. pp. 148–169. [Google Scholar]
- Hu B., Zeng L.P., Yang X.L., Ge X.Y., Zhang W., Li B. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. Epub 2017/12/01. PubMed PMID: 29190287; PubMed Central PMCID: PMCPMC5708621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4) doi: 10.3390/v12040372. Epub 2020/04/02. PubMed PMID: 32230900; PubMed Central PMCID: PMCPMC7232198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.T., de Roode J.C., Fenton A. Why infectious disease research needs community ecology. Science. 2015;349(6252) doi: 10.1126/science.1259504. Epub 2015/09/05. PubMed PMID: 26339035; PubMed Central PMCID: PMCPMC4863701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. PubMed PMID: 18288193; PubMed Central PMCID: PMCPMC5960580. Epub 2008/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.A., Grace D., Kock R., Alonso S., Rushton J., Said M.Y. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. U. S. A. 2013;110(21) doi: 10.1073/pnas.1208059110. 8399-404. Epub 2013/05/15. PubMed PMID: 23671097; PubMed Central PMCID: PMCPMC3666729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.A., Betson M., Pfeiffer D.U. Eco-social processes influencing infectious disease emergence and spread. Parasitology. 2017;144(1):26–36. doi: 10.1017/S0031182016001414. Epub 2016/09/10. PubMed PMID: 27609615. [DOI] [PubMed] [Google Scholar]
- Keesing F., Belden L.K., Daszak P., Dobson A., Harvell C.D., Holt R.D. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–652. doi: 10.1038/nature09575. Epub 2010/12/03. PubMed PMID: 21124449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc. Sci. Med. 1994;39(7):887–903. doi: 10.1016/0277-9536(94)90202-x. Epub 1994/10/01. PubMed PMID: 7992123. [DOI] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. Epub 2020/02/23. PubMed PMID: 32081636; PubMed Central PMCID: PMCPMC7127800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkin M., Miller A.I. On the challenge of comparing contemporary and deep-time biological-extinction rates. BioScience. 2016;66(9):785–789. [Google Scholar]
- Lammie S.L., Hughes J.M. Antimicrobial resistance, food safety, and one health: the need for convergence. Annu. Rev. Food Sci. Technol. 2016;7:287–312. doi: 10.1146/annurev-food-041715-033251. [DOI] [PubMed] [Google Scholar]
- Lister B.C., Garcia A. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. 2018;115(44) doi: 10.1073/pnas.1722477115. E10397-E406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J. Prisoners of the proximate: loosening the constraints on epidemiology in an age of change. Am. J. Epidemiol. 1999;149(10):887–897. doi: 10.1093/oxfordjournals.aje.a009732. Epub 1999/05/26. doi: PubMed PMID: 10342797. [DOI] [PubMed] [Google Scholar]
- McMichael A., Butler C., Dixon J. Climate change, food systems and population health risks in their eco-social context. Public Health. 2015;129(10):1361–1368. doi: 10.1016/j.puhe.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Menachery V.D., Graham R.L., Baric R.S. Jumping species-a mechanism for coronavirus persistence and survival. Curr. Opin. Virol. 2017;23:1–7. doi: 10.1016/j.coviro.2017.01.002. Epub 2017/02/20. PubMed PMID: 28214731; PubMed Central PMCID: PMCPMC5474123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz M., Levins R. Self-feedbacks determine the sustainability of human interventions in eco-social complex systems: impacts on biodiversity and ecosystem health. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0176163. Epub 2017/04/30. PubMed PMID: 28453548; PubMed Central PMCID: PMCPMC5409167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes M.W., Poland B., Allison S., Cole D.C., Culbert I., Gislason M.K. Preparing for the future of public health: ecological determinants of health and the call for an eco-social approach to public health education. Can. J. Public Health. 2020;111(1):60–64. doi: 10.17269/s41997-019-00263-8. Epub 2019/12/04. PubMed PMID: 31792844; PubMed Central PMCID: PMCPMC7046913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett K.E., Wilkinson R.G. Income inequality and health: a causal review. Soc. Sci. Med. 2015;128:316–326. doi: 10.1016/j.socscimed.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Rosenberg K.V., Dokter A.M., Blancher P.J., Sauer J.R., Smith A.C., Smith P.A. Decline of the North American Avifauna. Science. 2019;366(6461):120–124. doi: 10.1126/science.aaw1313. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. Epub 2020/03/03. PubMed PMID: 32113704; PubMed Central PMCID: PMCPMC7127067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Bayo F., Wyckhuys K.A. Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 2019;232:8–27. [Google Scholar]
- Schnorr S.L., Candela M., Rampelli S., Centanni M., Consolandi C., Basaglia G. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014;5(3654) doi: 10.1038/ncomms4654. Epub 2014/04/17. PubMed PMID: 24736369; PubMed Central PMCID: PMCPMC3996546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei L., Taymoori P., Maleki A., Nouri B. Effect of environmental intervention on the consumption of rice without toxic metals based on the health belief model and ecological-social model. J. Clin. Diagn. Res. 2017;11(7) doi: 10.7860/JCDR/2017/26784.10262. JC01-JC6. Epub 2017/09/13. PubMed PMID: 28892931; PubMed Central PMCID: PMCPMC5583945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. Epub 2020/04/08. PubMed PMID: 32257431; PubMed Central PMCID: PMCPMC7113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus Disease-2019 (COVID-19) Indian J. Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. Epub 2020/03/14. PubMed PMID: 32166607; PubMed Central PMCID: PMCPMC7090728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Wang L.-F. Bats and their virome: an important source of emerging viruses capable of infecting humans. Curr. Opin. Virol. 2013;3(1):84–91. doi: 10.1016/j.coviro.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.F., Goldberg M., Rosenthal S., Carlson L., Chen J., Chen C. Global rise in human infectious disease outbreaks. J. R. Soc. Interface. 2014;11(101) doi: 10.1098/rsif.2014.0950. Epub 2014/11/18. PubMed PMID: 25401184; PubMed Central PMCID: PMCPMC4223919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. Epub 2016/03/26. PubMed PMID: 27012512; PubMed Central PMCID: PMCPMC7125511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser M., Susser E. Choosing a future for epidemiology: II. From black box to Chinese boxes and eco-epidemiology. Am. J. Public Health. 1996;86(5):674–677. doi: 10.2105/ajph.86.5.674. Epub 1996/05/01. PubMed PMID: 8629718; PubMed Central PMCID: PMCPMC1380475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Wu C., Li X., Song Y., Yao X., Wu X. On the origin and continuing evolution of SARS-CoV-2. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP . 2020. Coronaviruses: Are They Here to Stay? UN Environment Programme (UNEP)https://wwwunenvironmentorg/news-and-stories/story/coronaviruses-are-they-here-stay [Google Scholar]
- Volpato G., Fontefrancesco M.F., Gruppuso P., Zocchi D.M., Pieroni A. Baby pangolins on my plate: possible lessons to learn from the COVID-19 pandemic. J. Ethnobiol. Ethnomed. 2020;16(1):19. doi: 10.1186/s13002-020-00366-4. Epub 2020/04/23. PubMed PMID: 32316979; PubMed Central PMCID: PMCPMC7171915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts N., Amann M., Arnell N., Ayeb-Karlsson S., Belesova K., Berry H. The 2018 report of the Lancet Countdown on health and climate change: shaping the health of nations for centuries to come. Lancet. 2018;392(10163):2479–2514. doi: 10.1016/S0140-6736(18)32594-7. Epub 2018/12/07. PubMed PMID: 30503045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E.O. 2016. Half-earth: Our Planet’s Fight for Life: Liveright: New York. [Google Scholar]
- Wolfe N.D., Daszak P., Kilpatrick A.M., Burke D.S. Bushmeat hunting, deforestation, and prediction of zoonotic disease. Emerging Infect. Dis. 2005;11(12):1822. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- Wynne J.W., Wang L.F. Bats and viruses: friend or foe? PLoS Pathog. 2013;9(10) doi: 10.1371/journal.ppat.1003651. Epub 2013/11/10. PubMed PMID: 24204253; PubMed Central PMCID: PMCPMC3814676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Zhou X.N., Jia T.W., Yang G.J., Wu X.H., Shi X.W. Eco-social determinants of Schistosoma japonicum infection supported by multi-level modelling in Eryuan county, People’s Republic of China. Acta Trop. 2015;141(Pt B):391–398. doi: 10.1016/j.actatropica.2014.04.013. Epub 2014/04/23. PubMed PMID: 24751418. [DOI] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. 2019. Probable Pangolin Origin of 2019-nCoV Associated With Outbreak of COVID-19. CURRENT-BIOLOGY-D-20-00299. [Google Scholar]