Aristotle first described emergent systems as being greater than the sum of their parts: “the totality is not, as it were, a mere heap, but the whole is something besides the parts.”1 Emergence occurs when an entity has properties that its individual components do not have, but are attributable to interactions among its components. Recognizing and understanding emergent systems is important because attempts to interpret and influence them by their individual components are often unsuccessful.
Emergent systems arise when individual units take simple actions, creating a transcendent pattern. Harvester ants systematically leave their colony searching for food, return at a rate correlating with its availability, and no longer depart if foragers do not return for an extended period, resembling the Transmission Control Protocol algorithm that manages internet data congestion.2 Autonomous actions by stock traders generate sophisticated market patterns that transcend individual decisions made within another emergent system: the human brain, composed of individual neurons whose coordinated actions produce consciousness and executive function.
Some diseases should be regarded as emergent systems. Frailty may be recognized by combinations of unintentional weight loss, exhaustion, weakness, slow walking speed, and low physical activity. This diagnostic approach fails to accurately represent complex interactions among physical, cognitive, social, and biologic factors producing accumulation of comorbidities, loss of compensatory reserve, and increased vulnerability to illness.3 Not surprisingly, frailty phenotyping and prognostication are often inconsistent and inaccurate.
Frailty increases perioperative and postoperative morbidity and mortality, along with hundreds of other risk factors whose relationships with outcomes are influenced by complex, non-linear interactions among diseases and host factors. Accordingly, preoperative risk stratification is evolving from additive scoring systems and regression-based calculators to machine learning algorithms which may be more accurate than online calculators and physicians in predicting adverse events, including another emergent system: sepsis.4,5 The definition of sepsis conveys depth and complexity: “life-threatening organ dysfunction caused by a dysregulated host response to infection.” Organ dysfunction is graded by the sequential organ failure assessment (SOFA) score which uses static variable thresholds for a small number of parameters without considering the associations among them. Immune dysregulation is absent from clinical sepsis phenotyping methods.
Frailty, operative risk, and sepsis emerge from complex, non-linear underlying pathology. Traditional phenotyping and risk assessment tools do not accurately represent these emergent systems, but artificial intelligence models do. State-of-the art phenotyping and surgical risk assessment could be achieved by expert classification of high-quality training datasets paired with artificial intelligence models that use natural language processing to capture written clinical assessments and computer vision to analyze imaging findings like psoas muscle indices, automatically detecting phenotypes by time-honored classification systems while discovering new phenotypes, identifying candidates for clinical trial enrollment, providing accurate risk assessments, and augmenting surgical decision-making.
This approach could be used in surgical wards and intensive care units (ICUs), which often depend on sporadic, manual assessments of important parameters like pain and physical function and require time-consuming review of massive amounts of data by busy surgeons making decisions during and between cases. Deep learning has been applied to sequential time-series electronic health record physiologic data, producing accurate real-time in-hospital mortality predictions with greater accuracy than traditional additive acuity scores.6 New technologies will allow deep models to see more data from more unique sources. An ICU patient assessment platform could continuously assess visual cues for pain and physical function, combine these inputs with live-streaming physiologic data anchored to a robust characterization of acute and chronic medical conditions derived from the electronic health record, and use deep models to predict imminent adverse events and long-term outcomes (Figure 1). A similar approach could surveil ward patients for decompensation and alert rapid response teams.
Figure 1:
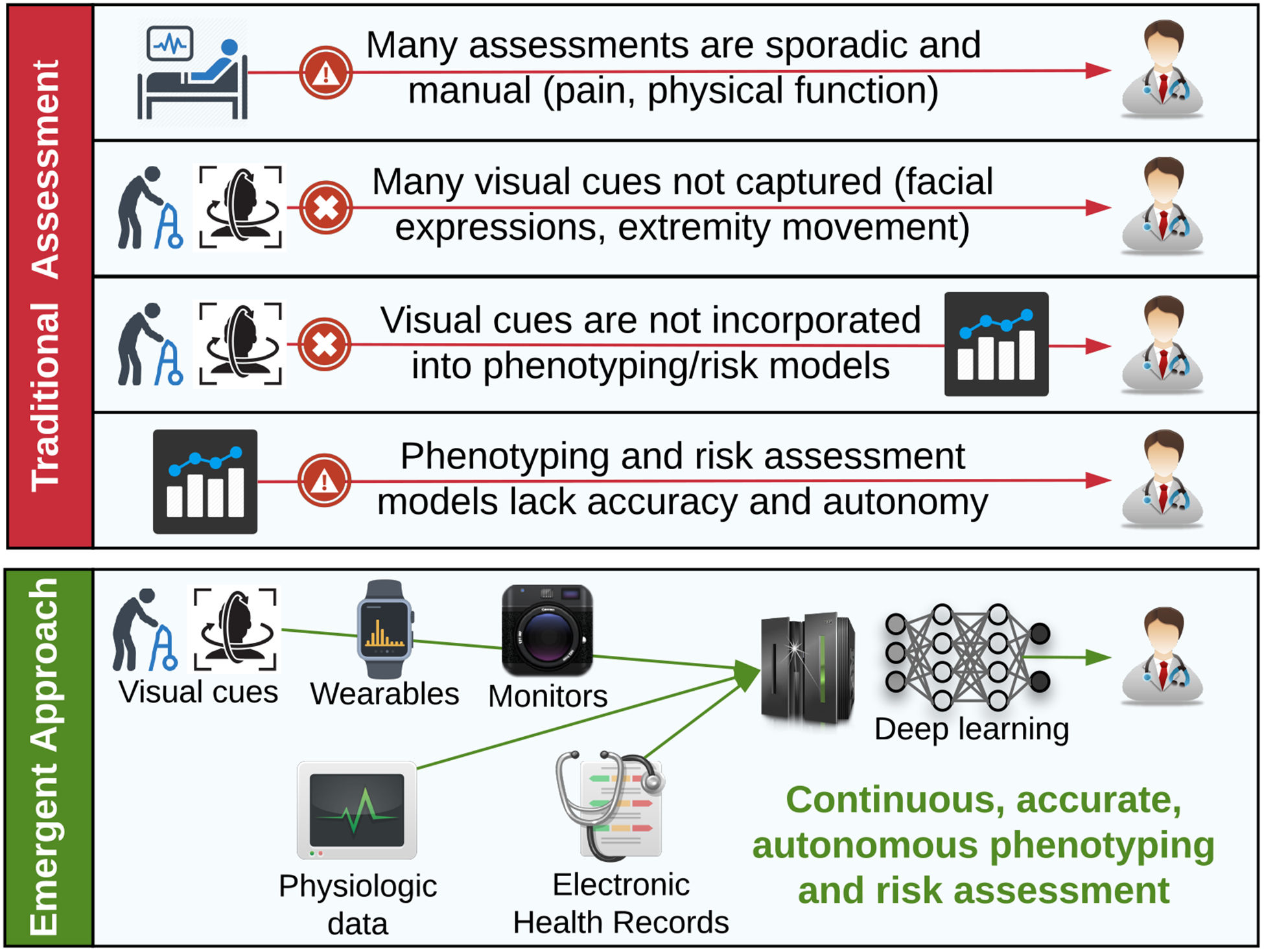
Traditional and emergent approaches to patient assessment on surgical wards and intensive care units.
Beyond phenotyping and risk assessment, artificial intelligence models are capable of recommending resuscitation and vasopressor administration strategies for septic patients by identifying the treatment strategy with the highest odds of survival.7 Similar methods could be used to augment surgical decision-making for source control of infection and any other clinical scenario for which big data are available. Decision-making under time constraints with incomplete information may force reliance on heuristics, or cognitive shortcuts, which are prone to bias and error. With years of training, human intuition, and use of de-biasing strategies like morbidity and mortality conferences and surgeon-specific registries, surgeons are in the best position to offer wisdom and advice about surgical diseases to patients and their caregivers. Artificial intelligence can augment this process by providing real-time recommendations for strategies with the highest probability of success.
Unfortunately, deep and reinforcement learning models offer little insight regarding the relative importance of model inputs in determining outputs. Self-attention mechanisms and probabilistic graphical models provide visual representations of model activities, but lack mechanistic detail. Technological advances and scientific rigor must answer this call. Artificial intelligence should be used to augment human decision-making rather than replace it. A surgeon makes mistakes one at a time, but an errant model could harm hundreds or thousands of patients around the world in a short period of time. Artificial intelligence models must be trained on high-quality data, outputs must be carefully interpreted by well-trained clinicians, and their performance must be directly compared with traditional scoring systems in prospective clinical trials to validate observations from retrospective studies. Because emergent systems affect medical and surgical patients of all ages, future investigations should test applications of artificial intelligence to emergent systems across the field of medicine.
Traditional methods for patient phenotyping, risk assessment, and augmented decision-making are ill-equipped to address the complex, non-linear underlying pathology of emergent systems. Thoughtful integration of deep and reinforcement learning harbors the potential to enhance shared decision-making regarding informed consent and resource utilization, facilitate early identification of patients at increased risk for adverse events, recommend optimal treatment strategies, and promote the development of targeted preventative and therapeutic measures for established and yet undiscovered phenotypes.
Acknowledgement
AB was supported by R01 GM110240 from the National Institute of General Medical Sciences (NIGMS). AB was supported by Sepsis and Critical Illness Research Center Award P50 GM-111152 from the NIGMS. TJL was supported by a post-graduate training grant (T32 GM-008721) in burns, trauma, and perioperative injury from the NIGMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Aristotle. Metaphysics, Book H.1045a 1048–1010. [Google Scholar]
- 2.Prabhakar B, Dektar KN, Gordon DM. The Regulation of Ant Colony Foraging Activity without Spatial Information. Plos Computational Biology. 2012;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267(13):1806–1809. [PubMed] [Google Scholar]
- 4.Bertsimas D, Dunn J, Velmahos GC, Kaafarani HMA. Surgical Risk Is Not Linear: Derivation and Validation of a Novel, User-friendly, and Machine-learning-based Predictive OpTimal Trees in Emergency Surgery Risk (POTTER) Calculator. Ann Surg. 2018;268(4):574–583. [DOI] [PubMed] [Google Scholar]
- 5.Brennan M, Puri S, Ozrazgat-Baslnati T, et al. Comparing clinical judgment with the MySurgeryRisk algorithm for preoperative riskassessment: A pilot usability study. Surgery. Accepted 2 January 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shickel B, Loftus TJ, Adhikari L, Ozrazgat-Baslanti T, Bihorac A, Rashidi P. DeepSOFA: A Continuous Acuity Score for Critically Ill Patients using Clinically Interpretable Deep Learning. Sci Rep. 2019;9(1):1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018. [DOI] [PubMed] [Google Scholar]


