ABSTRACT
Acellular liver scaffolds (ALS) have arisen as potential candidates for transplantation. Until now, all reports involving ALS transplantation failed in surgical method descriptions and do not offer support to scientists to reproduce the procedures used in experimental microsurgery to make the results comparable to literature. To overcome the lack of detail information, we described surgical steps details to perform heterotopic and partial orthotopic surgical models to promote ALS transplantation. After preservation and vessel cannulation steps, the liver grafts were decellularized. In addition, ex vivo blood perfusion tests were performed to obtain a successful anticoagulation treatment prior in vivo transplantation. Then, methods of partial liver resection, combination of hand-suture and cuff techniques to complete end-to-end anastomosis between the scaffold and the recipient animal were performed. These procedures which take 30–60 min and were efficient to allow acellular liver scaffold viability and recellularization of different types of cell post-surgery. In conclusion, our methods are practical and simple promising approach that provides the opportunity to investigate ways to achieve sufficient liver function post-transplantation in vivo.
KEYWORDS: acellular scaffold, decellularization, extracellular matrix, liver engineering, liver transplantation
Introduction
Chronic liver diseases affect more than 500 million people worldwide.1 Currently, transplantation is the only treatment available for liver failure. However, problems such as organ availability, graft viability and immune rejection create a long waiting list. Tissue engineering appears as a promising alternative with the production of acellular organs and tissues from the extracellular matrix (ECM) of potential use in Regenerative Medicine. ECM is a complex and dynamic environment characterized by biophysical, mechanical and biochemical properties specific to each organ. Acellular scaffolds can be produced by decellularization techniques.2 The decellularization process removes all cell content of an organ or tissue, leaving only the components of the extracellular matrix specific to the organ or tissue such as collagen, fibronectin, laminin and others.3 The technique of decellularization has already been used for several organs such as heart, lung, kidney, placenta and liver, as well as tissues such as skin, intestinal mucosa, heart valve, among others.4-11 Some studies have shown that these acellular organs can be transplanted in animals.12, 13 Unfortunately, regarding acellular liver transplantation several aspects have not yet been reported, such as endogenous ECM potency to cell recruitment in vivo, acellular liver graft long-term function and contribution to recipient rat liver functions post-transplantation.14
In general, acellular scaffolds can be used on regenerative medicine by orthotopic or heterotopic transplantation. Heterotopic liver transplantation (HLT) is usually used for the treatment of acute liver failure and metabolic liver disease. The aim of HLT is to support the patient’s failing liver for a period of time until the native liver has recovered or to maintain normal metabolic functions using a small proportion of the liver mass. The first HLT in rats was performed in 1966 by Lee et al.15 In this study, a hepatectomy of 70% of the graft native liver of the recipient was performed prior to graft right renal position of the animal. This type of surgical technique of experimental microsurgery is used as an alternative to orthotopic transplantation, a complex surgical technique considered the most difficult of the experimental surgery.16 Such orthotopic transplantation, heterotopic transplantation involves two animals, one of which is the graft donor animal to be transplanted and the other the recipient animal. This surgical technique occurs through submission of the donor animal to a surgical procedure of total or partial hepatectomy that provides complete removal of the liver from the animal for subsequent transplantation in a recipient animal.17 The recipient animal undergoes a surgical procedure in which its native liver is maintained intact and the hepatic graft is transplanted into a different anatomical region from the original position of the animal’s organ as an auxiliary organ. The anatomic region most used in heterotopic transplants in experimental microsurgery consists of one of the two renal positions.18–20 Thus, the graft is heterotopically transplanted into the left or right kidney position of the recipient animal. In this case, the renal vein (RV) and the renal artery (RA) of the recipient animal are preserved and dissected and from different types of anastomoses can be connected to the graft vessels to be transplanted. Since 1966, several groups have tried to improve the HLT model. Surgical strategies such as PV arterialization, PV reconstruction without arterial flow, dual arterial blood supply were performed.12, 19-21 Furthermore, after Cuff technique development, the procedure was simplified, decreasing the operation time and improving the survival rate. Focusing on translational medicine aspects, the heterotopic transplantation can be useful to some diseases, such as liver failure. Liver failure patient might be suitable to perform acellular liver scaffold (ALS) heterotopic transplantation, because while the native liver functions do not recover, the acellular liver scaffold graft transplanted heterotopically can supply the liver functions. On the other hand, patients with liver tumors are outside of this case. For these patients, hepatic resections are more attractive surgical procedure. However, the resections are limited by volume.22 Depending on the case, 30, 70 and 90% of the liver can be resected.23 Recently, Shimoda et al. evaluated the potential of regeneration promoted by ALS after hepatectomy. These results suggest a new frontier to explore tissue-engineered constructs based on decellularization. Based on these results, ALS partial orthoptic transplantation can be an efficient strategy to solve patient’s liver diseases.
To date, there are no reports about step-by-step of surgical approaches to perform ALS transplantation in rats. In all studies involving ALS transplantation, the surgical methods are briefly described and do not offer support to scientists that do not have experimental microsurgery background. So, here, we report for the first time, the key surgical techniques of two different methods to explore ALS transplanted in vivo. In our studies, we used a combination of hand-suture, cuff techniques, liver resection and suture to complete connections between acellular liver scaffold and recipient vasculature and to evaluate acellular liver-engineered construct potency to cell recruitment in vivo using two transplantation models in rats. The liver is a multi-functional, multilobulated and structural complex organ. Thanks to special liver anatomy, we can surgically explore this organ and perform surgeries using different parts of the liver, such as specific entire or partial lobes, or the whole liver. Therefore, our aim was to describe a surgical protocol to explore surgically the acellular whole and partial liver lobe. In the present study, we selected two different models to use acellular liver scaffolds (Figure 1). The first surgical technical (Model 1) promoted the connection between rat recipient’ arterial-venous system and ALS. So, we used an acellular liver scaffold for heterotopic transplantation. In this model, we placed the whole acellular liver scaffold (decellularized rat liver) in recipient left kidney position. The second surgical technical (Model 2) promoted the connection and tissue integration between recipient liver and ALS. So, we used an acellular liver scaffold for partial orthotopic transplantation post-recipient liver resection. Briefly, a 10% of liver resection was performed and then, the acellular liver scaffold was transplanted. This is a useful method to explore tissue and ECM connections. The step-by-step of two surgical procedures was described below and our objective is to provide a simple and useful protocol and to boost all scientists, including those who have no experimental microsurgery background.
Figure 1.
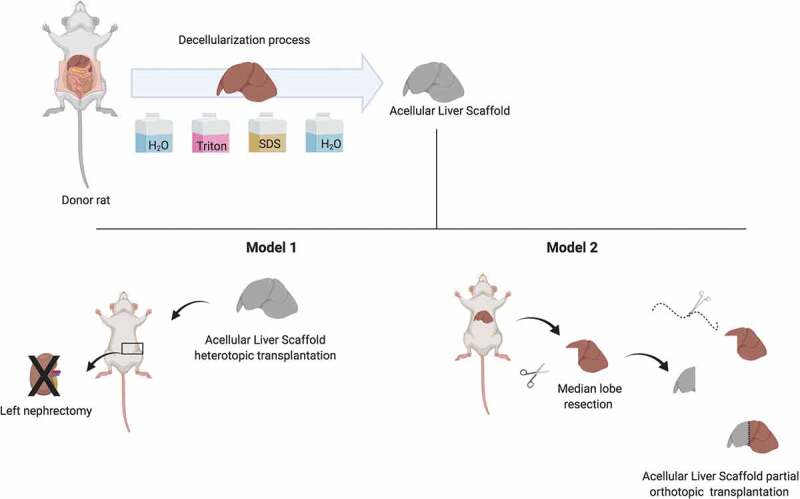
Experimental design. Rat liver grafts were obtained and submitted to decellularization protocol to produce acellular liver scaffold and then, this scaffold were transplanted in recipient rats using two different models (heterotopic and partial orthotopic acellular liver scaffold transplantation).
Materials and methods
Animals
Eight- to twelve-week-old female (donor) and male (recipient) Wistar rats were used (n = 10 to each model). The experimental protocol was approved by the Animal Ethics Committee of Health Science Center of Federal University of Rio de Janeiro, Brazil. All procedures were performed by two persons without a surgery microscopy. Anesthesia was induced by inhalation of 3‐4% isoflurane and maintained by inhalation of 1‐2% isoflurane. All rats were given oxygen at a dose of 0.3–0.5 l/min.
CAUTION All animal experiments must comply with laws and institutional regulations.
Experimental procedures
Reagents
Soap
Sterile Lactate Ringer’s solution
70% ethanol vol/vol
20% Hydrogen peroxide vol/vol
Heparin sodium
Organ storage Solution
Isoflurane, 1.5–2% (wt/vol) in oxygen! CAUTION Isoflurane is harmful if inhaled. It may cause nausea, vomiting, nose/throat/respiratory irritation, headache, drowsiness and skin irritation. Wear gloves and long sleeves to avoid skin contact. Carbon filters should be used to scavenge waste anesthetic gas
100% Oxygen tank
Equipment
Isoflurane vaporizer
Surgical table with heating pad
Heating lamp
SUGGESTION Surgical Microscope (x6-40 magnification) or magnification lens (x2-6 magnification). The use of this auxiliary equipments reduces the operation time.
Surgical materials
Razor blade
Sterile gauze sponges
Cotton tips
Sterile surgical gloves
Icebox
Rubber bands
Nugent forceps
Vannas Spring Scissors
Standard tip forceps
Surgical scissors
Scalpel
Mosquito forceps
Curved halsted-mosquito
Microclamp 8 × 5 mm (RS-5420, ROBOZ)
Microclamp 75 × 6 mm (RS-5422, ROBOZ)
Microvascular moveable clip 8 mm (RS-6488, ROBOZ)
Bulldog clamp 1.2 mm (RS-7438, ROBOZ)
5–0, 6–0 nylon silk
5–0 Vicryl
24 gauge-catheter
20 gauge-catheter
Insulin syringe U-100 27-gauge 5/8-inch 1.0 m
Syringe 20 ml
Petri dish (60 mm)
Model 1: Whole acellular liver scaffold heterotopic transplantation procedure
Allow the animals to acclimate for a minimum of 3 d in a temperature-controlled room with a 12-h light/dark cycle.
Rat liver graft procedure
Inject heparin sodium (5000 U/l) intraperitoneally 15 minutes before.
Place the donor rat in a chamber for induction of anesthesia with a mix of 2% isoflurane and oxygen (1–2 l/min).
CRITICAL Carefully observe frequency and depth of respiration of the animal and make sure the respiration does not arrest and check for a response to toe pinching every 1 min.
After induction of anesthesia, shave the abdominal wall with water and soap using a razor blade. After that, place the animal on the surgical table made and fix all four limbs to the table using rubber bands and with its face in the anesthesia system’s nozzle.
Disinfect the abdominal wall with disinfectant (70% ethanol)
CAUTION Wipe excess disinfectant with sterile gauze to avoid exposing internal organs to these disinfectants.
Start isoflurane inhalation with oxygen flow at 3–4% for the induction of anesthesia during laparotomy.
Do a transverse abdominal skin and muscle incision to expose the xiphoid process. Expose the abdominal cavity by retracting the lower abdominal walls bilaterally using forceps (Figure 2a).
After making the transverse abdominal incision, lower the isoflurane flow to 1–2% for the maintenance of anesthesia.
Figure 2.
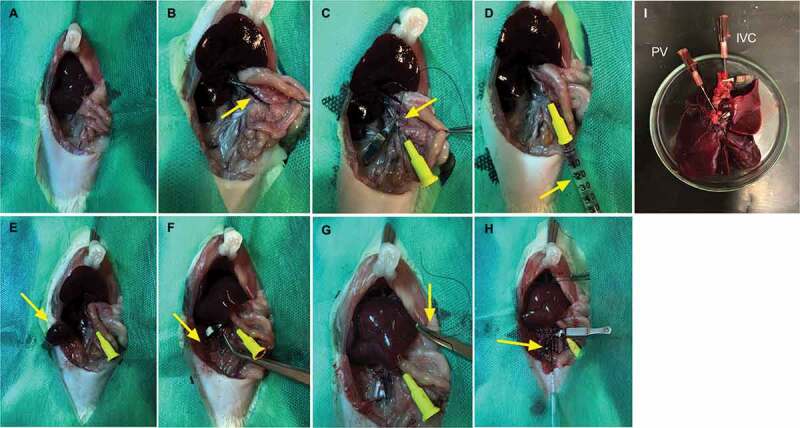
Rat liver graft procedures. Vision of the liver after transverse abdominal incision (a). Arrows indicate PV separation and cannulation (b, c) respectively. Liver perfusion with HTK solution + Ringer’s Lactate and heparin solution by portal vein (d). Renal right vein closure and renal excision. Arrow indicates right kidney presence and absence, respectively (e, f). SVC closure (5–0 silk indicated by arrow) (g) and VCI clamping and cannulation, indicate by arrow (h). Liver rat graft obtained after surgical procedure with main vessels preserved (i).
CRITICAL Continue to observe the respiration of the animal to make sure the animal continues breathing.
After laparotomy, wrap small intestine with moisten gauze and position small intestine on the left side of the abdominal cavity to expose the abdominal aorta.
Access the portal vein (PV) and make a dissection, isolating from surrounding tissue. Then, clamp PV with a microclamp and cannulate with the aid of a 20-gauge catheter (Descarpack®). Fix the catheter in place with 5–0 silk (Figure 2b, c).
CRITICAL Skeletonize the PV as much as possible to prepare for catheter insertion.
TROUBLESHOOTING!
Use a Nugent forceps in the left-hand to hold the surrounding’s intestine to skeletonize all PV prolongment. Hold the catheter in your right-hand e make a 90 angulation to promote PV exposure. Then, carefully, insert the catheter. Use a microclamp to prevent blood exposition. Furthermore, do a liver perfusion with organ storage solution or Ringer Lactate serum to avoid blood coagulation (Figure 2d).
Isolate the left and right kidneys. Ligate the left and right renal veins with a 5–0 nylon silk. Here, to perform renal vein ligation to allow IVC longer extension. After renal vein ligation, you will be able to skeletonize IVC and consequently performed anastomosis during recipient operation. Then, make a suture at the level of the lateral extremity of the inferior vena cava (IVC) and cut the kidneys before the left and right renal vein sutures to allow excision of the left and right kidney of the animal (Figure 2e, f).
Sequentially, make a rupture in the diaphragm and displaced from the animal’s rib cage to expose the entire end of the superior vena cava (SVC) and then wrapped with a 5–0 nylon suture (Figure 2g).
CAUTION Do not a closure now! Just wrap the SVC using a 5–0 nylon silk to permit normal blood circulation and avoid losing IVC stiffness before cannulation.
Isolate the IVC and make a dissection into regions near the lower limbs of the animal in order to ensure a greater venous extension and to separate it from the aorta.
After completely separated, cannulate the IVC with the aid of a 20 G catheter (Descarpack®). After cannulation, use a bulldog clamp to clamp IVC and fix the catheter in place with a 5–0 nylon suture wire (Figure 2h).
Finally, back to animal’s rib cage to close the VCS with a continuous suture using 5–0 nylon suture silk.
Release the liver of the donor animal carefully.
CAUTION Release from all tissue junctions that connect it to the other organs of the gastrointestinal system, keeping, carefully, the main vessels isolated (PV, IVC, SVC) and cannulate (PV, IVC). Gently pull upward, freeing it from surrounding structures. Take care to do not cut vessels.
TROUBLESHOOTING!
Use a Nugent forceps to hold de cannulated vessels and gently pull the graft liver upward with your hands.
After complete removal of the liver, place the liver on a 60 mm Petri dish (Figure 2i). Then, slowly perfuse the liver with 5 ml cold organ storage solution and 10 μl heparin. After that, remove the liver and submerge in cold sterile PBS solution or organ storage solution (4°C in a sterile container).
TROUBLESHOOTING!
The organ storage solution perfusion by PV will permit the efficient cannulation, sutures and closed vessel analysis. If all will be correct, you will observe a unidirectional flux inside the rat liver graft followed by PV perfusion, solution entrance inside the liver and blood release by IVC.
After perfusion, submit the liver rat graft to the decellularization procedure to generate an acellular liver scaffold.
Decellularization process
Harvested rat graft livers were decellularized. Briefly, liver was followed by cell-ECM detachment using perfusion with water and 1% w/v Triton X-100 solution, through the portal vein for 2 h at 3 mL/min. Subsequently, for 24 hours, a 1% w/v SDS solution was perfused at 6 mL/min until all cells were removed. Samples were washed with water for 2 d. Then, the decellularized liver scaffold was perfused with 0.1% peracetic acid, 1% penicillin and streptomycin for 1 h for sterilization. After decellularization, acellular liver scaffolds were stored in 5 ml of HTK (Histidine Tryptophan Ketoglutarate) solution containing heparin (10 ul) and perfused with solution (5 ml) containing HTK solution (2,5 ml) + Ringer’s Lactate (2,5 ml) + heparin (10 µl). Then, the ALS were submitted to transplantation in recipient rats (Figure 1).
DNA content analysis
DNA was isolated from 25 mg of control and acellular liver scaffold tissue and detected by DNeasy® Blood & Tissue Kit (Qiagen, Inc, Valencia, Ca). Then, the samples were submitted to read at NanoDrop 2000 C (Thermo Fisher Scientific).
Histological and immunohistochemistry analysis
Biopsies were formalin fixed (4%) for 24 h, paraffin embedded and sectioned (5 µm) for histological analysis. To analyze the general morphology, sections were stained with hematoxylin and eosin (H&E) and Sirius red.
TUNEL assay
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using ApopTag® In Situ Peroxidase Detection Kit (Merck Millipore, Massachusetts, USA) in according to instructions of the manufacture’s protocol.
Periodic Acid–Schiff (PAS) staining
Periodic Acid–Schiff (PAS) staining was performed through oxidation with 1% of periodic acid for 20 min and treatment with Schiff’s reagent for 30 min.
The images were analyzed and obtained using Pannoramic MIDI II microscopic and scanner (3DHISTECH Ltd, Hungria).
Acellular liver scaffold anticoagulation tests
Primarily, the ALS were placed in a 60 mm Petri dish and the PV catheter was attached to a 016 G pump tubing (Masterflex). Then, ex vivo blood perfusion was performed. To perform ALS blood perfusion, donor rats (n = 3) were submitted a blood collection (10 ml each) by superior vena cava access. After blood obtention, the collected blood (10 ml) was placed in tubes (50 ml) containing 40 ml of HTK (20 ml) + Ringer’s Lactate (20 ml) + heparin solution (10 µl) or 40 ml of PBS 1x. Then, we mix the tubes three times and subsequently, we performed ALS perfusion with 1) rat blood diluted in HTK + Ringer’s Lactate + heparin Solution (1:4) (n = 3) and 2) rat blood diluted in PBS 1x (1:4) (n = 3). The ALS blood perfusion was performed using peristatic pump (Masterflex Cole Parmer L/S, Model 7519–05) by portal vein at the speed of 3 ml/min for 1 hour, continually. After blood perfusion, in both cases, ALS perfused with blood diluted in HTK + Ringer’s Lactate + heparin or PBS samples were obtained and submitted to histological analysis.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (Prism 8 for Macintosh) (GraphPad Software, La Jolla, CA, USA). Data are described as means ± standard deviations (SD). The between-group comparations were performed by Student’s t test for paired variables.
Heterotopic acellular liver scaffold transplantation
Check Figure 3. Repeat Steps 1–8.
Dissect the left renal vein (RV) and renal artery (RA) at the height of the lateral branch of the IVC and the aorta to separate them from one another and facilitate the later stages (Figure 4a).
Clamp the IVC and the abdominal aorta at their upper extremities in order to interrupt blood flow in the left renal vein and artery using a bulldog clamp.
Cannulate the RV using a 24-gauge catheter. After cannulation, wrap the RV with a 5–0 nylon silk and clamp with a microclamp (Figure 4c).
Repeat step 22 to RA preparation. CRITICAL STEP: This procedure causes a lot of blood loss (Figure 4b).
Figure 3.
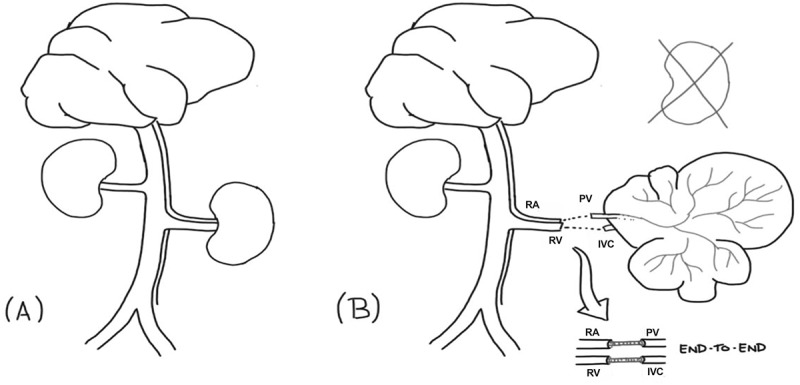
Schematic illustration of heterotopic liver transplantation model. Arterial-venous donor rat system before left nephrectomy (a). Acellular liver scaffold heterotopic transplantation in a recipient rat (b). RA, PV, IVC and RV abbreviations indicate renal artery, portal vein, inferior vena cava and renal vein, respectively.
Figure 4.
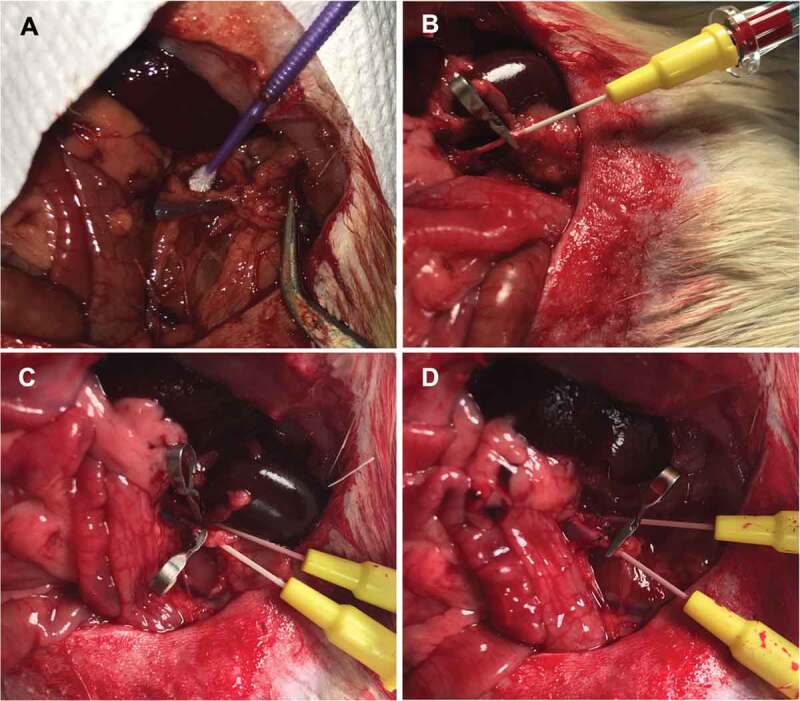
Surgical procedure prior heterotopic transplantation. Renal vein and artery isolating (a). Renal artery (b) and renal vein (c) cannulation. Left renal area post-nephrectomy (d).
TROUBLESHOOTING!
Blood volume lost during this step can be replaced with Lactated Ringer’s solution.
Perform left nephrectomy to create the space for heterotopic liver transplantation (Figures 3 and 4d).
Remove the upper extremities of the RA and RV catheters.
Subsequently, attach the PV (graft) and RA (recipient) catheters and IVC (graft) and RV (recipient) catheters. Use a combination of hand-suture and Cuff techniques to complete end-to-end anastomosis of the PV and RA and IVC and RV.
CRITICAL Anastomosis time >30 minutes would likely result in a poor survival rate.
TROUBLESHOOTING!
Check the anastomoses for bleeding and ensure that the vessels are not twisted, and that flow is not obstructed.
Place the small intestine back to abdominal cavity gently. Irrigate the abdomen with warm Ringer lactate solution’s and close the muscle and skin in two layers with 5–0 Vicryl. Additional saline can be administered to compensate for blood loss.
Wipe the skin surrounding the suture with 70% alcohol and place the animal on a warming pad for recovery.
Model 2: Acellular liver scaffold partial orthotopic transplantation postmedian lobe resection procedure
Allow the animals to acclimate for a minimum of 3 d in a temperature-controlled room with a 12-h light/dark cycle.
Place the donor rat in a chamber for induction of anesthesia with a mix of 2% isoflurane and oxygen (1–2 l/min).
CRITICAL Carefully observe frequency and depth of respiration of the animal and make sure the respiration does not arrest and check for a response to toe pinching every 1 min.
After induction of anesthesia, shave the abdominal wall with water and soap using a razor blade. After that, place the animal on the surgical table made and fix all four limbs to the table using rubber bands and with its face in the anesthesia system’s nozzle.
Disinfect the abdominal wall with disinfectant (70% ethanol).
CAUTION Wipe excess disinfectant with sterile gauze to avoid exposing internal organs to these disinfectants.
Start isoflurane inhalation with oxygen flow at 3–4% for the induction of anesthesia during laparotomy.
Do a transverse abdominal skin and muscle incision to expose the xiphoid process (Figure 9a, b). Expose the abdominal cavity by retracting the lower abdominal walls bilaterally using forceps (Figure 9c).
After making the transverse abdominal incision, lower the isoflurane flow to 1–2% for the maintenance of anesthesia.
Figure 9.
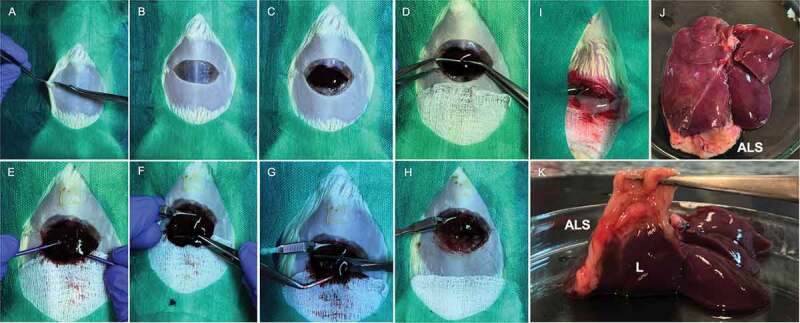
Acellular liver scaffold partial orthotopic transplantation. The rat is positioned in the supine position. A transverse abdominal incision is made, following Laparotomy steps (a, b). The median lobe is exposed (c) and pulling outside of abdominal cavity with Nugent forceps (d) or cotton tips (e). Then, a bulldog clamp is attached at the median lobe base (f). After that, a 10% of median lobe is resected (g). Macroscopic appearance postmedian lobe resection (h). Acellular liver scaffold partial transplantation. The liver scaffold is connected with recipient median lobe after suture with 7–0 silk (i). Recipient liver macroscopic appearance 30-d post-acellular liver scaffold orthotopic partial transplantation. “ALS” indicate acellular liver scaffold appearance post-transplantation (j). Acellular liver scaffold and recipient liver tissue integration and connection 30-d post-orthotopic partial transplantation (k).
CRITICAL Continue to observe the respiration of the animal to make sure the animal continues breathing.
After laparotomy, place sterile gauze below the opened abdominal cavity. Then, gently pull the median lobe down with a cotton tip or Nugent forceps (Figure 9d, e).
Use a bulldog clamp to clamp the median lobe base (Figure 9f).
CAUTION Place the clamp as close to the base of the lobe as possible. In few seconds note that the color of the median lobe will change. The lobe becomes dusky. This is a normal consequence of blood breakage. Soon, this part will be resected.
After median lobe clamping, use a scissor to cut 10% of median lobe (this amount can be variable taking into account the objective of your research) (Figure 9g, h).
#PLUS INFORMATION The median lobe represents 26 ± 2.5% of whole hepatic volume in rats. For this reason, different resections can be performed. This amount can be variate and more than two-thirds of the liver can be resected in mice, rats and humans. To more details check references.21-29
#PLUS INFORMATION Do not throw away the resected part of liver. The resected median lobe part can be useful to establish recipient liver histological analysis’ before transplantation.
Cut the acellular liver scaffold median lobe and place bellow of recipient median lobe resected area (Figure 9i).
Place the 7–0 silk to suture recipient median lobe with acellular liver scaffold.
CAUTION Take care to manipulate the liver post-resection. To avoid liver damage, gently place the 7–0 silk through the base of the median lobe.
TROUBLESHOOTING!
While suturing time, gently pulling down the bulldog clamp. So, to perform a continuous suture completely covering the resected area.
After suture, remove the clamp of the median lobe base.
Place the median lobe back to abdominal cavity gently. Irrigate the abdomen with warm Ringer lactate solution’s and close the muscle and skin in two layers with 5–0 silk. Additional lactated Ringer’s solution can be administered to compensate for blood loss.
Wipe the skin surrounding the suture with 70% alcohol and place the animal on a warming pad for recovery.
TIMING
To perform whole acellular liver scaffold heterotopic transplantation, our surgical procedures were performed in 90 and 110 minutes. To become technically proficient with Model 1 operation, we suggest approximately 30–40 cases to perform for a surgeon and suggest the use of microscope and lens of magnification during surgical procedure. To perform acellular liver scaffold postmedian lobe resection (Model 2), our surgical procedures were performed in 20–30 minutes. This surgical procedure is simple and fast. Wistar rats should recover within a few minutes after the end of the surgery. The Tramadol® (10 mg/kg) use is recommended to avoid pain and discomfort post-surgical.
Anticipated results
Here, the procurement steps performed were efficient to obtain the graft rat liver maintained the main vessels. PV, IVC and SVC cannulation and preservation were essential to form a unidirectional flux into the graft. Furthermore, our surgical technique was efficient to perform a liver graft and acellular liver scaffold transplantation. After graft liver obtention, the decellularization protocol was performed. Post-decellularization, an acellular liver scaffold was obtained. A translucid liver scaffold parenchyma was observed. Histological analysis revelated no nuclei or cytoplasm staining in acellular liver scaffold compared to normal liver. Furthermore, the DNA content was lower than 200 ng/mg of wet tissue (p < 0.001) in comparison with the DNA content present in normal liver samples (n = 3) (Figure 5). To avoid blood coagulation during transplantation into ASL, we performed ex vivo blood perfusion tests using a combination of solutions containing HTK solution, Ringer’s lactate and heparin. The results were compared with ALS perfused with PBS. After 1 h of rat blood perfusion with HTK + Ringer’s Lactate + heparin, no thrombus or clotting was microscopically detected into the scaffold. After histological analysis, this solution was able to avoid vessel congestion (Figure 6b) while ALS perfused with PBS do not prevent blood coagulation during blood perfusion (Figure 6a). Therefore, prior to our in vivo experiments, the ALS were perfused with a solution containing HTK + Ringer’s Lactate + heparin to avoid blood coagulation. To explore the whole acellular liver scaffold surgically we performed heterotopic transplantation (Figure 7). Here, the surgical procedure (Model 1) was efficient in promoting liver normal graft and acellular liver scaffold viability post-surgery. Some histologic analyses can be used to evaluate the graft post-transplantation (e.g., H&E staining, immunohistochemistry and immunofluorescence). H&E analysis showed some cell repopulation at acellular liver scaffold post-heterotopic transplantation (Figure 8c, d) and normal liver parenchyma post 22 hours after surgery (Figure 8a, b). A combination of hand-suture and Cuff techniques to complete end-to-end anastomosis of the PV (graft) and RA (recipient) and IVC (graft) and RV (recipient) permitted the connection between the graft and the recipient vasculature. After blood perfusion, the acellular liver scaffold shape resembled a native liver. Active blood flow within the acellular liver scaffold was observed indicating that the PV and IVC of the scaffolds were able to sustain the arterial blood pressure when the circulation was reestablished (Figure 7). No internal bleeding was observed prior to the rat abdomen closure. This surgical technique can be useful to clinical translational such as alternative therapy to patients suffering with acute hepatic failure. While the transplanted scaffold contributes to liver functions, the native liver can regenerate. In addition, other clinical benefits can be founded, such as drug therapy reduction time post-transplantation. On the other hand, the necessity of anatomical space to perform a transplantation and consequently establish blood supply into the liver scaffold represents a clinical challenge. To overcome this clinical challenge and to explore the partial acellular liver scaffold lobe, we explored a second surgical model of liver transplantation. In this case, the graft was transplanted in the same anatomical position of the native liver after partial liver lobe resection. This surgical technique can be useful to clinical translational because the scaffold can support the liver recovery post resections of injury or damage parts of the liver. It is possible because residential liver cells can recellularized the liver scaffold and regenerate. In addition, partial orthotopic transplantation post-liver resection represents a safe and simple surgical procedure. However, this technique can be limited by liver volume resections and by the risk of “small-for-size” syndrome development post-partial liver resections prior to transplantation. Using model 2, the potential for liver regeneration post-resection is supported by acellular liver scaffold transplantation. The partially hepatectomized recipient rat can be evaluated for different analyses, including macroscopic, histological and biochemistry analysis to check the regeneration and tissue-ECM connections. Here, we recommend performing this analysis before and after liver scaffold transplantation. In this present study, we macroscopically evaluate the transplanted liver rat 30-d post-partial orthotopic surgery (Figure 9j, k). Macroscopic evaluation of specimens collected on days 3 and 30 after surgery revelated an adhesion to peritoneum around the resection and sutured acellular liver scaffold area. Furthermore, we detected acellular liver scaffold and recipient liver completely integration (Figures 9 and 10). After Sirius red staining we are able to identify and delimited transplanted ALS and recipient liver area (white dotted line in Figure 10b, c). The collagen appeared more remarkable within the ASL area. H&E staining confirmed whole acellular liver scaffold recellularization post-transplantation (Figure 10). Infiltration of inflammatory cells was detected, and these cells were more distributed near the connection area between transplanted ALS and recipient liver (Figure 10d). Other cell types could be detected along the transplanted ALS (Figure 10f, g). Furthermore, we identify red blood cells into recipient liver sinusoids and the transplanted ALS parenchyma. Blood vessel-like structure was identified into the transplanted ALS (Figure 10c, f). No suture silk was detected 30-d post-acellular liver scaffold transplantation. Finally, after confirmed the ALS in vivo cell recruitment and whole recellularization, we evaluated the apoptotic nuclei presence into the ALS. TUNEL analysis reveals apoptotic cells in heterotopically and orthotopically transplanted ALS (Figure 11). To evaluate hepatocyte engraftment in transplanted ALS, we performed PAS staining. The glycogen storage was detected into the recipient liver and into the orthotopically transplanted ALS (Figure 12b). However, no evidence of glycogen storage was detected in ALS heterotopically transplanted (Figure 12c).
Figure 5.
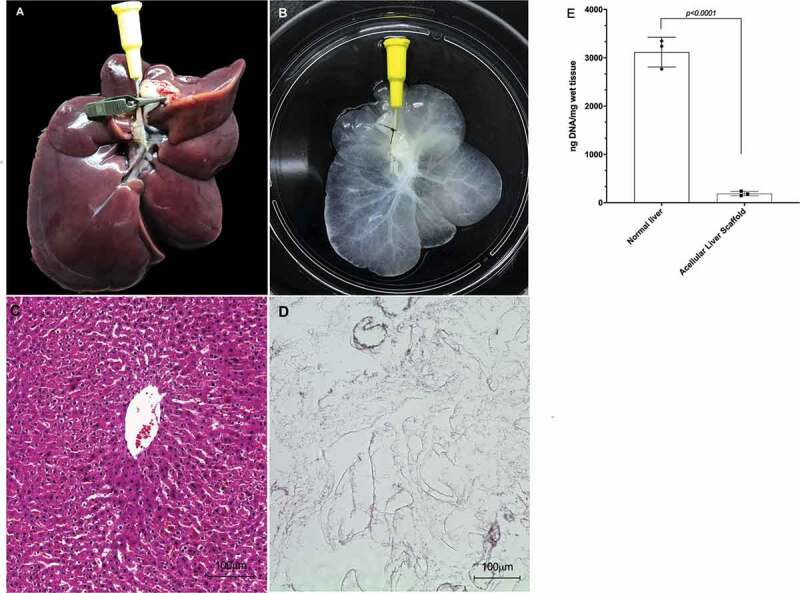
Macroscopic and Microscopic appearance of normal liver and acellular liver scaffold obtained by decellularization process. Normal (a) and acellular liver scaffold (b). Hematoxylin and eosin staining of the normal liver (c) and acellular liver scaffold (d). 10x and 20x magnification, respectively. Scale bars: 100 µm. DNA quantification demonstrated significant DNA reduction from 3118,4 ng/mg in normal liver to 189,06 ng/mg in acellular liver scaffold (e). N = 3 biological triplicates, *p < .05.
Figure 6.
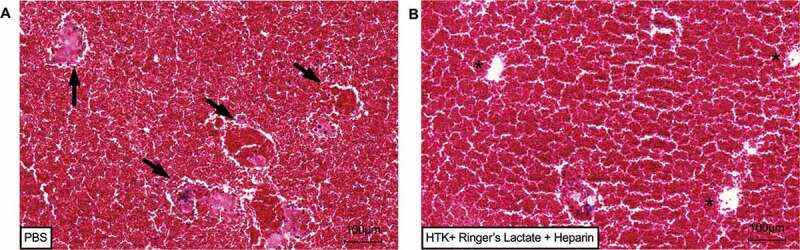
Histological evaluation of ALS submitted to ex vivo blood perfusion tests. H&E staining revelated whole blood vessel congestion (black arrows) in ALS perfused with rat blood diluted in PBS after 1 h of continuos perfusion (a). No blood vessel congestion or clotting were detected in ALS perfused with rat blood diluted in HTK solution + Ringer’s Lactate and heparin after 1 h of continuous perfusion (b). 10x, Scale bars: 100 µm.
Figure 7.
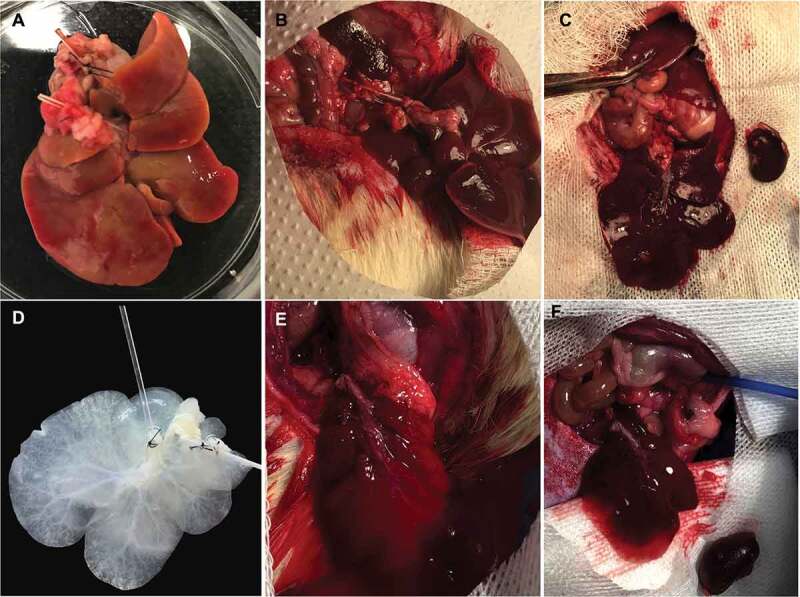
Liver heterotopic transplantation. Liver rat graft post-HTK perfusion (a). Liver graft post-anastomosis between PV and RA (1) and RV and IVC (2) (b). Macroscopy view of liver rat graft heterotopic transplantation on left renal position (c). Acellular liver scaffold prior transplantation (d). Acellular liver scaffold post anastomosis and microclamp removal (e). Macroscopy view of acellular scaffold heterotopic transplantation on left renal position (f).
Figure 8.
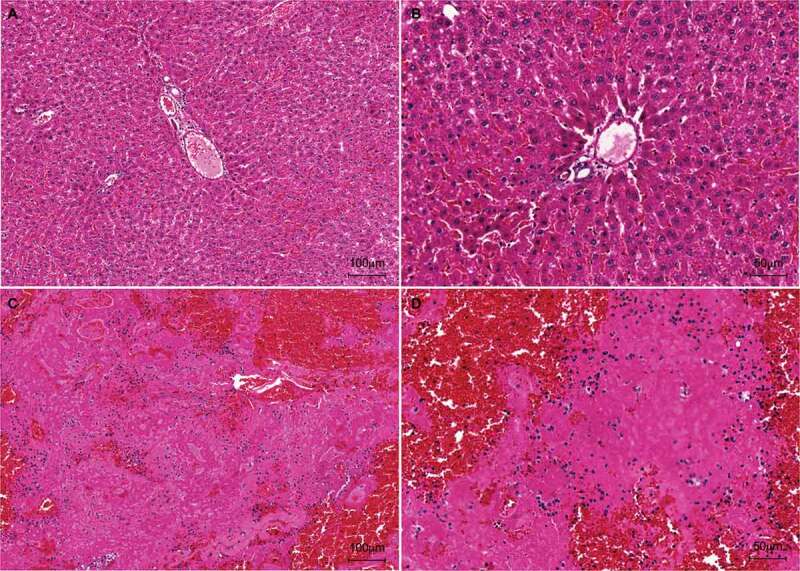
Histological analysis (H&E staining) of hepatic graft post-heterotopic transplantation. At 24 h after transplantation, the graft presented a normal hepatic parenchyma, composed of radially organized hepatocytes. 10x, scale bar: 100 µm (a) and 20x magnification, scale bar: 50 µm (b). H&E staining of acellular liver scaffold 2 h after transplantation. The transplanted acellular liver scaffold in the left renal position of the animal was recellularized by the cells of the recipient’s bloodstream after transplantation. 10x, scale bar: 100 µm (c) and 20x, scale bar: 50 µm (d).
Figure 10.
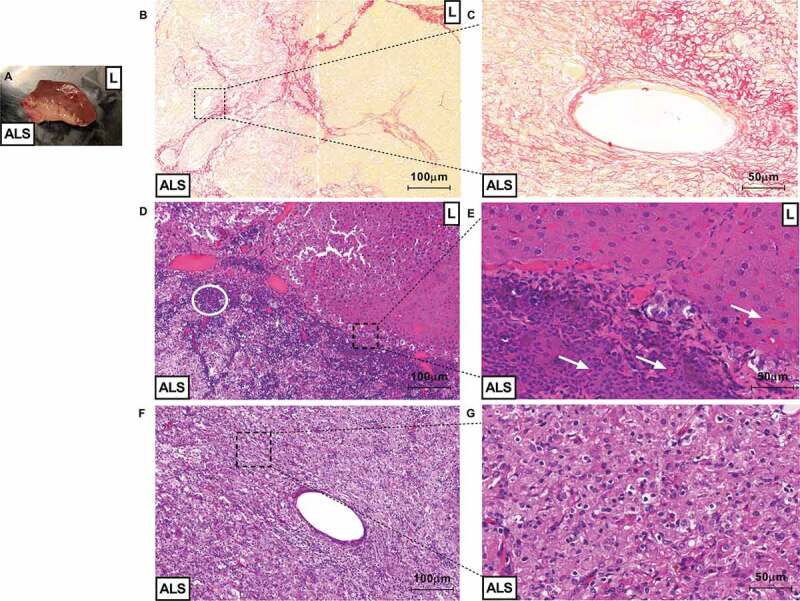
ALS could be recellularized after partial orthotopic transplantation. Macroscopic view of tissue connection area between recipient liver (represented by “L”) and acellular liver scaffold (represented by “ALS”) 3-d post-partial orthotopic transplantation (a). Microscopic view of liver sections obtained post 30 d of transplantation (B, C, D, E, F and G). Sirius red staining show delimitation between transplanted ALS area and recipient liver area. The white dotted line represents acellular liver scaffold and recipient liver tissue connection area (injured/fibrotic area) (b). Magnification of blood vessel-like structure formed into transplanted ALS (c). Recipient liver area (represented by “L”) appears a normal liver parenchyma 30-d post-transplantation (C). Infiltration of inflammatory cells (white circle) were detected into acellular scaffold area (represented by “ALS” (d). Red blood cells (white arrows) were detected into ALS vessel and recipient liver sinusoids (e). The acellular liver scaffold was completely recellularized post-orthotopic transplantation (f and g). Scale bars: 100 µm (B, D and F), 50 µm (C, E and G), 10x and 20x, respectively.
Figure 11.
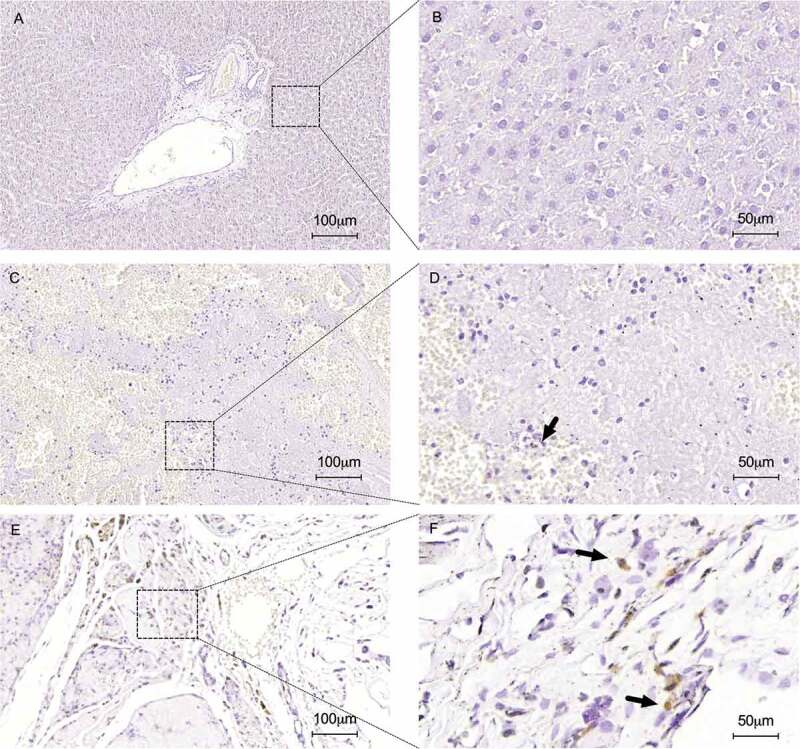
Immunohistochemical staining for apoptotic nuclei detection using the TUNEL method. No apoptotic nuclei were detected in normal liver sections represented in low (a) and high magnification (b). TUNEL analysis confirmed apoptotic cells into heterotopically (c and d) and orthotopically (e and f) transplanted ALS (black arrows). Normal liver samples (a and b) were used such as control. Scale bars: 100 µm (A, C and E), 50 µm (B, D and F), respectively.
Figure 12.
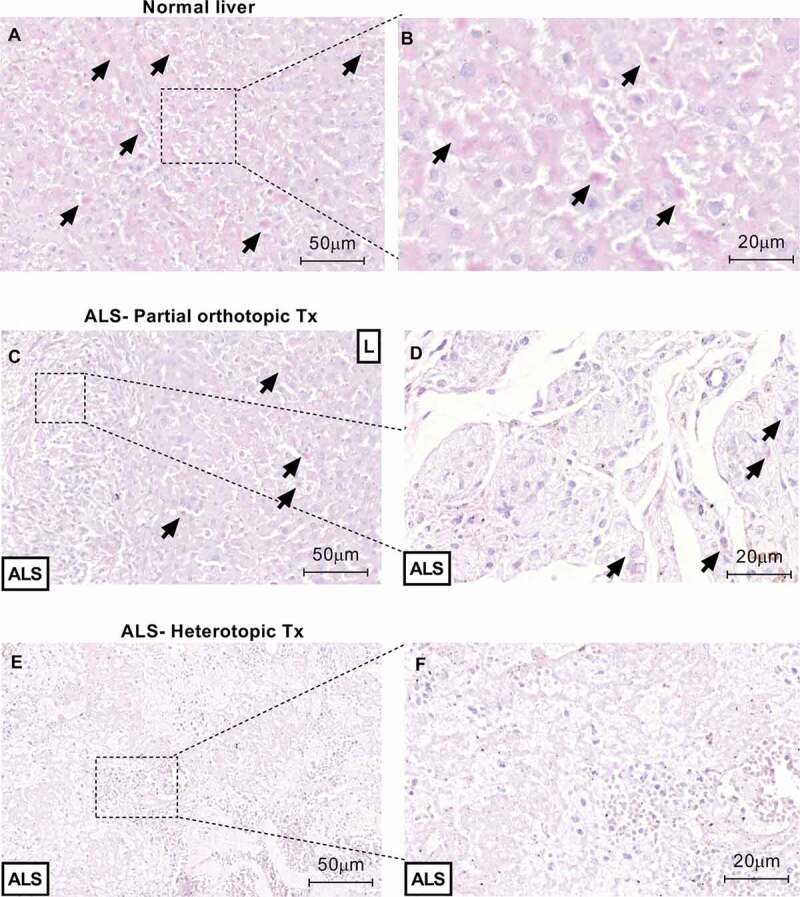
Transplanted ALS induce hepatocytes engraftment 30 d after transplantation. PAS staining confirms glycogen storage (black arrows) into orthotopically transplanted ALS (b). No glycogen storage was detected into heterotopically transplanted ALS. Normal liver samples (a and b) were used such as control. Scale bars: 50 µm (A, C and E), 20 µm (B, D and F), respectively.
To our knowledge, this is the first time that step-by-step of two different surgical techniques is describes to perform acellular liver scaffold transplantation and to explore the potency of their utilization to cell recruitment in vivo.
Discussion
Experimental microsurgery comprises one of the scientific developments inherent in the use of animals. Its development was important in elucidating medical, physiological and biophysical findings that together provided the realization of human medical practices, whether simple or complex. In particular, experimental microsurgery allows the realization of several techniques that can be applied to experimental models of studies involving hepatic regenerative medicine. Currently, some of these techniques have been widely used to provide the use of tissue engineering products in animal models. With the promising use of bioartificial organs generated by the decellularization technique, we can use some transplantation strategies created by experimental microsurgery, such as heterotopic and orthotopic transplantation. There are several protocols of these surgical techniques described in the literature. However, there is neither step-by-step description of the surgical technique to perform acellular liver scaffold transplantation or evaluation of acellular liver-engineered construct potency to cell recruitment. For this reason, step-by-step of a simple standard model to perform acellular liver transplantation is necessary.
According to Arias et al.,16 heterotopic transplantation techniques differ in aspects related to the type of graft vasculature that may be only arterial or portal or formed by the junction of both; to the type of venous drainage, which can be obtained by the superior vena cava (SVC) or inferior vena cava (IVC); the type of portal blood, which may be splenic or systemic; by biliary drainage, which can be reconstructed and obtained through bile duct duodenostomy or jejunum duodenostomy; the anatomical location of the graft, which may be intra- or extra-abdominal; besides the mass of the graft, which may be total or partial. Several surgical strategies may be adopted by manipulating each of these possibilities. As far as bioartificial organ transplantation is concerned, heterotopic transplantation is the most widely adopted type of surgical procedure.17,18,20 The first approach to acellular liver scaffold transplantation was published in 2010. Uygun et al.30 produced a functional scaffold from decellularized hepatic ECM obtained from rat livers, recellularized with murine hepatocytes for more than 5 d. The hepatic scaffold was heterotopically transplanted into the right renal position of recipient rats. Unfortunately, no details of the surgical steps of the surgical procedure adopted were published. Here, the acellular liver scaffold was heterotopically transplanted into the left renal position of recipient. We choose the left renal position because this area represents the largest anatomical space. Therefore, the renal vessel manipulation can be improved. In 2018, Yang et al.31 heterotopically transplanted the acellular liver scaffold obtained from mouse liver into C57BL/6 animals. Specifically, in this study, the authors developed a heterotopic-arterialized portal-renal type transplant. For this, end-to-end anastomosis was performed between RA of the recipient and the PV of the liver scaffold. By end-to-side anastomosis, the IVC was ligated. Heterotopic transplantation of the decellularized hepatic ECM was performed in the right renal position of the recipient animal in order to investigate the ECM’s potential to receive blood from the recipient animal and enable its recellularization. This surgical approach was an intelligent technique to perform mouse heterotopic transplantation. Recently, Meng et al.24 have developed a study to reconstruct the vasculature of decellularized hepatic ECM produced from livers of Sprague–Dawley rats. After an infusion-based treatment of decellularized ECM with gelatin-based hydrogels, the produced scaffold was heterotopically transplanted in the left renal position of the recipient animal. The connection between the scaffold and the recipient animal was possible by performing the end-to-side anastomoses between hepatic scaffold PV and the abdominal aorta of the recipient animal and between the hepatic scaffold and recipient animal scaffold IVC. This strategy surgical technique involving end-to-side is more laborious because involves the connecting end of one vessel with the side of the another one without the application and facility of Cuff technique. Therefore, the anastomosis time can be higher. Here, we describe a step-by-step to perform an acellular liver scaffold heterotopic transplantation. We developed a surgical procedure to make a rat liver graft procurement preserving the main vessels such portal vein, inferior vena cava and superior vena cava. The preservation of the vessels allowed the formation of unidirectional flow in the graft procurement and was essential to ensure the process of decellularization and subsequent transplantation in the recipient animal. We preserved the PV during liver graft procurement to use this vessel such as inflow zone during decellularization process and heterotopic transplantation. The IVC was used such as zone to allow blood and cell content removal during decellularization step. During the heterotopic transplantation, the PV was connected with RA of the recipient by end-to-end anastomosis and an adaptation of Cuff technique. Therefore, PV-RA anastomosis allowed the recipient blood flux motion to acellular liver scaffold. A few minutes after performed PV-RA anastomosis, the acellular liver scaffold assembled a native liver. Furthermore, the IVC and RV anastomosis allowed the blood flux return to recipient. Thanks to our anticoagulation treatment, no thrombus, clots or vessel congestion were detected during and after ALS in vivo transplantation. Our strategy involves a solution containing HTK solution, one of the most useful organ storage solution applied to organ transplantation, Ringer’s Lactate serum and heparin. This solution was efficient to avoid blood coagulation during our ex vivo experiments using rat blood perfusion and during in vivo transplantation. To confront our results using HTK + Ringer’s Lactate and heparin solution, the ALS were perfused with blood diluted in PBS. Our histological analysis confirmed whole blood vessel congestion and thrombus formation. Because that, before our in vivo transplantation experiments the ALS were perfused with HTK + Ringer’s Lactate and heparin solution.
The second surgical model described here represents a proof of concept that acellular liver scaffolds generated by decellularization process are useful to transplantation post-liver resections.25 Our surgical steps allowed direct connections between the acellular graft and recipient native liver. Furthermore, our approach was efficient to allow acellular liver scaffold recellularization post-transplantation. Shimoda et al.26 performed porcine acellular liver graft transplantation post-partial hepatectomy in pigs and confirmed that decellularized liver scaffolds contribute to liver regeneration. They found inflammatory cells, fibroblasts, hepatocytes and CD31 positive cells into scaffold after 28 d of transplantation. Naeem et al.27 performed a lobectomy to explore acellular graft recellularization in situ. The oval cells, stellate cells, cholangiocytes and hepatocyte were detected into the graft after 30 d of transplantation. Here, according to Naeem, our ALS were efficient to promote cell recruitment, including inflammatory cells after 2 h of transplantation and hepatocyte engraftment after 30-d post-transplantation in partial orthotopic transplantation. In addition, a few apoptotic nuclei cells were detected into the transplanted ALS.
To date, there are no studies in the literature addressing transplantation of acellular liver scaffolds in models of liver diseases such as fibrosis/cirrhosis. This approach represents an important point to be explored, since one of the objectives of regenerative hepatic medicine is to treat patients affected by the most diverse diseases that affect the liver. For this reason, we recommend our surgical models to realize it. Here, two step-by-step of two different models to transplant acellular liver scaffold was developed. In addition, cell recruitment in vivo was directed by transplanted engineered liver scaffolds. They are a feasible and reliable rat model for liver transplantation study involving tissue engineering products. Our models are expected to be very useful for future studies on the transplantation of bioengineered liver. This is strongly supported by the fact that numerous genetically manipulated strains of rats are currently available.
Conclusion
In summary, we have successfully established a step-by-step description regarding two models of acellular liver transplantation. Using these models, we have found cell repopulation into the acellular liver scaffold. Furthermore, our model allows to found liver graft viability 30-d post-transplantation. These models may prove valuable in addressing questions related to liver regeneration, cell recruitment and transplantation of bioengineered livers.
Funding Statement
This paper was funded by the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Foundation (FAPERJ), the Department of Science and Technology (DECIT)/Brazilian Ministry of Health, the Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Institute of Science and Technology for Regenerative Medicine.
Disclosure of potential conflicts of interest
The authors declare that they have no competing financial interests.
Author contributions statement
M. L. D.: PhD student involved with all experiments described here. Furthermore, this author was responsible for wrote this paper.
C. M. P. B.: Undergraduate student involved with surgeries. She performed all surgical procedures with M. L. D.
A. C. S.: Professor and gastric surgeon involved with elucidation of heterotopic and partial orthotopic surgical methods. He helped all surgeries described here.
V. J. K. S.: MdPhD student involved with surgical steps to donor liver procurement and posterior decellularization.
V. R. S. M.: PhD student responsible to perform immunohistochemistry.
L. A. F.: Postdoctoral responsible to do decellularization process.
R. C. S. G.: Principal investigator. Dr Regina was responsible for ideas, design of work, objectives, manuscript revision and coordination.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxma AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4.. [DOI] [PubMed] [Google Scholar]
- 2.Crapo PM, Gilbert TW, Badylak SF.. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233‐3243. doi: 10.1016/j/biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoshiba T, Chen G, Endo C, Maruyama H, Wakui M, Nemoto E, Kawazoe N, Tanaka M. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016;6397820. doi: 10.1155/2016/6397820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 5.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz J, Fiore V, Barker T, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Resp Crit Care Med. 2012;186(9):866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Bao J, Wu Q, Zhou Y, Li Y, Wu X, Shi Y, Li L, Bu H. Method for perfusion decellularization of porcine whole liver and kidney for use as a scaffold for clinical‐scale bioengineering engrafts. Xenotransplantation. 2015;22(1):48–6. doi: 10.1111/xen.12141. [DOI] [PubMed] [Google Scholar]
- 7.Kakabadze Z, Kakabadze A, Chakhunashvili D, Karalashvili L, Berishvili E, Sharma Y, Gupta S. Decellularized human placenta supports hepatic tissue and allows rescue in acute liver failure. Hepatology. 2018;67(5):1956–69. doi: 10.1002/hep.29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uygun BE, Price G, Saeidi N, Izamis ML, Berendsen T, Yarmush M, Uygun K. Decellularization and recellularization of whole livers. J Vis Exp. 2011;48:e2394. doi: 10.3791/2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrokhi A, Pakyari M, Nabai L, Pourghadiri A, Hartwell R, Jalili R, Ghahary A. Evaluation of detergent-free and detergent-based methods for decellularization of murine skin. Tiss Eng Part A. 2018;24(11-12:955–67. doi: 10.1089/ten.TEA.2017.0273. [DOI] [PubMed] [Google Scholar]
- 10.Badylak S, Liang A, Record R, Tullius R, Jason H. Endothelial cell adherence to small intestinal submucosa: an acellular bioscaffold. Biomaterials. 1999;20(23–24):2257–63. doi: 10.1016/s0142-9612(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 11.Zheng CX, Sui BD, Hu CH, Qiu XY, Zhao P, Jin Y. Reconstruction of structure and function in tissue engineering of solid organs: toward simulation of natural development based on decellularization. J Tiss Eng Reg Med. 2018;12(6):1432–47. doi: 10.1002/term.2676. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DA, Frazier OH, Elgalad A, Hochman‐Mendez C, Sampaio LC. Building a total bioartificial heart: harnessing nature to overcome the current hurdles. Art Org. 2018;42(10):970–82. doi: 10.1111/aor.13336. [DOI] [PubMed] [Google Scholar]
- 13.Cesaretti M, Le Bian AZ, Moccia S, Iannelli A, Schiavo L, Diaspro A. From deceased to bioengineered graft: new frontiers in liver transplantation. Transp Rev. 2019;33(2):72–76. doi: 10.1016/j.trre.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang A, Kuriata O, Xu F, Nietzsche S, Gremse F, Dirsch O, Settmacher U, Dahmen U. A survival model of in vivo partial liver lobe decellularization towards in vivo liver engineering. Tiss Eng Part C. 2019. doi: 10.1089/ten.TEC.2019.0194. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XY, Wheatley AM. Biological effect of heterotopic liver transplantation. In: Timmermann W, Gassel HJ, Ulrichs K, Zhong R, Thiede A, editors. Organ transplantation in Rats and Mice. Berlin (Heidelberg): Springer; 2018. p. 545–54. doi: 10.1007/978-3-642-72140-3_55. [DOI] [Google Scholar]
- 16.Lee S, Edgington TS. Heterotopic liver transplantation utilizing inbred rat strains. I. Characterization of allogeneic graft rejection and the effects of biliary obstruction and portal vein circulation on liver regeneration. Am J Path. 1968;52(3):649–69. PMC201325; PMID: 4868325. [PMC free article] [PubMed] [Google Scholar]
- 17.Aller M, Arias N, Prieto I, Agudo S, Gilsanz C, Lorente L, Arias JL, Arias J. A half century (1961-2011) of applying microsurgery to experimental liver research. W J Hep. 2012;4(7):199–208. doi: 10.4254/wjh.v4.i7.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono Y, Pérez-Gutiérrez A, Yovchev MI, Matsubara K, Yokota S, Guzman-Lepe J, Handa K, I’Hortet AC, Thomson AW, Geller, et al. Regeneration and cell recruitment in an improved heterotopic Auxiliary Partial Liver Transplantation (APLT) model in the rat. Transplantation. 2017;101(1):92–100. doi: 10.1097/TP.0000000000001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schleimer K, Stippel DL, Tawadros S, Hölzen J, Hölscher AH, Beckurts KT. Improved technique of heterotopic auxiliary rat liver transplantation with portal vein arterialization. Langenbecks Arch Surg. 2006;391(2):102‐107. doi: 10.1007/s00423-006-0032-x. [DOI] [PubMed] [Google Scholar]
- 20.Shleimer K, Kalder J, Grommes J, Jalaie H, Tawadros S, Greiner A, Jacobs M, Kokozidou M. Heterotopic auxiliary rat liver transplantation with flow-regulated portal vein arterialization in acute hepatic failure. J Vis Exp. 2014;91(e51115):1–9. doi: 10.3791/51115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi E, Yoshida Y, Nozawa M, Hishikawa S, Yamanaka T, Miyata M, Fujimura A. Auxiliary heterotopic liver transplantation in the rat: a simplified model using cuff technique and application for congenitally hyperbilirubimemic Gunn rat. Microsurgery. 1998;18(2):97–102. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz C, Kaczirek K, Bodingbauer M. Liver resection for non-colorectal metastases. Eur Surg - A Chi Aust. 2018;50(3):113–16. doi: 10.1007/s10353-018-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castaing D, Salloum C. Técnicas de hepatectomía por laparotomía. EMC - Técnicas Quirúrgicas - Aparato Digestivo. 2011;27(2):1–17. doi: 10.1016/S1282-9129(11)71063-7. [DOI] [Google Scholar]
- 24.Meng F, Almohanna F, Altuhami A, Assiri AM, Broering D. Vasculature reconstruction of decellularized liver scaffolds via gelatin‐based re‐endothelialization. J B M Res Part A. 2019;107(2):392–402. doi: 10.1002/jbm.a.36551. [DOI] [PubMed] [Google Scholar]
- 25.Hori T, Uemoto S, Zhao X, Chen F, Baine AT, Gardner LB, Ohashi N, Conkle F, Castanedes-Casey M, Phillips VR. Surgical guide including innovative techniques for orthotopic liver transplantation in the rat: key techniques and pitfalls in whole and split liver grafts. A Gastr. 2010;23:270–95. [Google Scholar]
- 26.Shimoda H, Yagi H, Higashi H, Tajima K, Kuroda K, Abe Y, Kitago M, Shinoda M, Kitagawa Y. Decellularized liver scaffolds promote liver regeneration after partial hepatectomy. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-48948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naeem EM, Sajad D, Talaei-Khozani T, Khajeh S, Azarpira N, Alaei S, Tanideh N, Reza TM, Razban V. Decellularized liver transplant could be recellularized in rat partial hepatectomy model. J Biomed Mater Res Part A. 2019;107(11):2576–88. doi: 10.1002/jbm.a.36763. [DOI] [PubMed] [Google Scholar]
- 28.Gaub J, Iversen J. Rat Liver Regeneration After 90% Partial Hepatectomy. Hepatology. 1984;4(5):902–04. doi: 10.1002/hep.1840040519. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3(7):1167–71. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 30.Uygun B, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Chen Q, Xia R, Zhang Y, Shuai L, Lai J, You X, Jiang Y, Bie P, Zhang L, et al. A novel bioscaffold with naturally-occurring extracellular matrix promotes hepatocyte survival and vessel patency in mouse models of heterologous transplantation. Biomaterials. 2018;177(1):52–66. doi: 10.1016/j.biomaterials.2018.05.026. [DOI] [PubMed] [Google Scholar]


