Ketamine was first synthesized in the early 1960s in search for anesthetic compounds and has been a mainstay for anesthesia since its approval by the United States Food and Drug Administration (USFDA) in 1970 [1]. Early clinical observations suggested that ketamine had a profile that differed from more traditional anesthetics, including producing a disconnected dreamlike state, and it was therefore referred to as a “dissociative” anesthetic [2,3]. For many years, the emphasis of ketamine research and clinical work focused on its anesthetic properties. More recently, however, there has been a growing interest in effects of ketamine at subanesthetic doses. This was sparked, in part, by the finding of rapid, long-lasting antidepressant properties at low doses, and to be effective in individuals who are resistant to traditional antidepressants [4]. It is therefore now categorized as a rapid-acting antidepressant (RAAD) effective in treatment-resistant (TRD) individuals. The rapid response is especially exciting since traditional antidepressants, such as serotonin reuptake inhibitors (SSRIs), can take several weeks to produce a therapeutic response. Moreover, ketamine’s pharmacological effects are unique compared to traditional antidepressants and it therefore represents the first of a potential novel class of psychotherapeutic drugs. After considerable clinical and preclinical work in the area, esketamine (the S(+) enantiomer of ketamine) was approved by the USFDA in 2019 for TRD [5]. There has been a burst of research into ketamine to better understand the mechanisms that underlie its antidepressant effects (see Fig. 1). Concerns about side effects, including dissociative effects and abuse potential, prevent its broad use and lead to hope that, by better understanding its mechanisms, alternatives can be developed.
Fig. 1.
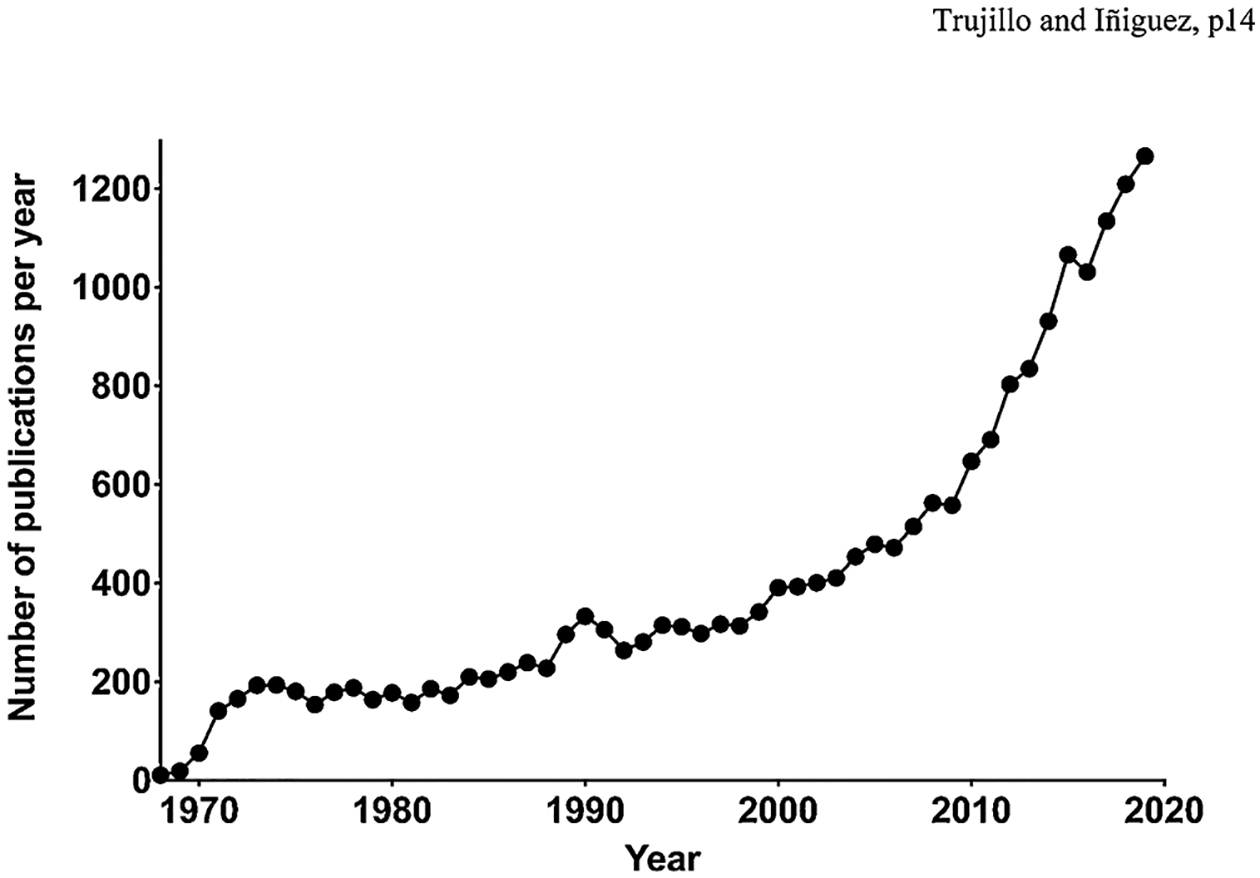
Number of publications on ketamine indexed in PubMed each year from 1965 to 2019, based on a simple keyword search. Note the upward incline beginning in the late 1990s, and then escalating in the past decade, illustrating the tremendous growth in research on the compound.
The use of ketamine for non-medical purposes emerged concurrently with its use as an anesthetic among young people seeking its unique dissociative effects [6,7]. Ketamine abuse remained at relatively low, but consistent levels for many years. However, a significant escalation in use began in the early 2000s, which was associated with the dance club and rave scene [8–10]. Because of its use in these settings, ketamine has sometimes been referred to as a “club drug.” Among the effects sought by users include a euphoric rush, altered senses, melting into surroundings, visual hallucinations, out-of-body experiences, and giggliness [8,9]. Although many use it intermittently in weekend party settings, the use of ketamine can escalate to a compulsive addiction-like pattern (see [11] for review). A better understanding of its effects will help with the prevention and treatment of ketamine abuse and addiction.
The focus of this special issue of Behavioural Brain Research is the effects of ketamine at subanesthetic doses, and in particular, the antidepressant effects and abuse potential. For this issue, we brought together an impressive group of scientists whose work leads to a better understanding of this fascinating compound. Eight of the contributions are literature reviews that address the mechanisms of ketamine action,while twelve are empirical papers that offer new insight into ketamine’s short and long-term effects at low doses. Together they provide a state-of-the-art look at the current status of research on subanesthetic ketamine.
Among the many top scientists who contributed to this Special Issue, we’d like to first acknowledge Dr. Edward Domino, who has been working on ketamine and related drugs for more than 60 years. Dr. Domino was a key scientist in the early development of ketamine, and was, in fact, the lead author on the first publication to examine the effects of ketamine (then known as CI-581) in humans [3]. In 2010, Dr. Domino summarized his personal decades-long scientific odyssey with ketamine in a paper aptly entitled “Taming the Ketamine Tiger” [2]. In the current review paper, Domino and his colleagues evaluate the pharmacological profile of ketamine and some of the key targets that are likely involved in its antidepressant actions [12].
Sial et al. (2020) offer an excellent overview of the current understanding of potential mechanisms that underlie ketamine’s antidepressant effects, including circuitry and intracellular signaling molecules [13]. They summarize clinical studies and preclinical work, address conflicts and controversies, as well as identify gaps in knowledge. This review paper is the perfect place to start for anyone interested in the broad view of the topic. Similarly, Carreno et al. (2020) review the antidepressant effects of ketamine, including brain circuits, synaptic mechanisms and intracellular signaling pathways [14]. They focus on a circuit from the ventral hippocampus to the medial prefrontal cortex that they believe is essential to the sustained antidepressant effects of the drug. Based on preclinical work, they point to novel drug classes that impact on GABAergic signaling in this pathway that may replicate the antidepressant effects of ketamine with fewer adverse effects.
Three of the review papers focus on the role of stress in the antidepressant effects of ketamine. Rincón-Cortés and Grace (2020) summarize preclinical research on the ability of ketamine to both prevent and reverse the impact of stress on brain and behavior [15]. They hone-in on the role of dopaminergic dysfunction in the mesolimbic system as a key pathway in depression-related symptoms. They suggest that affective regulation involves a balance between specific pathways that drive or reduce mesolimbic dopamine system function. The dysregulation of this system by stress, a key risk factor for the development of major depressive disorder (MDD), is stabilized by low doses of ketamine. Polis, Fitzgerald, Hale and Watson [16] also address stress in their review paper. The first half of their review summarizes the potential molecular targets and circuits involved in ketamine’s antidepressant actions. The second half addresses variability in responses to ketamine in animal models of antidepressant action and the potential role of stress and other factors in this variability. It is essential to better understand these factors in preclinical studies to assure that different laboratories are investigating the same phenomenon when trying to elucidate the antidepressant effects of ketamine. Abdallah and Krystal [17] provide a short review and perspective on human and animal research related to chronic stress pathology and its relationship to depression and the antidepressant effects of ketamine. They make the point that multiple psychiatric disorders – including MDD, bipolar depression, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD) and obsessive-compulsive disorder – are related to chronic stress and synaptic dysconnectivity, and that addressing synaptic dysfunction leads to positive therapeutic outcomes. They then offer a series of recommendations on how the field can move toward a cure, rather than simply treatments for MDD and other stress-related disorders.
Grabski, Borissova, Marsh, Morgan and Curran [18] address a very different and very important topic related to the antidepressant actions of ketamine – whether the psychoactive effects (such as the dissociative, psychotomimetic, hallucinogenic or mystical effects) contribute to the therapeutic response. These effects are often dismissed as unwanted side effects; however, if they are related to treatment efficacy, then it would be counterproductive to seek alternatives to ketamine that fail to produce these effects. Indeed, there is evidence that the psychoactive properties of psychedelic drugs, such as psilocybin and MDMA, contribute to their therapeutic actions [19,20] so it is reasonable to ask the same of the potent dissociative, ketamine. After reviewing the literature, Grabski and coworkers conclude that there is currently insufficient evidence to answer the question, and they offer guidance for future work on the topic.
The review paper from Kokane et al. [21] brings together the two major themes of this special issue by addressing the common molecular mechanisms that underlie the abuse of ketamine and its antidepressant effects. They begin by reviewing ketamine’s molecular targets and then summarize the evidence for its antidepressant effects and abuse potential. They then point to the mesolimbic dopamine system as key to both the antidepressant effects and the abuse potential of ketamine, and highlight specific downstream messengers, including brain-derived neurotrophic factor (BDNF), glycogen synthase kinase-3, and mammalian target of rapamycin.
Together, these review papers offer an excellent state-of-the-art overview on the pharmacological profile of subanesthetic ketamine, and the mechanisms that contribute to its antidepressant effects and abuse potential. Moreover, they point to gaps in the literature and fruitful directions for future research. In addition to these reviews, this special issue includes twelve empirical papers that add significantly to the current understanding of ketamine’s actions in preclinical models for the study of MDD and addiction related behavior.
The empirical papers in this special issue addressed three major overarching themes: RAAD properties of ketamine, short-term and long-term effects of ketamine during development, and effects of ketamine related to drug abuse and addiction. The RAAD properties of ketamine have been recapitulated in preclinical models of stress over the past decade [5,22,23]. Here, several lines of work corroborate and expand on the ability of subanesthetic doses of ketamine to reverse stress-induced maladaptive behavior across different animal models of depression-related symptomatology [24–26]. Specifically, Ecevitoglu and colleagues report that oral administration of ketamine (0.4 mg/day) decreases behavioral despair on the forced swim test, a traditional antidepressant-like response, without altering memory performance in adult male rats [27]. Likewise, Kim and Monteggia demonstrate that low (5 mg/kg), but not high (50 mg/kg), doses of ketamine increase BDNF, while facilitating synaptic potentiation within the CA1 region of the hippocampus. This work reveals novel neurobiological mechanisms that may underlie ketamine’s fast antidepressant-like effects in the mouse forced swim test [28]. Given that chronic and/or traumatic stress exacerbates the development of mood-related disorders, and MDD is a risk factor for low bone mineral density, Xiong and colleagues evaluated the effects of (R)-ketamine on depression-related behavior and inflammatory bone markers in male mice exposed to chronic social defeat stress [29]. Their work shows that (R)-ketamine (10 mg/kg), but not its metabolite (2R,6R)-hydroxynorketamine (10 mg/kg), is able to reverse the social defeat-induced behavioral alterations observed, as well as the stress-induced changes of molecular markers associated with bone formation and resorption. Similarly, Mastrodonato and colleagues assessed whether ketamine would protect against inflammatory stressors in a mouse model that screens for antidepressant-like effects [30]. Specifically, they tested if ketamine (30 mg/kg) would produce prophylactic-like effects against inflammatory-induced vulnerability (i.e., an injection of lipopolysaccharide) on contextual fear conditioning and the forced swim test in adult male mice [31]. They revealed that ketamine was effective in attenuating learned fear while inducing antidepressant-like effects on the forced swim test in mice undergoing inflammatory stress. Importantly, these preclinical findings suggest that ketamine not only reverses stress-induced maladaptive behavior, but that it may also be a treatment option for individuals suffering from TRD and comorbid osteoporosis and/or inflammation-related illnesses.
The short-term behavioral and neurobiological effects of ketamine exposure, in adult organisms, have been the focus of basic researchers over the years. However, less is known about the actions of ketamine across different stages of development. Addressing this issue, Wei and colleagues found that ketamine’s rapid antidepressant-like effects, using adolescent male mice as a model system, are mediated by its ability to facilitate fear memory extinction via hippocampal presynaptic-mediated plasticity processes [32]. Following on their prior work demonstrating that ketamine facilitates short-term fear conditioned memory processes in adult rodents [33], Radford and colleagues assessed whether ketamine would influence fear memory in a longer timeframe. Interestingly, they found that ketamine does not influence long-term conditioned fear learning/extinction [34]; yet, Radford and his team report that ketamine history mediates a sustained increase in progesterone and corticosterone, implicating potential enhanced vulnerability for the development of fear-related disorders, such as PTSD and GAD, later in life. This is an intriguing finding, given that ketamine is commonly administered to traumatically injured victims to provide sedation and analgesia [35]. As such, their findings highlight the need for future basic research to examine for enduring effects of ketamine exposure.
As discussed above, ketamine is a drug with abuse potential, and as such, basic researchers are exploring the mechanisms that underlie its addiction-related properties. For example, Trujillo and Heller examined behavioral sensitization, a phenomenon associated with motivation to seek drug, and demonstrated that both novel environments and social experiences facilitate the development of ketamine-induced locomotor sensitization [36]. Interestingly, the factors that influence the development of ketamine-induced locomotor sensitization are uniquely expressed across development and as a function of sex [37]. However, the neural mechanisms of ketamine’s locomotor activating effects are not fully understood. To address this gap in the literature, the laboratories of Crawford and McDougall, in two papers, evaluated the role of monoamine neurotransmission in ketamine-induced locomotor activity across age and sex [38,39]. Incorporating a series of elegant pharmacological experiments, they report that ketamine-induced locomotion is partially dependent on both dopamine and serotonin systems, since pharmacological depletion of these neurotransmitters (but not norepinephrine) attenuate the locomotor activating effects of ketamine differentially across the prepubertal, adolescent, and adult stages of development.
A fascinating paradox of ketamine is that it is a drug with abuse potential, however it also has potential for the treatment of addiction [40,41]. To investigate the neurobiological mechanisms that contribute to its therapeutic effects in addiction Piva et al. [42] explored the ability of ketamine to interfere with renewal or reconsolidation of a reward memory. Ketamine was given at a low subanesthetic dose 24 h prior to testing for renewal or reconsolidation of lever-pressing for sucrose reward following extinction. It was found that ketamine interfered with renewal of responding but did not interfere with reactivation of contextual memory. In parallel they demonstrated changes in glutamatergic receptors in amygdala, hippocampus and nucleus accumbens, which may contribute to the behavioral changes induced by ketamine.
While the short-term effects of subanesthetic doses of ketamine are being uncovered, less is known about the long-term effects that may arise in later life as a function of ontogenic ketamine exposure [43,44]. Extending on this important line of work, Bates and Trujillo evaluated how exposure to this NMDA receptor blocker, during adolescence or adulthood, influenced the expression of locomotor sensitization or cognitive functioning, 20 days after ketamine exposure [45]. They found that ketamine pretreatment induced persistent locomotor sensitization in animals pre-exposed to ketamine in adulthood, but not adolescence – thus, revealing that ketamine history induces long-lived alterations on addiction-related behavior, but not cognitive ability, as a function of age. Similarly, Franco and colleagues evaluated how pre-pubertal exposure to ketamine influenced responses to alcohol seeking behavior during adolescence [46]. Here, adopting the conditioned place preference approach [47], they demonstrated that early-life exposure to ketamine reduced the preference for environments paired with alcohol during adolescence. However, altered shifts in drug-reward response are likely dependent on the age of ketamine pre-exposure, as others have reported increases in cocaine and sucrose preference in adulthood as a function of adolescent ketamine pre-treatment [43]. Collectively, these basic research studies show that while ketamine exerts acute beneficial pharmacotherapeutic properties (on neuropsychiatric-related behavior) it also results in enduring reward-related side effects in later life. These studies point toward future research on both the therapeutic and unwanted effects of ketamine exposure.
Research into the subanesthetic effects of ketamine, to understand both its therapeutic effects in MDD and its abuse potential, is growing at a high rate (Fig. 1). The current special issue of Behavioural Brain Research contributes significantly to a better understanding of these areas and points in promising directions for future research. As we look toward the future it will be important for the field to arrive at a better understanding of preclinical models of ketamine’s antidepressant effects, and in particular, the variability in responses both across and within laboratories, and the potential role of stress in this variability. Further research into the intersection between ketamine, stress, learning, and depression will also be beneficial. It needs to be noted, however, that there are some effects of ketamine that cannot be easily studied in animal models, such as the mystical/spiritual states, that will require human subjects to fully understand the therapeutic effects of the drug. Finally, studies on the factors that contribute to non-medical use of ketamine and ketamine addiction are needed to prevent and treat problematic use, as is research on potential long-term consequences of ketamine use (both disadvantageous and beneficial). It is expected that research on ketamine will continue at a rapid pace with the potential to significantly benefit health and well-being.
Acknowledgements
This work was supported by the National Institute of General Medical Sciences: GM130467 and GM109811 to S.D.I. and GM136481 to K.A.T.
Contributor Information
Keith A. Trujillo, Department of Psychology and Office for Training, Research, and Education in the Sciences, California State University San Marcos, 333 S. Twin Oaks Valley Road, San Marcos, CA, 92096, United States.
Sergio D. Iñiguez, Department of Psychology, The University of Texas at El Paso, 500 West University Avenue, El Paso, TX, 79968, United States
References
- [1].FDA, FDA-Approved Drugs, New Drug Application (NDA): 016812 (Ketamine Hydrochloride), 1970, U.S. Food & Drug Administration, 1970. [Google Scholar]
- [2].Domino EF, Taming the ketamine tiger. 1965, Anesthesiology 113 (3) (2010) 678–684. [DOI] [PubMed] [Google Scholar]
- [3].Domino EF, Chodoff P, Corssen G, Pharmacologic effects of Ci-581, a new dissociative anesthetic, in man, Clin. Pharmacol. Ther 6 (1965) 279–291. [DOI] [PubMed] [Google Scholar]
- [4].Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, Antidepressant effects of ketamine in depressed patients, Biol. Psychiatry 47 (4) (2000) 351–354. [DOI] [PubMed] [Google Scholar]
- [5].FDA, FDA Approves New Nasal Spray Medication for Treatment-Resistant Depression; Available Only at a Certified Doctor’s Office or Clinic, FDA News Release, 2019. [Google Scholar]
- [6].Siegel RK, Phencyclidine and ketamine intoxication: a study of four populations of recreational users, NIDA Res. Monogr 21 (1978) 119–147. [PubMed] [Google Scholar]
- [7].Jansen KL, A review of the nonmedical use of ketamine: use, users and consequences, J. Psychoactive Drugs 32 (4) (2000) 419–433. [DOI] [PubMed] [Google Scholar]
- [8].Dillon P, Copeland J, Jansen K, Patterns of use and harms associated with non-medical ketamine use, Drug Alcohol Depend. 69 (1) (2003) 23–28. [DOI] [PubMed] [Google Scholar]
- [9].Morgan CJ, Curran HV, Independent D Scientific Committee on, Ketamine use: a review, Addiction 107 (1) (2012) 27–38. [DOI] [PubMed] [Google Scholar]
- [10].Ni A, Lee Cantrell F, Clark RF, Ketamine exposure demographics and outcomes over 16years as reported to US poison centers, Am. J. Emerg. Med 36 (8) (2018) 1459–1462. [DOI] [PubMed] [Google Scholar]
- [11].Jansen KL, Darracot-Cankovic R, The nonmedical use of ketamine, part two: a review of problem use and dependence, J. Psychoactive Drugs 33 (2) (2001) 151–158. [DOI] [PubMed] [Google Scholar]
- [12].Lavender E, Hirasawa-Fujita M, Domino EF, Ketamine’s dose related multiple mechanisms of actions: dissociative anesthetic to rapid antidepressant, Behav. Brain Res 390 (2020) 112631. [DOI] [PubMed] [Google Scholar]
- [13].Sial OK, Parise EM, Parise LF, Gnecco T, Bolaños-Guzmán CA, Ketamine: the final frontier or another depressing end? Behav. Brain Res 383 (2020) 112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carreno FR, Lodge DJ, Frazer A, Ketamine: leading us into the future for development of antidepressants, Behav. Brain Res 383 (2020) 112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rincón-Cortés M, Grace AA, Antidepressant effects of ketamine on depression related phenotypes and dopamine dysfunction in rodent models of stress, Behav. Brain Res 379 (2020) 112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Polis AJ, Fitzgerald PJ, Hale PJ, Watson BO, Rodent ketamine depression-related research: finding patterns in a literature of variability, Behav. Brain Res 376 (2019) 112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abdallah CG, Krystal JH, Ketamine and rapid acting antidepressants: are we ready to cure, rather than treat depression? Behav. Brain Res 390 (2020) 112628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grabski M, Borissova A, Marsh B, Morgan CJA, Curran HV, Ketamine as a mental health treatment: are acute psychoactive effects associated with outcomes? A systematic review, Behav. Brain Res 392 (2020) 112629. [DOI] [PubMed] [Google Scholar]
- [19].Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, Klinedinst MA, Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial, J. Psychopharmacol 30 (12) (2016) 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roseman L, Nutt DJ, Carhart-Harris RL, Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression, Front. Pharmacol 8 (2017) 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kokane SS, Armant RJ, Bolaños-Guzmán CA, Perrotti LI, Overlap in the neural circuitry and molecular mechanisms underlying ketamine abuse and its use as an antidepressant, Behav. Brain Res 384 (2020) 112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM, NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses, Nature 475 (7354) (2011) 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA Jr., Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice, Biol. Psychiatry 76 (7) (2014) 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Warren BL, Mazei-Robison MS, Robison AJ, Iñiguez SD, Can I get a witness? Using vicarious defeat stress to study mood-related illnesses in traditionally understudied populations, Biol. Psychiatry (2020) 300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Deslauriers J, Toth M, Der-Avakian A, Risbrough VB, Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation, Biol. Psychiatry 83 (10) (2018) 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Detke MJ, Lucki I, Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth, Behav. Brain Res 73 (1–2) (1996) 43–46. [DOI] [PubMed] [Google Scholar]
- [27].Ecevitoglu A, Canbeyli R, Unal G, Oral ketamine alleviates behavioral despair without cognitive impairment in Wistar rats, Behav. Brain Res 372 (2019) 112058. [DOI] [PubMed] [Google Scholar]
- [28].Kim JW, Monteggia LM, Increasing doses of ketamine curtail antidepressant responses and suppress associated synaptic signaling pathways, Behav. Brain Res 380 (2020) 112378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xiong Z, Fujita Y, Zhang K, Pu Y, Chang L, Ma M, Chen J, Hashimoto K, Beneficial effects of (R)-ketamine, but not its metabolite (2R,6R)-hydroxynorketamine, in the depression-like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model, Behav. Brain Res 368 (2019) 111904. [DOI] [PubMed] [Google Scholar]
- [30].Haroon E, Raison CL, Miller AH, Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior, Neuropsychopharmacology 37 (1) (2012) 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mastrodonato A, Cohensedgh O, LaGamma CT, McGowan JC, Hunsberger HC, Denny CA, Prophylactic (R,S)-ketamine selectively protects against inflammatory stressors, Behav. Brain Res 378 (2020) 112238. [DOI] [PubMed] [Google Scholar]
- [32].Wei MD, Wang YH, Lu K, Lv BJ, Wang Y, Chen WY, Ketamine reverses the impaired fear memory extinction and accompanied depressive-like behaviors in adolescent mice, Behav. Brain Res 379 (2020) 112342. [DOI] [PubMed] [Google Scholar]
- [33].Radford KD, Park TY, Jaiswal S, Pan H, Knutsen A, Zhang M, Driscoll M, Osborne-Smith LA, Dardzinski BJ, Choi KH, Enhanced fear memories and brain glucose metabolism ((18)F-FDG-PET) following sub-anesthetic intravenous ketamine infusion in Sprague-Dawley rats, Transl. Psychiatry 8 (1) (2018) 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Radford KD, Spencer HF, Zhang M, Berman RY, Girasek QL, Choi KH, Association between intravenous ketamine-induced stress hormone levels and long-term fear memory renewal in Sprague-Dawley rats, Behav. Brain Res 378 (2020) 112259. [DOI] [PubMed] [Google Scholar]
- [35].Morris C, Perris A, Klein J, Mahoney P, Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia 64 (5) (2009) 532–539. [DOI] [PubMed] [Google Scholar]
- [36].Trujillo KA, Heller CY, Ketamine sensitization: influence of dose, environment, social isolation and treatment interval, Behav. Brain Res 378 (2020) 112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McDougall SA, Moran AE, Baum TJ, Apodaca MG, Real V, Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose, Psychopharmacology (Berl.) 234 (18) (2017) 2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Crawford CA, Moran AE, Baum TJ, Apodaca MG, Montejano NR, Park GI, Gomez V, McDougall SA, Effects of monoamine depletion on the ketamine-induced locomotor activity of preweanling, adolescent, and adult rats: sex and age differences, Behav. Brain Res 379 (2020) 112267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McDougall SA, Rios JW, Apodaca MG, Park GI, Montejano NR, Taylor JA, Moran AE, Robinson JAM, Baum TJ, Teran A, Crawford CA, Effects of dopamine and serotonin synthesis inhibitors on the ketamine-, d-amphetamine-, and cocaine-induced locomotor activity of preweanling and adolescent rats: sex differences, Behav. Brain Res 379 (2020) 112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Krupitsky EM, Grinenko AY, Ketamine psychedelic therapy (KPT): a review of the results of ten years of research, J. Psychoactive Drugs 29 (2) (1997) 165–183. [DOI] [PubMed] [Google Scholar]
- [41].Witkin JM, Kranzler J, Kaniecki K, Popik P, Smith JL, Hashimoto K, Sporn J, R-(−)-ketamine modifies behavioral effects of morphine predicting efficacy as a novel therapy for opioid use disorder(1), Pharmacol. Biochem. Behav 194 (2020) 172927. [DOI] [PubMed] [Google Scholar]
- [42].Piva A, Caffino L, Padovani L, Pintori N, Mottarlini F, Sferrazza G, Paolone G, Fumagalli F, Chiamulera C, The metaplastic effects of ketamine on sucrose renewal and contextual memory reconsolidation in rats, Behav. Brain Res 379 (2020) 112347. [DOI] [PubMed] [Google Scholar]
- [43].Garcia-Carachure I, Flores-Ramirez FJ, Castillo SA, Themann A, Arenivar MA, Preciado-Pina J, Zavala AR, Lobo MK, Iñiguez SD, Enduring effects of adolescent ketamine exposure on cocaine- and sucrose-induced reward in male and female C57BL/6 mice, Neuropsychopharmacology 45 (9) (2020) 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Parise EM, Alcantara LF, Warren BL, Wright KN, Hadad R, Sial OK, Kroeck KG, Iñiguez SD, Bolaños-Guzmán CA, Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats, Biol. Psychiatry 74 (10) (2013) 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bates MLS, Trujillo KA, Long-lasting effects of repeated ketamine administration in adult and adolescent rats, Behav. Brain Res 369 (2019) 111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Franco D, Zamudio J, Blevins KM, Nunez-Larios EA, Ricoy UM, Iñiguez SD, Zavala AR, Early-life ketamine exposure attenuates the preference for ethanol in adolescent Sprague-Dawley rats, Behav. Brain Res 389 (2020) 112626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bardo MT, Bevins RA, Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl.) 153 (1) (2000) 31–43. [DOI] [PubMed] [Google Scholar]


