Abstract
Background
delirium is an event leading to negative health outcomes and increased mortality in patients. The aim of this study is to investigate the incidence, determinants and consequences of post-operative delirium (POD) in older patients undergoing transcatheter aortic valve implantation (TAVI).
Methods
The TAVI Care and Cure program is a prospective, observational registry in patients referred for TAVI at Erasmus University Medical Centre. The presence of delirium was evaluated by daily clinical assessment by a geriatrician pre- and up to 3 days post-TAVI. Mortality data were obtained from the Dutch Civil Registry.
Results
A total of 543 patients underwent TAVI between January 2014 and December 2017. Overall, the incidence of POD was 14% (75/543 patients) but declined from 18% in 2014 to 7% in 2017 (P = 0.009). Patients who developed POD were older (81.9 ± 5.8 versus 78.6 ± 8.3 years, P < 0.001), had higher prevalence of renal dysfunction and prior stroke (54% versus 40%, P = 0.02; 31% versus 18%, P = 0.01) and were more often frail (32% versus 25%, P = 0.02). From a procedural perspective, general anesthesia (odds ratios (OR), 2.31; 95% CI, 1.40–3.83; P = 0.001), non-transfemoral access (OR, 2.37; 95% CI, 1.20–4.70; P = 0.01) and longer procedural time (OR, 1.01; 95% CI, 1.01–1.02; P < 0.001) were significantly associated with POD. One-year survival rate was 68% among patients who had suffered a POD and was 85% in patients without a POD (hazard ratio’s 1.8 (95% CI 1.01–3.10), P = 0.045).
Conclusion
POD frequently occurs after TAVI and is associated with increased mortality. It might be speculated that patient selection and the minimalistic approach of TAVI may reduce the frequency of delirium.
Keywords: aortic stenosis, post-operative delirium, transcatheter aortic valve implantation, mortality
Key points
Delirium after transcatheter aortic valve implantation (TAVI) occurs frequently.
Delirium after TAVI is associated with increased mortality.
Patient selection and minimalistic approach of TAVI may reduce post-operative delirium.
Introduction
The incidence of delirium after transcatheter aortic valve implantation (TAVI) is reported to vary between 12 and 53% and proved to exert a significant and negative impact on hospital stay and long-term mortality, especially in frail and older patients [1–6]. TAVI is increasingly used to treat patients with aortic stenosis and as a result of increased experience, new generation heart valves and minimising the treatment itself, periprocedural complications have decreased [7–10]. The impact of better procedural outcomes on the incidence of post-operative delirium (POD) is thus far unknown.
Notwithstanding the expansion of the indication of TAVI to low-risk patients [9], the majority of patients who undergo TAVI have an advanced age with a high risk of POD given the presence of substantial co-morbidity and frailty [10–12].
The aim of this study is to investigate the incidence, determinants and consequences of POD in older patients undergoing TAVI, as well as the consequences of the changes in TAVI practice on the incidence on delirium.
Methods
Patient selection
The study population consists of 543 consecutive patients who received TAVI because of severe aortic stenosis between January 2014 and December 2018 and who were enrolled in the TAVI Care and Cure program [13]. In brief, the TAVI Care and Cure program is a prospective single-center multidisciplinary observational cohort study that was initiated in November 2013 and consists of a prospective collection of a comprehensive set of predefined cardiovascular and non-cardiovascular data including a comprehensive geriatric assessment (CGA), baseline characteristics in addition to procedural and post-operative data of all patients referred for and treated with TAVI. There were no specific exclusion criteria. Treatment decision and strategy was decided during the multidisciplinary heart team meeting (interventional cardiologists, cardiac surgeons, anesthesiologist, geriatricians and a TAVI-nurse coordinator) [14, 15]. The TAVI Care and Cure program was approved by the Medical Ethics Committee of the Erasmus Medical Center (MEC-2014-277) and was conducted according to the Helsinki Declaration. All patients provided written informed consent.
Study measurements
Cardiology assessment
Cardiology assessment included patient’s history, presence of symptoms using the New York Heart Association (NYHA) and the Canadian Cardiovascular Society (CCS) score, physical examination, electrocardiogram, transthoracic echocardiography, coronary angiography and multislice computed tomography, as described before [19, 20].
Geriatric assessment
Frailty was defined by the Erasmus Frailty Score (EFS) that has been reported to be associated with POD and one-year mortality [12]. The EFS uses five geriatric domains that are relevant for this specific population; cognition was measured by the mini-mental state examination [21], strength by the hand grip strength test [22], (mal) nutrition by the Malnutrition Universal Screening Tool [23], inactivity in basic activities of daily living (measured by the Katz index (Katz activities of daily living (ADL)) [24] and inactivity in instrumental activities of daily living (measured by the Lawton and Brody index) [25]. Patients were considered frail if the score on 3 or more domains were below predefined standard cut off points [12].
TAVI procedure
TAVI was initially performed under general anesthesia and from September 2015 onwards under local anesthesia, using the transfemoral approach as default choice. After TAVI, patients were admitted to the intensive care unit for early monitoring for a minimum of 4 h and then transferred, if no conduction disturbances or hemodynamic events occurred, to the general cardiology ward with telemonitoring facility.
Delirium assessment and management of delirium
One day before TAVI, all patients were seen by the geriatrician to assess the risk of delirium based on known risk factors of POD [1, 3]. All patients were given information on symptoms of delirium and non-pharmacological intervention for delirium prevention were taken. From the day of admission up to 4 days post-TAVI, a geriatrician evaluated patients on a daily basis. Delirium was defined according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).
Delirium features necessary for diagnoses were: acute onset and fluctuating coarse AND inattention AND/OR disorganised thinking AND/OR altered level of consciousness AND/OR hallucinations. The diagnosis of delirium was made by clinical diagnosis of a geriatrician and was based on the psychiatric examination of the patient. Patients were seen by a geriatrician at least once a day on a daily basis and besides the evaluation of the patient, we continuously evaluated with the nursing staff on the cardiology ward to learn if there was any fluctuations in symptoms during the day or overnight. When caregivers of patients were present, we evaluated with the caregivers symptoms and fluctuations of symptoms.
Severity of delirium was defined by the delirium observation score [26]. In case of delirium, patients were treated according to current national guideline ‘Delirium in adults’, Dutch Geriatrics Society [16].
Outcome measures
The primary objective of this study is the incidence and predictors of delirium after TAVI. Secondary objective was the impact of delirium on all-cause mortality within 1 year after TAVI. The Dutch Civil Registry was used for the collection of all-cause mortality data.
Data management and statistical analysis
All data were entered into a dedicated online database hosted on an internal server. Categorical variables are presented as numbers and percentages. Differences between patients with and without delirium were compared with the chi-square or Fisher’s exact test, as appropriate. Continuous variables are expressed as means with standard deviation (±SD) or median values with corresponding interquartile ranges (IQR). Differences within the two groups were compared using the independent t-test or its non-parametric equivalents, respectively. Univariate analysis was performed, variables with a P-value < 0.10 was entered in the multivariate regression models.
For the multivariate model, covariates were operationalised as follows: (i) prior stroke: transient ischemic attack or cerebrovascular accident prior to TAVI, (ii) renal dysfunction: estimated glomerular filtration rate < 50 mL/min, (iii) limitation of mobility (gait speed): walking speed < 1.0 m/s; (iv) frailty measured with the EFS: five component score, frail when three components are scored below predefined cut off points.
For the outcome incidence of delirium, odds ratios (OR) and corresponding 95% confidence interval (CI) were computed with multivariate logistic regression analysis. We tested multicollinearity for the multivariate regression model. Obtained variance inflation factor (VIF) values ranged between 1 and 2. Cox regression analysis was performed to investigate the association between delirium and mortality. The multivariate model was based on the results form a univariate analysis for the outcome ‘one-year mortality’. Variables with a P-value < 0.10 were entered in the multivariate regression models. Hazard ratio’s (HR) and corresponding 95% CI were computed. To investigate the changes over time, patients were divided into four cohorts, depending on the year in which the TAVI took place: cohort 1: 2014; cohort 2: 2015; cohort 3: 2016; cohort 4: 2017. Categorical variables were compared using the chi-square test, while continuous variables were compared with the ANOVA test. Statistical significance across the years was calculated using the chi-square method adjusting the P-values after the Bonferroni correction for the proportions. For the continue variables, the Games-Howell method was used as a post-hoc test. Analyses were performed using SPSS version 21 for Windows (SPSS, Inc., Chicago, IL).
Results
Patient characteristics
Between January 2014 and December 2017, a total of 543 patients underwent a TAVI for symptomatic aortic valve stenosis. Clinical baseline characteristics and procedural features are shown in Table 1. Mean age of the population was 79.1 ± 8.0 years and 55% were men. Hypertension (80%), hypercholesterolemia (65%), atrial fibrillation (32%) and diabetes mellitus (32%) were prevalent comorbidities. In this cohort, there were no patients with existing diagnosis of dementia (diagnosis noted in primary care records or records from referring hospitals). There were seven patients (=1.3%) with mild cognitive impairment, but with no interference in daily activities.
Table 1.
Baseline characteristics and procedural features of the total study population
| Variable (n, %) | All patients | Delirium | Association with delirium | |||
|---|---|---|---|---|---|---|
| n = 543 | Present (n = 75) | Absent (n = 468) | OR | 95% CI | P-value | |
| Male sex (n, %) | 297 (55%) | 44 (59%) | 253 (54%) | 0.83 | 0.51–1.36 | 0.48 |
| Age (mean ± SD) | 79.1 ± 8.0 | 81.9 ± 5.8 | 78.6 ± 8.3 | 1.06 | 1.02–1.10 | 0.001 |
| BMI (mean, SD) | 27.3 ± 4.9 | 27.1 ± 4.2 | 27.3 ± 5.1 | 0.99 | 0.93–1.05 | 0.76 |
| Aortic valve area, cm2 | 0.77 ± 0.25 | 0.74 ± 0.24 | 0.78 ± 0.25 | 0.54 | 0.18–1.60 | 0.27 |
| Cardiovascular risk factors | ||||||
| Diabetes mellitus | 176 (32%) | 20 (27%) | 156 (33%) | 0.73 | 0.42–1.26 | 0.25 |
| Hypertension | 432 (80%) | 62 (83%) | 370 (79%) | 1.26 | 0.67–2.39 | 0.47 |
| Hypercholesterolemia | 352 (65%) | 45 (60%) | 307 (66%) | 0.79 | 0.48–1.30 | 0.35 |
| Comorbidities | ||||||
| Prior MI | 118 (22%) | 18 (24%) | 100 (21%) | 1.16 | 0.65–1.06 | 0.61 |
| Prior stroke | 108 (20%) | 23 (31%) | 85 (18%) | 1.92 | 0.98–3.76 | 0.01 |
| Renal dysfunction | 227 (42%) | 40 (54%) | 187 (40%) | 1.76 | 1.07–2.88 | 0.02 |
| COPD | 117 (22%) | 13 (18%) | 104 (22%) | 0.74 | 0.39–1.40 | 0.35 |
| Symptoms | ||||||
| NYHA classes III/IV | 358 (66%) | 53 (72%) | 305 (65%) | 1.34 | 0.78–2.30 | 0.25 |
| Angina CCS classes III/IV | 64 (13%) | 8 (11%) | 56 (13%) | 0.88 | 0.40–1.93 | 0.74 |
| Frailty indices (n = 330) | ||||||
| Cognitive impairment | 107 (31%) | 26 (48%) | 81 (28%) | 2.40 | 1.34–4.39 | 0.003 |
| Malnutrition probable | 38 (11%) | 8 (15%) | 30 (10%) | 1.60 | 0.69–3.70 | 0.29 |
| Reduced gait speed | 219 (67%) | 41 (79%) | 178 (38%) | 2.09 | 1.03–4.26 | 0.04 |
| Reduced muscle strength | 160 (48%) | 302(62%) | 128 (45%) | 1.94 | 1.06–3.55 | 0.03 |
| Limitation in ADL activity | 107 (30.4%) | 19 (35%) | 88 (30%) | 1.30 | 0.70–2.39 | 0.41 |
| Limitation in IADL activity | 189 (54%) | 37 (69%) | 152 (51%) | 2.09 | 1.13–3.88 | 0.02 |
| Frailty identified (EFS) | 97 (28.5%) | 24 (32%) | 71 (25%) | 2.60 | 1.41–4.80 | 0.002 |
| Procedural features | ||||||
| General anesthesia | 232 (43%) | 45 (59%) | 187 (40%) | 2.31 | 1.40–3.83 | 0.001 |
| Non-transfemoral access | 51 (9%) | 13 (17%) | 38 (8%) | 2.37 | 1.20–4.70 | 0.01 |
| Cerebral protection | 274 (51%) | 36 (49%) | 238 (51%) | 0.93 | 0.57–1.53 | 0.78 |
| Procedure time, minutes (mean ± SD) | 140 ± 61.5 | 184 ± 93.4 | 133 ± 51.6 | 1.01 | 1.01–1.02 | <0.001 |
Values are n(%), mean ± SD.
BMI, body mass index; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease.
The incidence of delirium was 14% (75/543 patients). Patients who developed POD were older (82.1 ± 5.8 versus 78.6 ± 8.3 years, P < 0.001), had higher prevalence of renal dysfunction and prior stroke (54% versus 40%, P = 0.02; 31% versus 18%, P = 0.01) and were more often frail (32% versus 25%, P = 0.02). Cognitive impairment (OR, 2.28; 95% CI, 1.25–4.13; P = 0.007), reduced gait speed (OR, 3.69; 95% CI, 1.68–10.72; P = 0.02), limitation in instrumental activities of daily living (IADL) activity (OR, 2.15; 95% CI, 1.16–3.98; P = 0.02) and the presence of frailty (OR, 2.50; 95% CI, 1.30–4.82; P = 0.006) were significantly associated with POD (Table 1).
From a procedural perspective, general anesthesia (OR, 2.31; 95% CI, 1.40–3.83; P = 0.001), non-transfemoral access (OR, 2.37; 95% CI, 1.20–4.70; P = 0.01) and longer procedural time (OR, 1.01; 95% CI, 1.01–1.02; P < 0.001) were significantly associated with POD (Table 1).
Information on the onset of POD was available for all patients. POD occurred on day 0 and 1 in 68% of the patients. The median onset of POD was day 1 (IQR 0.0–2.0 days) (Supplementary Material A1). The incidence of post-procedural stroke (11% versus 3%; P = 0.001), new pacemaker implantation (24% versus 14%; P = 0.02), vascular complication (24% versus13%; P = 0.009), post-operative urinary tract infection (13% versus 2%; P < 0.001) and post-operative pneumonia (9% versus 4%; P = 0.03) was higher in patients with POD than those without POD. Yet, independent variables predicting POD were found to be prior stroke (OR, 4.29; 95% CI, 1.85–9.96; P = 0.001), the presence of frailty (OR, 2.37; 95% CI, 1.12–5.07; P = 0.025) and the length of the procedure (OR, 1.02; 95% CI, 1.01–1.03; P < 0.001) (Table 2).
Table 2.
Predictors for POD within a multivariate model
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Age | 1.06 | 0.99–1.13 | 0.097 |
| Prior stroke | 3.10 | 1.49–6.47 | 0.003 |
| Renal dysfunction | 1.18 | 0.57–2.48 | 0.655 |
| Limitation of mobility (Gait speed) | 1.04 | 0.44–2.46 | 0.926 |
| Frailty identified (EFS) | 2.37 | 1.12–5.07 | 0.025 |
| General anesthesia | 1.71 | 0.70–4.20 | 0.24 |
| Non-transfemoral access | 1.07 | 0.35–3.31 | 0.905 |
| Procedural time (min) | 1.02 | 1.01–1.03 | <0.001 |
The presence of POD was associated with prolonged in-hospital stay, independent of other periprocedural complications (uncomplicated TAVI 8.2 versus 7.5 days, P = 0.003 and complicated TAVI 17.8 versus 11.1 days, P < 0.001).
Impact of POD on Mortality during follow-up
Median follow-up was 1.4 years (IQR: 0.6–2.5 years), overall mortality was 29.5% (n = 190). One-year survival rate was 68% among patients who had suffered POD and 85% in patients without POD (HR 1.8 (1.01–3.10), P = 0.045) (Figure 1).
Figure 1.
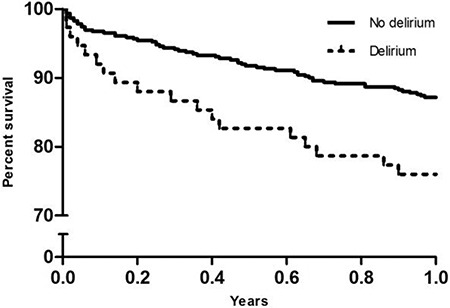
One-year mortality of patients with and without a delirium. One-year mortality of patients with and without delirium. HR 1.8 (1.01–3.10), P = 0.045. Adjusted for age, sex, dyslipidemia, atrial fibrillation, New York Heart Association classes 3 or 4, renal dysfunction
Changes over time
In the period from January 2014 to December 2017, the mean age, prevalence of frailty and prior stroke of patients who underwent TAVI remained the same (P = 0.332, P > 0.999 and P = 0.467, respectively). Yet, the proportion of patients with renal dysfunction was higher in those treated in 2014 and 2015 versus those treated in 2016 and 2017 (53% and 52% versus 37% and 32%, respectively, P < 0.001). The main change was found to be of procedural nature; the use of general anesthesia fell from 100% in 2014 to 19% in 2017 (P < 0.001), non-transfemoral access dropped from 18% in 2014 to 8% in 2017 (P = 0.08) and procedure time was reduced from 191.6 ± 65.7 minimum in 2014 to 91.0 ± 37.8 minimum in cohort 2017 (P < 0.001) (Supplementary Material A2). During this observation period, the incidence of delirium decreased from 18% in 2014 to 7% in 2017 (P = 0.009).
Discussion
In this study, we found that delirium frequently occurs after TAVI in older patients and is associated with increased mortality. Frailty and prior stroke as well as procedural time were independent predictors of POD.
Frailty is known to be a risk factor of POD in patients undergoing TAVI and can be explained by the fact that frailty is the result of reduced physical, cognitive and social functioning and, thus, associated with a reduction of an adaptive or reserve capacity in case of stressors [11, 12, 17, 18]. Despite the minimal invasive nature of TAVI, this procedure remains a stressful event in particular when considering the profile of patients undergoing TAVI who are in general old and have multiple comorbidities and frailty. Age remains and will remain advanced in patients undergoing TAVI, as shown in this study, in addition to the ageing society, notwithstanding the expansion of indication of TAVI to lower risk patients [10].
According with previous studies, we found prior stroke to be a predictor of POD [1, 2, 5, 19]. In the present analysis, prior stroke was associated with a 4-fold increased risk of POD. The risk factors for stroke overlap with those of cognitive impairment and dementia [20], which are also associated with increased risk for delirium [12, 21].
Not unexpectedly, procedural factors predicted POD. Previous studies have shown that the use of general anesthesia and the non-transfemoral access may lead to a 4-fold increase on POD [2, 17]. In the present study, we found by multivariable analysis that procedure time was the only independent procedural factor predicting POD.
There is a still controversy on the effect of the anesthetic technique (general versus local anesthesia) on POD [2, 22]. General anesthesia has been linked to post-operative cognitive dysfunction, but it has not been shown to have the same effect on POD, when considering POD a form of temporary cognitive dysfunction [23]. In the present study, the use of general anesthesia dropped from 100% in 2014 to 20% in 2017, in conjunction with a similar decrease in procedure time and a slight increase of transfemoral TAVI to 92% following a global trend in minimising the invasive nature of TAVI [24–26]. Yet, the effect of these procedural changes have not systematically been assessed in relation to POD, although one intuitively would expect a beneficial effect on the occurrence of POD, as shown in this study (i.e. a reduction of >10% over 4 years).
One of the possible confounders in the assessment of the incidence of POD in the present study is the fact that the geriatrician assessed the risk of POD before TAVI in the framework of the TAVI Care and Cure program and implemented preventive measures if deemed necessary. It has been shown that preventive strategies can lead to a 30% reduction in POD [27–29]. We have no control group with patients who did not receive delirium preventive measures, and therefore we could not add the presence or absence of delirium prevention into the multivariate model. Yet, in this study, we believe that both this delirium preventive strategy in conjunction with minimising the TAVI procedure to a PCI-like intervention explains the reduction in POD [30]. In addition to the limitation already addressed above, one must also acknowledge that the present analysis stems from a single centre observational study conducted in a tertiary referral centre. The population and findings may, therefore, not be representative for a general population of patients.
In addition to observation bias inherent to an observational study without independent adjudication of outcomes, some variables associated with POD, such as cognitive impairment and dependence in IADL activity, were not available for all patients. There were no patients in the cohort with existing diagnosis of dementia found in primary care records or records from referring hospitals. We performed a cognitive analysis in 330 out of 543; therefor, there is a chance that we underestimated the presence of mild cognitive impairment in our study cohort.
Concerning the data extracted from our CGA, a complete data set was available in 330 patients. The current study numbers are based on complete case analysis. There were no baseline differences on cardiovascular and non-cardiovascular data between those with and without CGA.
In conclusion, delirium occurs frequently after TAVI in older patients and is associated with impaired prognosis. Given the ageing of the general population, the burden of delirium post-TAVI will remain a matter of concern notwithstanding the expansion of TAVI to lower risk patients. It can be speculated whether patient selection and the minimalistic approach on TAVI might lower the incidence of delirium.
Supplementary Material
Declaration of Conflicts of Interest
None.
Funding
None.
References
- 1. Tse L, Bowering JB, Schwarz SK, Moore RL, Burns KD, Barr AM. Postoperative delirium following transcatheter aortic valve implantation: a historical cohort study. Can J Anaesth 2015; 62: 22–30. [DOI] [PubMed] [Google Scholar]
- 2. Abawi M, Nijhoff F, Agostoni P et al. Incidence, predictive factors, and effect of delirium after transcatheter aortic valve replacement. J Am Coll Cardiol Intv 2016; 9: 160–8. [DOI] [PubMed] [Google Scholar]
- 3. Eide LS, Ranhoff AH, Fridlund B et al. Comparison of frequency, risk factors, and time course of postoperative delirium in octogenarians after transcatheter aortic valve implantation versus surgical aortic valve replacement. Am J Cardiol 2015; 115: 802–9. [DOI] [PubMed] [Google Scholar]
- 4. Bestehorn K, Bestehorn M, Fleck E. Influence of different approaches of aortic valve replacement on the incidence of post-operative delirium in intermediate risk patients—a matched pair analysis. Curr Med Res Opin 2015; 31: 2157–63. [DOI] [PubMed] [Google Scholar]
- 5. Huded CP, Huded JM, Sweis RN et al. The impact of delirium on healthcare utilization and survival after transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2017; 89: 1286–91. [DOI] [PubMed] [Google Scholar]
- 6. Tilley E, Psaltis PJ, Loetscher T et al. Meta-analysis of prevalence and risk factors for delirium after transcatheter aortic valve implantation. Am J Cardiol 2018; 122: 1917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barbanti M, Webb JG, Gilard M, Capodanno D, Tamburino C. Transcatheter aortic valve implantation in 2017: state of the art. EuroIntervention 2017; 13: AA11–21. [DOI] [PubMed] [Google Scholar]
- 8. de Jaegere PPT. How to move toward the least invasive transfemoral transcatheter aortic valve implantation procedure? Circ Cardiovasc Interv 2014; 7: 439–40. [DOI] [PubMed] [Google Scholar]
- 9. Mack MJ, Leon MB, Thourani VH et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019; 380: 1695–705. [DOI] [PubMed] [Google Scholar]
- 10. Osnabrugge RLJ, Mylotte D, Head SJ et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013; 62: 1002–12. [DOI] [PubMed] [Google Scholar]
- 11. Assmann P, Kievit P, van der K et al. Frailty is associated with delirium and mortality after transcatheter aortic valve implantation. Open Heart 2016; 3: e000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goudzwaard JA, de Ronde-Tillmans MJAG, El N et al. The Erasmus Frailty Score is associated with delirium and 1-year mortality after transcatheter aortic valve implantation in older patients. The TAVI Care & Cure program. Int J Cardiol 2019; 276: 48–52. [DOI] [PubMed] [Google Scholar]
- 13. De Ronde-Tillmans MJAG, Goudzwaard JA, El Faquir N et al. TAVI care and cure, the Rotterdam multidisciplinary program for patients undergoing transcatheter aortic valve implantiation: design and rationale. Int J Cardiol 2020; 302: 36–41. doi: 10.1016/j.ijcard.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 14. de MJ, Lenzen MJ, Abawi M, Van NM, Zijlstra F, De PP. 10 years of transcatheter aortic valve implantation: an overview of the clinical applicability and findings. Ned Tijdschr Geneeskd 2013; 158: A7768–8. [PubMed] [Google Scholar]
- 15. Baumgartner H, Falk V, Bax JJ et al. ESC/EACTS guidelines for the management of valvular heart disease. Rev Esp Cardiol (Engl Ed) 2018 2017; 71: 110. [DOI] [PubMed] [Google Scholar]
- 16. Van der RC, Huyse FJ, Rosier P. Richtlijn'Delirium. Ned Tijdschr Geneeskd 2005; 149: 1027–32. [PubMed] [Google Scholar]
- 17. Bagienski M, Kleczynski P, Dziewierz A et al. Incidence of postoperative delirium and its impact on outcomes after transcatheter aortic valve implantation. Am J Cardiol 2017; 120: 1187–92. [DOI] [PubMed] [Google Scholar]
- 18. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma V, Katznelson R, Horlick E et al. Delirium after transcatheter aortic valve implantation via the femoral or apical route. Anaesthesia 2016; 71: 901–7. [DOI] [PubMed] [Google Scholar]
- 20. Coco DL, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag 2016; 12: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009; 195: 61–6. [DOI] [PubMed] [Google Scholar]
- 22. Maniar HS, Lindman BR, Escallier K et al. Delirium after surgical and transcatheter aortic valve replacement is associated with increased mortality. J Thorac Cardiovasc Surg 2016; 151: 815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis 2010; 22: S67–79. [DOI] [PubMed] [Google Scholar]
- 24. Gurevich S, Oestreich B, Kelly RF et al. Outcomes of transcatheter aortic valve replacement using a minimalist approach. Cardiovasc Revasc Med 2018; 19: 192–5. [DOI] [PubMed] [Google Scholar]
- 25. Barbanti M, Gulino S, Costa G, Tamburino C. Optimization and simplification of transcatheter aortic valve implantation therapy. Expert Rev Cardiovasc Ther 2018; 16: 287–96. [DOI] [PubMed] [Google Scholar]
- 26. Lauck SB, Wood DA, Baumbusch J et al. Vancouver transcatheter aortic valve replacement clinical pathway: minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes 2016; 9: 312–21. [DOI] [PubMed] [Google Scholar]
- 27. Inouye SK, Bogardus ST Jr, Charpentier PA et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340: 669–76. [DOI] [PubMed] [Google Scholar]
- 28. Hshieh TT, Yue J, Oh E et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med 2015; 175: 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49: 516–22. [DOI] [PubMed] [Google Scholar]
- 30. Van B, Flamaing J, Dierckx de Casterlé B et al. Effectiveness of in-hospital geriatric co-management: a systematic review and meta-analysis. Age Ageing 2017; 46: 903–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


