Abstract
Context
The identification of adjunct safe, durable, and cost-effective approaches to reduce the progression from prediabetes to type 2 diabetes (T2D) is a clinically relevant, unmet goal. It is unknown whether cinnamon’s glucose-lowering properties can be leveraged in individuals with prediabetes.
Objective
The objective of this work is to investigate the effects of cinnamon on measures of glucose homeostasis in prediabetes.
Design, Setting, Participants, and Intervention
This double-blind, placebo-controlled, clinical trial randomly assigned adult individuals meeting any criteria for prediabetes to receive cinnamon 500 mg or placebo thrice daily (n = 27/group). Participants were enrolled and followed at 2 academic centers for 12 weeks.
Main Outcome Measures
Primary outcome was the between-group difference in fasting plasma glucose (FPG) at 12 weeks from baseline. Secondary end points included the change in 2-hour PG of the oral glucose tolerance test (OGTT), and the change in the PG area under the curve (AUC) derived from the OGTT.
Results
From a similar baseline, FPG rose after 12 weeks with placebo but remained stable with cinnamon, leading to a mean between-group difference of 5 mg/dL (P < .05). When compared to the respective baseline, cinnamon, but not placebo, resulted in a significant decrease of the AUC PG (P < .001) and of the 2-hour PG of the OGTT (P < .05). There were no serious adverse events in either study group.
Conclusions
In individuals with prediabetes, 12 weeks of cinnamon supplementation improved FPG and glucose tolerance, with a favorable safety profile. Longer and larger studies should address cinnamon’s effects on the rate of progression from prediabetes to T2D.
Keywords: prediabetes, prevention, cinnamon, glucose tolerance, oxidative stress
Diabetes affects more than 460 million adults globally [1] and is the seventh leading cause of disability worldwide [2], accounting for more than $320 billion in health care costs in the United States alone [3]. The rise in prevalence of diabetes over the past 3 decades, primarily type 2 diabetes (T2D), has occurred at a particularly fast rate in East and South Asia [4].
Using current diagnostic criteria set forth by the American Diabetes Association [5], approximately 38% of the US population has prediabetes [6], which remains unrecognized in the vast majority of cases [7]. Prediabetes portends an annual progression to T2D ranging from 3% to 11% depending on the population [8-10]. The rate of incident T2D is higher in individuals with impaired glucose tolerance (IGT) who are also at increased risk of cardiovascular disease, as compared to patients with impaired fasting glucose (IFG) [11, 12].
As previously reviewed [13], the conversion from prediabetes to T2D can be effectively prevented using lifestyle modifications [9, 14-16], antidiabetic drugs [9, 16-19], weight loss medications [20], and metabolic surgery [21, 22]. However, such approaches to T2D prevention have several limitations. First, long-term follow-up, which is available for only a subset of interventions, demonstrates a progressive attenuation of benefits on T2D conversion rate over time [23-25]. In addition, both the cost and potential side effects (eg, weight gain with pioglitazone) can limit adherence to drugs longitudinally. Finally, up to 50% of at-risk patients will develop T2D even within the structured framework of randomized clinical trials (RCTs) [13]. Therefore, identification of efficacious, durable, safe, and cost-effective strategies for T2D prevention remains a clinically relevant unmet need, especially in low- and middle-income countries.
Complementary and alternative medicine practices, including the use of nutraceuticals, have flourished over recent decades. Based on a 2012 National Health Interview Survey, 18% of adults in the United States had used a nonvitamin, nonmineral dietary supplement during the past year [26]. Approximately one-third of patients with T2D have used complementary and alternative medicine, either alone or as adjunct, for treatment of this condition. The evidence for safety and efficacy of nutraceuticals in T2D treatment is sparse and in many instances of low quality [27, 28].
Extracts of the inner bark of the Cinnamomum genus (“cinnamon”) of aromatic trees, commonly used as flavoring agents, have been employed since ancient times for treating arthritis and other inflammatory diseases [29], and are currently marketed as therapeutic supplements for T2D. In experimental models of diabetes, mechanisms invoked for the glucose-lowering activity of cinnamon include increased glucose transporter type 4 (GLUT4) membrane translocation [30], stimulation of postprandial levels of glucagon-like peptide-1 [31], inhibition of α-glucosidase activity [32], and antioxidant properties [33].
Several RCTs in adult patients with T2D have addressed the effects of various powdered monopreparations of cinnamon, predominantly Cinnamomum cassia, on changes in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and lipid profile [34, 35]. The duration of these studies ranged from 4 weeks to 4 months with daily dosages of compounded cinnamon varying from 500 mg to 6000 mg. Notwithstanding significant heterogeneity in study design, the majority of RCTs demonstrated a 10% to 15% reduction in FPG from baseline whereas changes in HbA1c were inconsistent and did not reach statistical significance in the aggregate [35]. Cinnamon’s beneficial effects were more pronounced in drug-naive patients than as add-on therapy, and in patients with high baseline HbA1c (> 8%) [34].
In individuals with prediabetes, the evidence from RCTs addressing the impact of cinnamon on glucose homeostasis is more limited. Specifically, it remains unclear whether in this population: a) cinnamon affects FPG, glucose tolerance, or both, and b) the response to cinnamon is conserved across ethnic groups.
Here, we present the results of efficacy and safety in a 2-country, placebo-controlled RCT of cinnamon treatment conducted in participants with prediabetes.
1. Materials and Methods
A. Participants
This study was a binational, double-blind, placebo-controlled RCT. Participants were recruited at Kyung Hee University Medical Center (Seoul, Republic of Korea) and the Joslin Diabetes Center (Boston, Massachusetts, US) between 2017 and 2018. The study was approved by the ethical board of Kyung Hee University Korean Medicine Hospital, (No. KOMCIRB-160318-HR-010) and the Joslin Diabetes Center (JDC) (No. 2017-15). This study was exempted from the Investigational New Drug Program by the US Food and Drug Administration. All participants provided written informed consent. Studies were conducted using Good Clinical Practice and in accordance with the Declaration of Helsinki.
Participants between ages 20 and 70 years were recruited for screening through advertisement brochures posted at the 2 enrolling clinical centers or if they had a history of dysglycemia consistent with prediabetes. Individuals met study inclusion criteria for prediabetes if they demonstrated one of the following abnormalities [5]: IFG, defined as FPG between 100 and 125 mg/dL; IGT, as demonstrated by a 2-hour PG level of 140 to 199 mg/dL based on 75-g oral glucose tolerance test (OGTT); and HbA1c of 5.7% to 6.4%. Patients were excluded from screening and enrollment if they had evidence of diabetes mellitus or other significant endocrine, cardiovascular, pulmonary, renal, or liver disease, or intentional weight loss of 10 pounds (0.45 kg) or more within the past 6 months. Further exclusion criteria included pregnancy, therapy with corticosteroids, any drug or supplement with glucose-lowering activity, or any investigational drugs. The full list of exclusion criteria is available at the trial registration webpage at ClinicalTrials.gov (see “Study Funding and Oversight”).
B. Study Funding and Oversight
The study was funded by a grant of the Ministry of Health and Welfare (Republic of Korea) awarded to the Korean Medicine Clinical Trial Center (K-CTC) at Kyung Hee University Medical Center as part of a collaboration with the JDC. Data and safety monitoring was conducted by an independent committee from K-CTC with periodic on-site review of study centers. The trial was registered with ClinicalTrials.gov on July 17, 2017 (NCT03219411).
C. Study Protocol
Screening procedures occurred after a minimum 8-hour overnight fast and included anthropometric measurements, HbA1c, FPG, fasting insulin (FI), fasting lipid panel, comprehensive chemistry, and a standard 75-g OGTT. Eligible participants were randomly assigned 1:1 to receive cinnamon capsule 500 mg or placebo 3 times a day for 12 weeks.
Randomization was performed by a statistician using PROC PLAN of SAS 9.4 (SAS Institute Inc). To ensure a 1:1 allocation ratio of patients to cinnamon and placebo groups at each site, randomization was stratified by each site employing a random block design. All study participants and staff were blinded to treatment assignment until study completion.
The cinnamon capsules contained 300 mg of cinnamon extract (Cinnamomum spp) and 200 mg of Cinnamomum burmannii powder (Solgar Inc). The placebo, which contained cellulose (91.5%), caramel food coloring (8.4%), and cinnamon incense (0.1%) (BioFood Technology Center) did not contain any active substances. The overall appearance, weight, and organoleptic properties of cinnamon and placebo capsules were identical.
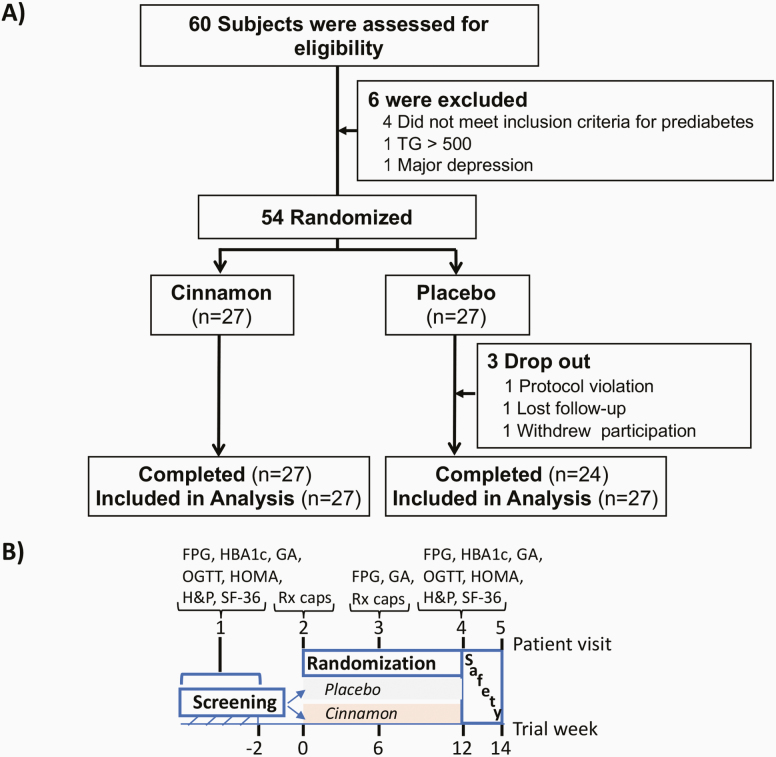
Of the 60 screened participants, 54 were enrolled in the study, which was completed by 51 participants (39 at Kyung Hee University Medical Center and 12 at JDC) (Fig. 1A). As shown in Fig. 1B, screening was performed at visit 1, and eligible participants were randomly assigned to placebo or cinnamon within 2 weeks (visit 2). Visits 3 and 4 occurred 6 and 12 weeks after randomization, respectively. The safety follow-up (visit 5) was performed 1 to 2 weeks after visit 4. Compliance with capsule administration was assessed at visit 3 and visit 4 by counting capsules returned by the patient, if any, and self-reported adherence to the thrice-daily regimen.
Figure 1.
A, Screening, randomization, and follow-up through the 12-week treatment period. Six out of the 60 screened individuals were excluded. Three out of 27 participants randomly assigned to placebo did not complete the study. B, Study design and main assessments over the 5 patient visits. FPG, fasting plasma glucose; GA, glycated albumin; H&P, complete history and physical exam; HBA1c, glycated hemoglobin; HOMA, Homeostatic Model Assessment, HOMA-IR and HOMA-B; IR, insulin resistance; OGTT, oral glucose tolerance test; Rx caps, dispensing of placebo or cinnamon capsules; SF-36, 36-Item Short Form Survey.
D. Primary and Secondary End Points
The primary outcome measure was the between-group difference in FPG at 12 weeks from baseline. Prespecified efficacy secondary end points included the change at 6 weeks from baseline in FPG; the change at 12 weeks from baseline in PG at the 2-hour time point of the OGTT; and the change at 12 weeks from baseline in the area under the curve (AUC) of PG derived from the OGTT. Other relevant measure of glucose homeostasis included HbA1c, glycated albumin (GA), homeostatic model assessment for insulin resistance (HOMA-IR), which was calculated as FI (μU/mL) × FPG (mg/dL)/405, and (HOMA)–β-cell function (HOMA-B) percentage, which was calculated as 360 × FI (μU/mL)/FPG (mg/dL) – 63. Finally, a quality of life indicator test was completed using the 36-Item Short Form Survey.
E. Statistical Analysis
Statistical analyses were conducted using SPSS for Windows, version 21.0. Data are expressed as mean ± SD unless otherwise specified. The study was powered for detection of clinically significant differences in the primary end point of change in FPG. Briefly, the protocol was designed with the plan to enroll 60 participants with an expected 10% dropout, which would provide 90% power to detect a between-treatment difference in FPG means of 9 mg/dL with an SD of 10 mg/dL, with type 1 error of 0.05.
Primary and secondary outcomes were analyzed principally using an intent-to-treat approach, unless otherwise noted. The t test (or Mann-Whitney test) and the chi-square test (or Fisher exact test) were used to compare general characteristics within groups, and between the cinnamon and placebo groups. To adjust for potential between-groups differences in PG at baseline, analysis of covariance was used to compare changes in FPG, and AUC and PG at all time points of the OGTT. Statistical tests were 2-sided with a significance level of α equal to .05.
F. Safety Analysis
Frequency and severity of adverse events (AEs), including serious AEs, were recorded and rated as mild, moderate, or severe. In addition, we monitored clinically relevant changes in vital signs and physical examination, and laboratory safety indicators such as complete blood cell count, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, and creatinine levels.
G. Assessments
OGTTs were performed in the morning after an overnight fast and PG levels were measured at baseline and then at 30, 60, 90, and 120 minutes after glucose administration using a YSI 2300 Stat Plus device (Xylem Inc). Within the plasma glucose range of 75 to 250 mg/dL, the intra-assay and interassay coefficients of variation were 0.9% and 1.5%, respectively. PG, and serum insulin and lipoprotein levels were measured in samples collected at baseline, and after 6 and 12 weeks of randomization at an accredited clinical chemistry laboratory at Kyung Hee University Hospital and JDC. GA was measured using a commercially available kit in serum samples at baseline, and at the 6- and 12-week time point (Lucica GA-L assay; Asahi Kasei Pharma Corporation). Total protein carbonylation was quantitated in serum samples (50 μL), according to the manufacturer’s instructions (Sigma-Aldrich; catalog No. MAK094). Serum proteins carbonyl groups were derivatized with 2,4-dinitrophenyl (DNP)-hydrazine (DNPH) to form stable protein-DNP adducts followed by detection by fluorometry (375 nm). Carbonyl content (nanomoles, nmol) was calculated as absorbance/6.364 × 100 (6.364 = extinction coefficient), then normalized by microliter (μL) of serum. Only samples collected in participants enrolled at JDC were available for this post hoc analysis.
2. Results
A. Participant Characteristics
A total of 54 individuals meeting the criteria for prediabetes [5] were enrolled at 2 sites and randomly assigned to placebo (n = 27) or cinnamon (n = 27). The 12-week study period was completed by all participants in the cinnamon group and 24/27 participants enrolled in the placebo group (Fig. 1A). Baseline characteristics were well matched in the 2 groups (Table 1) with the exception of body mass index, which was higher in the group randomly assigned to cinnamon. Also, triglycerides’ mean at baseline was higher in the placebo group owing to relatively elevated levels in 3 participants, who however did not meet the statistical definition of outliers. The proportion of participants with IFG, IGT, and IFG/IGT was comparable in the cinnamon (22%, 18%, and 48%, respectively) and placebo groups (20%, 13%, and 58%, respectively). Three participants in the cinnamon group and 2 in the placebo group were enrolled solely based on the HbA1c criterion. Adherence to the thrice-daily capsule regimen was greater than 90% in both treatment groups and study centers.
Table 1.
Characteristics of participants at baselinea
| Placebo | Cinnamon | ||
|---|---|---|---|
| n | 27 | 27 | |
| Age, y | 54.1 ± 8.0 | 50.4 ± 11.5 | |
| Sex (M:F) | 16:8 | 12:15 | |
| Raceb | |||
| White | 4 | 4 | |
| Asian | 19 | 23 | |
| African American | 1 | 0 | |
| Weight, kg | 69.8 ± 11.4 | 77.7 ± 20.9 | |
| BMI, kg/m2 | 25.5 ± 3.3 | 28.2 ± 5.0 | e |
| SBP, mm Hg | 125 ± 10 | 127 ± 11 | |
| DBP, mm Hg | 75 ± 7 | 77 ± 9 | |
| FPG, mg/dL | 110 ± 11.5 | 109 ± 12.4 | |
| Glycated albumin, % | 13.9 ± 1.2 | 13.8 ± 2.0 | |
| HbA1c, % | 5.7 ± 0.4 | 6.0 ± 0.5 | |
| OGTT, min | |||
| 0 | 105 ± 12 | 111 ± 12 | |
| 30 | 185 ± 23 | 171 ± 34 | |
| 60 | 211 ± 40 | 202 ± 38 | |
| 90 | 208 ± 45 | 194 ± 43 | |
| 120 | 174 ± 55 | 169 ± 55 | |
| AUC | 22 330 ± 4090 | 21 389 ± 3858 | |
| Insulin, µIU/mL | 12.7 ± 4.4 | 11.7 ± 7.1 | |
| HOMA-IRc | 3.17 ± 1.17 | 3.21 ± 2.09 | |
| HOMA-B %d | 92.4 ± 40.8 | 91.4 ± 50.6 | |
| Total cholesterol, mg/dL | 194 ± 37 | 180 ± 39 | |
| Triglycerides, mg/dL | 170 ± 95 | 109 ± 53 | f |
| HDL, mg/dL | 45 ± 11 | 52 ± 13 | |
| LDL cholesterol, mg/dL | 129 ± 33 | 113 ± 37 | |
| Total protein, g/dL | 7.3 ± 0.5 | 7.4 ± 0.5 | |
| ALT, U/L | 25 ± 11 | 27 ± 21 | |
| AST, U/L | 24 ± 5 | 25 ± 10 | |
| γ-GT, U/L | 36 ± 29 | 36 ± 40 | |
| BUN, mg/dL | 16 ± 4 | 15 ± 3 | |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.7 ± 0.2 | |
| Uric acid, mg/dL | 5.9 ± 1.4 | 5.3 ± 1.2 | |
| Creatinine kinase, U/L | 146 ± 114 | 118 ± 41 | |
| Alkaline phosphatase, IU/L | 71 ± 23 | 69 ± 18 | |
| WBC, 109/L | 5.4 ± 1.2 | 6.0 ± 1.4 | |
| RBC, 1012/L | 4.6 ± 0.4 | 4.6 ± 0.4 | |
| Hemoglobin, g/dL | 14 ± 1 | 14 ± 1 | |
| Hematocrit, % | 42 ± 3 | 41 ± 3 | |
| Platelets, 109/L | 243 ± 33 | 235 ± 68 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; F, female; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA, homeostatic model assessment; IR, insulin resistance; LDL, low-density lipoprotein; M, male; OGTT, oral glucose tolerance test; RBC, red blood cells; SBP, systolic blood pressure; WBC, white blood cells.
aPlus-minus values are means ± SD.
bRace was self-reported.
cHOMA-IR or dβ-cell function (HOMA-B) were calculated as described in “Materials and Methods.”
e P less than .5.
f P less than .01.
B. Efficacy
FPG levels were similar at enrollment in the placebo and cinnamon groups (110 ± 11 vs 109 ± 12 mg/dL, respectively) and were not different after 6 weeks of treatment in either group (109 ± 11 with placebo vs 108 ± 13 mg/dL with cinnamon). Conversely, the assessment at 12 weeks (visit 4) showed that FPG had increased from baseline by an average of 4.5 ± 6 mg/dL with placebo treatment without any significant difference in the cinnamon group, resulting in a statistically significant between-group mean difference of approximately 5 mg/dL (114 ± 8 vs 108 ± 11; P < .01; Table 2). Although the present study was not powered to detect outcome differences between the 2 study centers, a similar trend in FPG was noted in participants independently randomly assigned at K-CTC and JDC.
Table 2.
Changes between baseline and 12 weeksa
| Placebo | Cinnamon | ||
|---|---|---|---|
| FPG, mg/dL | 4.2 ± 6.6 | –0.7 ± 6.7 | e |
| Glycated albumin, % | 0.3 ± 0.6 | –0.5 ± 0.6 | f |
| HbA1c, % | 0.1 ± 0.2 | –0.1 ± 0.3 | e |
| Insulin, µIU/mL | –0.7 ± 5.7 | 2.2 ± 3.5 | d |
| HOMA-IRb | –0.1 ± 1.6 | 0.5 ± 1.0 | |
| HOMA-B %c | –13.2 ± 40 | 20.1 ± 36.8 | e |
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HOMA, homeostatic model assessment; IR, insulin resistance.
aPlus-minus values are means ± SD.
bHOMA-IR or cβ-cell function (HOMA-B) were calculated as described in “Materials and Methods.”
d P less than .5.
e P less than .01.
f P less than .001.
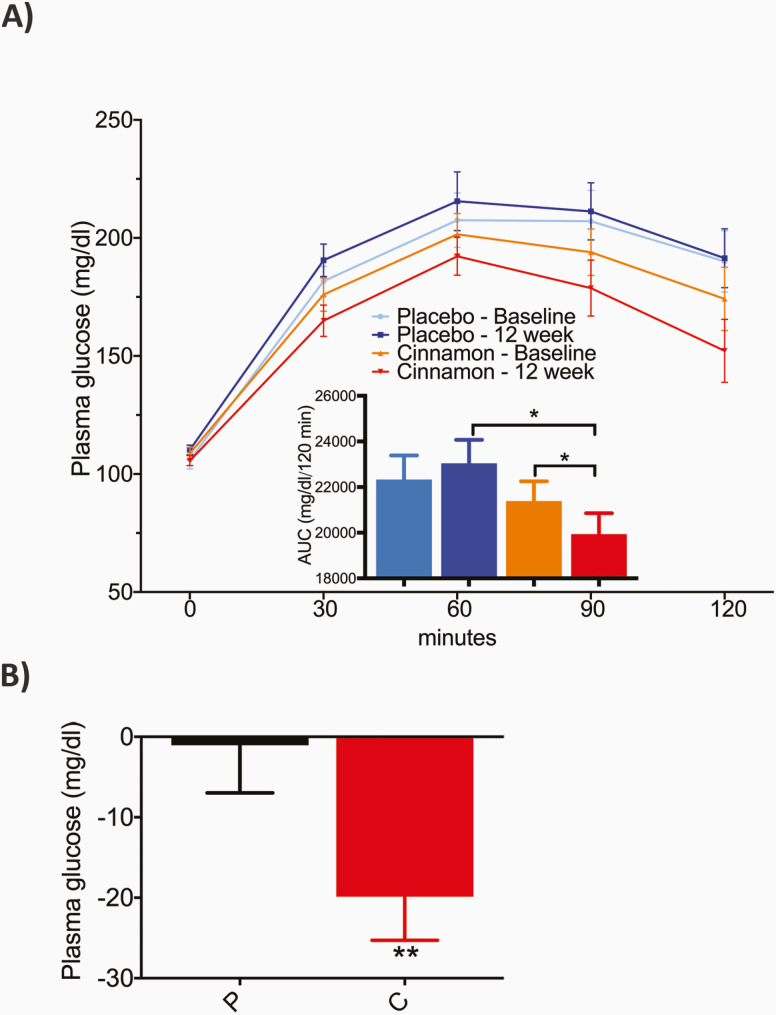
With regard to prespecified secondary end points, cinnamon resulted in a significant reduction in the AUC of PG during the OGTT from baseline to 12 weeks (21 389 ± 3858, baseline vs 19 946 ± 4070 mg/dL/120 min, 12 weeks; P < .05) whereas no change was observed in the placebo group (Fig. 2A). Also, cinnamon but not placebo resulted in a significant decrease in PG at the 2-hour time point of the OGTT, from baseline to 12 weeks (–20 ± 27 mg/dL; Fig. 2B; P < .01 for between-group difference).
Figure 2.
Change in A, overall plasma glucose (PG) profile, and B, 2-hour plasma glucose of the oral glucose tolerance test (OGTT), from baseline to 12 weeks. A, PG profile and corresponding area under the curve (AUC, inset) of the OGTT, at baseline and after 12 weeks, on placebo and cinnamon. B, Mean PG change at 2-hour time point from respective baseline OGTT (0) to OGTT at 12 weeks with placebo (P) and cinnamon (C). Data are presented as mean ± SEM. Between-group differences were analyzed by analysis of covariance. Within-group differences were analyzed by paired t test. *P less than .05; **P less than .01; ***P less than .001.
Analysis of HOMA-B showed an increase from baseline to 12 weeks in the cinnamon group (20.1 ± 36.8%, Table 2; P < .01), but not with placebo, with a 32% between-group difference at the end of the study period. HOMA-B measurements were comparable at baseline (92 ± 40.8% for placebo and 91.4 ± 50.6% for cinnamon, Table 1). In contrast, cinnamon supplementation had no effect on HOMA-IR (Table 2).
In addition, we observed improvements in long-term measures of glucose levels routinely used in clinical practice. Cinnamon treatment was associated with a modest drop in HbA1c at 12 weeks (–0.13 ± 0.25% from 5.98 ± 0.49% at baseline), with a 0.2% between-group mean difference (Table 2; P < .01). Similarly, randomization to cinnamon was accompanied by a between-group difference of 0.7% in GA at 12 weeks (P < .01), which was primarily accounted for by a decrease of 0.45 ± 0.6% from baseline with cinnamon (Table 2). In keeping with the results for FPG, changes in GA—a marker of glucose levels over 3 weeks—were not observed at 6 weeks in either group.
Within the combined pool of participants with IGT and IFG/IGT at baseline, 4 out of 18 in the cinnamon group vs 1 out of 18 with placebo had normalization of the 2-hour PG during OGTT to levels less than 140 mg/dL. Also, within the group of participants with an HbA1c of 5.7% to 6.4% at baseline, 7 out of 14 in the cinnamon group vs 1 out of 10 with placebo had reductions to levels of less than 5.7% at the end of the 12-week randomization period (χ 2 [1, N = 24] = 4.02, P < .05). None of the participants with baseline an HbA1c of 5.7% to 6.4%, in either group, progressed to an HbA1c greater than 6.5%. Although the present study was not designed to assess remission of prediabetes, these preliminary findings suggest that cinnamon treatment could result in recategorization of a number of people with prediabetes to the nonprediabetic population.
Weight, blood pressure, complete blood count, and measures of liver and kidney function were not affected by cinnamon or placebo.
With regard to health-related quality of life measures, none of the 36-Item Short Form Survey components (ie, Physical Component and Mental Component) was changed after 12 weeks on either placebo or cinnamon, from baseline measurements that were comparable between groups.
C. Exploratory Studies
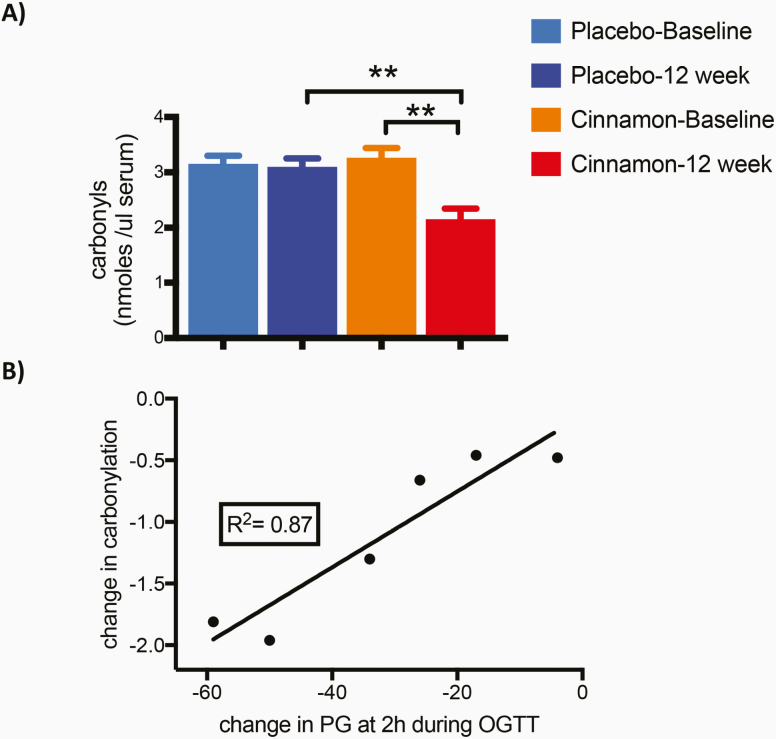
Because of the previously reported effects of cinnamon supplementation on circulating markers of oxidative stress [33], we tested the level of serum carbonylation—a form of oxidation characterized by the introduction of carbon monoxide into a substrate—before and after 12 weeks of placebo or cinnamon. This post hoc analysis was performed using remaining samples that were available at only 1 of the 2 study centers. Serum protein carbonylation was comparable in the 2 groups at baseline, and was reduced by cinnamon treatment (3.3 ± 0.43, baseline vs 2.2 ± 0.47, 12 weeks; n = 6/group; P < .01) but not by placebo treatment (3.2 ± 0.36, baseline vs 3.1± 0.37, 12 weeks; n = 6/group) (Fig. 3A). In addition, the reduction of carbonylation closely correlated with the change in the 2-hour PG during OGTT (R2 = 0.87; P < .01) (Fig. 3B), suggesting a potential link between the cinnamon-mediated effects on glucose homeostasis and oxidative stress.
Figure 3.
A, Serum protein carbonylation, and B, correlation of changes in carbonylation and in 2-hour plasma glucose (PG) during oral glucose tolerance test (OGTT). A, Total protein serum carbonylation at baseline and after 12 weeks on placebo or cinnamon. Levels of carbonyls were reduced from baseline by cinnamon but not by placebo. Samples were available and analyzed from participants enrolled at 1 of the 2 study centers. Data are presented as mean ± SEM (n = 6/group; **P < .01). B, Correlation between the change in serum carbonyls and the decrease in PG at 2-hour time point of the OGTT in the cinnamon group (R2 = 0.87; **P < .01).
D. Safety
The rate of any AEs was comparable in participants taking cinnamon, 29.2% (8/27), and placebo, 29.2% (7/24). No serious AEs were reported in either group; all AEs were rated mild with the exception of nausea and vomiting in 2 patients on placebo that were moderate. All AEs were self-limiting and were deemed unlikely to be related to the study treatment.
No clinically relevant changes in vital signs, physical exam, or laboratory tests results were observed in either group.
3. Discussion
This RCT of individuals with prediabetes showed that treatment with cinnamon 500 mg thrice daily resulted in a statistically significant between-group mean difference in FPG of approximately 5 mg/dL at 12 weeks, which was the primary outcome measure of the study. No change in FPG was noted at 6 weeks, which was 1 of the 3 prespecified secondary end points.
Our data are consistent and extend the results of the study by Roussel et al [33], which demonstrated a decrease in FPG with 500 mg daily of Cinnulin (aqueous extract of C cassia) in a small group of participants with IFG. Similar to our trial, effects on FPG were observed after 12 weeks of supplementation, but not at 6 weeks. Of note, our larger study enrolled participants meeting any of the criteria for prediabetes [5], thus expanding the relevance of these findings to a larger population.
Cinnamon treatment also exerted beneficial effects on several other end points. OGTT results showed a significant drop of the PG at 2 hours and of the overall AUC of PG profile after 12 weeks of cinnamon, but not placebo, thus indicating an improvement in glucose tolerance. Our findings are in keeping with previous studies showing that cinnamon extract induces membrane translocation of GLUT4 in 3T3-L1 adipocytes and partial correction of glucose intolerance in a rat model of T2D [30]. The significant decrease in the AUC of PG suggests that reductions in HbA1c and GA with cinnamon, discussed as follows, result in part from lessening of postprandial hyperglycemia.
Although the lack of information on insulin concentrations during the OGTT prevents any statement regarding the effect of cinnamon on insulin release in response to a glucose load, cinnamon-treated participants displayed higher FI levels and an ample increase in HOMA-B percentage at 12 weeks when compared to the respective baseline (Table 2). Notwithstanding limitations of static models [36], our findings point to an effect of cinnamon on β-cell function that should be further investigated with more mechanistic studies. In this context, ingestion of rice pudding with 3 g of cinnamon (C cassia) in healthy participants resulted in a higher peak of glucagon-like peptide-1, when compared to the meal without cinnamon [31].
The correlation between the lowering in the AUC of PG during the OGTT and the reduction in serum protein carbonylation is intriguing. Mohanty and colleagues first reported an increase in reactive oxygen species in polymorphonuclear cells of normal individuals following a glucose challenge [37]; also, fiber intake blunted reactive oxygen species production induced by a high-fat, high-calorie diet in mononuclear cells, in parallel with an increase in insulin secretion [38]. Finally, orange juice reduced markers of oxidative stress induced by a high-fat, high-calorie meal in polymorphonuclear cells of healthy participants [39]. In this view, our exploratory studies suggest that a reduction in serum protein carbonylation could be a marker both for cinnamon’s metabolic effects and its antioxidant activity.
Furthermore, cinnamon had a significant impact on long-term indexes of dysglycemia, including a between-group difference of –0.2% in HbA1c and –0.7% in GA at 12 weeks. The concordant trend of HbA1c and GA is noteworthy because participants in the cinnamon group had a small reduction in hemoglobin, which could be a caveat when interpreting HbA1c [40]. Within a population with prediabetes, relatively small changes in HbA1c can be clinically meaningful. The EPIC-Norfolk study estimated that 10% of total mortality could be prevented by lowering HbA1c by 0.2% [41], from a baseline of 5.0% to 6.9% that overlaps the HbA1c range in our trial (ie, 5.7%-6.0%).
Cinnamon was overall well tolerated with mild AEs reported in 29% of participants, with a frequency nearly identical to the placebo group and without any prevailing pattern in the frequency of specific AEs.
Our study has several strengths. First, this is the largest RCT testing the effects of cinnamon on glucose homeostasis in participants with prediabetes, along a spectrum of abnormalities (IFG, IGT, or both). Although our trial was not powered to compare the extent of changes between the 2 study centers, the overall trend on relevant end points appeared comparable in participants enrolled at K-CTC (all of self-reported Asian ethnicity) and at JDC (primarily white). More targeted, powered studies will be required to assess whether age and/or sex are factors that modify the response to cinnamon on metabolic outcomes.
Second, the study protocol had a ‘light-touch’ approach, without any recommendation for structured lifestyle interventions beyond general counseling at the time of enrollment. Although this experimental design could also be construed as a limitation, it allows the better isolation of the effect of cinnamon from superimposed investigator-mandated lifestyle modifications.
Third, the correlation between the reduction in serum protein carbonylation and in PG at 2 hours of the OGTT lends initial support to the use of total carbonylation as a marker of the effect of cinnamon on glucose tolerance.
This trial also has certain limitations. The relatively short duration does not allow us to draw conclusions regarding the durability of cinnamon’s effects or the rate of progression from prediabetes to T2D. Also, the potential emergence of tachyphylaxis, noted with certain mainstay diabetes drugs [42], has to be evaluated rigorously. In addition, the present study was not powered to discriminate differences in the response to cinnamon between participants with IFG vs IGT. Finally, our experimental design did not directly aim to clarify the mechanism(s) of action of cinnamon, which should be investigated in future studies.
In conclusion, treatment with cinnamon for 12 weeks, compared to placebo, resulted in favorable changes in measures of glucose homeostasis in a representative population of participants with prediabetes. These findings should set the foundation for a longer and larger RCT that directly addresses the impact of cinnamon on incident T2D and/or remission of prediabetes.
Acknowledgments
We thank Charlene Coneys and the staff of the Clinical Research Center at JDC, and Wonsook Kim and the staff of the K-CTC for coordinating study visits and for their assistance throughout the trial. Aden Lee (Phillips Exter Academy, New Hampshire) contributed to data analysis. We thank Dr M.E. Patti at JDC for reading our manuscript and providing valuable feedback. Finally, we are grateful to all patients who participated in this study.
Financial Support: This work was supported by the Traditional Korean Medicine Research and Development program funded by the Ministry of Health and Welfare through the Korea Health Industry Development Institute (Grant No. HI13C0700).
Clinical Trial Information: ClinicalTrials.gov registration number NCT03219411 (registered July 17, 2017).
Author Contributions: G.R.R., C.M.M., J.L., and B.-C.L. developed the study design, supervised the research, analyzed and interpreted the data, and wrote the manuscript; Y.N. and C.H. acquired the clinical data. All authors agreed to be accountable for all aspects of the work.
Glossary
Abbreviations
- AEs
adverse events
- AUC
area under the curve
- FI
fasting insulin
- FPG
fasting plasma glucose
- GA
glycated albumin
- HbA1c
glycated hemoglobin
- HOMA-B
homeostatic model assessment–β-cell function
- HOMA-IR
homeostatic model assessment for insulin resistance
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- JDC
Joslin Diabetes Center
- K-CTC
Korean Medicine Clinical Trial Center
- OGTT
oral glucose tolerance test
- PG
plasma glucose
- T2D
type 2 diabetes
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1.International Diabetes Federation. ProMED-mail website. www.IDF.org. https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. 2019. Accessed December 3, 2019.
- 2. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of medical care—2019. Diabetes Care. 2019;42(Suppl 1):S1-193.30559224 [Google Scholar]
- 6. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. [DOI] [PubMed] [Google Scholar]
- 7. The National Prediabetes Awareness Campaign. ProMED-mail website. https://www.cdc.gov/diabetes/campaigns/national-prediabetes-awareness-campaign.html. Accessed March 12, 2019.
- 8. Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343-1350. [DOI] [PubMed] [Google Scholar]
- 9. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heianza Y, Hara S, Arase Y, et al. HbA1c 5·7-6·4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011;378(9786):147-155. [DOI] [PubMed] [Google Scholar]
- 11. Abdul-Ghani M, DeFronzo RA, Jayyousi A. Prediabetes and risk of diabetes and associated complications: impaired fasting glucose versus impaired glucose tolerance: does it matter? Curr Opin Clin Nutr Metab Care. 2016;19(5):394-399. [DOI] [PubMed] [Google Scholar]
- 12. Haffner SM, Mykkänen L, Festa A, Burke JP, Stern MP. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000;101(9):975-980. [DOI] [PubMed] [Google Scholar]
- 13. Stevens JW, Khunti K, Harvey R, et al. Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pract. 2015;107(3):320-331. [DOI] [PubMed] [Google Scholar]
- 14. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537-544. [DOI] [PubMed] [Google Scholar]
- 15. Lindström J, Louheranta A, Mannelin M, et al. ; Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26(12):3230-3236. [DOI] [PubMed] [Google Scholar]
- 16. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289-297. [DOI] [PubMed] [Google Scholar]
- 17. DeFronzo RA, Tripathy D, Schwenke DC, et al. ; ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104-1115. [DOI] [PubMed] [Google Scholar]
- 18. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072-2077. [DOI] [PubMed] [Google Scholar]
- 19. Holman RR, Haffner SM, McMurray JJ, et al. ; NAVIGATOR Study Group Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1463-1476. [DOI] [PubMed] [Google Scholar]
- 20. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155-161. [DOI] [PubMed] [Google Scholar]
- 21. Pontiroli AE, Folli F, Paganelli M, et al. Laparoscopic gastric banding prevents type 2 diabetes and arterial hypertension and induces their remission in morbid obesity: a 4-year case-controlled study. Diabetes Care. 2005;28(11):2703-2709. [DOI] [PubMed] [Google Scholar]
- 22. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695-704. [DOI] [PubMed] [Google Scholar]
- 23. Knowler WC, Fowler SE, Hamman RF, et al. ; Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tripathy D, Schwenke DC, Banerji M, et al. Diabetes incidence and glucose tolerance after termination of pioglitazone therapy: results from ACT NOW. J Clin Endocrinol Metab. 2016;101(5):2056-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015(79):1-16. [PMC free article] [PubMed] [Google Scholar]
- 27. Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26(4):1277-1294. [DOI] [PubMed] [Google Scholar]
- 28. Fischer F, Lewith G, Witt CM, et al. A research roadmap for complementary and alternative medicine—what we need to know by 2020. Forsch Komplementmed. 2014;21(2):e1-16. [DOI] [PubMed] [Google Scholar]
- 29. Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen Y, Honma N, Kobayashi K, et al. Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PloS One. 2014;9(2):e87894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hlebowicz J, Hlebowicz A, Lindstedt S, et al. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89(3):815-821. [DOI] [PubMed] [Google Scholar]
- 32. Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol. 2010;101(12):4676-4689. [DOI] [PubMed] [Google Scholar]
- 33. Roussel AM, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28(1):16-21. [DOI] [PubMed] [Google Scholar]
- 34. Medagama AB. The glycaemic outcomes of cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;2012(9):CD007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487-1495. [DOI] [PubMed] [Google Scholar]
- 37. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85(8):2970-2973. [DOI] [PubMed] [Google Scholar]
- 38. Ghanim H, Batra M, Abuaysheh S, et al. Antiinflammatory and ROS suppressive effects of the addition of fiber to a high-fat high-calorie meal. J Clin Endocrinol Metab. 2017;102(3):858-869. [DOI] [PubMed] [Google Scholar]
- 39. Ghanim H, Sia CL, Upadhyay M, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and toll-like receptor expression. Am J Clin Nutr. 2010;91(4):940-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184-S190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ. 2001;322(7277):15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60(5):1561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.