Abstract
Over the last decade, there have been substantial advances in our understanding about how viral infections regulate asthma (Table 1). Important lessons have been learned from birth cohort studies examining viral infections and subsequent asthma, understanding the relationships between host genetics and viral infections, the contributions of respiratory viral infections to patterns of immune development, the impact of environmental exposure on severity of viral infections, and how the viral genome influences host immune responses to viral infections. Further, there has been major progress in our knowledge about how bacteria regulate host immune responses in asthma pathogenesis. In this article, we also examine the dynamics of respiratory tract bacterial colonization during viral upper respiratory tract infection, in addition to the relationship of the gut and respiratory microbiomes with respiratory viral infections. Finally, we focus on potential interventions that could decrease virus-induced wheezing and asthma. There are emerging therapeutic options to decrease severity of wheezing exacerbations caused by respiratory viral infections. Primary prevention is a major goal and a strategy toward this end is considered.
Keywords: virus, asthma, genetics, immune, microbiome
Introduction
Over the last decade, there have been substantial advances in our understanding about how viral infections regulate asthma (Table 1). Important lessons have been learned from birth cohort studies examining viral infections and subsequent asthma, understanding the relationships between host genetics and viral infections, the contributions of respiratory viral infections to patterns of immune development, the impact of environmental exposure on severity of viral infections, and how the viral genome influences host immune responses to viral infections. Further, there has been major progress in our knowledge about how bacteria regulate host immune responses in asthma pathogenesis. In this article, we also examine the dynamics of respiratory tract bacterial colonization during viral upper respiratory tract infection, in addition to the relationship of the gut and respiratory microbiomes with respiratory viral infections. Finally, we focus on potential interventions that could decrease virus-induced wheezing and asthma. There are emerging therapeutic options to decrease severity of wheezing exacerbations caused by respiratory viral infections. Primary prevention is a major goal and a strategy toward this end is considered.
Table 1.
Review of most salient points
|
The viral genome and how it influences host immune responses to viral infections
Respiratory syncytial virus (RSV) and rhinovirus (RV) are important causes of wheezing in early life and wheezing illness with these viruses have been associated with increased asthma risk later in childhood. At age 6, there is an increased risk of asthma if children had wheezing illness with RSV (odds ratio 2.6), RV (odds ratio 9.8), or both RSV and RV (odds ratio 10.0) in the first 3 years of life. RSV is a negative-sense, single stranded RNA virus that is a member of the Paramyxoviridae family and is the leading cause of hospitalization each year in the United States in children under 1 year of age.1 There are three species of RV in the enterovirus genus, and all are positive-sense, single-stranded RNA viruses that have protein capsids. RV are the most frequently detected viruses in wheezing children over the age of 1 year, and from children and adults with acute exacerbations of asthma.
The clinical manifestations of a viral infection in the respiratory tract result from a complex interplay of the host, environment, and virus. To make comparisons between different immune responses elicited by diverse viruses, host and the environmental conditions must be held constant in order to prevent the introduction of confounding factors. This requires artificial conditions, such as the use of human cell lines for in vitro infection studies, the infection of genetically identical animals, such as mice, housed in the same environment, and the use of a standard viral inoculum. Determining the effect of specific genes within a virus requires that all other viral genes are identical. Such studies have begun but are still relatively new.
Experiments in models of RSV genomes have provided important insights into how the viral genome influences host immune responses to infection. Three RSV strains commonly used in pathogenesis studies are A2, line 19, and Long. RSV A2 infection in BALB/c mice resulted in a predominant IFN-γ immune response, no production of the Th2 cytokine IL-13 in the lung, an absence of airway mucus, and no airway responsiveness (AR) to methacholine.2 Infection with RSV Long similarly did not result in host IL-13 production in the lung nor was there airway mucus.3 However, line 19 infection in genetically identical mice in the same environment caused the host immune response to produce IL-13, decreased IFN-γ compared to A2 infection, airway mucus, and heightened AR.2 Sequencing of the A2, line 19, RSV Long strains revealed six amino acid differences between line 19 and the A2 and Long strains, of which 5 amino acid differences were in the fusion (F) protein.3 To determine the contribution of the F gene of each virus to disease pathogenesis, a reverse genetics approach was undertaken by creating chimeric viruses whereby an A2 virus was manipulated to replace the A2 F gene with either the F gene of either line 19 or Long. Infection with the chimeric virus containing the line 19 F gene caused decreased host IFN-α lung levels, higher viral load in the lungs, greater lung IL-13 protein, augmented airway mucus, and increased AR, compared to the chimeric viruses containing either A2 or Long F proteins.3 Therefore, this reverse genetic approach provided the opportunity to not only discover which genes in RSV line 19 strain were responsible for the lung IL-13, airway mucus, and AR, but also the identification of the specific amino acids that caused airway remodeling. These techniques not only provide the knowledge of unique components of the viral genome that contribute to specific pathogenic features but may also assist in vaccine and therapeutic strategies aimed at the proteins responsible for specific disease characteristics.
Future perspectives:
To date, there have not been studies that reveal a relationship between RSV genotypes and the presence of wheezing in hospitalized children with bronchiolitis or bronchopneumonia; however, this may be a function of the lack of application of technology to sequence strains because of cost. Studies relating viral genetics to severity of illness in mice have demonstrated the intricate interactions between viral genome, viral proteins, host cell function and metabolism and immune response. Developing a greater understanding of this chain of events could highlight several therapeutic opportunities, including identification of high-priority pathogens, inhibition of viral or host proteins that are critical for replication, and strategies to inhibit virus-induced skewing of immune responses that favors viral replication over host defense.
Host genetics and viral respiratory infections
A number of studies have begun to shed light on the relationships among host genetics, viral infections and acute and long-term respiratory outcomes. Candidate gene approaches have been utilized to identify associations between genetic polymorphisms and viral respiratory illness outcomes. Polymorphisms in several antiviral and innate immune genes have been linked to susceptibility to respiratory viruses, infection severity, and virus-induced asthma exacerbations, and have been replicated across multiple cohorts (Table 2). These genes include STAT4, JAK2, MX1, VDR, DDX58, and EIF2AK2.4 Additionally, whole exome sequencing has been utilized to identify rare variants in innate immune responses linked to severe respiratory viral infections. Autosomal recessive IRF7 deficiency has been observed in one patient in association with severe influenza infection and acts through impairment of interferon amplification.5 Dominant negative loss-of-function variants in IFIH1, critical to viral RNA sensing, have been shown to be a risk for intensive care unit hospitalization due to viral infections in previously healthy children.6
Table 2.
Polymorphisms in several antiviral and innate immune genes have been linked to susceptibility to respiratory viruses, infection severity, and virus-induced asthma exacerbations, and have been replicated across multiple cohorts.
| Gene | Function |
|---|---|
| STAT4 | Transcription factor required for IL-12 signaling in the development of Th1 cells from naïve CD4 T cells |
| JAK2 | A non-receptor tyrosine kinase critical for signaling of the GM-CSF, gp130, and single chain receptor families. |
| MX1 | Responsible for the antiviral state against influenza infection |
| VDR | Vitamin D receptor |
| DDX58 | Involved in antiviral signaling in response to viruses containing a dsDNA genome |
| EIF2AK2 | Innate antiviral immune response to viral infection that can trigger apoptosis via FADD-mediated caspase 8 |
| IRF7 | Critical role in the innate immune response against DNA and RNA viruses |
| IFIH1 | Provides instructions for making the MDA5 protein that has a critical role in innate antiviral immunity |
| IFNA5 | One of the type I IFN-α isoforms that has antiviral activities |
| NOS2 | Nitric oxide synthase gene that mediates the antiviral activity of IFN-γ |
| ADAM33 | Member of the ADAM (a disintegrin and metalloprotease domain) family identified as a major susceptibility gene in asthma |
| IL4R | Interleukin 4 receptor through which IL-4 and IL-13 signal to induce IgE class switching and airway mucus metaplasia |
| CD14 | Multiple functions, one of which is critical for TLR signaling in host defense |
| TNF | Tumor necrosis factor that is produced in abundance by mast cells and has roles in cell survival and proliferation |
| IL13 | Important in airway mucous cell metaplasia, airways responsiveness, VCAM expression |
| IL1RL1 | One subunit of the receptor for IL-33, which can activate ILC2 and promote CD4 T cell differentiation toward Th2 phenotype |
| CDHR3 | Cadherin that is the receptor for rhinovirus |
A number of polymorphisms have been specifically associated with increased severity of illnesses associated with hospitalization from RSV infection. A candidate gene approach identified SNPs in the innate immune genes VDR, IFNA5, and NOS2 as risk factors for RSV bronchiolitis.7 In order to further elucidate associations of host genetics with RSV illness severity and asthma risk, a recent review examined overlap amongst genes associated with both outcomes. This approach identified a number of genes involved with both innate immunity and type 2 (Th2) inflammatory responses (ADAM33, IL4R, CD14, TNF, IL13 and IL1RL1) that are highly relevant to these outcomes.8
The most replicated association between host genetics and asthma risk is the 17q21 locus. In two birth cohort studies, variants in this locus, including ORMDL3 and GSDMB, were also associated with increased risk of wheezing with RV infections in early life.9 Interestingly, these variants were only associated with increased risk of subsequent asthma in children who developed RV wheezing in the first 3 years of life. In contrast, early life RSV wheezing was not linked to 17q21 variants in these cohorts. In addition, farm exposures10 and pets11 in the home lessen the risk of asthma for children with high-risk 17q21 genotypes. In each case, the genetic risk associated with 17q21 was buffered by protective environmental exposures.
RV virulence varies by species; RV-A and RV-C are more likely to cause illnesses, wheezing and lower respiratory tract infection compared to RV-B,12 which has a slower rate of replication and induces muted cytokine and chemokine responses.13 Whether there are individual types within RV species that are more virulent is unknown, and difficult to study given the genetic diversity of these viruses and high mutation rate. A functional polymorphism in cadherin-related family member 3 (CDHR3) has been associated by genome wide association study (GWAS) with early childhood asthma and severe wheezing episodes.14 Interestingly, CDHR3 is a receptor that enables binding and replication of RV-C, suggesting that this link between CDHR3 and asthma risk may be mediated by RV-C infections.15 In support of this hypothesis, children with the risk polymorphism in CDHR3 were recently found to have greater risk of RV-C illnesses, but not illnesses associated with other viruses.16
Future perspectives:
These genetic associations among respiratory virus susceptibility, infection severity and subsequent asthma risk may prove to be important to risk stratify populations, and potentially provide new therapeutic targets for reducing illness severity and subsequent risk. Further, unbiased approaches have been employed recently to identify pathways of gene expression in the upper airway that differentiate a viral cold that resolves from one that leads to an asthma exacerbation.1 Efforts are ongoing to understand how these gene expression patterns are regulated in hopes of identifying new personalized therapeutic strategies to prevent asthma exacerbation. The integration of multiple “omics” approaches holds promise to provide the ability to unravel these complex relationships.
Environmental factors affecting the inception and severity of asthma exacerbations
The exposome, defined as the measure of all exposures that influence the health of an individual, is an important determinant of asthma risk during the lifespan of an individual.17 Early exposures can set in motion pathways that will ultimately define illnesses and symptom exacerbations, which is especially true when considering the ontogeny of asthma. From birth through school years, children are frequently exposed to a variety of respiratory pathogens, allergens, microbes and airway irritants. The pathogenic or beneficial effects of these exposures and their interactions remain the focus of research to develop new interventions and preventive therapies.
Most of the initial research devoted to the ontogeny of asthma focused on RSV infections which are frequently detected by culture and tests for RSV antigen in nasal washes from wheezing infants during the mid-winter months. Studies in the past reported that flares of wheezing caused by RSV leading to hospitalization during infancy increased the risk for developing asthma and allergy.18, 19 However, recent studies indicate that the more severe episodes (i.e., those requiring hospitalization) of infantile wheezing caused by RSV increase the risk for subsequent wheeze in infants and toddlers, but it is less certain whether RSV-induced wheeze influences the development of atopy or asthma as children grow older.20
In contrast, flares of wheezing caused by RV are more strongly linked to persistent wheezing and the development of asthma, especially in children who are sensitized to allergens at an early age.21–23 In keeping with this, the dominant risk factors for asthma attacks that require hospital care among children after 3 years of age is the combination of allergic airway inflammation and RV infection.24–26 As a result, several host factors should be considered in efforts to treat asthma exacerbations more effectively and to reduce the risk for asthma development, For example:
There may be phenotypes of asthmatic children who would benefit from development of vaccines to RV or RSV. For example, genetic variations at the 17q21 locus and a coding SNP in CDHR3 (the receptor for RV-C genotypes) increase the risk for wheezing with RV during childhood.9, 14
There is current interest in whether the administration of a biologic such as omalizumab (anti-IgE antibody) during early childhood will have a disease modifying effect after this intervention is discontinued (i.e., the Preventing Asthma in High Risk Kids (PARK) trial; NCT02570984).
There is evidence that the asthmatic airway, especially epithelial cells and innate lymphoid cells, has a Type 2 bias with enhanced production of TSLP, IL-13, and IL-25 in parallel with decreased type I and III IFN responses that are needed for effective antiviral killing and clearance.27–34 This bias may increase the susceptibility of allergic asthmatics to RV infections. Once infected, however, in vivo studies have shown that during RV infections viral loads and clearance are similar among children and young adults with asthma compared to non-allergic individuals without asthma.31 At present, mechanisms to explain these differences are poorly understood. A better understanding is likely to come from research focused on the cascade of early, innate cellular and molecular events that follow RV infection of epithelial cells.
Future perspectives:
Airway inflammation caused by recurrent infections (predominantly with RV) in the allergic host will continue to be the focus of research designed to develop new therapies to help children and young adults with asthma. Whether treatments targeting allergic inflammation will be sufficient to reduce the frequency and severity of exacerbations (e.g., using new monoclonal antibody-based biologics), or whether additional therapeutics will be needed to decrease the frequency of RV infections, or enhance viral killing, remains to be determined. Looking to the future, the evaluation of other interventions such as the administration of antibiotics to treat secondary bacterial pathogens, or azithromycin to reduce wheezing following virus infection also deserve further study, along with investigations to determine whether the administration of vitamin D, probiotics, and dietary modifications (e.g., fish oil) will have benefits. In contrast, the adverse effects of airway irritants such as environmental tobacco smoke (ETS) and air pollution (e.g., diesel fuel) on the severity and persistence of RV-induced asthma remain poorly understood.
Effects of respiratory viral infections on patterns of immune development
Acute wheezing illnesses with respiratory tract viruses in infancy and early childhood represent an important risk factor for childhood recurrent wheezing and later asthma development. This link is particularly well-established with RV and RSV, suggesting that these viruses may have a causative role, and significant research is directed towards understanding how these viruses can alter immune development to contribute to asthma pathogenesis. That said, causation remains unproven and asthma prevention strategies targeting viral illnesses do not currently exist.
RV-associated wheezing, in particular, is associated with a higher asthma risk than other viruses; this has been consistently demonstrated across multiple studies.21, 23, 35–38 Many of these studies have linked RV-induced wheezing with other asthma risk factors, in particular markers of atopy including allergic sensitization, increased eosinophils, and atopic eczema, suggesting possible additive or synergistic effects in increasing asthma risk. Experimental models have demonstrated alterations in type-2 immune responses to RV that may account for this risk (Figure 1). Mouse models have demonstrated that neonatal RV (RV1B) infection results in persistent airway hyperresponsiveness, mucous cell metaplasia, and IL-13 production that does not occur in adult mice. Furthermore, knockout of IL4R prevents this response, consistent with an IL13-dependent process.39 Subsequent work demonstrated that RV infection leads to expression of epithelial derived cytokines IL-25, IL-33, and TSLP and an increase in ILC2s as an important source of airway IL-13; blocking these pathways with anti-IL-25 attenuates neonatal RV-induced AHR and mucous cell metaplasia.40, 41 While there is no equivalent human evidence regarding the immune effects of RV in early life, these same pathways are known to play a key role in the response to RV leading to exacerbations in established asthma.29
Figure 1.
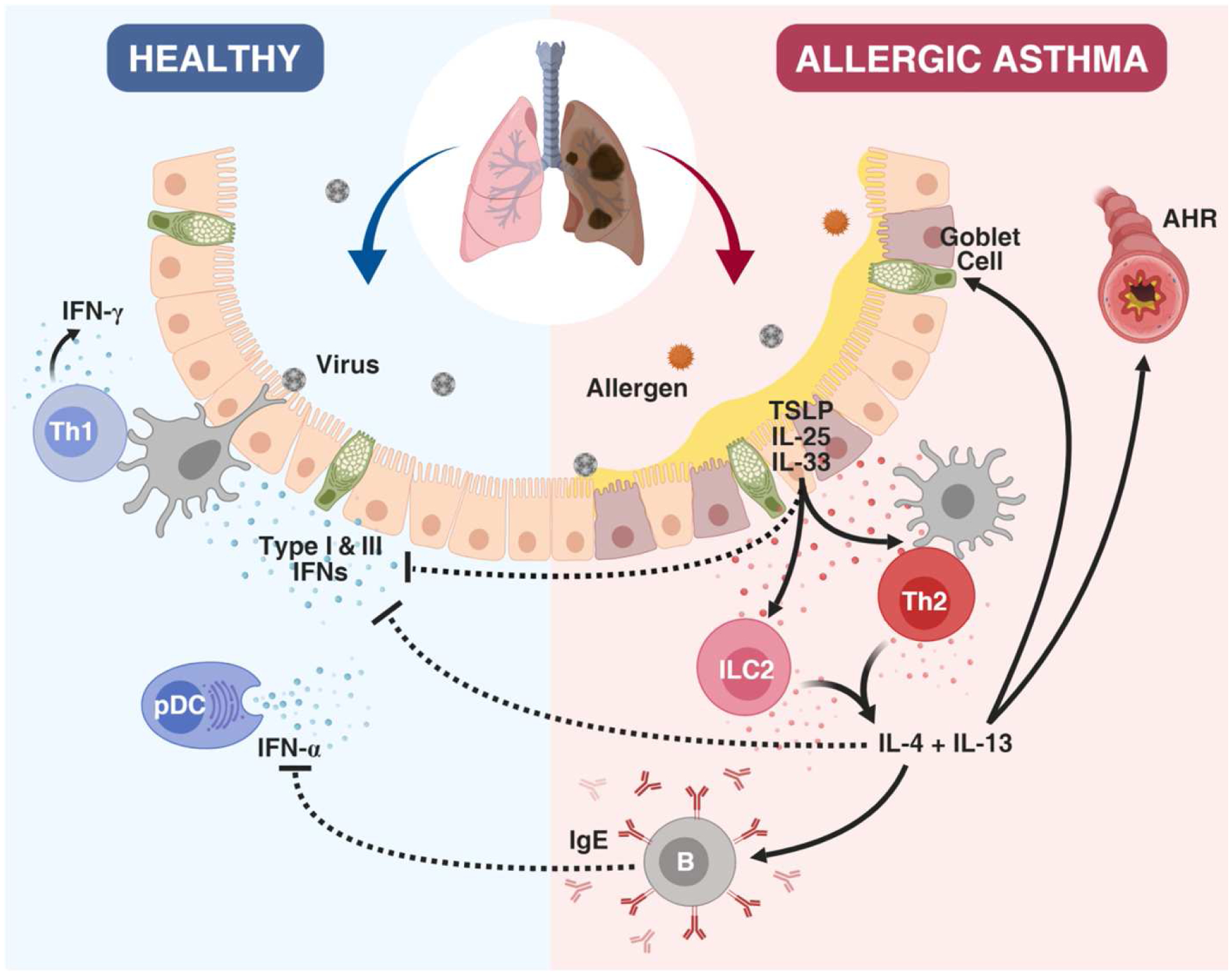
Immune responses to virus in the allergic asthmatic host. In the healthy host, anti-viral IFN responses control and clear respiratory viral infections. In allergic asthmatics, the release of the type 2-skewing cytokines TSLP, IL-25, and IL-33 promote the induction of Th2 cytokines and the suppression of IFN responses, in addition to promoting airway hyperreactivity (AHR) and increased mucus and IgE production. Furthermore, IgE has the capacity to suppress IFN-α production by Plasmacytoid DCs (pDCs).
A key question however, is whether underlying Th2 inflammation or RV associated wheezing comes first. A prospective birth cohort analysis has shown that allergic sensitization generally precedes RV wheezing but not the other way around, suggesting allergic sensitization may lead to more severe RV illnesses and the development of asthma.42 Supporting this observation, in vitro studies have shown that Th2 inflammation can inhibit type I and III interferon antiviral responses to RV infections,43, 44 which may increase susceptibility to more severe RV infections. However in contrast, several human studies have demonstrated increased IFN signatures in asthmatic children with virus infections, as well as in severe asthma in adults;45–47 these might represent different disease states, as a recent report found that early life exacerbation-prone asthma was correlated with low IFN signatures, while the highest IFN signatures were associated with later-onset asthma.48
Allergy is a major risk factor for the progression from wheezing illnesses to asthma, and this has been a very consistent finding across multiple cohorts.21, 49, 50 Allergic sensitization precedes wheezing illnesses in most young children,42 and allergic inflammation can impair antiviral responses in vitro51 and in vivo.52 This suggests that allergic airway inflammation can increase susceptibility to and severity of viral respiratory illnesses. Allergic sensitization in early childhood may also modify the relationship between microbial colonization and respiratory outcomes. In preschool children whose airways were colonized with pathogen-dominated microbiomes, sensitized children were at increased risk for chronic asthma while non-sensitized children were likely to have transient wheeze that resolved by age 4 years.53
It is well established that hospitalization for RSV bronchiolitis in the first year of life is associated with later development of asthma.38, 54–56 RSV induces a broad innate immune response in infants including systemic interferon, neutrophil, and inflammatory pathways, and distinct RSV strains and concomitant airway bacteria can influence the severity of infection.57, 58 The risk for more severe RSV-illnesses has also been linked to polymorphisms in several immune regulatory genes,59 many of which also can influence asthma risk. However, whether RSV is causal remains a subject of debate with two large cohort studies showing different conclusions,59, 60 one suggesting causation and the other an underlying genetic predisposition. Notably, two prevention studies using palivizumab (a monoclonal antibody directed against RSV) in high-risk infants found that prevention of more severe RSV-illnesses decreased the risk of childhood recurrent wheezing but not asthma development.61, 62 Ultimately RSV infection appears to have the greatest impact on asthma risk during a critical window of lung development for infants born during the fall (in the Northern hemisphere) who are at ~4 months of age during the peak of the winter RSV season. It has been well-established that RSV infection can induce pathologic Th2 immune responses, especially within the context of formalin-inactivated RSV vaccination.63–65 More recently, studies in mice have demonstrated the ability for RSV-related Pneumonia Virus of Mice as well as human RSV infection to break tolerance to allergens in neonatal mice.66 Furthermore, it is now appreciated that RSV triggers release of epithelial-derived cytokines that promote Th2 responses and can induce ILC2 responses following infection.67, 68 These same epithelial cytokines have also been implicated in RV infection, perhaps suggesting a shared innate Th2-skewing mechanism during viral infection.27, 40
Future perspectives:
Fully understanding the patterns of immune development that lead to asthma inception, and how such patterns are affected by exogeneous exposures including viral infections, will direct asthma prevention research. Ongoing studies are focused on altering Th2-skewing in early life including through blocking IgE and through altering microbial exposures. If effective, decreasing Th2-inflammation in early life may function in part through enhancing antiviral responses.51, 69 However, antiviral specific therapies including RV and RSV vaccines, may also prove to be critical in asthma prevention.
Dynamics of respiratory tract bacterial colonization during viral upper respiratory tract infection
Detection of viruses in the upper airway during peak viral seasons can be as high as 90% in prospective studies.70 However, rates of illness are significantly lower, leading researchers to question why some patients are more susceptible to increased morbidity when they have a viral upper respiratory tract infection (URI). One factor that has been shown to increase upper and lower airway symptoms during viral infections are bacteria.71
These bacteria collectively constitute the microbiota. The upper airway microbiota develops over the first year of life with alterations in the natural development associated with increased risk for URIs during the first few years of life.72, 73 The most abundant bacteria within the upper airway of infants and children are Staphylococcus, Streptococcus, Moraxella, Haemophilus, Dolosigranulum, and Corynebacterium.71, 72, 74–78
In several infant cross-sectional and cohort studies, the presence of Streptococcus, Moraxella, or Haemophilus during upper respiratory infection increases the likelihood that the infant will have lower airway symptoms.72, 76 Studies examining airway bacteria during RSV bronchiolitis have reported links between an increased abundance of Streptococcus9 and Haemophilus.78 In contrast, RV-bronchiolitis is associated with an increased abundance of Moraxella and Haemophilus.9 While these studies suggest that a bacteria-virus interaction occurs during infancy, only a few studies have examined the association between virus and bacteria in school-age children. One such study revealed that children with Streptococcus or Moraxella present in their airway are more likely to have cold and asthma symptoms during a naturally occurring RV infection.71 Collectively, these studies demonstrate that an association exists between specific bacteria and illness severity.
While Streptococcus, Moraxella and Haemophilus are often associated with an increase in viral-associated symptoms, a higher abundance of Corynebacterium, Staphylococcus and Dolosigranulum is often present in the airway in the absence of viral detection and clinical symptoms.75, 77, 78 In addition, when the latter three bacteria are enriched in the upper airway, infants are less likely to have an acute respiratory illness,72 and school-age children are less likely to have a symptomatic illness during RV infection.75 Furthermore, high abundance of Lactobacillus in the upper airway during RSV illness is associated with a decreased risk of childhood wheeze,77 suggesting that bacteria present in the airway during viral illnesses may contribute to both illness severity and long term sequela.
Future perspectives:
Because most studies examining airway bacteria during viral infection have been cross-sectional, observational studies, it remains unclear how airway microbes affect the epithelium, and whether these interactions contribute to the causation of wheezing illnesses, asthma development in young children, and exacerbations of established asthma. Greater insight is needed into metabolic, immunologic and toxic effects of bacteria on epithelial cells that could contribute to acute illnesses and asthma risk. While many studies have examined changes in bacteria that occur during viral infection, few have examined how the airway microbiome influences susceptibility vs. resilience to viral infection. Some bacteria could promote a “pro-inflammatory” environment thereby making the airway susceptible to viral infection. The presence of H. influenzae in the infant airway prior to viral infection is associated with increased expression of local inflammatory cytokines suggesting a link between bacteria and airway inflammation.79 In contrast, mice receiving intranasal administration of Lactobacillus rhamnosus prior to viral infection have enhanced antiviral immune responses,80 suggesting that some bacteria protect the airway and reduce the risk of symptomatic viral infection. Greater understanding of these relationships may lead to new preventive approaches to acute viral-bacterial illnesses and perhaps the development of childhood asthma.
The influence of the gut microbiome on viral infections of the respiratory tract
The gut microbiome represents the most abundant and diverse microbial environment in the human body, comprised of approximately 40 trillion bacteria.81 These bacteria have coevolved with humans over millennia to contribute to a symbiosis in which humans consume prebiotic fiber which is metabolized by resident microbes in the gut to create short chain fatty acids (SCFA) which in turn regulate immune responses.82, 83 Alterations in this relationship are occurring in modern times due to practices such as the frequent use of antibiotics, and the consumption of a high-sugar, low fiber diet. As a consequence, a state of microbial dysbiosis, or an ecological imbalance, may result, which leads to the loss of metabolic capabilities and predisposes infants to both the development of atopic diseases as well as an increased susceptibility to viral infections.84
Although epidemiologic evidence strongly supports a role of the gut microbiome in the development of asthma, the mechanisms remain unclear.85, 86 The most popular theory to explain these observations is that colonization with certain gut bacteria have a direct anti-inflammatory effect on the respiratory tract decreasing the likelihood of airway hyperreactivity.87 However, there is evidence that certain species of microbiota in the gastrointestinal tract prime the respiratory immune system to effectively fight viral pathogens. Immunologic factors in early life such as low blood cell interferon responses88–91 and attenuated cytokine production92 have been associated with increased risk for wheezing in infancy. Furthermore, patterns of metabolites (which can regulate immune responses93) at birth are associated with the risk for wheezing illnesses.94 The idea that delayed immune maturation might contribute to wheezing is supported by studies showing that early life exposures to dogs,95, 96 farm life,10, 97 and increased microbes and allergens98 are inversely related to the risk of wheezing illnesses. Furthermore, exposure to these microbes and allergens during the prenatal period or infancy may be immunostimulatory.99, 100 A loss, therefore, of these resident microbes may then lead to a predisposition to viral infections and in turn, the development of asthma.
Several studies have proposed mechanisms for the influence of the gut microbiota on both local and distant immune functions. SCFA have been shown to have a local effect on immune responses through their influence on mucosal barrier function, and a loss of SCFA-producing bacteria has been implicated in the development of food allergy.101 Recent advances have also shown that this symbiosis also influences vital immune responses in other systemic tissues. For example, in the absence of SCFA, mucosal barrier function can break down and allow for translocation of gut pathobionts, bacteria that are symbiotic under normal conditions but pathogenic when removed from their normal environment, which, in turn, can drive autoimmunity.102 Similarly, in a murine model intact commensal bacteria in the gut were required for adaptive immune responses to respiratory influenza virus infection. Specifically, when mice were treated with antibiotics, they had reduced virus-specific antibody titers, CD4+ T-cell responses, and cytokine secretion which consequently resulted in elevated viral titers post infection. This impairment, however, was rescued by local or distal injection of Toll-like receptor ligands.103 Further, exposure to house dust from homes with dogs enriched the cecal microbiome in a murine model with L. johnsonii, which protected them against infection with RSV.104
Future Perspectives:
Although the pathways remain incomplete, evidence continues to mount that the gut microbiome can influence the maturation of the immune system in viral defense and therefore the development of asthma.105 Future therapies look to a role of probiotics for the prevention and treatment of allergic disorders, with recent evidence that atopy risk may be associated with a dysbiosis of the gut microbiome. Studies have shown that in asthma, MMP9 (members of a family of enzymes that cleave extracellular matrix proteins) levels were significantly increased and treatment with the probiotic, L. rhamnosus GG (LCC), decreased MMP9 expression in lung tissue and inhibited inflammatory cell infiltration, as well as reducing exhaled nitric oxide among 4- to 7-year olds in pediatric asthma.106
In early childhood, total fecal IgE levels appear to be specifically correlated with house dust mite-specific IgE levels, indicating that fecal IgE levels represent markers of allergic response to aeroallergens. A significant correlation of fecal IgE levels with Dorea spp. and Clostridium spp. related to allergic rhinitis and asthma, respectively, suggest that modulation of particular subsets of gut microbial dysbiosis could contribute to the susceptibility to allergic airway diseases.107 Future work is required for identification of specific species and functional studies to understand the strength and mechanism of these associations. In the future, it is critical to understand more precisely the microbiota composition. Optimized biomarker studies of the microbial taxa and the metabolites involved in asthma-associated dysbiosis could help identify infants at risk of asthma before symptoms. This would also provide a scientific rationale for future therapeutic strategies aimed at restoring an altered infant gut microbiome. Future studies need to revolve around state-of-the-art methods for the evaluation of the microflora to better define indications, the probiotic strains and the type of prebiotic to be used.
Potential for primary prevention: clinical trials aiming to prevent the development of the episodic wheeze phenotype
The inception of childhood asthma is tightly related to early life events such as respiratory infections and the development of aeroallergens sensitization. Other co-factors (e.g., vitamin D) may modulate asthma inception pathways. Previous and on-going clinical trials, geared for asthma prevention, have targeted these pathways and co-factors.
Early life respiratory infections are significant determinants of childhood asthma108. In young toddlers, prevention of severe RSV bronchiolitis may reduce the risk of episodic wheeze/asthma development109, 110. In preterm infants (33–35 wks), palivizumab treatment during the RSV season resulted in a 73% reduction in the number of wheezing days during the first year of life, and outside of the RSV season109. A follow up study from the same cohort, revealed that at the age of 6 years the intervention resulted in a 41% relative risk reduction in parent-reported asthma, but the forced expiratory volume in 0.5 s (FEV0.5) percentage predicted values, which was an additional primary outcome, were similar between the palivizumab and placebo treated infants110.
Since early life respiratory infections cannot be completely prevented, attenuation of the immune/inflammatory processes during these infections may be another pathway for asthma prevention. This concept is illustrated by the results of a proof-of concept clinical trial in 40 infants hospitalized with RSV bronchiolitis. In this trial, azithromycin treatment for 2 weeks, during acute RSV bronchiolitis, reduced the likelihood of developing recurrent wheeze during the subsequent year111. Azithromycin effects were attributed to anti-inflammatory properties and/or its effects on the airway microbiome112. A larger confirmatory trial is ongoing (APW-RSV II; NCT02911935; Table 3).
Table 3.
Future/ongoing interventional studies examining treatments for viral triggered asthma
| Study Title | Study Population | Intervention | Primary Outcome Measurement | Estimated Completion Date |
|---|---|---|---|---|
| Vitamin D In the Prevention of Viral-Induced Asthma in preschoolers | children age 1-<6 with recurrent cold triggered asthma attacks, expected enrollment 865 subjects | Baseline and 3.5 month high dose vitamin D 100,000 IU and daily Vitamin D dose 400 IU OR placebo | Number of courses of rescue oral steroids (OCS) over 7 months | December 2022 enrolling |
| Azithromycin to Prevent Wheezing Following Severe RSV Bronchiolitis II | children 1–18 months of age, hospitalized due to RSV bronchiolitis, expected enrollment 200 subjects | Azithromycin (10 mg/kg × 7 days followed by 5 mg/kg × 7days) OR placebo | Time to occurrence of a 3rd episode of post-RSV wheezing, observation over 48 months | December 2021 not yet enrolling |
Based on observational studies that linked maternal vitamin D deficiency to childhood asthma, two clinical trials (VDAART113, COPSAC2010114) investigated whether maternal vitamin D supplementation (2400 IU/day113, 4000IU/day114) during pregnancy would prevent asthma/recurrent wheeze in their children. A recent meta-analysis that combined these two trials revealed that this intervention resulted in a 25% significant reduction in asthma/recurrent wheeze risk during the first 3 years of life115. The effect was most profound among women with sufficient serum vitamin D levels at randomization highlighting the importance of normal preconception vitamin D levels115. It was suggested that vitamin D beneficial effects may be related to enhancement of in-utero lung growth and development and promotion of antimicrobial effects, thereby reducing early life respiratory infections, and/or providing immune modulation effects116.
Omega-3 fatty acids were suggested to have anti-inflammatory effects, potentially due to decreased production of arachidonic acid metabolites. In a recent clinical trial, high dose Omega-3 fatty acids supplementation (2.4 g daily) to pregnant women, beginning at 24 week of gestation, resulted in a 30% relative risk reduction of persistent wheeze or asthma at age 3 years117. These positive effects were driven by subgroups of children born to mothers with a variant of the gene encoding fatty acid desaturase, predisposing to low ability to produce omega-3 fatty acids, and by infants born to mothers with low omega-3 fatty acids baseline blood levels. These sub-group analyses suggest the plausibility of a precision-medicine approach of this potential future intervention. Nevertheless, it is important to assure that high dose omega-3 fatty acids does not possess any safety issues, before omega-3 fatty acids may be utilized for asthma prevention.
The ongoing ORBEX clinical trial ( NCT02148796) is attempting to modulate the infant immune system by treating high-risk preschool children with Broncho-Vaxom® for 2 years to prevent/delay the development of wheezing lower respiratory tract illness (WLRI) during a third observation year. Broncho-Vaxom® contains bacterial lysates and was previously shown to reduce the rate of respiratory infections118. Hence, it is postulated that prevention of early life WLRI will prevent the development of the recurrent wheeze phenotype. Finally, the ongoing PARK clinical trial ( NCT02570984) is targeting the association between allergic sensitization and asthma inception. PARK investigates whether treatment of high-risk preschool children with Omalizumab for 2-years would prevent asthma development, and whether the treatment would decrease asthma severity among infants who will develop asthma, during an additional 2-year observation period.
Future perspectives:
This is an exciting time for all involved in childhood asthma prevention: recent clinical trials have shown the feasibility of asthma prevention, and multiple clinical trials are ongoing toward this goal. In addition to targeting type 2 immune responses, new interventions are needed to inhibit viral replication, either with specific inhibitors or strategies to boost the development of global antiviral responses in the airways. Finally, studies in farming environments strongly suggest that environmental exposure can lower the risk of viral respiratory illness in addition to reducing allergy.10, 97 Identifying relevant mechanisms is likely to lead to new preventive approaches to virus-induced wheeze and asthma.
New therapeutic options to decrease severity of asthma exacerbations caused by respiratory viral infection
Recent studies have focused on short-term increases in standard asthma therapy, vitamin D supplementation, azithromycin and anti-IgE therapy. However, mixed efficacy results limit the widespread application of many of these therapies in clinical practice. Maintenance inhaled corticosteroids (ICS) are effective in reducing the risk of asthma exacerbations and, when combined with inhaled long acting beta agonists (LABA), this decreases the risk further. However, exacerbations continue to occur. Attempts to increase dosing of inhaled steroid with early signs of loss of asthma control with viral infection, termed the “yellow zone”, to decrease exacerbation risk, have yielded mixed results. GINA guidelines suggest increasing ICS at onset of symptoms as part of a self-management plan (http://www.ginasthma.org). A Cochrane database review (including five studies in adults and three studies in children) concluded that current evidence does not support increasing ICS in mild to moderate asthma patients as part of a self-management plan to treat exacerbations.119
A clinical trial examined this question further in 254 children aged 5–11 with history of mild to moderate persistent asthma with at least one previous exacerbation in the past year.120 Children were treated for 48 weeks with low dose inhaled steroid and assigned to either continue this or quintuple the dose for 7 days at onset of loss of asthma control. There was no significant difference in the rate of severe exacerbations in the groups. The total corticosteroid exposure in the high dose group was 16% higher (including both inhaled corticosteroid use and prednisone) and there was an effect on linear growth velocity between the high dose and low dose group (−0.23 cm/year), suggesting potential risk without identifiable benefit of the therapy.
Vitamin D levels have been inversely associated with asthma severity, including hospitalization for severe infections.121 A large study aimed at optimizing low Vitamin D levels through supplementation did not reduce rates of colds or treatment failures in adults with asthma.122, 123 In contrast, a meta-analysis of seven randomized trials demonstrated a significant reduction in asthma exacerbations, with the effect seen only in patients with low vitamin D at baseline.124 There are ongoing studies in children with asthma examining the possible role of vitamin D supplementation in preventing asthma exacerbations (Table 3).
Current guidelines do not recommend the use of antibiotic treatment for episodes of asthma-like symptoms in children, yet they are commonly used. A randomized, double-blind, placebo-controlled trial conducted in the US, evaluated the role of early administration of azithromycin in prevention of progression to severe lower respiratory tract illness (LRTI) symptoms.125 Preschool children, age 12–71 months, with history of recurrent severe wheezing in the setting of LRTI, were randomized to azithromycin 12 mg/kg for 5 days (307 patients) or placebo (300 patients). The medications were to be started as soon as the children developed signs or symptoms that typically preceded the development of a severe LRTI. The primary outcome measure was the number of respiratory tract infections not progressing to a severe LRTI. The azithromycin group experienced a lower risk of progression to a severe LRTI than the placebo group.
In the COPSAC2010 (Copenhagen Prospective Studies on Asthma in Childhood 2010 cohort), children (age 1–3) with recurrent asthma-like symptoms within this cohort were enrolled in a study to assess the duration of episodes when treated with azithromycin.126 With each episode of 3 days of consecutive symptoms (wheeze, cough, dyspnea), children were randomized to receive 10 mg/kg azithromycin or placebo for 3 days. Seventy-two children from the recurrent asthma-like symptoms group had 158 episodes. The azithromycin treatment shortened the days of symptoms, 3.4 days compared with 7.7 days after placebo, corresponding to a calculated reduction in episode length of 63.3%. More improvement was seen when the treatment was started earlier in the episode; however, treatment did not significantly affect the time to next episode of troublesome lung symptoms in children.
With these episodes, a hypopharyngeal aspirate was collected and cultured for common bacterial pathogens and a nasopharyngeal aspirate was collected for viral PCR. Overall, the presence of any cultured pathogenic bacteria did not significantly alter the treatment effect compared to episodes without bacteria present; however, azithromycin was more effective in those whose culture grew H. influenzae. The treatment effect in these studies is promising; however, resistance to these antibiotics and eliminating commensal microbes along with pathogens are concerns with repeated treatment.
Birth cohort studies have shown allergic sensitization to be a risk factor for RV-induced wheeze.42 Additionally, in one prospective cohort study, the severity of RV-triggered asthma exacerbation increased as the degree of allergen sensitization increased, with serum IgE levels (total IgE and allergen specific IgE) increasing from baseline during the exacerbation.127 Persistence of asthma by age 13 was most strongly associated with wheezing illness with RV and aeroallergen sensitization in early life37 suggesting a role for both viral infection and allergic sensitization in the development of asthma.
A possible mechanism for impaired response to viral infections in allergic asthmatics is a decreased secretion of IFN in response to viral infection. Purified plasmacytoid dendritic cells (pDC) from patients with allergic asthma were shown to secrete less IFN-α in response to exposure with influenza A virus.51 Increased FcεRIα expression and serum IgE levels were inversely associated with IFN- α secretion. The increased susceptibility to viral wheeze in atopic patients and impaired antiviral response in these patients suggests a role for possible therapeutic intervention to decrease allergic inflammation with the goal of decreasing asthma exacerbations in response to viral infection.
Omalizumab, a humanized monoclonal antibody that selectively binds to IgE, has recently been studied as an add-on therapy to prevent fall asthma exacerbations in atopic asthmatics in the Preventative Omalizumab or Step-Up Therapy for Fall Exacerbations (PROSE) study.128 The PROSE study included 478 children, age 6–17, with respiratory allergy and asthma, randomized to either inhaled corticosteroid boost, add on omalizumab or placebo. All patients had guidelines-based care in addition to the add-on treatment (ICS boost, omalizumab or placebo). Treatment was begun 4–6 weeks before the participant’s school start day and ended 90 days after school start date. Omalizumab treatment significantly decreased the odds of having at least 1 exacerbation, whereas boosting ICS did not reduce risk. Omalizumab increased IFN-α responses to RV ex vivo. Within the omalizumab group, greater restoration of IFN-α responses were associated with fewer exacerbations. In this trial, omalizumab was associated with a decreased frequency of RV illnesses, decreased duration of RV infection as well as decreased frequency of overall respiratory illness, and reduced peak RV shedding.52 Omalizumab reduced expression of FcεRIα on the surface of pDC and this reduction was associated with lower exacerbation rates and correlated with enhanced IFN-α production, suggestion a possible mechanism for the interaction between allergic sensitization and virus-induced asthma exacerbations.69 However, the connection between the pDC type I IFN production and asthma exacerbation will benefit from further study.
In an observational study following children with asthma presenting with an acute asthma exacerbation triggered by RV, the use of omalizumab for at least 4 weeks prior to presentation was associated with reduced severity of exacerbation compared with patients primarily treated with ICS.129 This suggests a benefit in not only frequency and duration of asthma exacerbation, but also severity of exacerbation.
Another possible mechanism for the interaction between allergic sensitization and virus-induced asthma exacerbations is the presence of anti-viral IgE in response to infection. In RSV infection in infants, RSV specific IgE was detected in nasopharyngeal secretions, with significantly higher titers in subjects with wheezing.130, 131 Correlation of the peak titers with degree of hypoxia was also noted. Following known exposure to a specific laboratory strain, RV-specific IgE could be detected in human sera.132 While the IgE response to RV and RSV are associated with infection, the role of IgE in the host response to these infections is not fully understood. Given the decreased exacerbations with use of omalizumab, further investigation into the role of anti-viral IgE is indicated.
Future perspectives:
Given the morbidity of RSV and RV infections in patients with asthma, a consistent and effective treatment approach is highly desirable. While studies have found possible benefits to treatment with azithromycin and omalizumab, the widespread use of these treatment approaches is not currently justified. Further characterization of risk in this patient population and additional work to delineate the mechanisms by which these drugs are effective may lead to selection of patients most appropriate for these therapies.
Conclusion
There have been important advances in our knowledge of the relationship between viruses and asthma over the last decade. Advances in scientific methods have provided innovative opportunities to examine host, environment, and viral interactions that either protect against or increase vulnerability to asthma development and exacerbations. The exploration of the contribution of the respiratory and gut microbiome to virally-induced asthma is in its infancy and we suspect that over the next 5 years there will be major advances in this area. Finally, primary prevention is a major goal to diminish the morbidity of virally-mediated wheezing, asthma and exacerbations. Until primary prevention becomes a reality, clinical trials examining the impact of established medications, as well as novel therapies, will be critical to diminish the impact of viral infections on wheezing and asthma.
Abbreviations:
- RSV
Respiratory syncytial virus
- RV
Rhinovirus
- AR
Airway responsiveness
- F
Fusion
- Th2
Type 2
- CDHR3
Cadherin-related family member 3
- GWAS
Genome wide association study
- ETS
Environmental tobacco smoke
- URI
Upper respiratory tract infection
- SCFA
Short chain fatty acids
- FEV0.5
Forced expiratory volume in 0.5 s
- WLRI
Wheezing lower respiratory tract illness
- ICS
Inhaled corticosteroids
- LABA
Long acting beta agonists
- LRTI
Lower respiratory tract illness
- pDC
Plasmacytoid dendritic cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have no financial conflict of interest with the manuscript that has been submitted.
Contributor Information
Matthew C. Altman, Department of Medicine, University of Washington, Seattle, Washington
Avraham Beigelman, Division of Pediatric Allergy, Immunology & Pulmonary Medicine, Washington University School of Medicine, St. Louis MO The Kipper Institute of Allergy and Immunology, Schneider Children’s Medical Center of Israel, Petach Tikvah, Israel.
Christina Ciaccio, Allergy/Immunology and Pediatric Pulmonology and Sleep Medicine, University of Chicago School of Medicine, Chicago, IL
James E. Gern, Division of Pediatric Allergy, Immunology and Rheumatology, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI
Peter W. Heymann, Department of Pediatrics, University of Virginia Medical Center, Charlottesville, VA
Daniel J. Jackson, Division of Pediatric Allergy, Immunology and Rheumatology, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI
Joshua L. Kennedy, Division of Allergy/Immunology, University of Arkansas for Medical Sciences, Little Rock, AR
Kirsten Kloepfer, Division of Pediatric Pulmonology, Allergy and Sleep Medicine, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN
Robert F. Lemanske, Jr., Division of Pediatric Allergy, Immunology and Rheumatology, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI
Laurie M. McWilliams, Allergy Partners of the Triangle, Raleigh, N.C.
Lyndsey Muehling, Department of Medicine, University of Virginia Medical Center, Charlottesville, VA
Christy Nance, Departments of Pediatrics and Immunology/Pathology, Baylor College of Medicine, Houston, TX
R. Stokes Peebles, Jr., Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, TN
Bibliography
- 1.Stier MT, Peebles RS Jr. Host and Viral Determinants of Respiratory Syncytial Virus-induced Airway Mucus. Ann Am Thorac Soc 2018; 15:S205–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, et al. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J Pathol 2006; 169:977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, et al. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol 2009; 83:4185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loisel DA, Du G, Ahluwalia TS, Tisler CJ, Evans MD, Myers RA, et al. Genetic associations with viral respiratory illnesses and asthma control in children. Clin Exp Allergy 2016; 46:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciancanelli MJ, Huang SX, Luthra P, Garner H, Itan Y, Volpi S, et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 2015; 348:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asgari S, Schlapbach LJ, Anchisi S, Hammer C, Bartha I, Junier T, et al. Severe viral respiratory infections in children with IFIH1 loss-of-function mutations. Proc Natl Acad Sci U S A 2017; 114:8342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, Doornbos G, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis 2007; 196:826–34. [DOI] [PubMed] [Google Scholar]
- 8.Larkin EK, Hartert TV. Genes associated with RSV lower respiratory tract infection and asthma: the application of genetic epidemiological methods to understand causality. Future Virol 2015; 10:883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood onset asthma. N Engl J Med 2013; 368:1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loss GJ, Depner M, Hose AJ, Genuneit J, Karvonen AM, Hyvarinen A, et al. The Early Development of Wheeze. Environmental Determinants and Genetic Susceptibility at 17q21. Am J Respir Crit Care Med 2016; 193:889–97. [DOI] [PubMed] [Google Scholar]
- 11.Stokholm J, Chawes BL, Vissing N, Bonnelykke K, Bisgaard H. Cat exposure in early life decreases asthma risk from the 17q21 high-risk variant. J Allergy Clin Immunol 2018; 141:1598–606. [DOI] [PubMed] [Google Scholar]
- 12.Lee WM, Lemanske RF Jr., Evans MD, Vang F, Pappas T, Gangnon R, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med 2012; 186:886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagome K, Bochkov YA, Ashraf S, Brockman-Schneider RA, Evans MD, Pasic TR, et al. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol 2014; 134:332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Moller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 2014; 46:51–5. [DOI] [PubMed] [Google Scholar]
- 15.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A 2015; 112:5485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnelykke K, Coleman AT, Evans MD, Thorsen J, Waage J, Vissing NH, et al. Cadherin-related Family Member 3 Genetics and Rhinovirus C Respiratory Illnesses. Am J Respir Crit Care Med 2018; 197:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vrijheid M The exposome: a new paradigm to study the impact of environment on health. Thorax 2014; 69:876–8. [DOI] [PubMed] [Google Scholar]
- 18.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–7. [DOI] [PubMed] [Google Scholar]
- 19.Welliver RC. RSV and chronic asthma. Lancet 1995; 346:789–90. [DOI] [PubMed] [Google Scholar]
- 20.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol 2009; 123:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008; 178:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemanske RF Jr., Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005; 116:571–7. [DOI] [PubMed] [Google Scholar]
- 23.Lukkarinen M, Koistinen A, Turunen R, Lehtinen P, Vuorinen T, Jartti T. Rhinovirus-nduced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol 2017; 140:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004; 114:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med 1999; 159:785–90. [DOI] [PubMed] [Google Scholar]
- 26.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol 2012; 129:1499–505 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med 2014; 6:256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 2006; 12:1023–6. [DOI] [PubMed] [Google Scholar]
- 29.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-Dependent Type 2 Inflammation during Rhinovirus-induced Asthma Exacerbations In Vivo. Am J Respir Crit Care Med 2014; 190:1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy JL, Koziol-White CJ, Jeffus S, Rettiganti MR, Fisher P, Kurten M, et al. Effects of rhinovirus 39 infection on airway hyperresponsiveness to carbachol in human airways precision cut lung slices. J Allergy Clin Immunol 2018; 141:1887–90 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy JL, Shaker M, McMeen V, Gern J, Carper H, Murphy D, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med 2014; 189:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, et al. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy 2009; 64:375–86. [DOI] [PubMed] [Google Scholar]
- 33.Laza-Stanca V, Message SD, Edwards MR, Parker HL, Zdrenghea MT, Kebadze T, et al. The role of IL-15 deficiency in the pathogenesis of virus-induced asthma exacerbations. PLoS Pathog 2011; 7:e1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A 2008; 105:13562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Pan Y, Zhu Y, Song Y, Su X, Yang L, et al. Association between rhinovirus wheezing illness and the development of childhood asthma: a meta-analysis. BMJ Open 2017; 7:e013034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Midulla F, Nicolai A, Ferrara M, Gentile F, Pierangeli A, Bonci E, et al. Recurrent wheezing 36 months after bronchiolitis is associated with rhinovirus infections and blood eosinophilia. Acta Paediatr 2014; 103:1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol 2017; 139:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruotsalainen M, Hyvarinen MK, Piippo-Savolainen E, Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol 2013; 48:633–9. [DOI] [PubMed] [Google Scholar]
- 39.Schneider D, Hong JY, Popova AP, Bowman ER, Linn MJ, McLean AM, et al. Neonatal rhinovirus infection induces mucous metaplasia and airways hyperresponsiveness. J Immunol 2012; 188:2894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol 2014; 134:429–39 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han M, Rajput C, Hong JY, Lei J, Hinde JL, Wu Q, et al. The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice. J Immunol 2017; 199:1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med 2012; 185:281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 2015; 70:910–20. [DOI] [PubMed] [Google Scholar]
- 44.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol 2014; 52:2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller EK, Hernandez JZ, Wimmenauer V, Shepherd BE, Hijano D, Libster R, et al. A Mechanistic Role for Type III IFN-lambda1 in Asthma Exacerbations Mediated by Human Rhinoviruses. Am J Respir Crit Care Med 2012; 185:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altman MC, Reeves SR, Parker AR, Whalen E, Misura KM, Barrow KA, et al. Interferon response to respiratory syncytial virus by bronchial epithelium from children with asthma is inversely correlated with pulmonary function. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest 2015; 125:3037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Custovic A, Belgrave D, Lin L, Bakhsoliani E, Telcian AG, Solari R, et al. Cytokine Responses to Rhinovirus and Development of Asthma, Allergic Sensitization, and Respiratory Infections during Childhood. Am J Respir Crit Care Med 2018; 197:1265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 2006; 368:763–70. [DOI] [PubMed] [Google Scholar]
- 50.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol 2007; 119:1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 2010; 184:5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esquivel A, Busse WW, Calatroni A, Togias AG, Grindle KG, Bochkov YA, et al. Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am J Respir Crit Care Med 2017; 196:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inouye M, Teo SM, Tang H, Mok D, Judd L, Watts S, et al. Dynamics of airway microbiota identify a critical window for interplay of pathogenic bacteria and allergic sensitization in childhood respiratory disease. Cell Host & Microbe 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. The Lancet 1999; 354:541–5. [DOI] [PubMed] [Google Scholar]
- 55.Henderson J, Hilliard TN, Sherriff A, Stalker D, Shammari NA, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: A longitudinal birth cohort study. Pediatric Allergy and Immunology 2005; 16:386–92. [DOI] [PubMed] [Google Scholar]
- 56.Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedon JC. Risk and Protective Factors for Childhood Asthma: What Is the Evidence? J Allergy Clin Immunol Pract 2016; 4:1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Fernandez R, Tapia LI, Yang CF, Torres JP, Chavez-Bueno S, Garcia C, et al. Respiratory Syncytial Virus Genotypes, Host Immune Profiles, and Disease Severity in Young Children Hospitalized With Bronchiolitis. J Infect Dis 2017; 217:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Steenhuijsen Piters WA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez-Arrabal MC, et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am J Respir Crit Care Med 2016; 194:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med 2008; 178:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomsen SF, van der Sluis S, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med 2009; 179:1091–7. [DOI] [PubMed] [Google Scholar]
- 61.Carroll KN, Gebretsadik T, Escobar GJ, Wu P, Li SX, Walsh EM, et al. Respiratory syncytial virus immunoprophylaxis in high-risk infants and development of childhood asthma. Journal of Allergy and Clinical Immunology 2017; 139:66–71.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mochizuki H, Kusuda S, Okada K, Yoshihara S, Furuya H, Simoes EAF, et al. Palivizumab Prophylaxis in Preterm Infants and Subsequent Recurrent Wheezing. Six-Year Follow-up Study. Am J Respir Crit Care Med 2017; 196:29–38. [DOI] [PubMed] [Google Scholar]
- 63.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol 1994; 68:5321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol 1996; 70:2852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol 1998; 72:2871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med 2012; 18:1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 2016; 138:814–24 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegle JS, Hansbro N, Herbert C, Rosenberg HF, Domachowske JB, Asquith KL, et al. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir Res 2010; 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol 2018; 141:1735–43 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol 2010; 125:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol 2014; 133:1301–7, 7, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015; 17:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyde ER, Petrosino JF, Piedra PA, Camargo CA Jr., Espinola JA, Mansbach JM. Nasopharyngeal Proteobacteria are associated with viral etiology and acute wheezing in children with severe bronchiolitis. J Allergy Clin Immunol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bosch A, de Steenhuijsen Piters WAA, van Houten MA, Chu M, Biesbroek G, Kool J, et al. Maturation of the Infant Respiratory Microbiota, Environmental Drivers, and Health Consequences. A Prospective Cohort Study. Am J Respir Crit Care Med 2017; 196:1582–90. [DOI] [PubMed] [Google Scholar]
- 75.Kloepfer KM, Sarsani VK, Poroyko V, Lee WM, Pappas TE, Kang T, et al. Community-acquired rhinovirus infection is associated with changes in the airway microbiome. J Allergy Clin Immunol 2017; 140:312–5 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hasegawa K, Mansbach JM, Ajami NJ, Espinola JA, Henke DM, Petrosino JF, et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J 2016; 48:1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, et al. Nasopharyngeal Lactobacillus is associated with a reduced risk of childhood wheezing illnesses following acute respiratory syncytial virus infection in infancy. J Allergy Clin Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, et al. Differences in the Nasopharyngeal Microbiome During Acute Respiratory Tract Infection With Human Rhinovirus and Respiratory Syncytial Virus in Infancy. J Infect Dis 2016; 214:1924–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Folsgaard NV, Schjorring S, Chawes BL, Rasmussen MA, Krogfelt KA, Brix S, et al. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med 2013; 187:589–95. [DOI] [PubMed] [Google Scholar]
- 80.Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H, et al. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol 2013; 14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016; 164:337–40. [DOI] [PubMed] [Google Scholar]
- 82.Moeller AH, Caro-Quintero A, Mjungu D, Georgiev AV, Lonsdorf EV, Muller MN, et al. Cospeciation of gut microbiota with hominids. Science 2016; 353:380–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 1991; 70:443–59. [DOI] [PubMed] [Google Scholar]
- 84.Laforest-Lapointe I, Arrieta MC. Patterns of Early-Life Gut Microbial Colonization during Human Immune Development: An Ecological Perspective. Front Immunol 2017; 8:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe 2015; 17:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sokolowska M, Frei R, Lunjani N, Akdis CA, O’Mahony L. Microbiome and asthma. Asthma Res Pract 2018; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 2018; 48:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am.J.Respir.Crit Care Med. 2004; 170:175–80. [DOI] [PubMed] [Google Scholar]
- 89.Sumino K, Tucker J, Shahab M, Jaffee KF, Visness CM, Gern JE, et al. Antiviral IFN-gamma responses of monocytes at birth predict respiratory tract illness in the first year of life. J.Allergy Clin.Immunol. 2012; 129:1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol 2006; 117:72–8. [DOI] [PubMed] [Google Scholar]
- 91.Ly NP, Rifas-Shiman SL, Litonjua AA, Tzianabos AO, Schaub B, Ruiz-Perez B, et al. Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics 2007; 119:e171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macaubas C, de Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, et al. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 2003; 362:1192–7. [DOI] [PubMed] [Google Scholar]
- 93.Pelgrom LR, Everts B. Metabolic control of type 2 immunity. Eur J Immunol 2017; 47:1266–75. [DOI] [PubMed] [Google Scholar]
- 94.Donovan BM, Ryckman KK, Breheny PJ, Gebretsadik T, Turi KN, Larkin EK, et al. Association of newborn screening metabolites with risk of wheezing in childhood. Pediatr Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bufford JD, Reardon CL, Li Z, Roberg KA, Dasilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy 2008; 38:1635–43. [DOI] [PubMed] [Google Scholar]
- 96.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 2002; 288:963–72. [DOI] [PubMed] [Google Scholar]
- 97.Ludka-Gaulke T, Ghera P, Waring SC, Keifer M, Seroogy C, Gern JE, et al. Farm exposure in early childhood is associated with a lower risk of severe respiratory illnesses. J Allergy Clin Immunol 2018; 141:454–6 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 2014; 134:593–601 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gern JE, Calatroni A, Jaffee KF, Lynn H, Dresen A, Cruikshank WW, et al. Patterns of immune development in urban preschoolers with recurrent wheeze and/or atopy. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schaub B, Liu J, Hoppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 2009; 123:774–82 e5. [DOI] [PubMed] [Google Scholar]
- 101.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A 2014; 111:13145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018; 359:1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 2011; 108:5354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A 2014; 111:805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ciaccio CE. Modulating the microbiome: The future of allergy therapeutics? Ann Allergy Asthma Immunol 2019; 122:233–5. [DOI] [PubMed] [Google Scholar]
- 106.Wang HT, Anvari S, Anagnostou K. The Role of Probiotics in Preventing Allergic Disease. Children (Basel) 2019; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiu CY, Chan YL, Tsai MH, Wang CJ, Chiang MH, Chiu CC. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organ J 2019; 12:100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beigelman A, Bacharier LB. Early-life respiratory infections and asthma development: role in disease pathogenesis and potential targets for disease prevention. Curr Opin Allergy Clin Immunol 2016; 16:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–9. [DOI] [PubMed] [Google Scholar]
- 110.Scheltema NM, Nibbelke EE, Pouw J, Blanken MO, Rovers MM, Naaktgeboren CA, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med 2018; 6:257–64. [DOI] [PubMed] [Google Scholar]
- 111.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2015; 135:1171–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Y, Bacharier LB, Isaacson-Schmid M, Baty J, Schechtman KB, Sajol G, et al. Azithromycin therapy during respiratory syncytial virus bronchiolitis: Upper airway microbiome alterations and subsequent recurrent wheeze. J Allergy Clin Immunol 2016; 138:1215–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chawes BL, Bonnelykke K, Stokholm J, Vissing NH, Bjarnadottir E, Schoos AM, et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA 2016; 315:353–61. [DOI] [PubMed] [Google Scholar]
- 114.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016; 315:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wolsk HM, Chawes BL, Litonjua AA, Hollis BW, Waage J, Stokholm J, et al. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS One 2017; 12:e0186657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mirzakhani H, Al-Garawi A, Weiss ST, Litonjua AA. Vitamin D and the development of allergic disease: how important is it? Clinical and Experimental Allergy 2015; 45:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med 2016; 375:2530–9. [DOI] [PubMed] [Google Scholar]
- 118.Del-Rio-Navarro BE, Espinosa Rosales F, Flenady V, Sienra-Monge JJ. Immunostimulants for preventing respiratory tract infection in children. Cochrane Database Syst Rev 2006:CD004974. [DOI] [PubMed] [Google Scholar]
- 119.Kew KM, Quinn M, Quon BS, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst Rev 2016:CD007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jackson DJ, Bacharier LB, Mauger DT, Boehmer S, Beigelman A, Chmiel JF, et al. Quintupling Inhaled Glucocorticoids to Prevent Childhood Asthma Exacerbations. N Engl J Med 2018; 378:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in costa rica. Am J Respir Crit Care Med 2009; 179:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Denlinger LC, King TS, Cardet JC, Craig T, Holguin F, Jackson DJ, et al. Vitamin D Supplementation and the Risk of Colds in Patients with Asthma. Am J Respir Crit Care Med 2016; 193:634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 2014; 311:2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA Jr., Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med 2017; 5:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA 2015; 314:2034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2016; 4:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kantor DB, Stenquist N, McDonald MC, Schultz BJ, Hauptman M, Smallwood CD, et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J Allergy Clin Immunol 2016; 138:1467–71 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr., Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 2015; 136:1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kantor DB, McDonald MC, Stenquist N, Schultz BJ, Smallwood CD, Nelson KA, et al. Omalizumab Is Associated with Reduced Acute Severity of Rhinovirus-triggered Asthma Exacerbation. Am J Respir Crit Care Med 2016; 194:1552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Welliver RC, Kaul TN, Ogra PL. The appearance of cell-bound IgE in respiratory-tract epithelium after respiratory-syncytial-virus infection. N Engl J Med 1980; 303:1198–202. [DOI] [PubMed] [Google Scholar]
- 131.Welliver RC, Wong DT, Sun M, Middleton E Jr., Vaughan RS, Ogra PL. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med 1981; 305:841–6. [DOI] [PubMed] [Google Scholar]
- 132.Tam JS, Jackson WT, Hunter D, Proud D, Grayson MH. Rhinovirus specific IgE can be detected in human sera. J Allergy Clin Immunol 2013; 132:1241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


