Abstract
Background/Aims
This study was aimed to investigate the protective effects of swimming on nonalcoholic fatty liver disease associated with high-fat-diet-induced obesity using microscopic and biochemical parameters.
Materials and Methods
Sprague–Dawley male rats were fed either standard chow (STD group; 6% fat) or a high-fat diet (HFD group; 45% fat) for 18 weeks. The animals were divided into 4 groups: STD, STD+exercise (EXC), HFD, and HFD+EXC. The exercise groups were given swimming training for 5 days of week, 1 hour per day, during the last 6 weeks of the experiment. At the end of the experiment, the liver samples were evaluated morphologically and ultrastructurally, and malondialdehyde (MDA) and glutathione (GSH) levels were evaluated in the liver samples.
Results
Normal morphology of the liver parenchyma with hepatocytes and sinusoids was observed in the STD and STD+EXC groups. Steatosis, lipid accumulation, ballooned hepatocytes, decrease of glycogen deposits, and fibrosis in the periportal area were observed in the HFD group. Liver MDA level was increased and GSH level was decreased in the HFD group. Exercise ameliorated these morphologic and oxidative changes in the HFD-induced liver damage.
Conclusion
On the basis of morphologic and biochemical analysis, we could conclude that swimming ameliorated obesity-induced liver damage by regulating lipid accumulation and oxidative damage.
Keywords: Exercise, obesity, fatty liver, oxidative stress, electron microscopy
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is defined as excess fat accumulated in the liver, which is generally associated with excessive calorie intake. NAFLD is a very common liver disease in the Western countries because of lifestyle factors, such as high-fat diet and decreased physical activity, and these factors are also associated with obesity and type 2 diabetes. Obese people are more prone to NAFLD (1), are not physically active, and eat more than healthy individuals (2). NAFLD is defined as significant lipid deposition in the hepatocytes of the liver parenchyma, and patients with NAFLD are also usually observed to have a previous history of excessive alcohol consumption (1). This disease shows different histopathologic appearances, such as simple steatosis, nonalcoholic steatohepatitis, fibrosis, and cirrhosis (3–7).
Exercise is an important way to alleviate the impaired energy metabolism and obesity. Increasing fatty acid oxidation is accepted as one of the most effective strategies for the nonpharmacologic treatment of obesity (8, 9).
Oxidative stress (OS) is an important factor in lethal hepatocyte injury associated with NAFLD (10). Increased OS was observed in patients with NAFLD compared with individuals with nonalcoholic steatohepatitis (11). Malondialdehyde (MDA) levels, the final product of lipid peroxidation (12), are frequently referred to as an oxidative stress marker (12). Glutathione (GSH) is an endogen antioxidant that directly reduces most of the reactive oxygen species (ROS) (13). It was shown that MDA level increased and GSH level decreased in the liver of high-fat- and high-fructose-diet-induced obese male rats (14). It was also shown that moderate exercise decreased MDA level and elevated GSH level in human (15). Therefore, this study aimed to evaluate the effects of swimming on high-fat-diet (HFD)-induced liver damage by means of histologic, ultrastructural, and biochemical methods.
MATERIALS AND METHODS
Animals
Sprague–Dawley male rats (200–300 g), 8 weeks old, were kept in standard plastic cages with free food and water access. These animals were housed in a room with controlled temperature (21°C) and light-dark cycle (12:12 hours). The experimental protocols were approved by the animal ethics committee of the Marmara University (05.2019.mar).
Experimental Design
Sprague–Dawley male rats (n=6 in each group) were randomly divided into 4 groups as standard (STD), STD+exercise (EXC), HFD, and HFD+EXC. STD and STD+EXC groups were fed STD diet (6% of calories as fat) and HFD and HFD+EXC groups were fed HFD (45% of calories as fat) for 18 weeks. STD+EXC and HFD+EXC groups were given swimming exercises from the 12th week until the 18th week of the feeding period. The swimming happened in a plastic tank (60 cm×150 cm×45 cm) with warm water at 32°C±1°C for 1 hour, 5 days a week. The weight of the rats was measured periodically once a week. At the end of the experiment, the animals were euthanized with an intraperitoneal injection of ketamine and xylazine. The liver tissue samples were processed for histologic, ultrastructural, and biochemical evaluations.
Light Microscopy Preparation and Histopathologic Scoring
For light microscopic investigations, the liver tissue samples were fixed in 10% neutral buffered formalin solution. These samples were dehydrated with alcohol, cleared with xylene, and incubated in paraffin. The paraffin sections were stained with hematoxylin and eosin (H&E) for histopathologic evaluation, stained with periodic acid-Schiff (PAS) for glycogen distribution, and stained with Masson trichrome for fibrosis. Modified histopathologic scoring criteria (16), including steatosis, hepatocellular ballooning, inflammation, fibrosis, and glycogen content, are summarized in Table 1.
Table 1.
Modified histopathologic score parameters (16).
| Histologic appearance | Score | Type / Localization |
|---|---|---|
| Steatosis | 0: < 5% 1: 5–33% 2: > 33–66% 3: > 66% |
Macrovesicular, microvesicular or mixed Zone 1, 2, 3 |
| Hepatocellular Ballooning | 0: None 1: Few balloon cells 2: Many cells/ prominent ballooning |
Hydropic degeneration |
| Inflammatory foci per 200× field, mean of 3 fields (NAS) | 0: 0 1: < 2 2: 2–4 3: > 4 |
Lobular or portal |
| Fibrosis | 0: None 1: Perisinusoidal or periportal 2: Perisinusoidal and portal/ periportal 3: Bridging 4: Cirrhosis |
Subgroups of score 1: 1A: Mild, zone 3, perisinusoidal 1B: Moderate, zone 3, perisinusoidal 1C: Portal/ periportal |
| PAS positive staining | 0: − −1: + 2: ++ 3: +++ |
Zone 1, 2, 3 |
Transmission Electron Microscopic Preparation
The liver samples were fixed with 2.5% glutaraldehyde in phosphate buffered saline (PBS) (0.1 M, pH 7.2), then post-fixed in 1% osmium tetroxide in PBS (0.1 M, pH 7.2), dehydrated in increasing concentrations of ethyl alcohol series, and embedded in Epon 812 (Fluka, Sigma–Aldrich Chemical, Steinheim, Switzerland). Semi-thin sections were stained with toluidine blue. Ultra-thin sections were contrasted with uranyl acetate and lead citrate. Ultra-thin sections were evaluated under a transmission electron microscope (JEOL 1200 EXII, Tokyo, Japan).
Measurement of MDA and GSH levels
Levels of MDA, as an index of lipid peroxidation, were measured using the spectrophotometric method, which determined the thiobarbituric acid reactive substances, and GSH levels in liver tissue were determined using the Ellman method (17). Results of MDA and GSH levels were expressed as nmol/g.
Statistical Analysis
Data were analyzed with 1-way or 2-way analysis of variance, followed by Tukey’s multiple comparison tests. Statistical analysis was performed using the Prism 6.0 GraphPad Software (San Diego, CA, USA). The statistical significance level was regarded as p<0.05.
RESULTS
Body Weight of Experimental Animals
The body weight of rats in the HFD and HFD+EXC groups (p<0.001) increased significantly on a weekly basis compared with that of the rats in the STD group. There was a negligible decrease in the weight of the rats of STD+EXC and HFD+EXC groups compared with that of the rats in the STD and HFD groups, respectively (Figure 1).
Figure 1.
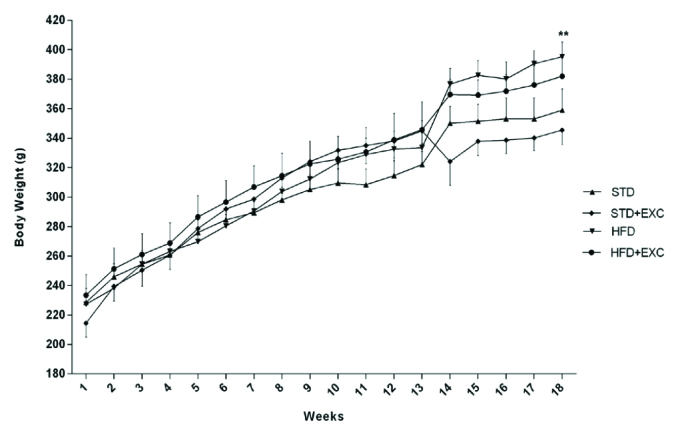
Body weight of the experimental animals.
**p<0.01 vs standard (STD) group.
Histopathologic Findings
Normal liver morphology, including hepatocytes, glycogen distribution and sinusoids, was observed in the STD (Figures 2a–5a) and STD+EXC groups (Figures 2b–5b). Lipid accumulation, microvesicular steatosis, ballooned hepatocytes, decreased glycogen distribution and increased fibrosis in the periportal area were detected in the HFD group (Figures 2c–5c). Degenerated hepatocytes with lipid accumulation, microvesicular steatosis and fibrosis were decreased in HFD+EXC group (Figures 2d–5d).
Figure 2. a–d.
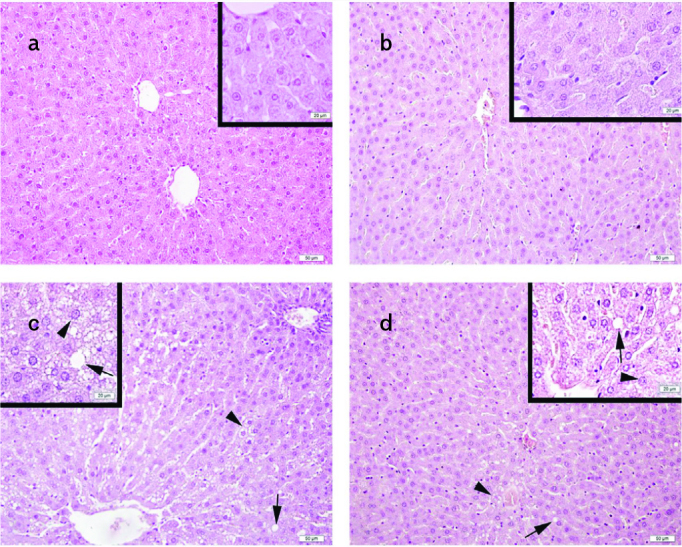
Normal liver morphology with hepatocytes and sinusoids in the standard (STD) (a) and STD+exercise (EXC) (b) groups; lipid vacuoles (arrow) and microvesicular steatosis (arrowhead) in the high-fat diet (HFD) group (c); and decrease in the lipid vacuoles (arrow) and microvesicular steatosis (arrowhead) in the HFD+EXC group (d) are seen on hematoxylin and eosin staining.
Figure 3. a–d.
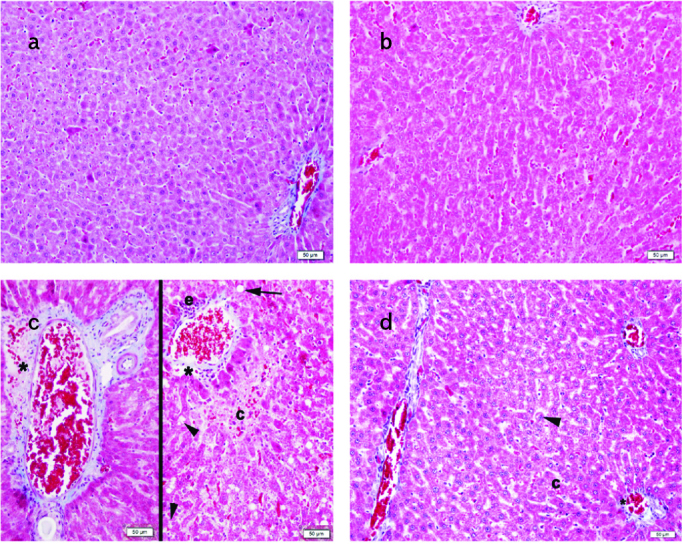
Normal liver parenchyma in periportal area in the standard (STD) (a) and STD+exercise (EXC) (b) groups; lipid vacuoles (arrow), ballooned hepatocytes (arrowhead), vascular congestion (c), and fibrosis (asterix) in the periportal area of the high-fat diet (HFD) group (c); decrease of ballooned hepatocytes (arrowhead), vascular congestion (c), and fibrosis (asterix) in the periportal area of the HFD+EXC group (d) are seen on Masson trichrome staining.
Figure 4. a–d.
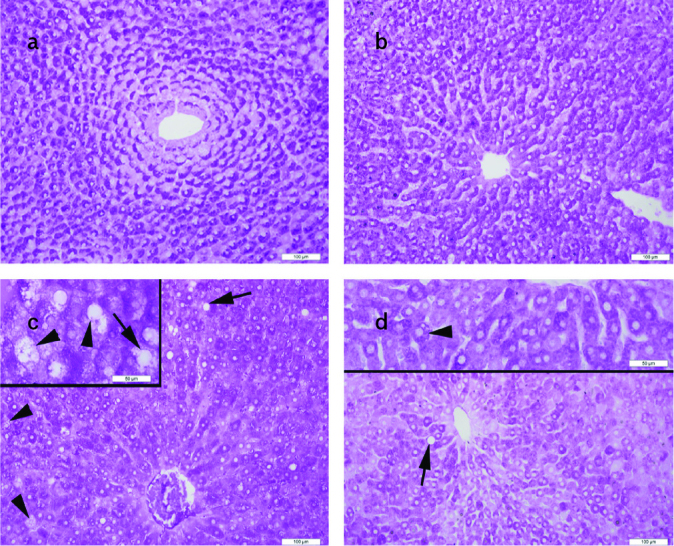
Normal liver parenchyma with regular distribution of glycogen in the hepatocytes and sinusoids in the standard (STD) (a) and STD+exercise (EXC) (b) groups; decrease of glycogen (g) distribution, lipid vacuoles (arrow), and microvesicular steatosis (arrowhead) in the high-fat diet (HFD) group (c); increase in glycogen (g) distribution, decrease in lipid vacuoles (arrow), and microvesicular steatosis (arrowhead, inset) in the HFD+EXC group (d) seen on periodic acid-Schiff staining.
Figure 5. a–d.
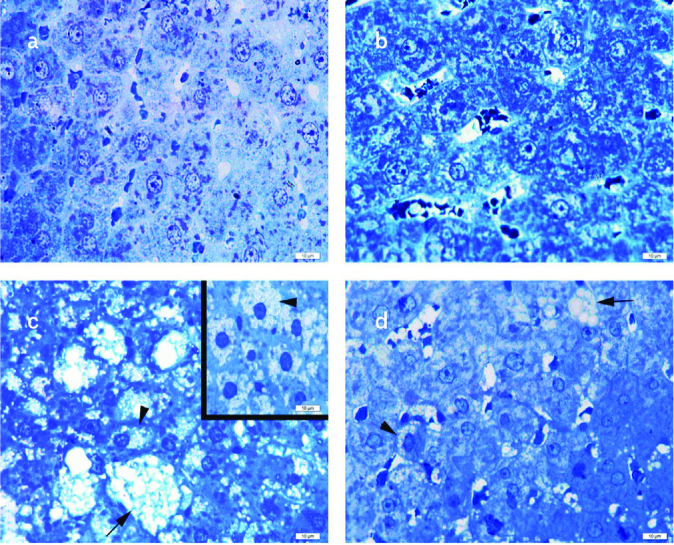
Normal liver parenchyma with hepatocytes and sinusoids in the standard (STD) (a) and STD+exercise (EXC) (b) groups; ballooned hepatocytes (arrow) and microvesicular steatosis (arrowhead) in the high-fat diet (HFD) group (c); decreased ballooned hepatocytes (arrow) and microvesicular steatosis (arrowhead) in the HFD+EXC group (d) seen on toluidine blue staining.
The histopathologic scores (Figure 6) for steatosis, inflammation, and fibrosis were significantly increased in the HFD group (p<0.001) compared with those in the STD and STD+EXC groups. However, the steatosis and fibrosis scores were significantly decreased in the HFD+EXC group (p<0.01) compared with those in the HFD group. Although the inflammation score decreased in the HFD+EXC group, this decrease was not significant. PAS-positive staining score of the HFD group was lower than that of the STD and STD+EXC groups (p<0.001) and higher in the HFD+EXC group (p<0.05).
Figure 6. a–d.
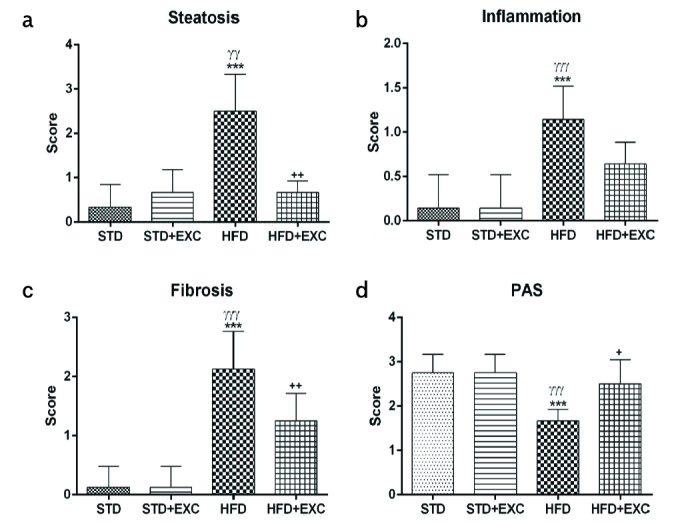
Histopathologic scores for steatosis (a), inflammation (b), fibrosis (c), and periodic acid-Schiff-positive staining (d) for experimental groups. ***p<0.001 vs. standard (STD) group; γγp<0.01 and γγγp<0.001 vs. STD+exercise, +p<0.05 and ++p<0.01 vs. high-fat diet group.
Ultrastructural Findings
The STD (Figure 7a) and the STD+EXC groups (Figure 7b) reflected a regular hepatocyte ultrastructure. An increased number of microvesicular and macrovesicular lipid droplets and degenerated nuclear structures were noticed in the HFD group (Figure 7c). Considerable decrease of microvesicular and macrovesicular lipid droplets and regular nuclear ultrastructure were observed in the HFD+EXC group (Figure 7d).
Figure 7. a–d.
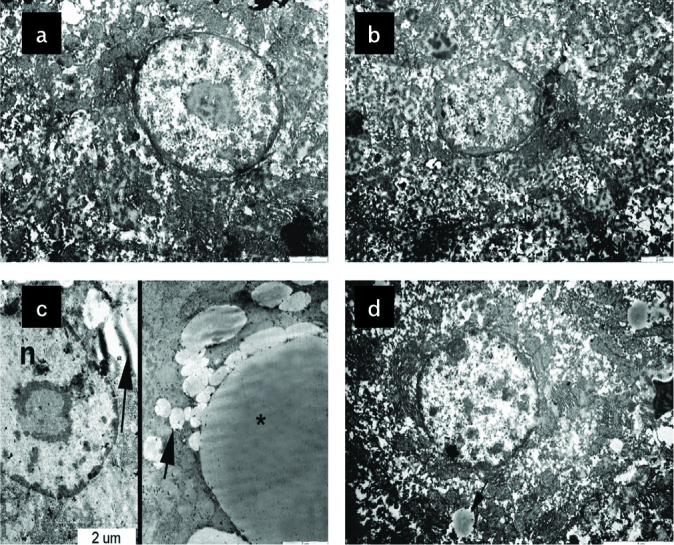
Normal ultrastructure of hepatocytes in the standard (STD) (a) and STD+exercise (EXC) (b) groups; degenerated nucleus (n), many small (arrow) and large (asterix) lipid droplets in the high-fat diet (HFD) group (c); quite regular hepatocyte ultrastructure with nucleus and decrease of small lipid droplets (arrow) in the HFD+EXC group (d) are seen.
MDA and GSH Levels
The MDA level (Figure 8a) of the HFD group (p<0.001) was significantly higher than that of the STD group. The MDA level was lower in the HFD+EXC group (p<0.001) than that of the HFD group. The GSH level (Figure 8b) (p<0.001) in the HFD group was lower than that of the STD group (p<0.01).
Figure 8. a–d.
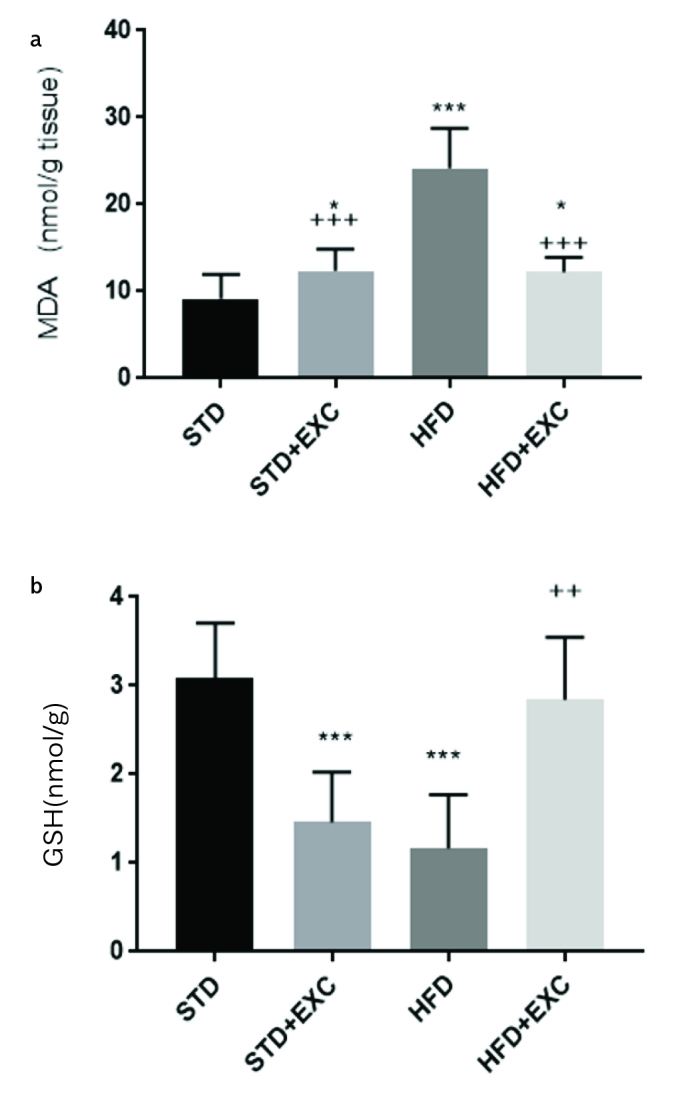
Malondialdehyde (a) and glutathione (b) levels of experimental groups. ***p<0.05 and ***p<0.001 vs. STD group; ++p<0.01 and +++p<0.001 vs. high-fat diet group.
DISCUSSION
This study demonstrated that high-fat diet caused weight gain in the rats in the HFD and HFD+EXC groups. Elevated MDA levels and decreased GSH levels were seen in HFD-induced obesity. HFD-induced obesity also showed prominent histopathologic damage with hepatic tissue degeneration, such as steatosis, lipid accumulation, ballooned hepatocytes, fibrosis, and decreased glycogen distribution in the HFD group. Furthermore, moderate swimming exercise ameliorated HFD-induced liver oxidative injury and morphologic damage.
Liver fat accumulation in NAFLD and obesity cause adipose tissue dysfunction and insulin resistance (18). NAFLD affects approximately two-thirds of the individuals who are obese (18) and is closely linked to obesity (19). HFD results in increased body mass and altered triglyceride and total cholesterol concentrations (20). Moreover, it was observed that even without weight loss, regular physical exercise resulted in a reduction in the total and visceral fat and in skeletal muscle lipid levels in obese individuals (21). In this study, HFD caused weight gain, and swimming led to weight loss in the HFD+EXC group that was not significant compared with the weight of the animals in the HFD group. However, the amelioration of liver damage might be related to the reduction of lipid accumulation in total and visceral fat.
NAFLD may be the most prevalent type of chronic liver disease in adolescents in several developed countries of the continents (22). The pathology subcommittee, including pathologists from different clinical research centers, agreed that only H&E and Masson trichrome stains are required to carry out the assessment of NAFLD (22). Histopathologic semi-quantitative scoring, including steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis, is used for evaluation of NAFLD (22). NAFLD is described histologically as the existence of liver steatosis in excess of 5% of hepatocytes, whether macrovesicular, microvesicular, or mixed (23). Excessive storage of lipids in the adipose tissue, which is associated with obesity (24), results in modification of its metabolic and endocrine functions, resulting in stress signals and carbohydrate metabolism derangement (25). In humans (25) and the diet-induced obese Göttingen Minipig model (16), glycogen deposition in the liver parenchyma and skeletal muscles was reduced under pathological circumstances connected with obesity and was considered a sign of metabolic stress (25). In this study, we observed histopathologic damage with steatosis, lipid accumulation, ballooning of hepatocyte, fibrosis, and decrease of cytoplasmic glycogen deposits in the HFD group. Exercise reduced the histopathologic damage score and increased the distribution of glycogen in the HFD+EXC group by a regulatory pathway of glycogen metabolism regulation.
A recent ultrastructural study showed that a high-fat and -sugar diet for 15 weeks caused hepatocyte damage, which was ameliorated by swimming 3 times a week for 4 weeks (26). Similarly, we observed that swimming reduced the ultrastructural damage in the hepatocytes caused by increased lipid droplets in the hepatocyte cytoplasm and degenerated nucleus in the HFD group. In our study, we fed the animals with HFD for 18 weeks and then they were made to swim 5 days a week for 6 weeks. As a result of that exercise, we observed reduced and smaller lipid droplets and regular organelles in the cell cytoplasm of the hepatocytes in the HFD+EXC group.
NAFLD develops as a consequence of HFD and could progress to an increased production of oxidants (27). These oxidants are known as significant factors in the pathology and progression of steatosis (27). The progression from simple steatosis to nonalcoholic steatohepatitis includes increased the production of ROS (27). Excessive ROS results in lipid peroxidation, for which MDA is a key indicator (28). GSH has also been shown to be involved in ROS scavenging as a nonenzymatic scavenger, and GSH dysfunction may exacerbate organ injury (28). It was reported that MDA levels increased and GSH levels decreased in the blood serum of obese mice; but exercises, such as treadmill, improved these parameters (29). A previous study on an obesity model observed an increase in MDA and a decrease in GSH levels in the heart and aorta of HFD-induced obese rats. However, swimming ameliorated these oxidative parameters in the mentioned tissues (30). Similar to the previous studies, we observed that MDA level increased and GSH level decreased in HFD-induced liver damage, and these oxidative parameters were enhanced by swimming.
In conclusion, our results showed that HFD-induced obesity increased oxidative stress and histopathologic damage in the liver tissue. Swimming could ameliorate obesity-induced liver damage by regulating the lipid accumulation and inhibiting the oxidative stress.
MAIN POINTS.
Moderate swimming exercise (EXC) enhanced high fat diet induced liver damage.
Moderate EXC regulates lipid accumulation, inhibits oxidative stress and histopathological liver damage.
EXC may protect obesity-induced liver damage by regulating the balance of oxidants/antioxidants.
Footnotes
This study was presented at the 13th Multinational Congress on Microscopy, September 24–29, 2017, Rovinj, Croatia and 24th National Electron Microscopy Congress, April 24–26, 2019, Edirne, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the Animal Ethics Committee of Marmara University, Date of Approval: 07.01.2019 (05.2019.mar).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.A.E., F.E.; Design - M.A.E., Ö.B.Ö., S.A., M.K., G.Ş., F.E.; Supervision M.A.E., F.E.; Resources - M.A.E., Ö.B.Ö., S.A., M.K., G.Ş., F.E.; Materials - M.A.E., N.A., Ö.B,Ö., S.A., M.K., G.Ş., F.E.; Data Collection and/or Processing - M.A.E., F.E.; Analysis and/or Interpretation - M.A.E., Ö.B.Ö., S.A., M.K., G.Ş., F.E.; Literature Search - M.A.E., Ö.B.Ö., S.A., M.K., G.Ş., F.E.; Writing Manuscript - M.A.E., F.E.; Critical Review - M.A.E., Ö.B.Ö., S.A., M.K., G.Ş., F.E.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Kim HJ, Kim HJ, Lee KE, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–75. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 2.Kwak JH, Jun DW, Lee SM, et al. Lifestyle predictors of obese and non-obese patients with nonalcoholic fatty liver disease: A cross-sectional study. Clin Nutr. 2018;37:1550–7. doi: 10.1016/j.clnu.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin YJ, Kim KM, Hwang S, et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol. 2012;27:1341–7. doi: 10.1111/j.1440-1746.2012.07165.x. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Yasutake K, Kohjima M, Kotoh K, Nakashima M, Nakamuta M, Enjoji M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1756–67. doi: 10.3748/wjg.v20.i7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz A, Mendonca LS, Aguila MB, Mandarim-de-Lacerda CA. Swimming training beneficial effects in a mice model of nonalcoholic fatty liver disease. Exp Toxicol Pathol. 2012;64:273–82. doi: 10.1016/j.etp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Sene-Fiorese M, Duarte FO, Scarmagnani FR, et al. Efficiency of intermittent exercise on adiposity and fatty liver in rats fed with high-fat diet. Obesity (Silver Spring) 2008;16:2217–22. doi: 10.1038/oby.2008.339. [DOI] [PubMed] [Google Scholar]
- 10.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205–18. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leghi GE, Domenici FA, Vannucchi H. Influence of Oxidative Stress and Obesity in Patients with Nonalcoholic Steatohepatitis. Arq Gastroenterol. 2015;52:228–33. doi: 10.1590/S0004-28032015000300014. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Qian ZY, Zhou PH, et al. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018;17:165. doi: 10.1186/s12944-018-0815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra UK, Kalita J, Singh SK, Rahi SK. Oxidative Stress Markers in Vitamin B12 Deficiency. Molecular neurobiology. 2017;54:1278–84. doi: 10.1007/s12035-016-9736-2. [DOI] [PubMed] [Google Scholar]
- 14.Zaki SM, Fattah SA, Hassan DS. The differential effects of high-fat and high-fructose diets on the liver of male albino rat and the proposed underlying mechanisms. Folia Morphol (Warsz) 2019;78:124–36. doi: 10.5603/FM.a2018.0063. [DOI] [PubMed] [Google Scholar]
- 15.Chen JD. Benefits of physical activity on nutrition and health status: studies in China. Asia Pac J Clin Nutr. 1995;4(Suppl 1):29–33. [PubMed] [Google Scholar]
- 16.Schumacher-Petersen C, Christoffersen BO, Kirk RK, et al. Experimental non-alcoholic steatohepatitis in Gottingen Minipigs: consequences of high fat-fructose-cholesterol diet and diabetes. J Transl Med. 2019;17:110. doi: 10.1186/s12967-019-1854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghorbanzadeh V, Mohammadi M, Mohaddes G, Dariushnejad H, Chodari L, Mohammadi S. Protective effect of crocin and voluntary exercise against oxidative stress in the heart of high-fat diet-induced type 2 diabetic rats. Physiol Int. 2016;103:459–68. doi: 10.1556/2060.103.2016.4.6. [DOI] [PubMed] [Google Scholar]
- 18.Dhir G, Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J Investig Med. 2018;66:7–10. doi: 10.1136/jim-2017-000554. [DOI] [PubMed] [Google Scholar]
- 19.Machado MV, Cortez-Pinto H. Diet, Microbiota, Obesity, and NAFLD: A Dangerous Quartet. Int J Mol Sci. 2016;17:481. doi: 10.3390/ijms17040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallou-Kabani C, Vige A, Gross MS, et al. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity (Silver Spring) 2007;15:1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol (1985) 2005;99:1220–5. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 23.Kucera O, Cervinkova Z. Experimental models of non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2014;20:8364–76. doi: 10.3748/wjg.v20.i26.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milic S, Lulic D, Stimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330–7. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceperuelo-Mallafre V, Ejarque M, Serena C, et al. Adipose tissue glycogen accumulation is associated with obesity-linked inflammation in humans. Mol Metab. 2016;5:5–18. doi: 10.1016/j.molmet.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallak MA, Bin-Jaliah I, Albawardi A, et al. Swim exercise training ameliorates hepatocyte ultrastructural alterations in rats fed on a high fat and sugar diet. Ultrastruct Pathol. 2018;42:155–61. doi: 10.1080/01913123.2017.1422581. [DOI] [PubMed] [Google Scholar]
- 27.Kakimoto PA, Kowaltowski AJ. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol. 2016;8:216–25. doi: 10.1016/j.redox.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang JT, Jiang YJ. The influence of palatable high-energy diet in diet-induced obesity pregnant rats on offspring oxidative stress in liver. Eur Rev Med Pharmacol Sci. 2018;22:2468–76. doi: 10.26355/eurrev_201804_14841. [DOI] [PubMed] [Google Scholar]
- 29.Susantiningsih T, Perdani RRW, Berawi K, Hadi S. The Effect of Treadmill Treatment on Oxidative Stress Markers and Endogenous Antioxidant Status in Obesity Mice. Open Access Maced J Med Sci. 2018;6:1803–8. doi: 10.3889/oamjms.2018.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acikel Elmas M, Cakici SE, Dur IR, et al. Protective effects of exercise on heart and aorta in high-fat diet-induced obese rats. Tissue Cell. 2019;57:57–65. doi: 10.1016/j.tice.2019.01.005. [DOI] [PubMed] [Google Scholar]


