Abstract
Little is known about the SARS-CoV-2 contamination of environmental surfaces and air in non-health care settings among COVID-19 cases. We explored the SARS-CoV-2 contamination of environmental surfaces and air by collecting air and swabbing environmental surfaces among 39 COVID-19 cases in Guangzhou, China. The specimens were tested on RT-PCR. The information collected for COVID-19 cases included basic demographic, clinical severity, symptoms at onset, radiological testing, laboratory testing and hospital admission. A total of 641 environmental surfaces and air specimens were collected among 39 COVID-19 cases before disinfection. Among them, 20 specimens (20/641, 3.1%) were tested positive from 9 COVID-19 cases (9/39, 23.1%), with 5 (5/101, 5.0%) positive specimens from 3 asymptomatic cases, 5 (5/220, 2.3%) from 3 mild cases, and 10 (10/374, 2.7%) from 3 moderate cases. All positive specimens were collected within 3 days after diagnosis, and 10 (10/42, 23.8%) were found in toilet (5 on toilet bowl, 4 on sink/faucet/shower, 1 on floor drain), 4 (4/21, 19.0%) in anteroom (2 on water dispenser/cup/bottle, 1 on chair/table, 1 on TV remote), 1 (1/8, 12.5%) in kitchen (1 on dining-table), 1 (1/18, 5.6%) in bedroom (1 on bed/sheet pillow/bedside table), 1 (1/5, 20.0%) in car (1 on steering wheel/seat/handlebar) and 3 (3/20, 21.4%) on door knobs. Air specimens in room (0/10, 0.0%) and car (0/1, 0.0%) were all negative. SARS-CoV-2 was found on environmental surfaces especially in toilet, and may survive for several days. We provided evidence of potential for SARS-CoV-2 transmission through contamination of environmental surfaces.
Author summary
The Coronavirus Disease 2019 (COVID-19) pandemic has precipitated a global crisis. It is important to understanding the SARS-CoV-2 contamination of environmental surfaces and air in non-health care settings among COVID-19 cases. In this study, we explored the SARS-CoV-2 contamination of environmental surfaces and air by collecting air and swabbing environmental surfaces among 39 COVID-19 cases in Guangzhou, China. We found that 20 specimens were tested positive from 9 COVID-19 cases. All positive specimens were collected within 3 days after diagnosis, and 10 were found in toilet. Air specimens in room and car were all negative. SARS-CoV-2 was found on environmental surfaces especially in toilet, and may survive for several days. We provided evidence of potential for SARS-CoV-2 transmission through contamination of environmental surfaces.
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has precipitated a global crisis, and it has resulted in 5,404,512 confirmed cases including with 343,514 deaths globally as of May 26, 2020 [1]. Reported transmission modes of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) among humans were mainly through respiratory droplets produced by infected cases with sneezes or coughs [2]. People may be infected by inhalation of virus laden liquid droplets, and infection is more likely when someone are in close contact with COVID-19 cases [2–4]. However, the importance of indirect contact transmission, such as environmental contamination, is uncertain [5–7]. Evidences suggested that environmental contamination with SARS-CoV-2 is likely to be high, and it is supported by recent researches focused on environmental contamination from COVID-19 cases in hospital [5–9]. Hospitals have already perfect disinfection measures, and are less likely to appear super-spreaders compared with community and household [4,10–12]. However, the role of air and surface contamination in non-health care settings is still need to be explored. Therefore, it is important to understand the environmental contamination of infected cases by SARS-CoV-2 in non-health care settings, which is a vital aspect of controlling the spread of the epidemic.
To address this question, in this study, we sampled total of 641 surfaces environmental and air specimens among 39 cases in Guangzhou, China, to explore the surrounding environmental surfaces and air contamination by SARS-CoV-2 in non-health care settings.
Methods
Study design and setting
Based on COVID-19 case reports, environmental surfaces and air specimens were collected by Guangzhou CDC (GZCDC) from Feb 6 to Apr 10, 2020. The environmental surfaces specimens of COVID-19 cases sampled in home, hotel, public area, restaurant, marketplace, car and pet, which was associated with COVID-19 cases’ life trajectory before hospitalization. Air specimens of COVID-19 cases were also sampled in their room (home or hotel). All specimens were collected before disinfection.
Demographic and epidemiological data
Epidemiological investigations were performed for COVID-19 cases including basic demographic (age, sex, imported cases or not), date of symptoms onset, date of diagnosis, date of environmental specimens collection, clinical severity, symptoms at onset (fever, dry cough, expectoration, fatigue, myalgia and diarrhea), radiological examinations (chest computed tomography [CT]), laboratory testing (white blood cell count, lymphocyte, lymphocyte percentage, neutrophilic granulocyte and neutrophilic granulocyte percentage) and hospital admission.
Sample collection
The environmental specimens of SARS-CoV-2 were collected by qualified technicians who had received biosafety training (who had passed the training) and were equipped with corresponding laboratory skills. Personal protective equipment (PPE) were required for sampling personnel including N95 masks or masks with higher filtration efficiency, goggles, protective clothing, double-layer latex gloves and waterproof boot covers [13]. Sterile premoistened swabs were used for sampling, and then were put into a tube containing 2 to 3 mL virus preservation solution (or isotonic saline solution, tissue culture solution or phosphate buffer) with swabs’ tail discarded and cap tightened. According to Technical Guidelines for COVID-19 Laboratory Testing in China [13], three kinds of positions for environmental sampling were recommended: high frequency contact sites, surface of daily necessaries and toilet related specimens. Two to four specimens should be collected for each category. We further classified the three categories into 9 sub-categories: (1) toilet (sink, faucet, shower, toilet bowl, floor drain); (2) anteroom (chair, table, TV remote, water dispenser, cup, bottle, TV bench, handrail); (3) kitchen (cutting board, bowl, chopsticks); (4) bedroom (bed, sheet pillow, bedside table, telephone, computer, mouse, air conditioner, fan); (5) door knobs and switch buttons in room; (6) air in room; (7) outside room (elevator, elevator button, stair armrest); (8) car (steering wheel, seat, handlebar, air); (9) pet (mouth, nose, anus). Door knobs and switch buttons in room were not classified into specific room locations due to the specimens were collected with mixed form, where were commonly used by COVID-19 cases. And specimens in car (steering wheel, seat and handlebar) were also mixed form.
Air specimens were sampled using an MD8 microbiological sampler (Sartorius, Germany) and sterile gelatin filters (3 μm pores and 80 mm diameter, Sartorius, Germany) [7,14]. Air sampling was set with a volume of 1000 L at a speed of 50 L/minute, and it took about 20 minutes to complete the sampling process. The filters were dissolved aseptically in 30 mL virus preservation solution (or isotonic saline solution, tissue culture solution, or phosphate buffer).
Laboratory procedures
The environmental surfaces and air specimens for nucleic acid detection were stored at -70°C or below (specimens may be temporarily stored in -20°C refrigerators in the absence of -70°C storage condition) and were tested as soon as possible. Laboratory confirmation of environmental surfaces and air specimens was performed by qualified staff, and results were identified through open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) of SARA-CoV-2 by RT-PCR testing in accordance with the protocol established by China CDC [13]. Details on laboratory processes are provided in Appendix (appendix 1 p1-2).
Definitions
The asymptomatic persons infected with SARA-CoV-2 (asymptomatic cases in short) are referring to those who have no relevant clinical manifestations including clinically detectable signs or self-perceived symptoms such as fever, cough or sore throat, but who have tested positive for SARA-CoV-2 in respiratory specimens or other specimens [15]. Clinical classification of symptomatic cases includes 4 categories [16]: mild, moderate, severe, and critical (appendix 2 p3). In this study, the COVID-19 cases included asymptomatic, mild, moderate, severe, and critical cases. Fever was defined as an axillary temperature of 37.3°C or higher. Public area included elevator, elevator buttons and stair armrests. An imported case is a person who was infected with SARA-CoV-2 in a foreign country and diagnosed in Guangzhou.
Statistical analysis
Categorical variables were presented with frequency (percentage) and continuous variables were summarized with median (interquartile range [IQR]). Figures were drawn using Office Excel (version 2019). Analyses were all performed with the SAS software (version 9.4 for Windows, SAS Institute, Inc., Cary, NC, USA).
Ethics statement
This study was based on the data from the work for an ongoing public health response to COVID-19 by GZCDC as required by the National Health Commission of China, and hence individual informed consent was waived. The study was determined not to be human subjects research and therefore was considered exempt from ethical approval after consultation with the ethics committee of GZCDC. Analytical datasets were constructed in an anonymized manner, and all analysis of personally identifiable data took place onsite at the GZCDC.
Results
Characteristics of 39 COVID-19 cases
Among 39 COVID-19 cases, 23 (23/39, 59.0%) were male, 11 (11/39, 28.2%) were imported and the median age was 36.0 years (IQR, 31.0 to 48.0 years). A total of 9 (9/39, 23.1%) asymptomatic cases and 30 (30/39, 76.9%) symptomatic cases with 10 (10/30, 33.3%) mild, 19 (19/30,63.4%) moderate and 1 (1/30, 3.3%) severe cases were identified, and all (39/39, 100.0%) were cured and discharged. Among them, the common symptoms at onset was fever (24/39, 61.5%), dry cough (21/39, 53.9%), expectoration (9/39, 23.1%), fatigue (6/39, 15.4%), myalgia (3/39, 7.7%), diarrhea (2/39, 5.1%), and 31 (31/37, 79.5%) with CT lung abnormalities (Table 1).
Table 1. Characteristics of 39 COVID-19 cases in Guangzhou, China.
| Characteristics | COVID-19 cases (n = 39) |
|---|---|
| Median age (IQR) (years) | 36.0 (31.0–48.0) |
| Age group (%) | |
| <18 | 1/39 (2.5) |
| 18–44 | 22/39 (56.4) |
| 45–59 | 12/39 (30.8) |
| ≥60 | 4/39 (10.3) |
| Male (%) | 23/39 (59.0) |
| Imported (%) | 11/39 (28.2) |
| Severity (%) * | |
| Asymptomatic | 9/39 (23.1) |
| Symptomatic | 30/39 (76.9) |
| Mild | 10/30 (33.3) |
| Moderate | 19/30 (63.4) |
| Severe | 1/30 (3.3) |
| Hospital admission (%) † | |
| Death | 0/39 (0.0) |
| Discharge | 39/39 (100.0) |
| Highest temperature (%) (°C) | |
| <37.3 | 15/39 (38.5) |
| 37.3–38.0 | 19/39 (48.7) |
| 38.1–39.0 | 4/39 (10.2) |
| >39.0 | 1/39 (2.6) |
| Symptoms at onset (%) | |
| Fever | 24/39 (61.5) |
| Dry cough | 21/39 (53.9) |
| Expectoration | 9/39 (23.1) |
| Fatigue | 6/39 (15.4) |
| Myalgia | 3/39 (7.7) |
| Diarrhea | 2/39 (5.1) |
| CT lung abnormalities (%) | 31/37 (79.5) |
| Median blood biochemical index (IQR) | |
| WBC (109/L) ‡, § | 6.2 (4.7–7.4) |
| Ly (109/L) § | 1.2 (0.9–1.8) |
| Ne (109/L) § | 4.3 (3.1–5.2) |
| Ly% § | 22.6 (16.1–25.8) |
| Ne% § | 69.4 (65.7–72.5) |
WBC = white blood cell count; Ly = lymphocyte; Ly% = lymphocyte percentage; Ne = neutrophilic granulocyte; Ne% = neutrophilic granulocyte percentage.
* Severity of COVID-19 at the time of diagnosis.
† Data on May 10.
‡ Data at hospital admission
§ Missing Values: WBC = 6, Ly (%) = 8, Ne = 14, Ne% = 11.
Distribution of environmental specimens among 39 COVID-19 cases
A total of 641 environmental surfaces and air specimens were collected among 39 COVID-19 cases, and 20 specimens (20/641, 3.1%) were tested positive on RT-PCR from 9 COVID-19 cases (9/39, 23.1%), with 5 (5/101, 5.0%) positive specimens from 3 asymptomatic cases, 5 (5/220, 2.3%) from 3 mild cases and 10 (10/374, 2.7%) from 3 moderate cases (Table 2). The COVID-19 cases without fever (8/16 [50.0%] vs. 1/23 [4.3%]), dry cough (7/18 [38.9%] vs 2/21 [9.5%]), expectoration (8/30 [26.6%] vs. 1/9 [11.1%]), fatigue (9/33 [27.2%] vs. 0/6 [0.0%]) and myalgia (20/36 [55.6%] vs. 0/3 [0.0%]), diarrhea (9/37 [24.3%] vs. 0/2 [0.0%]) symptoms were more likely to have positive specimens of SARS-CoV-2 (Table 2).
Table 2. Distribution of environmental specimens among COVID-19 cases.
| Characteristic | Total | Environmental specimens in 9 positive COVID-19 cases, n/N (%) ‡ | |
|---|---|---|---|
| Positive environmental specimens, n/N (%) § | Positive COVID-19 cases, n/N (%) | ||
| Total | 20/641 (3.1) | 9/39 (23.1) | 20/136 (14.7) |
| Sampling site | |||
| Home | 13/259 (5.0) | 6/20 (30.0) | 13/68 (19.1) |
| Hotel | 6/113 (5.3) | 3/8 (37.5) | 6/50 (12.0) |
| Restaurant | 0/30 (0.0) | 0/3 (0.0) | 0/0 (0.0) |
| Public area * | 0/108 (0.0) | 0/13 (0.0) | 0/13 (0.0) |
| Marketplace | 0/122 (0.0) | 0/6 (0.0) | 0/0 (0.0) |
| Car | 1/5 (20.0) | 1/1 (100.0) | 1/5 (20.0) |
| Pet | 0/4 (0.0) | 0/1 (0.0) | 0/0 (0.0) |
| Days from symptom onset to sampling † | |||
| 0–3 | 9/110 (8.2) | 4/7 (57.1) | 9/67 (13.4) |
| 4–9 | 6/130 (4.6) | 4/11 (36.4) | 6/37 (16.2) |
| ≥10 | 0/300 (0.0) | 0/16 (0.0) | 0/0 (0.0) |
| Days from diagnosis to sampling | |||
| 0–3 | 20/338 (5.9) | 9/25 (36.0) | 20/136 (14.7) |
| 4–9 | 0/94 (0.0) | 0/5 (0.0) | 0/0 (0.0) |
| ≥10 | 0/209 (0.0) | 0/12 (0.0) | 0/0 (0.0) |
| Severity | |||
| Asymptomatic | 5/101 (5.0) | 3/9 (33.3) | 5/32 (15.6) |
| Mild | 5/220 (2.3) | 3/10 (30.0) | 5/53 (9.4) |
| Moderate | 10/374 (2.7) | 3/20 (15.0) | 10/51 (19.6) |
| Severe | 0/24 (0.0) | 0/1 (0.0) | 0/0 (0.0) |
| Imported | |||
| No | 9/423 (2.1) | 5/28 (17.9) | 9/52 (17.3) |
| Yes | 11/218 (5.1) | 4/11 (36.4) | 11/84 (13.1) |
| Symptoms at onset, yes | |||
| Fever | |||
| No | 18/234 (7.7) | 8/16 (50.0) | 18/98 (18.4) |
| Yes | 2/407 (0.5) | 1/23 (4.3) | 2/38 (5.3) |
| Dry cough | |||
| No | 15/240 (6.3) | 7/18 (38.9) | 15/118 (12.7) |
| Yes | 5/401 (1.3) | 2/21 (9.5) | 5/18 (27.8) |
| Expectoration | |||
| No | 16/493 (3.3) | 8/30 (26.6) | 16/123 (13.0) |
| Yes | 4/148 (2.7) | 1/9 (11.1) | 4/13 (30.8) |
| Fatigue | |||
| No | 20/535 (3.7) | 9/33 (27.2) | 20/136 (14.7) |
| Yes | 0/106 (0.0) | 0/6 (0.0) | 0/0 (0.0) |
| Myalgia | |||
| No | 20/591 (3.4) | 20/36 (55.6) | 20/136 (14.7) |
| Yes | 0/50 (0.0) | 0/3 (0.0) | 0/0 (0.0) |
| Diarrhea | |||
| No | 20/597 (3.4) | 9/37 (24.3) | 20/136 (14.7) |
| Yes | 0/44 (0.0) | 0/2 (0.0) | 0/0 (0.0) |
* Public area included elevator, elevator button, stair armrest.
† A total of 9 asymptomatic COVID-19 cases with 101 environmental specimens were excluded.
§ The number of positive environmental specimens divided by the number of total environmental specimens with 39 COVID-19 cases.
‡ The number of positive environmental specimens divided by the number of environmental specimens with 9 positive COVID-19 cases.
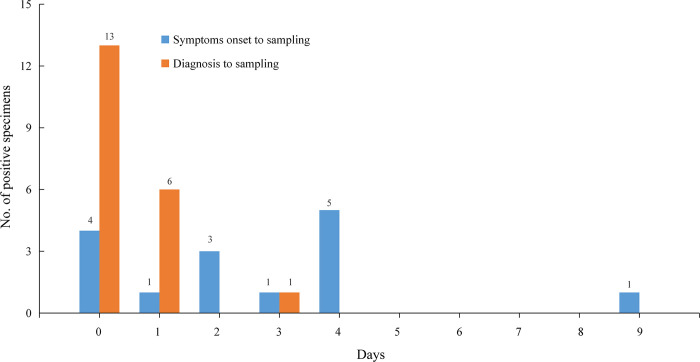
All the 20 positive specimens were collected within 3 days (≤3 days) from diagnosis to sampling (Fig 1), and 13 (13/259, 5.0%) positive environmental surfaces specimens were collected from home, 6 (6/113, 5.3%) from hotel and 1 (1/5, 20.0%) from car that had driven. While, specimens in restaurant that had eaten (0/30, 0.0%), marketplace that had visited (0/122, 0.0%), pet that had lived with (0/4, 0.0%) and public area that had stayed (0/108, 0.0%) were all tested negative on RT-PCR (Table 2).
Fig 1. Distribution of positive environmental specimens by days from date of symptoms onset or diagnosis to sampling.
Day 0 means sampling at the same day with symptom onset or diagnosis. Environmental specimens were tested on RT-PCR. Of 5 positive environmental specimens of asymptomatic COVID-19 cases were excluded in bars of symptoms onset to sampling.
Distribution of 20 positive environmental specimens among 9 COVID-19 cases
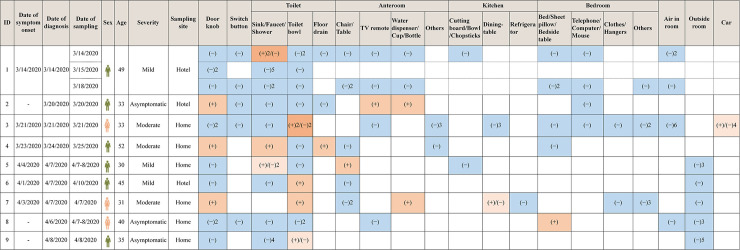
A total of 136 environmental surfaces and air specimens were collected from 9 COVID-19 cases who with at least one positive environmental specimen. Among them, 20 specimens (20/136, 14.7%) of specimens were tested positive on RT-PCR. Among 20 positive environmental specimens, 10 (10/42, 23.8%) were found in toilet (5 on toilet bowl, 4 on sink/faucet/shower, 1 on floor drain), 4 (4/21, 19.0%) in anteroom (2 on water dispenser/cup/bottle, 1 on chair/table, 1 on TV remote), 1 (1/8, 12.5%) in kitchen (1 on dining-table), 1 (1/18, 5.6%) in bedroom (1 on bed/sheet pillow/bedside table), 1 (1/5, 20.0%) in car (1 on steering wheel/seat/handlebar) and 3 (3/20, 21.4%) on door knobs. Surfaces specimens collected from outside room (0/13, 0.0%), air specimens collected from room (0/10, 0.0%) and car (0/1, 0.0%) were tested negative on RT-PCR (Table 3 and Fig 2).
Table 3. Distribution of 20 positive environmental specimens among 9 COVID-19 cases.
| Characteristic | Total, n/N (%) |
|---|---|
| Total | 20/136 (14.7) |
| Toilet | 10/42 (23.8) |
| Toilet bowl | 5/17 (29.4) |
| Sink/Faucet/Shower | 4/22 (18.2) |
| Floor drain | 1/3 (33.3) |
| Anteroom | 4/21 (19.0) |
| Water dispenser/Cup/Bottle | 2/4 (50.0) |
| Chair/Table | 1/8 (12.5) |
| TV remote | 1/5 (20.0) |
| Others (TV Bench/Handrail) | 0/4 (0.0) |
| Kitchen | 1/8 (12.5) |
| Dining-table | 1/5 (20.0) |
| Cutting board/Bowl/Chopsticks | 0/2 (0.0) |
| Refrigerator | 0/1 (0.0) |
| Bedroom | 1/18 (5.6) |
| Bed/Sheet pillow/Bedside table | 1/6 (16.7) |
| Telephone/Computer/Mouse | 0/4 (0.0) |
| Clean Clothes/Hangers | 0/2 (0.0) |
| Others (Air conditioner/Fan) | 0/6 (0.0) |
| Door knob in room * | 3/14 (21.4) |
| Switch buttons in room * | 0/5 (0.0) |
| Air in room | 0/10 (0.0) |
| Outside room (Elevator/ Elevator buttons/Stair armrest) | 0/13 (0.0) |
| Car | 1/5 (20.0) |
| Steering wheel/Seat/Handlebar † | 1/4 (25.0) |
| Air in car | 0/1 (0.0) |
* Door knobs and switch buttons in room were not classified into specific room locations due to the specimens were collected with mixed form, where was commonly used by COVID-19 cases.
† Specimens in car (steering wheel, seat and handlebar) were mixed form.
Fig 2. Distribution of 136 environmental specimens among 9 COVID-19 cases.
(+) represents positive environmental surfaces specimens, and (−) represents negative environmental surfaces and air specimens. The number represents the count of negative/positive environmental specimens. White blank represents without specimens.
Discussion
In the current study, we evaluated the environmental contamination by SARS-CoV-2 among 641 environmental surfaces and air specimens belong to 39 COVID-19 cases before they were admitted to hospital. SARS-CoV-2 was found on environmental surfaces especially in toilet, and may survive for several days. Our study provided evidence of potential for SARS-CoV-2 transmission through contamination of environmental surfaces. All air specimens were negative. To our known, this study is the largest sample of environmental specimens with COVID-19 cases.
Previous studies showed that if one person contacted frequently with COVID-19 cases, he or she would have high incidence of infected SARS-CoV-2 [3,17–19], presumably due to inhalation of droplets or contact transmission. Mathematical models, animal models and intervention researches suggested that contact transmission was the most important route in some scenarios [20,21]. In current study, several environmental specimens sampled from home, hotel and car were tested positive on RT-PCR, where were the sites that COVID-19 cases contacted frequently. Our results further demonstrated the possibility of contact transmission through environmental contamination probably due to self-inoculation of mucous membranes of mouth, nose or eyes through hands, resulting in high chance to infect SARS-CoV-2. Similar findings were made during SARS-CoV [22] and MERS-CoV [23] outbreak, in which RT-PCR positive swab specimens were confirmed from frequently touched environmental surfaces in patients’ rooms, such as medication refrigerator door, bed table and a television remote control. In other sites, such as restaurant and marketplace, there was no positive environmental specimens on RT-PCR testing in our study. However, all surrounding environmental contamination from COVID-19 cases should be alert. Although, the RT-PCR testing among specimens in restaurant and marketplace was negative, the huge number of people exposed to there also existed great risk. Previous study showed that the COVID-19 outbreak was associated with environmental contamination in restaurant [24]. In addition, some studies reported that all identified COVID-19 outbreaks of cluster confirmed cases occurred in an indoor environment [25], suggesting sharing indoor space was a major risk of SARS-CoV-2 infection.
All environmental specimens were collected before they were diagnosed in our study, which indicated that a person who exposed to environmental contamination from COVID-19 cases had a high infected risk unknowingly. In addition, SARS-CoV-2 had a great opportunity of surviving for a while on surfaces such as toilet, anteroom and kitchen in current study. Therefore, we suggested that home quarantine for suspected COVID-19 cases might be not a good control strategy. It was difficult to ensure that cluster infection did not occur in household during the quarantine period for at least fourteen days because they shared areas like toilets during quarantine. Previous study also suggested that home quarantine required personal protective equipment and professional training, but for ordinary people and families, especially those living together in a narrow space, was obviously hard to implement excellent infection control, causing other families to be infected [12,26,27] and centralized quarantine was recommended in this condition [28].
Previous study showed that COVID-19 cases with severe disease had significantly higher viral loads than that with mild disease in respiratory specimens [29]. We tried to explore whether the more serious the COVID-19, the more contaminated to the environment surfaces. In this study, 24 environmental surfaces specimens were collected in marketplace from one severe COVID-19 cases, and the specimens’ RT-PCR testing was negative, which was probably due to the sampling site was where he or she came into contact occasionally. In other cases, several environmental surfaces specimens were tested positive with 5 (5/32, 15.6%) for 3 asymptomatic cases, 5 (5/53, 9.4%) for 3 mild cases, 10 (10/51, 19.6%) for 3 moderate cases, suggesting all cases would contaminate environmental surfaces. In addition, COVID-19 cases without symptoms like fever, dry cough, expectoration, fatigue, myalgia, diarrhea, were more likely to have positive specimens of SARS-CoV-2. While, cases with symptoms may be prone to wear face mask, which may reduce the risk of contaminating the environment. It might also due to that people with symptoms were quarantined more quickly in general, while, people without symptoms would continue to contaminated the environmental surfaces and air before they were admitted to hospital. We highly recommend that persons no matter COVID-19 cases or general public should conduct hand hygiene and personal protective equipment to minimize self-contamination and to protect against inoculation of mucosal surfaces and the respiratory tract [30].
Evidence suggested that influenza virus, MERS-CoV, and SARS-CoV could survive on environmental surfaces for extended periods, sometimes up to months [20,31–33]. It suggested that prolonged potential transmission of coronavirus might be existed via contact or fomite. Among environmental surfaces specimens in our study, all positive specimens were collected within 3 days (≤3 days) after diagnosis. It means that without disinfection, the SARS-CoV-2 may survive on environmental surfaces for at least 3 days, and in other studies, the survival time was 0 to 14 days [34–37], which was consistent with current understanding. Therefore, enhance surfaces cleaning and disinfection were important and essential [20].
Whether SARS-CoV-2 can be transmitted by aerosols remains controversial [5,38,39]. Fortunately, all air specimens from COVID-19 cases were tested negative in our study, and other studies also showed that air specimens were tested negative despite the extent of environmental contamination in hospital [7,38]. However, aerosol specimens in two Wuhan hospitals were tested positive collected from toilet areas, areas prone to crowding, medical staff areas [5], and suggested that SARS-CoV-2 might have potential to be transmitted via aerosols. Therefore, routine disinfection and cloth masks were recommended and some study suggested that cloth masks could potentially provide significant protection against the transmission of aerosol particles when socializing [30].
Limitations
This study has several limitations. Firstly, the results of RT-PCR testing do not indicate the amount of viable virus, and viral culture was not done to demonstrate viability. Secondly, due to operational limitations during the outbreak, days of sampling interval was inconsistent. However, it also provided additional information about SARS-CoV-2 survival. Thirdly, the number of air specimens represented only a small fraction of total specimens, and air exchanges in room would have diluted the presence of SARS-CoV-2 in the air. Further studies are required to confirm these preliminary results.
Conclusions
SARS-CoV-2 was found on environmental surfaces especially in toilet, and may survive for several days. Our study provided evidence of potential for SARS-CoV-2 transmission through contamination of environmental surfaces.
Supporting information
(DOCX)
(XLS)
Data Availability
Readers interested in obtaining survey data for this study are available from CM at maochen9@smu.edu.cn.
Funding Statement
This study was sponsored by the Project Supported by Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (2019), Young Elite Scientists Sponsorship Program by CAST (2019QNRC001), the Construction of High-level University of Guangdong (G619339521 and G618339167) and the Zhejiang University special scientific research fund for COVID-19 prevention and control (K920330111). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Coronavirus disease 2019 (COVID-19) situation report. Accessed at https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200526-covid-19-sitrep-127.pdf?sfvrsn=7b6655ab_8 on 27 May 2020.
- 2.Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science (New York, N.Y.). 2020; 368: eabb6936 10.1126/science.abb6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infectious Diseases. 2020. 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England). 2020; 395: 514–523. 10.1016/s0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020. 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 6.Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerging infectious diseases. 2020; 26 10.3201/eid2607.200885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020. 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature communications. 2020; 11: 2800 10.1038/s41467-020-16670-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye G, Lin H, Chen S, Wang S, Zeng Z, Wang W, et al. Environmental contamination of SARS-CoV-2 in healthcare premises. The Journal of infection. 2020; 81: e1–e5. 10.1016/j.jinf.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braden CR, Dowell SF, Jernigan DB & Hughes JM. Progress in global surveillance and response capacity 10 years after severe acute respiratory syndrome. Emerging infectious diseases. 2013; 19: 864–869. 10.3201/eid1906.130192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gire SK, Goba A, Andersen KG, Sealfon RSG, Park DJ, Kanneh L, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science (New York, N.Y.). 2014; 345: 1369–1372. 10.1126/science.1259657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang R, Xia J, Chen Y, Shan C & Wu C. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. Lancet Infectious Diseases. 2020; 20: 534–535. 10.1016/S1473-3099(20)30147-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinese Center for Disease Control and Prevention. Technical Guidelines for COVID-19 Laboratory Testing. Accessed at http://weekly.chinacdc.cn/en/article/doi/10.46234/ccdcw2020.085 on 10 April 2020. [DOI] [PMC free article] [PubMed]
- 14.Kim SH, Chang SY, Sung M, Park JH, Bin Kim H, Lee H, et al. Extensive Viable Middle East Respiratory Syndrome (MERS) Coronavirus Contamination in Air and Surrounding Environment in MERS Isolation Wards. Clinical infectious diseases. 2016; 63: 363–369. 10.1093/cid/ciw239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinese Center for Disease Control and Prevention. Protocol for the Management of Asymptomatic Persons Infected with COVID-19 Virus. Accessed at http://weekly.chinacdc.cn/en/article/doi/10.46234/ccdcw2020.117 on 6 March 2020.
- 16.National Health Commission of the People’s Republic of China. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (Edition 6). Accessed at http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf on 6 March 2020 (in Chinese).
- 17.Kakimoto K, Kamiya H, Yamagishi T, Matsui T, Suzuki M & Wakita T. Initial Investigation of Transmission of COVID-19 Among Crew Members During Quarantine of a Cruise Ship—Yokohama, Japan, February 2020. Morb Mortal Wkly Rep. 2020; 69: 312–313. 10.15585/mmwr.mm6911e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Litvinova M, Liang Y, Wang Y, Wang W, Zhao S, et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science (New York, N.Y.). 2020. 10.1126/science.abb8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Huang Y, Tu C, Bi C, Chen Z, Luo L, et al. Household Transmission of SARS-CoV-2, Zhuhai, China, 2020. Clinical infectious diseases. 2020. 10.1093/cid/ciaa557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD & Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. The Journal of hospital infection. 2016; 92: 235–250. 10.1016/j.jhin.2015.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Ai S, Song S, Zhu G, Tian F, Li H, et al. Population movement, city closure in Wuhan and geographical expansion of the 2019-nCoV pneumonia infection in China in January 2020. Clinical infectious diseases. 2020. 10.1093/cid/ciaa422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booth TF, Kournikakis B, Bastien N, Ho J, Kobasa D, Stadnyk L, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. The Journal of infectious diseases. 2005; 191: 1472–1477. 10.1086/429634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bin SY, Heo JY, Song MS, Lee J, Kim EH, Park SJ, et al. Environmental Contamination and Viral Shedding in MERS Patients During MERS-CoV Outbreak in South Korea. Clinical infectious diseases. 2016; 62: 755–760. 10.1093/cid/civ1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, Gu J, Li K, Xu C, Su W, Lai Z, et al. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerging infectious diseases. 2020; 26 10.3201/eid2607.200764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian H, Miao T, Liu L, Zheng X, Luo D & Li Y. Indoor transmission of SARS-CoV-2. medRxiv. 2020: 2020. 2004.2004.20053058. 10.1101/2020.04.04.20053058 [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Wang C, Dong L & Xiao M. Home quarantine or centralized quarantine, which is more conducive to fighting COVID-19 pandemic? Brain, behavior, and immunity. 2020. 10.1016/j.bbi.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan R, Mao ZQ, He LY, Hu YC & Wei C. Evidence from two cases of asymptomatic infection with SARS-CoV-2: Are 14 days of isolation sufficient? International journal of infectious diseases. 2020; 95: 174–175. 10.1016/j.ijid.2020.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Zhang Z, Yang J, Wang J, Zhai X, Barnighausen T, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet (London, England). 2020; 395: 1305–1314. 10.1016/s0140-6736(20)30744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ (Clinical research ed.). 2020; 369: m1443 10.1136/bmj.m1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konda A, Prakash A, Moss GA, Schmoldt M, Grant GD & Guha S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano. 2020. 10.1021/acsnano.0c03252 [DOI] [PubMed] [Google Scholar]
- 31.van Doremalen N, Bushmaker T & Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro surveillance. 2013; 18 10.2807/1560-7917.es2013.18.38.20590 [DOI] [PubMed] [Google Scholar]
- 32.Casanova LM, Jeon S, Rutala WA, Weber DJ & Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Applied and environmental microbiology. 2010; 76: 2712–2717. 10.1128/AEM.02291-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai MY, Cheng PK & Lim WW. Survival of severe acute respiratory syndrome coronavirus. Clinical infectious diseases. 2005; 41: e67–71. 10.1086/433186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin A, Chu J, Perera M, Hui K, Yen H-L, Chan M, et al. Stability of SARS-CoV-2 in different environmental conditions. medRxiv. 2020: 2020. 2003.2015.20036673. 10.1101/2020.03.15.20036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang T, Lien C, Liu S & Selveraj P. Effective Heat Inactivation of SARS-CoV-2. medRxiv. 2020: 2020.2004.2029.20085498. 10.1101/2020.04.29.20085498 [DOI] [Google Scholar]
- 36.Carducci A, Federigi I, Liu D, Thompson JR & Verani M. Making waves: Coronavirus detection, presence and persistence in the water environment: State of the art and knowledge needs for public health. Water research. 2020; 179: 115907 10.1016/j.watres.2020.115907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. The New England journal of medicine. 2020; 382: 1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. The Science of the total environment. 2020; 725: 138401 10.1016/j.scitotenv.2020.138401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan L, Zhou L, Zhang J, Peng W & Chen R. More awareness is needed for severe acute respiratory syndrome coronavirus 2019. transmission through exhaled air during non-invasive respiratory support: experience from China. The European respiratory journal. 2020; 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLS)
Data Availability Statement
Readers interested in obtaining survey data for this study are available from CM at maochen9@smu.edu.cn.