Abstract
Background
Attention deficit hyperactivity disorder (ADHD) affects approximately 3% of adults globally. Many pharmacologic treatments options exist, yet the comparative benefits and harms of individual treatments are largely unknown. We performed a systematic review and network meta-analysis to assess the relative effects of individual pharmacologic treatments for adults with ADHD.
Methods
We searched English-language published and grey literature sources for randomized clinical trials (RCTs) involving pharmacologic treatment of ADHD in adults (December 2018). The primary outcome was clinical response; secondary outcomes were quality of life, executive function, driving behaviour, withdrawals due to adverse events, treatment discontinuation, serious adverse events, hospitalization, cardiovascular adverse events, and emergency department visits. Data were pooled via pair-wise meta-analyses and Bayesian network meta-analyses. Risk of bias was assessed by use of Cochrane’s Risk of Bias tool, and the certainty of the evidence was assessed by use of the GRADE framework.
Results
Eighty-one unique trials that reported at least one outcome of interest were included, most of which were at high or unclear risk of at least one important source of bias. Notably, only 5 RCTs were deemed at overall low risk of bias. Included pharmacotherapies were methylphenidate, atomoxetine, dexamfetamine, lisdexamfetamine, guanfacine, bupropion, mixed amphetamine salts, and modafinil. As a class, ADHD pharmacotherapy improved patient- and clinician-reported clinical response compared with placebo (range: 4 to 15 RCTs per outcome); however, these findings were not conserved when the analyses were restricted to studies at low risk of bias, and the certainty of the finding is very low. There were few differences among individual medications, although atomoxetine was associated with improved patient-reported clinical response and quality of life compared with placebo. There was no significant difference in the risk of serious adverse events or treatment discontinuation between ADHD pharmacotherapies and placebo; however, the proportion of participants who withdrew due to adverse events was significantly higher among participants who received any ADHD pharmacotherapy. Few RCTs reported on the occurrence of adverse events over a long treatment duration.
Conclusions
Overall, despite a class effect of improving clinical response relative to placebo, there were few differences among the individual ADHD pharmacotherapies, and most studies were at risk of at least one important source of bias. Furthermore, the certainty of the evidence was very low to low for all outcomes, and there was limited reporting of long-term adverse events. As such, the choice between ADHD pharmacotherapies may depend on individual patient considerations, and future studies should assess the long-term effects of individual pharmacotherapies on patient-important outcomes, including quality of life, in robust blinded RCTs.
Registration
PROSPERO no. CRD 42015026049
Introduction
Attention deficit hyperactivity disorder (ADHD) is a chronic, multifaceted condition that affects approximately 3% of adults [1] and contributes to important functional impairment and reduced quality of life [2, 3]. Prescriptions for ADHD medication have steadily increased over the last decade [4], leading to important expenditure to public payer health care systems [5]. Practice guidelines recommend a multi-modal approach, including psychosocial interventions and pharmacologic treatments, with continued support and follow-up; the choice between pharmacologic treatments should be based on efficacy and tolerability, as well as duration of effect, affordability, and use of co-medications [2].
Pharmacologic treatment options for ADHD in adults comprise both psychostimulant (e.g., methylphenidate- and amphetamine-based products) and non-stimulant options (e.g., atomoxetine, guanfacine). The availability of individual pharmacotherapies varies by jurisdiction [6], and treatment guidelines similarly differ by region [2, 6]. Canadian guidelines recommend long-acting amphetamine mixture, methylphenidate, or lisdexamfetamine as first-line pharmacologic options, with atomoxetine or short-acting dextroamphetamine or methylphenidate as second-line options for patients whose ADHD does not respond to first-line treatments [7]. British guidelines recommend psychostimulants as first-line treatment for adults, with atomoxetine considered as first-line treatment in some clinical situations (substance use disorder, contraindications to stimulants) [6].
Systematic reviews of randomized clinical trials (RCTs) are generally considered to be the gold standard for decision making in evidence-based medicine. Ideally, RCTs would simultaneously compare all available interventions to treat a given condition; however, in practice, such direct evidence from head-to-head treatment comparisons may be limited or insufficient. Further, pair-wise meta-analyses compare the benefits and harms of two treatments that have been directly compared in head-to-head trials, which may not provide decision-makers (i.e., patients, clinicians, policy makers) with information about the relative benefits and harms of all individual treatments of interest. In contrast, network meta-analyses (NMAs) use direct and indirect comparisons of interventions (i.e., treatments that have not necessarily been studied in head-to-head trials) and provide estimates of the relative effects of each treatment. As such, these types of analyses provide a more robust evidence base for decision-making.
There are multiple available treatment options for ADHD, and few have been directly compared in head-to-head trials. To inform clinical and policy decision-making, we performed a systematic review and NMA of pharmacotherapies available to treat ADHD in adults to assess the relative benefits and harms of each treatment.
Methods
This review was registered a priori (PROSPERO no. CRD 42015026049) and followed guidance for the conduct and reporting of systematic reviews from the Cochrane handbook [8] and the PRISMA NMA checklist (S1 File) [9].
Search strategy
We updated the search of an earlier evidence synthesis that met the population, intervention, and comparator requirements [10] through an iterative process by an experienced medical information specialist in consultation with the review team. Using the Ovid platform, we searched Ovid MEDLINE®, including In-Process and Other Non-Indexed Citations EMBASE and PsycINFO. We also searched the Cochrane Central Register of Controlled Trials (CENTRAL) using the Wiley version of the Cochrane Library. Grey literature was sought by use of Grey Matters Light [11], which includes the TRIP database, ClinicalTrials.gov and the ICTRP Search Portal The searches were updated from January 2011 onward, except for bupropion, for which there was no date restriction. The initial search was conducted in April 2015 and was updated twice (July 2017, December 2018). The 2008 Cochrane Highly Sensitive Search Strategy, sensitivity and precision-maximizing version, slightly amended, was applied to restrict publications to RCTs. The search strategy is available in Appendix A in S2 File.
Study selection
We included English-language RCTs, including cross-over trials, that included adult (≥ 18 yr) outpatients with attention deficit disorders (attention deficit disorder or ADHD) administered an ADHD pharmacotherapy (Appendix B in S2 File). Eligible comparators were placebo (or no treatment), another pharmacotherapy, or the same pharmacotherapy at a different dose. Titles and abstracts identified by the literature search were screened in duplicate, and the full text of any potentially relevant record was evaluated. Records that met the population, intervention, comparator, and design criteria were selected for inclusion; notably, studies were not selected for inclusion on the basis of reported outcomes.
Data extraction
Data were extracted using DistillerSR software (Evidence Partners Inc.) by one reviewer using piloted standardized abstraction forms; extracted data were checked for accuracy and completeness by a second reviewer. First-period data were extracted from cross-over trials. The primary publication of each unique study was used for data extraction, with additional information obtained from companion publications, supplements, and clinical trial registries, if available.
We extracted information on study design (e.g., first author, year of publication, funding source, country of study), participant characteristics (e.g., age, sex), intervention and comparator details (e.g., type of treatment, dose, duration), and primary and secondary outcomes. If an RCT used more than one scale to assess an outcome (e.g., clinical response), we extracted data from the scale defined by the author as the primary measure or the scale used to inform the sample size calculation. If neither of these were stated, we extracted data from the scale mentioned first in the results section.
Risk of bias and certainty of the evidence
Risk of bias (ROB) was assessed by two independent reviewers using the Cochrane Collaboration’s ROB tool for RCTs [8]. Disagreements were resolved by discussion. When sufficient data were available, we examined the impact of blinding on subjective outcomes in post-hoc sensitivity analyses involving only studies judged to be at low risk of bias for blinding because of the potential for bias due to patient or investigator expectations. Specifically, studies were deemed to be at low risk of bias for blinding if they involved use of a matched placebo and described that the placebo was indistinguishable from the intervention (e.g., in terms of taste, smell, appearance) or involved a double-dummy placebo. Studies that involved an open-label design or used a method of blinding that was deemed insufficient to prevent knowledge of group assignment were considered to be at high risk of bias. Finally, studies that mentioned blinding but did not provide details about the method of blinding were judged to be at unclear risk of bias. To be considered at overall low risk of bias, trials had to be at low risk of bias for each of the following domains: allocation concealment, blinding, incomplete data.
The risk of publication bias was addressed by conducing comprehensive searches for both published and grey literature and including all eligible studies, regardless of publication status. We also sought additional information from companion publications (e.g., supplements, clinical trial registries, post-hoc analyses), when available, and assessed the source of study funding. Furthermore, we looked for evidence that positive findings were more likely to have been reported in earlier-published studies and visually inspected the funnel plots of outcomes that included data from at least 10 RCTs for obvious signs of asymmetry [8].
The certainty of the evidence for each outcome was assessed by use of the GRADE method [12, 13]. For outcomes based on network meta-analyses, the GRADE assessment was operationalized by use of the CINEMA approach [14].
Outcomes
The primary outcome was clinical response (patient- or clinician-reported). Secondary outcomes were quality of life, executive function, driving behaviour, withdrawals due to adverse events, treatment discontinuation, serious adverse events, hospitalization, cardiovascular adverse events, and emergency department visits. Eligible scales for clinical response, quality of life, and executive function were based on a previous systematic review [10] (Appendix C in S2 File).
Data analysis and synthesis of results
Before analyses were conducted, included studies with available outcome data were compared to identify obvious sources of between-study heterogeneity. Clinical heterogeneity was assessed by comparing data on study participants, interventions, and comparators; methodological diversity was assessed by comparing study designs and the results of ROB assessments. Based on input from a clinical expert, a decision was made to restrict all analyses of benefits to data reported by RCTs with a treatment duration of at least 12 weeks, whenever possible; an approach that is in keeping with the current clinical recommendations to follow up every three months [2]. Two sets of analyses were conducted for harms; data reported by RCTs with a treatment duration of at least 12 weeks and data reported by RCTs of any treatment duration.
We performed pair-wise meta-analyses (MAs) by use of RevMan (v.5.3; Cochrane Collaboration) and NMAs were performed by use of WinBUGS (v.1.4.3; MRC Biostatistics Unit). All network diagrams were constructed using NodeXL (v.1.0.1.251). Outcomes with insufficient data for analysis are summarized narratively (hospitalizations, visits to the emergency department, cardiovascular events). Evidence suggests that the drug release mechanism and dose of at least some ADHD medications may impact their efficacy in adults [15]. As such, all analyses in this study compared individual ADHD medications, which were further categorized in terms of both drug release formulation (e.g., extended release, immediate release) and dose classification (e.g., low-, standard- or high-dose). Dose classification was based on the Health Canada-approved product monograph, if available, otherwise recommended doses from other jurisdictions were used (Appendix B in S2 File).
For MAs, between-study heterogeneity was assessed using the I2 statistic and a visual inspection of forest plots. I2 values of between 75% and 100% were interpreted as possibly representing a considerable amount of heterogeneity [8]; thus, all MAs with I2 values ≥75% were explored to determine if the heterogeneity could be explained by a clinical or methodological feature of one or more studies contributing data to the analysis. In a sensitivity analysis, such studies were removed, and their impact on the summary statistics and I2 value was evaluated [16].
The decision to conduct NMA was based on the sufficiency of data available to derive robust and consistent network models. We used a binomial likelihood model for dichotomous outcomes and a normal likelihood model for continuous outcomes, allowing for the inclusion of multi-arm trials [17]. NMAs were conducted using vague priors (e.g., N[0,1002]) assigned for basic parameters throughout [17]. To verify model convergence, trace plots and Brooks-Gelman-Rubin statistics were assessed [18]. Three chains were fit for each analysis with at least 20,000 iterations and a burn-in of at least 20,000 iterations. For all NMAs, we explored both random- and fixed-effects models. When closed loops (i.e., mixed evidence informed by more than one RCT [19]) were present, we assessed inconsistency by comparing deviance information criterion (DIC) statistics in fitted consistency and inconsistency models and examined the resulting inconsistency plot [20].
The choice of model was determined by assessing the goodness of fit statistics, i.e., we compared the mean residual deviance with the number of unconstrained data points (the total number of trials for outcomes analyzed using standardized mean difference (SMDs) and the total number of trial arms for binary data), deviance information criterion (DIC), and between study variance. We considered the most appropriate model to be the one that reported the lowest DIC, and where the total residual deviance was closest to the number of unconstrained data points.
Analyses were based on SMD from baseline as the measurement scale for clinical response, executive function, and quality of life. The total number of participants randomized was used as the denominator for clinical response (number of responders), and the number who received treatment was used for harms (serious adverse events, withdrawals due to adverse events, treatment discontinuations).
Summary measures for continuous outcomes (clinical response, quality of life, executive function) are reported as MDs and 95% confidence intervals (CIs) for pairwise meta-analyses and median MDs and 95% credible intervals (CrIs) for NMAs. SMDs were converted to a common scale by multiplication of the pooled SMD by the pooled standard deviation of baseline scores [8] (patient-reported clinical response: Conners’ Adult ADHD Rating Scale–Self, Short Version [CAARS-S-SV]; clinician-reported clinical response: CAARS–Observer, Short Version [CAARS-O-SV]; executive function: Brief Rating Inventory of Executive Function for Adults [BRIEF-A]; quality of life: Adult ADHD Quality of Life Scale [AAQoL]). Negative MDs indicate improvement in clinical response and executive function, while positive MDs indicate improvement in quality of life. Summary measures for binary data (number of responders, serious adverse events, withdrawals due to adverse events, treatment discontinuations) are reported as relative risks (RRs) with 95% CIs.
Results
Study characteristics
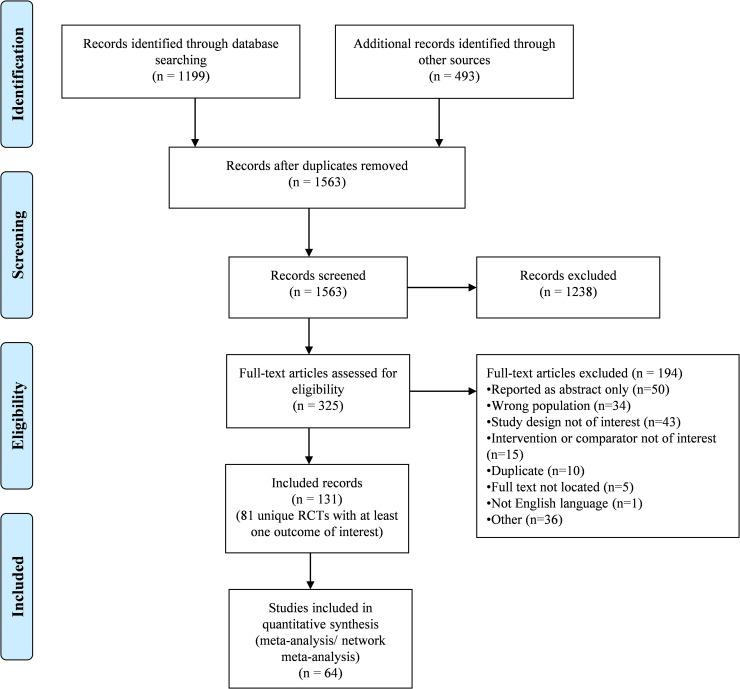
The literature search yielded 1563 unique titles and abstracts (Fig 1) of which 131 records met the eligibility criteria (Appendix D in S2 File). These records correspond to a total of 84 unique RCTs, owing to multiple companion records for most RCTs (e.g., one RCT described across multiple publications). Of the 84 unique RCTs, 81 reported at least one outcome of interest (Table 1). Two records each reported on two RCTs [21, 22].
Fig 1. PRISMA flowchart of study selection.
Table 1. Characteristics of RCTs with at least one reported outcome of interest.
| Author year, page no. (companion publications) | Country or region | No. of study sites | Duration | Diagnosis of ADHD | Population | Intervention (no. randomized) | Age, mean (SD) | Male, % | Funding source | |
|---|---|---|---|---|---|---|---|---|---|---|
| PARALLEL GROUP DESIGN | ||||||||||
| Paterson 1999, p. 494 [65] | Australia | NR | 6 wk | DSM-IV ADHD symptom checklist | Adults who reported the presence of at least four inattentive and/or five hyperactive symptoms during the previous 6 months (on the DSM-IV ADHD symptom checklist) | Placebo (21) | 35.5 | 60 | Non-pharma | |
| DEX (24) | ||||||||||
| Butterfield 2016, p. 136 [43] | US | 1 | 12 wk | DSM-IV-TR | Adults with suboptimal response to stimulant ADHD at time of screening | Placebo | 37.5 (12.2) | 46.2 | Pharma | |
| GUAN 1–4 mg | ||||||||||
| Total: 26 | ||||||||||
| Biederman 2012, p. 484 [23] (Biederman 2012) | US | NR | 6 wk | DSM-IV | 18–26 yr with childhood-onset ADHD | Placebo (34) | 21.6 (2.1) | Total: 62 | Pharma | |
| LIS-DEX 30–70 mg/d (35) | ||||||||||
| Sutherland 2012, p. 445 [74] | US | 8 | 8 wk | DSM-IV-TR, ACDS v1.2 | 18–60 yr with ADHD | Placebo (47) | Total: | Total: | Pharma | |
| ATX 40–100 mg/d (97) | 37 (NR) | 59 | ||||||||
| Adler 2009, p. 212 [35] | US | 30 | 12 wk | DSM-IV-TR by CAADID | 18–65 yr with ADHD and social anxiety disorder | Placebo (218) | Total: | Total: | Pharma | |
| ATX 40–100 mg/d (224) | 38 (NR) | 53.6 | ||||||||
| Weiss 2006, p. 611 [79] | US, Canada | 5 | 12 wk | DSM-IV | 18–66 yr with ADHD | Placebo (26) | NR | 64 | Pharma | |
| DEX-IR 10–40 mg/d (23) | ||||||||||
| Biehl 2016, p. 1 [42] | Germany | 1 | 6 wk | DSM-IV-TR | Adult patients with ADHD | Placebo (16) | 35.5 (10.1) | 57.1 | Mixed | |
| MPH IR up to 60mg/d (19) | 36.7 (10.0) | 66.7 | ||||||||
| Schrantee 2016, p. 955 (Bottelier 2014) [26] | Netherlands | 3 | 16 wk | DSM-IV; confirmed with the Diagnostic Interview for ADHD in Adults | 23–40 yr old stimulant treatment-naïve males with ADHD | Placebo (24) | 29.0 (4.9) | 100 | Non-pharma | |
| MPH (25) | 28.6 (4.6) | 100 | ||||||||
| Fan 2017, p. 4850 [46] | Taiwan | 1 | 10 wk | DSM-IV | Adults with ADHD | Placebo (12) | 32.5 (9.8) | 41.7 | Non-pharma | |
| ATX 1.2 mg/kg (12) | 28.9 (7.8) | 41.7 | ||||||||
| Goodman 2017, p. 105 [49] | US | 35 | 6 wk | DSM-IV | 18–65 yr with ADHD | Placebo (179) | 34.7 (11.60) | 54.9 | Pharma | |
| MPH-OROS up to 72 mg/d (178) | 36.8 (11.95) | 50.9 | ||||||||
| Goto 2017, p. 100 [50] | Japan, South Korea, Taiwan | 45 | 10 wk | DSM-IV-TR | Asian patients aged 18 years and older with a childhood diagnosis of ADHD | Placebo (196) | 31.7 (7.8) | 48.7 | Pharma | |
| ATX 80–120 mg/d (195) | 32.8 (8.1) | 46.6 | ||||||||
| Philipsen 2010, p. 203 [66] (Philipsen 2014, Philipsen 2015) | Germany | 7 | 1 yr | DSM-IV | 18–60 yr with childhood diagnosis of ADHD | Placebo (107) | 35 (10) | 43.7 | Mixed | |
| MPH-SR up to 60 mg/kg/d (110) | 35 (10) | 40.5 | ||||||||
| Hamedi 2014, p. 675 [51] | Iran | 1 | 6 wk | DSM-IV-TR | 20–60 yr with ADHD | Placebo (21) | 33.19 (4.00) | 71.4 | Non-pharma | |
| BUP-SR 75–150 mg/d (21) | 33.90 (4.83) | 57.1 | ||||||||
| Lee 2014, p. 386 [56] | Korea, Japan, Taiwan | 45 | 10 wk | DSM-IV-TR, CAADID | Adults with childhood-onset ADHD | Placebo (37) | 31.5 (8.6) | 35.1 | Pharma | |
| ATX 40–120 mg/d (37) | 35.2 (8.8) | 41.7 | ||||||||
| Kollins 2014, p. 158 [53] | US | NR | 4 wk | DSM-IV | 18–50 yr smokers with ADHD | Placebo (15) | 33.5 (9.1) | 60.0 | Pharma | |
| LIS-DEX 30–70 mg/d (17) | 29.6 (8.4) | 64.7 | ||||||||
| Konstenius 2014, p. 440 [28] | Sweden | 1 | 24 wk | DSM-IV | 18–65 yr prison inmates with ADHD | Placebo (27) | 42 (11.7) | 100 | Non-pharma | |
| MPH-OROS 18–180 mg/d (27) | 41 (7.5) | 100 | ||||||||
| Takahashi 2014, p. 488 [75] | Japan | 39 | 8 wk | DSM-IV, CAADID, CAARS-O:SV | 18–64 yr with childhood-onset ADHD | Placebo (141) | 34.1 (9.05) | 48.2 | Pharma | |
| MPH-OROS 18–72 mg/d (143) | 33.4 (8.85) | 49.7 | ||||||||
| Adler 2013, p. 694 [33] (Adler 2013,Weisler 2017, p.1198) | US | 35 | 10 wk | DSM-IV-TR | 18–85 yr with ADHD | Placebo (81) | 34.9 (11.02) | 53.8 | Pharma | |
| LIS-DEX 30–70 mg/d (80) | 34.2 (10.58) | 50.6 | ||||||||
| Durell 2013, p. 45 [25](Leuchter 2014, Adler 2014) | US | 32 | 12 wk | DSM-IV-TR, ACDS v1.2 | 18–30 yr with ADHD | Placebo (225) | 24.7 (3.5) | 56.4 | Pharma | |
| ATX 40–100 mg/d (220) | 24.7 (3.4) | 58.2 | ||||||||
| Ni 2013, p. 1959 [64] (Ni 2016, Ni 2017) | Taiwan | 1 | 8–10 wk | DSM-IV, Adult ADHD Self-Report Scale v1.1 | 18–55 yr with ADHD | MPH-IR 30–60 mg/d (31) | 31.4 (7.2) | 58.1 | Non-pharma | |
| ATX 0.5–1.2mg/kg/d (32) | 31.2 (8.4) | 59.4 | ||||||||
| Ginsberg 2012, p. 68 [48] (Ginsberg 2012, p. 705) | Sweden | 1 | 5 wk | DSM-IV | 21–61 yr male prison inmates with ADHD | Placebo (15) | 35.3 (NR) | 100 | Non-pharma | |
| MPH-OROS 36–72 mg/d (15) | 33.5 (NR) | 100 | ||||||||
| Retz 2012, p. 48 [68] | Germany | 10 | 8 wk | DSM-IV ADHD-DC | ≥18 yr with childhood-onset ADHD | Placebo (78) | 38.2 (9.9) | 56 | Pharma | |
| MPH-ER 10–120 mg/d (84) | 36.6 (10.4) | 38 | ||||||||
| Sobanski 2012, p. 100 [70] (Sobanski 2013) | Germany | 1 | 12 wk | DSM-IV, CAARS | 18–50 yr with ADHD | Placebo (37) | 37.4 (9.5) | 42.9 | Pharma | |
| ATX 18–80 mg/d (27) | 34.7 (8.8) | 59.1 | ||||||||
| Young 2011, p. 51 [32] (Wietecha 2012) | US | 42 | 24 wk | DSM-IV-TR, CAADID | ≥18 yr with childhood-onset ADHD | Placebo (234) | 41.4 (7.5) | 43.6 | Pharma | |
| ATX 40–100 mg/d (268) | 41.2 (6.9) | 51.1 | ||||||||
| Konstenius 2010, p. 130 [54] | Sweden | 1 | 12 wk | DSM-IV, CAARS-O:SV | Amphetamine addiction clinic outpatients with newly diagnosed ADHD | Placebo (12) | 39.7 (9.8) | 83 | Non-pharma | |
| MPH-OROS 18–72 mg/d (12) | 34.6 (10.1) | 75 | ||||||||
| Biederman 2010, p. 549 [41] (Biederman 2011, Beiderman 2012) | US | NR | 6 wk | DSM-IV, AISRS | 19–60 yr with childhood-onset ADHD | Placebo (115) | 36.4 (8.6) | 52 | Pharma | |
| MPH-OROS 36 to > 100 mg/d (112) | 34.7 (9.2) | 39 | ||||||||
| McRae-Clark 2010, p. 481 [62] | US | NR | 12 wk | DSM-IV | 18–65 yr with ADHD and marijuana dependence | Placebo (39) | 30.4 (13.0) | 68 | Non-pharma | |
| ATX 25–100 mg/d (39) | 29.4 (10.0) | 84 | ||||||||
| Winhusen 2010, p. 1680 [82] (Heffner 2013, Westover 2013, Nunes 2013, Berlin 2012, Covey 2011, Covey 2010, Covey 2011, Mamey 2017) | US | 6 | 11 wk | DSM-IV, ACDS v1.2, ADHD-RS | 18–55 yr smokers with ADHD | Placebo (128) | 37.5 (9.6) | 52.3 | Mixed | |
| MPH-OROS 18–72 mg/d (127) | 38.1 (10.4) | 60.6 | ||||||||
| Adler 2009, p. 239 [35] | US | 27 | 7 wk | DSM-IV, AISRS | 18–65 yr with childhood-onset ADHD | Placebo (116) | 38.2 (11.40) | 55.2 | Pharma | |
| MPH-OROS (113) | 39.9 (12.27) | 57.3 | ||||||||
| Adler 2009, p. 44 [36] (Brown 2011) | US | 21 | 6 mo | DSM-IV-TR, ACDS v1.2 | 18–54 yr with ADHD | Placebo (251) | 37.4 (9.9) | 51.8 | Pharma | |
| ATX 25–100 mg/d (250) | 37.7 (10.4) | 49.6 | ||||||||
| Rosler 2009, p. 120 [69] (Rosler 2010) | Germany | 28 | 24 wk | DSM-IV, ADHD RS-IV | ≥18 yr with ADHD | Placebo (118) | 33.8 (10.6) | 50 | Pharma | |
| MPH-ER 10–60 mg/d (241) | 35.2 (10.1) | 50 | ||||||||
| Adler 2008, p. 720 [34] | US | 22 | 6 mo | DSM-IV-TR | 18–50 yr with childhood-onset ADHD | Placebo (139) | 36.0 (8.4) | 63.3 | Pharma | |
| ATX 40–100 mg/d (271) | 37.1 (8.3) | 56.1 | ||||||||
| Spencer 2008, p. 1437 [73] (Spencer 2008) | US | 39 | 7 wk | DSM-IV-TR, ADHD-RS-IV | 18–55 yr with ADHD | Placebo (137) | 37.0 (10.3) | 49.6 | Pharma | |
| MAS-XR 12.5–75 mg/d (137) | 36.1 (10.1) | 50.4 | ||||||||
| Wilens 2008, p. 145 [80] (Wilens 2011) | US, Canada | 14 | 12 wk | DSM-IV-TR, AISRS | ≥18yr with ADHD | Placebo (75) | 34.8 (9.9) | 85.3 | Pharma | |
| ATX 25–100 mg/d (72) | 34.3 (10.2) | 84.7 | ||||||||
| Levin 2007, p. 20 [58] | US | 2 | 13 wk | DSM-IV | Adults with ADHD seeking outpatient treatment for cocaine use | Placebo (53) | 37 (6) | 83 | Non-pharma | |
| MPH-SR 10–60 mg/d (53) | 37 (7) | 83 | ||||||||
| Biederman 2006, p. 829 [40] | US | NR | 6 wk | DSM-IV | 19–60 yr with ADHD | Placebo (77) | 37.6 (8.4) | 47 | Pharma | |
| MPH-OROS 36 to > 100 mg/d (72) | 32.7 (18.5) | 57 | ||||||||
| Reimherr 2005, p. 245 [67] | US | NR | 6 wk | DSM-IV, Utah Criteria, WRAADDS | Childhood-onset ADHD | Placebo (24) | 34.6 (11.2) | 75.0 | Pharma | |
| BUP-SR 100–400 mg/d (35) | 34.3 (14.8) | 71.4 | ||||||||
| Spencer 2005, p. 456 [71] | US | NR | 6 wk | DSM-IV | 19–60 yr with ADHD | Placebo (42) | 40.3 (10.0) | 54.8 | Mixed | |
| MPH-IR 1.3 mg/kg/d (max) (104) | 35.6 (9.7) | 59.6 | ||||||||
| Wilens 2005, p. 793 [31] | US | 16 | 8 wk | DSM-IV | 18–60 yr with childhood-onset ADHD | Placebo (81) | 41.4 (10.0) | 59 | Pharma | |
| BUP-ER 150–450 mg/d (81) | 39.1 (10.3) | 60 | ||||||||
| Michelson 2003, p. 112 [22] (Faraone 2005) | US | 17 | 10 wk | DSM-IV by CAADID | Adults with at least moderate ADHD symptoms | Placebo (139) | 40.3 (11.6) | 62.6 | Pharma | |
| ATX 60–120 mg/d (141) | 40.2 (11.7) | 64.5 | ||||||||
| Trial 1 | ||||||||||
| Michelson 2003, p. 112 [22] (Faraone 2005) | US | 14 | 10 wk | DSM-IV by CAADID | Adults with at least moderate ADHD symptoms | Placebo (127) | 41.2 (11.2) | 68.5 | Pharma | |
| ATX 60–120 mg/d (129) | 43.0 (10.3) | 64.3 | ||||||||
| Trial 2 | ||||||||||
| Levin 2001, p. 83 [57] | US | 1 | 4 wk | DSM-IV-TR, CAADID, WRAADDS | 19–56 yr with ADHD | Placebo (10) | 35.3 (2.1) | 50 | NR | |
| MPH-SR 20 mg/d (10) | 40.2 (3.6) | 80 | ||||||||
| Wilens 2001, p. 282 [81] | US | 1 | 6 wk | DSM-IV | 20–59 yr with ADHD | Placebo (19) | 39.6 (10.4) | 53 | Mixed | |
| BUP-SR 100–400 mg/d (21) | 37.0 (11.8) | 57 | ||||||||
| Matochik 1994, p. 658 [61] | US | 2 | NR | DSM-III-R, Utah Criteria, CAARS-O:SV | Adults with childhood-onset ADHD | MPH 10–50 mg/d (19) | 35.5 | 68.4 | NR | |
| DEX-IR 10–30 mg/d (18) | 35.6 | 44.4 | ||||||||
| Weisler 2017, p. 685 [77] | US | 43 | 4 wk | DSM-V | 18–55 yr with ADHD and ADHD-RS-AP total score ≥28 | Placebo (91) | 34.5 (10.8) | 47.2 | Pharma | |
| Triple bead MAS 12.5 mg/d (92) | 33.0 (10.4) | 62.0 | ||||||||
| Triple bead MAS 37.5 mg/d (92) | 32.4 (10.0) | 56.7 | ||||||||
| Levin 2015, p. 593 [60] (Levin 2018) | US | 2 | 13 wk | DSM-IV-TR | Adults with co-occurring ADHD and cocaine use disorder | Placebo (43) | 39.3 (7.4) | 88.4 | Non-pharma | |
| MAS-ER 60 mg (40) | 43.9 (7.5) | 82.5 | ||||||||
| MAS-ER 80 mg (43) | 38.4 (8.6) | 81.4 | ||||||||
| Casas 2013, p. 268 [44](Kooij 2013) | Europe | 42 | 12 wk | DSM-IV-TR by CAADID, CAARS-O:SV | 18–65 yr with ADHD | Placebo (97) | 35.5 (8.8) | 53.6 | Pharma | |
| MPH-OROS 54 mg/d (90) | 35.8 (11.7) | 48.9 | ||||||||
| MPH-OROS 72 mg/d (92) | 35.8 (10.1) | 54.3 | ||||||||
| Weisler 2012, p. 421 [78] | US | 37 | 6 wk | DSM-IV-TR by CAADID, CAARS-S:SV | 18–55 yr with ADHD | Placebo (74) | 33.4 (10.34) | 58.9 | Pharma | |
| MPH-OROS 36–54 mg/d (68) | 33.2 (9.73) | 66.2 | ||||||||
| ATX 40–80 mg/d (74) | 34.6 (10.43) | 53.4 | ||||||||
| Levin 2006, p. 137 [59] | US | 5 | 12 wk | DSM-IV | 18–60 yr with ADHD and opiate dependence | Placebo (33) | 39 (8) | 55 | Non-pharma | |
| MPH-SR 20–80 mg/d (32) | 40 (6) | 59 | ||||||||
| BUP-SR 100–400 mg/d (33) | 38 (8) | 66 | ||||||||
| Kuperman 2001, p. 129 [55] | US | 1 | 7 wk | DSM-IV | Childhood-onset ADHD | Placebo (12) | 32.2 (9.8) | 73 | Pharma | |
| MPH-IR 0.9 mg/kg/d (max) (12) | 31.4 (7.3) | 75 | ||||||||
| BUP-SR 300 mg/d (max) (13) | 33.2 (10.8) | 64 | ||||||||
| Frick 2017, p. 1 [47] | US | 48 | 6 wk | DSM-IV-TR | 18–55 yr | Placebo (104) | 35.6 (9.79) | 55.8 | Pharma | |
| Triple bead MAS 25 mg (104) | 38.0 (9.92) | 51.9 | ||||||||
| Triple bead MAS 50 mg (101) | 37.2 (9.51) | 65.3 | ||||||||
| Triple bead MAS 75 mg (102) | 37.9 (9.70) | 53.9 | ||||||||
| Huss 2014, p. 44 [52] | Belgium, Colombia, Denmark, Germany, Norway, Singapore, South Africa, Sweden, US | NR | 9wk | DSM-IV, ADHD-RS | 18–60 yr with childhood-onset ADHD | Placebo (181) | 36.8 (12.15) | 55.8 | Pharma | |
| MPH-LAC 40 mg/d (181) | 35.1 (11.37) | 51.9 | ||||||||
| MPH-LAC 60 mg/d (182) | 34.8 (10.79) | 57.7 | ||||||||
| MPH-LAC 80 mg/d (181) | 34.9 (11.13) | 52.5 | ||||||||
| Adler 2008, p. 1364 [34] (Faraone 2012, Mattingly 2013, Babcock 2012, Kollins 2011, Ginsberg 2011, Adler 2009, Adler 2009) | US | 48 | 4 wk | DSM-IV-TR, ADHD by ADHD-RS-IV-Inv | 18–55 yr with ADHD | Placebo (62) | 35.2 (10.9) | 52 | Pharma | |
| LIS-DEX 30 mg/d (119) | 35.3 (10.1) | 56 | ||||||||
| LIS-DEX 50 mg/d (117) | 34.2 (10.0) | 56 | ||||||||
| LIS-DEX 70 mg/d (122) | 35.8 (10.5) | 52 | ||||||||
| Medori 2008 [63] (Buitelaar 2012, Rosler 2013, Buitelaar 2011) | Europe | 51 | 5 wk | DSM-IV by CAADID | 18–65 yr with childhood-onset ADHD | Placebo (96) | 34.5 (NR) | 61.5 | Pharma | |
| MPH-OROS 18 mg/d (101) | 34.2 (NR) | 57.4 | ||||||||
| MPH-OROS 36 mg/d (102) | 33.8 (NR) | 45.1 | ||||||||
| MPH-OROS 72 mg/d (102) | 33.6 (NR) | 53.9 | ||||||||
| Weisler 2006, p. 625 [76] | US | Multisite | 4 wk | DSM-IV-TR, CAARS-O:SV | ≥ 18yr with ADHD | Placebo (64) | 39.3 (NR) | 68 | Pharma | |
| MAS-XR 20 mg/d (66) | 38.8 (NR) | 64 | ||||||||
| MAS-XR 40 mg/d (64) | 38.9 (NR) | 59 | ||||||||
| MAS-XR 60 mg/d (61) | 39.9 (NR) | 48 | ||||||||
| Spencer 2007, p. 1380 [72] | US | 18 | 5 wk | DSM-IV, ADHD-RS | 18–60 yr with childhood-onset ADHD | Placebo (53) | 38.1 (NR) | 50.9 | Pharma | |
| Total DEX-MPH-ER: 168 | 38.8 (NR) | 59.5 | ||||||||
| DEX-MPH-ER 20 mg (58) | ||||||||||
| DEX-MPH-ER 30 mg (55) | ||||||||||
| DEX-MPH-ER 40 mg (55) | ||||||||||
| Arnold 2014, p. 133 [39] | US | 18 | 9 wk | DSM-IV-TR, ACDS v1.2 | Childhood-onset ADHD | Placebo (74) | 38.6 (11.15) | 53 | Pharma | |
| MODA 255 mg/d (73) | 41.1 (10.52) | 70 | ||||||||
| MODA 340 mg/d (73) | 37.6 (11.49) | 58 | ||||||||
| MODA 425 mg/d (74) | 39.6 (12.05) | 55 | ||||||||
| MODA 510 mg/d (44) | 39.6 (12.64) | 66 | ||||||||
| CROSS-OVER DESIGN | ||||||||||
| Herring 2012, p. E891 [88] | US | 6 | 2 x 4 wk; 1 wk washout | DSM-IV, ACDS v1.2 | 18–55 yr with childhood-onset ADHD | Placebo (58) | 38.4 (11.4) | 64.1 | Pharma | |
| MPH-OROS 36–72 mg/d (45) | ||||||||||
| Total: 103 | ||||||||||
| Wigal 2018, p. 111 [27] | US | 4 | 2 x 1 wk | DSM-IV-TR (with ≥6 of 9 subtype criteria met) established by DSM-IV-TR [SCID] | Adults aged 18–55 yrs with baseline ADHD-RS-IV total score ≥24 and an intelligence quotient ≥80 based on the Kaufman Brief Intelligence Test | Placebo | 29.1 (10.4) | 55.1 | Pharma | |
| Triple bead MAS 25 mg | ||||||||||
| Total: 79 | ||||||||||
| Bron 2014, p. 519 [87] | The Netherlands | 1 | 2 x 2 wk; 1 wk washout | DSM-IV | Drug-naïve adults with childhood-onset ADHD | Placebo | 30.5 (7.4) | 77.3 | Non-pharma | |
| MPH-OROS 36–72 mg/d | ||||||||||
| Total: 22 | ||||||||||
| Martin 2014, p. 147 [91] | US | 1 | 3 x 1 wk | ACDS v1.2, ADHD-RS-IV | Adults with ADHD and previous successful treatment with an amphetamine-based agent | Placebo | 30.8 (10.75) | 61.1 | Pharma | |
| LIS-DEX 50 mg/d | ||||||||||
| Total: 18 | ||||||||||
| Bain 2013, p. 405 [83] | US | 20 | 2 x 4 wk; 2 wk washout | DSM-IV-TR, ACDS v1.2, CAARS:Inv | 18–60 yr with ADHD | Placebo | 36.2 (11.85) | 53 | Pharma | |
| ATX 40–80 mg/d | ||||||||||
| Total: 53 | ||||||||||
| Wender 2011, p. 36 [98] | US | NR | 2 x 2 wk | Utah Criteria, WRAADDS | 21–55 yr with ADHD | Placebo | 36.9 (8.5) | 72.4 | Non-pharma | |
| MPH-IR 30–60 mg/d | ||||||||||
| Total: 116 | ||||||||||
| Wigal 2010, p. 34 [101] (Wigal 2011, Brams 2011) | US | 5 | 2 x 1 wk | DSM-IV-TR, ACDS v1.2 | 18–55 yr with ADHD; | Placebo | 30.5 (10.7) | 62.0 | Pharma | |
| LIS-DEX 30–70 mg/d | ||||||||||
| Total: 127 | ||||||||||
| Kay 2009, p. 316 [21] | US | 1 | 2 x 3 wk | DSM-IV-TR | 19–25 yr with ADHD | MAS-XR 20–50 mg/d | 22.3 (2.1) | 89.5 | Pharma | |
| Placebo | ||||||||||
| Trial 1 | Total: 19 | |||||||||
| Kay 2009, p. 316 [21] | US | 1 | 2 x 3 wk | DSM-IV-TR | 19–25 yr with ADHD | ATX 40–80 mg/d | 22.4 (1.8) | 87.5 | Pharma | |
| Placebo | ||||||||||
| Trial 2 | Total: 16 | |||||||||
| Verster 2008, p. 230 [97] (Verster 2014) | The Netherlands | NR | 2 x 1 d; 6–7 d washout | DSM-IV | Adults with ADHD | Placebo | 38.3 (7.7) | 61.1 | Non-pharma | |
| MPH | ||||||||||
| Total: 18 | ||||||||||
| Barkley 2007, p. 306 [84] | US | 1 | 2 x 4 wk | DSM-IV | 21–65 yr with childhood-onset ADHD | Placebo | 36.1 (12.2) | 44 | Mixed | |
| ATX 0.6 to 1.2 mg/kg/d | ||||||||||
| Total: 22 | ||||||||||
| Jain 2007, p. 268 [89] | Canada | Multisite | 2 x 2-3wk | DSM-IV | Adults with childhood-onset ADHD | Placebo | 37.2 (11.2) | 62.5 | Pharma | |
| MPH-MR-STD | ||||||||||
| Total: 50 | ||||||||||
| Reimherr 2007, p. 93 [92] (Robison 2010) | US | NR | 2 x 4 wk | DSM-IV-TR. Utah Criteria | 18–65 yr adults with ADHD | Placebo | 30.6 (10.8) | 66 | Pharma | |
| MPH-OROS 18–90 mg/d | ||||||||||
| Total: 47 | ||||||||||
| Kooij 2004, p. 973 [90] (Boonstra 2007, Boonstra 2005) | The Netherlands | 1 | 2 x 3 wk; 1 wk washout | DSM-IV | Adults with childhood-onset ADHD | Placebo (20) | 39.1 (20–56) | 53.3 | Non-pharma | |
| MPH-IR, 0.5–1 mg/kg/d (25) | ||||||||||
| Total: 45 | ||||||||||
| Bouffard 2003, p. 546 [86] | Canada | NR | 2 x 4 wk; 1 wk washout | DSM-IV, CAADID | 17–51 yr with childhood onset ADHD | Placebo | 34 (NR) | 80.0 | Non-pharma | |
| MPH-IR 15–45 mg/d | ||||||||||
| Total: 38 | ||||||||||
| Tenenbaum 2002, p. 49 [30] | US | NR | 3 x 3 wk; 2x 1 wk washout | DSM-IV, ADSA | 24–53 yr with ADHD | Placebo | 42 (NR) | 45.8 | Pharma | |
| MPH-IR 10–45 mg/d | ||||||||||
| Total: 33 | ||||||||||
| Spencer 2001, p. 775 [93] | US | NR | 2 x 3 wk; 1 wk washout | DSM-IV | 19–60 yr with childhood-onset ADHD | Placebo | 38.8 (9.27) | 56 | Mixed | |
| MAS-XR 20–60 mg/d | ||||||||||
| Total: 30 | ||||||||||
| Spencer 1998, p. 693 [94] | US | NR | 2 x 3 wk; 1 wk washout | DSM-III-R | 19–60 yr with ADHD | Placebo | 34 (9) | 47.6 | Mixed | |
| ATX 40–80 mg/d | ||||||||||
| Total: 22 | ||||||||||
| Spencer 1995, p. 434 [95] | US | 1 | 2 x 3 wk; 1 wk washout | DSM-III-R | 18–60 yr with childhood-onset ADHD | Placebo | 40 (2.1) | 43 | NR | |
| MPH-IR up to 1 mg/kg/d | ||||||||||
| Total: 25 | ||||||||||
| Wender 1985, p. 547 [99] | US | 1 | 2 x 2 wk; 1 wk washout | Utah criteria | ADD, residual type | Placebo | 31.1 (6.7) | 54.1 | Non-pharma | |
| MPH-IR 10–90 mg/d | ||||||||||
| Total: 37 | ||||||||||
| Barkley 2005, p. 121 [85] | US | 1 | 1 x 3 doses; 2 x 7–14 day washout | DSM-IV | 18–65 yr with childhood onset ADHD | Placebo | 31.3 (11.3) | 74 | Non-pharma | |
| MPH-IR- 10 mg | ||||||||||
| MPH-IR- 20 mg | ||||||||||
| Total: 56 | ||||||||||
| Taylor 2001, p. 223 [29] | US | 1 | 3 x 2 wk; 2x 4 d washout | DSM-IV | Adults with childhood onset of ADHD | Placebo | 41.2 (11.4) | 41 | NR | |
| GUAN-IR 0.25–2 mg/d | ||||||||||
| DEX-IR 2.5–20 mg/d | ||||||||||
| Total: 17 | ||||||||||
| Taylor 2000, p. 311 [96] | US | 1 | 3 x 2 wk; 2x 4 d washout | DSM-IV | ≥21 yr with childhood-onset ADHD | Placebo | 40.8 (12.5) | 59 | NR | |
| MODA 100–400 mg/d | ||||||||||
| DEX-IR 10–40 mg/d | ||||||||||
| Total: 22 | ||||||||||
| Wigal 2018, p. 481 [100] | US | 4 | 3 x 1 wk | DSM-IV-TR | Adults aged 18–55 yrs with baseline ADHD-RS-IV total scores ≥24 and an intelligence quotient ≥80 | Placebo | 33.1(9.0) | 61.6 | Pharma | |
| MAS IR 25 mg | ||||||||||
| Triple bead MAS 50 mg/ Triple bead MAS 75 mg | ||||||||||
| Total: 86 | ||||||||||
ADHD = Attention deficit hyperactivity disorder, ADD = attention deficit disorder, ATX = atomoxetine, BUP = bupropion, DEX = dexamphetamine, HD = high dose, ER = extended release, GUAN = guanfacine, LD = low dose, LIS = lisdexamfetamine, MAS = mixed amphetamine salts, MODA = modafinil, MPH = methylphenidate, NR = not reported, OROS = osmotic-release oral system SD = standard deviation, SR = sustained release, STD = standard dose.
In total, 12,423 participants (between 16 and 725 participants randomized per trial) were included in the 81 RCTs that reported an outcome of interest. All included studies required that participants have a clinical diagnosis of ADHD, which was predominantly based on either the 3rd, 4th, or 5th edition of the Diagnostic Statistical Manual (DSM) for Mental Disorders (96%). Participants were both treatment naïve and experienced; most studies required a washout period if ADHD pharmacotherapy had been used before enrollment, although the duration of washout varied by route of administration (Appendix E in S2 File). The mean age of participants in the RCTs was generally between 30 and 40 years of age, although six RCTs involved participants with a mean age of less than 30 years [21, 23–27] and six involved participants with a mean age of 41 years and older [22, 28–32].The interventions and comparators were varied among the included RCTs, with 98% involving a placebo control. The interventions considered included methylphenidate (36 RCTs), atomoxetine (20 RCTs), mixed amphetamine salts (9 RCTs), bupropion (6 RCTs), dexamfetamine (6 RCTs), lisdexamfetamine (6 RCTs), guanfacine (2 RCTs), and modafinil (2 RCTs). Most RCTs (70%) involved a parallel-group design [22–24, 26, 28, 31–82], while 30% involved a cross-over design [21, 27, 29, 30, 83–101] (Table 1). In total, funding by a pharmaceutical company was reported by 57 trials (49 studies fully funded by pharmaceutical industry sources [60%]; 8 with mixed industry and non-industry sources) [21–25, 27, 30–36, 39–45, 47, 49, 50, 52, 53, 55, 56, 63, 66–84, 88, 89, 91–94, 100, 101]. The treatment period of all included studies ranged from one day to 52 weeks; however, approximately 75% of studies involved treatment for less than 12 weeks. Most were conducted in the United States (72%).
Risk of bias
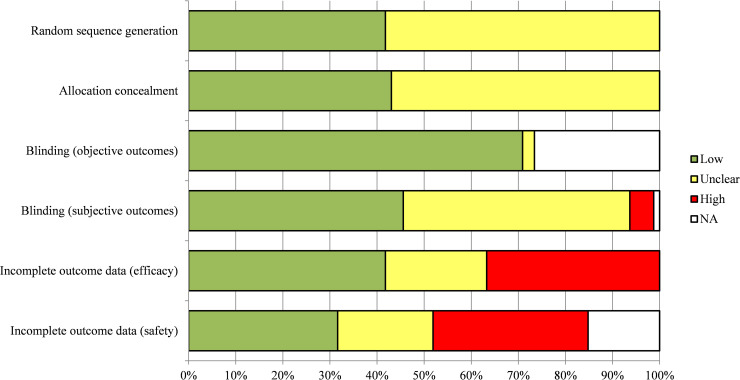
Five RCTs [26, 47–49, 75] were judged to be at low risk of bias across the six domains assessed (Fig 2). Less than half (46%) of the included RCTs were at low ROB for blinding, which is an important consideration for subjective outcomes. Fifty-eight percent of studies were judged to be at unclear or high ROB because of incomplete data pertaining to benefit outcomes (e.g., studies were missing outcome data, authors performed as-treated analyses, or incompletely reported discontinuations across study groups). Similarly, just over half of trials (53%) were judged to be at unclear or high ROB because of incomplete harms data. Overall, most studies (91%) were at high or unclear risk of at least one important risk of bias (allocation concealment, blinding, incomplete data). A detailed summary of all ROB judgements across all domains is located in Appendix F in S2 File.
Fig 2. Risk of bias of included randomized controlled trials.
Publication bias was not detected for outcomes with at least 10 included RCTs (clinical response, executive function, treatment discontinuation, withdrawal due to adverse events, and serious adverse events) based on visual inspections of funnel plots for the presence of a skewed or asymmetric shape (Appendix G in S2 File). Furthermore, we did not identify clear evidence that earlier-published studies were more likely to have published positive results compared to studies published more recently.
Synthesis of results
Both NMAs and MAs were performed for both patient- and clinician-reported clinical response using data reported by studies with a treatment duration of 12 weeks or longer. These analyses were based on clinical response as measured on a continuous scale and as a dichotomous measure (treatment response yes versus no). MAs were performed for quality of life (≥ 12-week data only), executive function (all treatment durations), and all harms outcomes (Table 2). A summary of findings is presented in Table 3.
Table 2. Summary of analyses completed.
| Outcome | Treatment durations analyzed | Type of measure | No. of trials* | No. of participants | Duration (range, wk) |
|---|---|---|---|---|---|
| BENEFITS£ | |||||
| Patient-reported clinical response | ≥12 wk | Continuous | 9 | 1462 | 12–52 |
| Dichotomous | 4 | 322 | 12–24 | ||
| Clinician-reported clinical response | ≥12 wk | Continuous | 15 | 3365 | 12–52 |
| Dichotomous | 8 | 1902 | 12–24 | ||
| Quality of Life† | ≥12 wk | Continuous | 5‡ | 1862 | 12–26 |
| Executive Function† | Any duration** | Continuous | 11 | 3024 | 2–10 |
| HARMS | |||||
| Serious adverse events† | ≥12 wk | Dichotomous | 5 | 1380 | 12–24 |
| Any duration | Dichotomous | 33 | 7161 | 1–24 | |
| Withdrawals due to adverse events† | ≥12 wk | Dichotomous | 16 | 3650 | 12–52 |
| Any duration | Dichotomous | 52 | 10726 | 2–52 | |
| Treatment discontinuation† | ≥12 wk | Dichotomous | 16 | 3568 | 12–26 |
| Any duration | Dichotomous | 51 | 9959 | 3–26 | |
* Data are for trials included in analyses. Two included publications each reported data from two unique trials, thus, the number of trials is greater than the number of included publications.
£ Patient and clinician-reported clinical response were analyzed by both pairwise meta-analysis and Bayesian network meta-analysis; quality of life and executive function were analyzed by pairwise meta-analysis only.
†Analyzed by pair-wise meta-analysis.
‡All studies involved atomoxetine.
**No studies with a treatment duration of at least than 12 weeks assessed executive function.
Table 3. Summary of findings.
| A. PATIENT-REPORTED CLINICAL RESPONSE (Continuous; Conner's ADULT ADHD Rating Scales—Self-report, short version) | ||||||
| Intervention options (9 RCTs; 1462 participants in total) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | ||
| (network estimates) | (studies) | (GRADE) | ||||
| ATX-STD | MD: -5.9 (-12.6, -0.4) | 388 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (4 RCTs) | VERY LOW | |||||
| BUP-SR-STD | MD: -0.8 (-13.5, 11.4) | 33 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence | ||
| (1 RCT) | VERY LOW | |||||
| MPH-OROS-STD | MD: -4.7 (-14.1, 3.7) | 194 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (2 RCTs) | VERY LOW | |||||
| MPH-OROS-HD | MD: -6.3 (-19.2, 6.4) | 27 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (1 RCT) | VERY LOW | |||||
| MPH-SR-STD | MD: -4.8 (-16.5, 7.1) | 110 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (1 RCT) | VERY LOW | |||||
| MPH-SR-HD | MD: 3.3 (-9.3, 15.6) | 32 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (1 RCT) | VERY LOW | |||||
| PLACEBO | Reference comparator | NA | NA | Network reference comparator | ||
| B. PATIENT-REPORTED CLINICAL RESPONSE (Dichotomous) | ||||||
| Intervention options (4 RCTs; 322 participants in total) | Anticipated absolute effects* (95% CI) | Relative effect; (95% CI) | № of participants | Certainty of the evidence | Comments | |
| Without intervention† | Risk with ADHD pharmacotherapies | (network estimates) | (studies) | (GRADE) | ||
| ATX-STD | 347 per 1,000 | 1000 per 1,000 | RR 3.34 | 27 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, heterogeneity, incoherence |
| (652 to 1,000) | (1.88, 5.85) | (1 RCT) | VERY LOW | |||
| BUP-SR-STD | 347 per 1,000 | 378 per 1,000 | RR 1.09 | 33 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (139 to 811) | (0.40,2.34) | (1 RCT) | VERY LOW | |||
| MPH-OROS-HD | 347 per 1,000 | 832 per 1,000 | RR 2.40 | 27 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, heterogeneity, incoherence |
| (361 to 1,000) | (1.10,4.06) | (1 RCT) | VERY LOW | |||
| MPH-SR-STD | 347 per 1,000 | 277 per 1,000 | RR 0.80 | 53 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (104 to 600) | (0.30,1.73) | (1 RCT) | VERY LOW | |||
| MPH-SR-HD | 347 per 1,000 | 239 per 1,000 | RR 0.69 | 32 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (80 to 600) | (0.23,1.73) | (1 RCT) | VERY LOW | |||
| PLACEBO | Reference comparator | NA | NA | NA | NA | Network reference comparator |
| C. CLINICIAN-REPORTED CLINICAL RESPONSE (Continuous) | ||||||
| Intervention options (15 RCTs; 3365 participants in total) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | ||
| (network estimates) | (studies) | (GRADE) | ||||
| MAS-XR-HD | MD: -4.2 (-12.1, 3.5) | 83 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (1 RCT) | VERY LOW | |||||
| ATX-STD | MD: -3.7 (-6.7, -0.9) | 1100 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (7 RCTs) | VERY LOW | |||||
| GUAN-STD | MD: -0.6 (-9.4, 8.3) | 13 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence | ||
| (1 RCTs) | VERY LOW | |||||
| MPH-OROS-STD | MD: -1.4 (-7.0, 4.4) | 194 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (2 RCTs) | VERY LOW | |||||
| MPH-ER-STD | MD: -3.9 (-11.5, 3.7) | 241 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (1 RCT) | VERY LOW | |||||
| MPH-SR-STD | MD: -5.7 (-11.2, -0.3) | 163 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (2 RCTs) | VERY LOW | |||||
| MPH-LD | MD: -10.4 (-19.0, -2.1) | 25 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence | ||
| (1 RCTs) | VERY LOW | |||||
| PLACEBO | Reference comparator | NA | NA | Network reference comparator | ||
| D. CLINICIAN-REPORTED CLINICAL RESPONSE (Dichotomous) | ||||||
| Intervention options (8 RCTs; 1902 participants in total) | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
| Without intervention† | Risk with ADHD pharmacotherapies | (network estimates) | (studies) | (GRADE) | ||
| ATX-STD | 298 per 1,000 | 594 per 1,000 | RR 1.99 | 1336 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, incoherence |
| (286 to 1,000) | (0.96 to 4.14) | (3 RCTs) | VERY LOW | |||
| BUP-SR-STD | 298 per 1,000 | 215 per 1,000 | RR 0.72 | 854 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (18 to 976) | (0.06 to 3.27) | (1 RCT) | VERY LOW | |||
| MPH-OROS-STD | 298 per 1,000 | 451 per 1,000 | RR 1.51 | 1003 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (54 to 1,000) | (0.18 to 3.95) | (1 RCT) | VERY LOW | |||
| MPH-ER-STD | 298 per 1,000 | 471 per 1,000 | RR 1.58 | 1062 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (125 to 889) | (0.42 to 2.98) | (1 RCT) | VERY LOW | |||
| MPH-SR-STD | 298 per 1,000 | 340 per 1,000 | RR 1.14 | 874 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (33 to 1,000) | (0.11 to 3.78) | (1 RCT) | VERY LOW | |||
| MPH-SR-HD | 298 per 1,000 | 122 per 1,000 | RR 0.41 | 853 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, imprecision, incoherence |
| (9 to 806) | (0.03 to 2.70) | (1 RCT) | VERY LOW | |||
| MPH-LD | 298 per 1,000 | 1000 per 1,000 | RR 3.57 | 846 | ⨁◯◯◯ | Downgraded because of within-study bias, indirectness, heterogeneity, incoherence |
| (337 to 1,000) | (1.13 to 5.44) | (1 RCT) | VERY LOW | |||
| PLACEBO | Reference comparator | NA | NA | NA | NA | Network reference comparator |
*Full GRADE assessment for all outcome is available in Appendix H in S2 File.
Benefits
Patient-reported clinical response. Among RCTs with a treatment duration of at least 12 weeks, nine trials (n = 1462) [28, 36, 44, 54, 59, 62, 66, 70, 80] assessed patient-reported clinical response by use of a continuous scale, and four trials (n = 322) [28, 58, 59, 70] assessed the outcome as a dichotomous measure (treatment response yes versus no) (Table 2). Only one [59] of the four trials contributing data to the analysis of patient-reported clinical response as a dichotomous measure was judged to be of low ROB for blinding compared to three of nine (33%) trials [44, 59, 62] contributing data to the analysis of continuous data for this outcome. None of the studies that reported this outcome were at low risk of bias across all domains. Additional information is contained within Appendix I in S2 File.
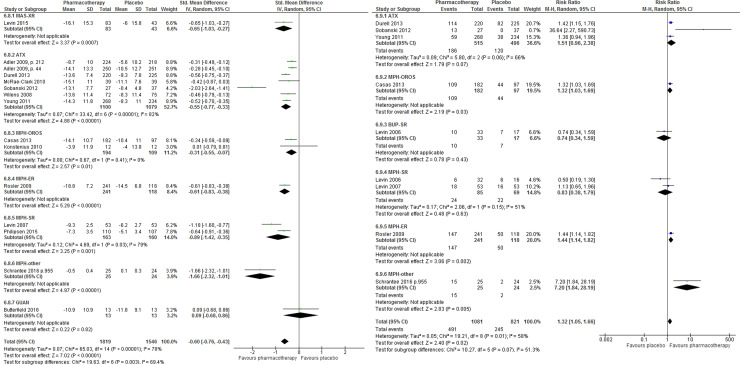
The results of pairwise MA showed that, compared with placebo, treatment with any ADHD pharmacotherapy was associated with a small but statistically significant improvement in patient-reported clinical response (MD –4.34, 95% CI –6.34 to –2.23) (Fig 3A); with no statistically significant difference in the number of clinical responders (RR 1.38, 95% CI 0.73 to 2.59; Fig 3B). Moderate to high heterogeneity was noted for both continuous and dichotomous measures of clinical response (I2 = 52% and 76%, respectively).
Fig 3. Patient-reported clinical response.
Clinical response among patients who received any ADHD pharmacotherapy (versus placebo) in trials with a treatment duration of at least 12 weeks. (A) Continuous measure of response. (B) Dichotomous (treatment response: yes versus no).
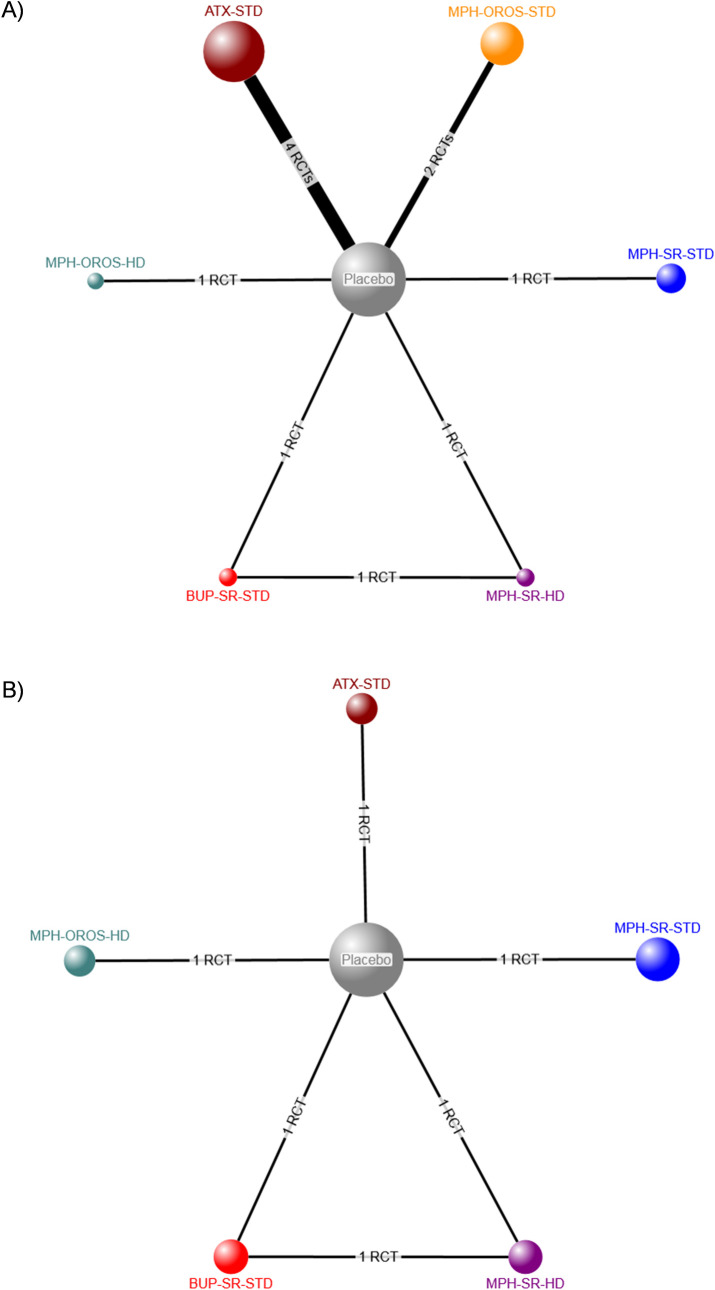
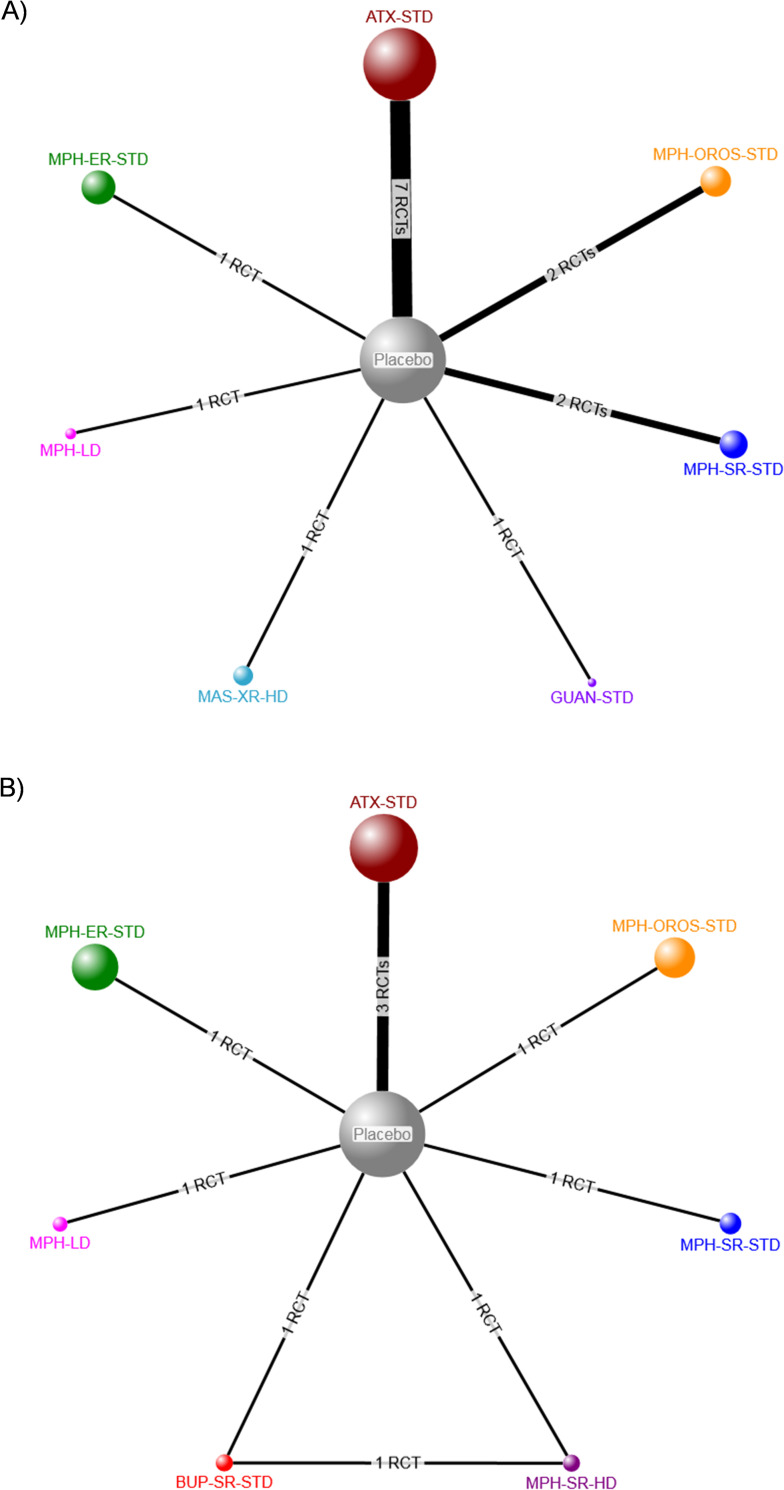
The evidence network for patient-reported clinical response as measured by a continuous scale included nine RCTs (eight two-arm, one three-arm) [28, 36, 44, 54, 59, 62, 70, 80, 102] and 1462 participants randomized to six pharmacotherapies, placebo, or no treatment (Fig 4A), while the network for clinical response assessed as a dichotomous variable included four RCTs (three two-arm, one three-arm) [28, 58, 59, 70] and involved 322 participants (Fig 4B). Consistency could not be formally evaluated for either NMA because of a lack of closed loops informed by more than one RCT [19].
Fig 4. Network diagrams for patient-reported clinical response.
Treatment nodes are proportional to the number of patients who took the corresponding treatment, while the width of each edge is proportional to the number of trials included in the comparison. Intervention abbreviations: ATX = atomoxetine, BUP-SR = sustained release bupropion, HD = high dose, MPH = methylphenidate, OROS = osmotic-release oral system, STD = standard dose, SMD = standardized mean difference, SR = sustained release. (A) Continuous measure of response. (B) Dichotomous (treatment response: yes versus no).
When clinical response to individual ADHD pharmacotherapies was assessed as a continuous measure, only standard-dose atomoxetine resulted in moderate significant improvements versus placebo (MD –5.9, 95% CrI –12.6 to –0.4). There were no significant differences in patient-reported continuous response between any of the ADHD pharmacotherapies (Table 4A). When assessed as a dichotomous outcome, clinical response with standard-dose atomoxetine and high-dose osmotic-release oral-system methylphenidate were significantly better than placebo at improving the number of patients who reported a clinical response (RR 3.34, 95% CrI 1.88 to 5.85 and RR 2.40, 95% CrI 1.10 to 4.06, respectively).
Table 4. Patient-reported clinical response in trials with a treatment duration of at least 12 weeks–indirect comparison of ADHD pharmacotherapies.
| A) CONTINUOUS measure of response | |||||||
| Mean difference (95% credible interval)* | |||||||
| Placebo | ATX-STD | BUP-SR-STD | MPH-OROS-STD | MPH-OROS-HD | MPH-SR-STD | MPH-SR- HD | |
| Placebo | — | ||||||
| ATX-STD | -5.9 | — | |||||
| (-12.6, -0.4) | |||||||
| BUP-SR-STD | -0.8 | 5.2 | — | ||||
| (-13.5, 11.4) | (-8.3, 19.3) | ||||||
| MPH-OROS-STD | -4.7 | 1.2 | -3.9 | — | |||
| (-14.1, 3.7) | (-9.6, 11.9) | (-19.4, 11.3) | |||||
| MPH-OROS-HD | -6.3 | -0.4 | -5.6 | -1.6 | — | ||
| (-19.2, 6.4) | (-14.0, 14.3) | (-23.5, 12.4) | (-17.0, 14.1) | ||||
| MPH-SR-STD | -4.8 | 1.2 | -4 | 0 | 1.6 | — | |
| (-16.5, 7.1) | (-11.5, 15.1) | (-20.7, 13.5) | (-14.4, 15.3) | (-15.4, 19.3) | |||
| MPH-SR-HD | 3.3 | 9.3 | 4.1 | 8.1 | 9.7 | 8.1 | — |
| (-9.3, 15.6) | (-4.0, 23.6) | (-8.1, 16.6) | (-6.6, 23.7) | (-8.4, 27.5) | (-9.2, 25.1) | ||
| Note: ATX = atomoxetine, BUP-SR = sustained release bupropion, HD = high dose, MPH = methylphenidate, OROS = osmotic-release oral system, STD = standard dose, SR = sustained release. | |||||||
| *Random-effects model. A negative value indicates improvement in clinical response. Pooled mean differences expressed on the Conners’ ADULT ADHD Rating Scale-Self (CAARS-S), short form. Statistically significant changes are indicated by use of bold and colour (green indicates that the row treatment is significantly better than the column treatment). White indicates no significant difference between treatments. | |||||||
| B) DICHOTOMOUS (treatment response yes versus no) | |||||||
| Relative risk (95% credible interval)* | |||||||
| Placebo | ATX-STD | BUP-SR-STD | MPH-OROS-HD | MPH-SR-STD | MPH-SR-HD | ||
| Placebo | — | ||||||
| ATX-STD | 3.34 | — | |||||
| (1.88, 5.85) | |||||||
| BUP-SR-STD | 1.09 | 0.33 | — | ||||
| (0.40,2.34) | (0.11,0.78) | ||||||
| MPH-OROS-HD | 2.4 | 0.73 | 2.21 | — | |||
| (1.10,4.06) | (0.32,1.26) | (0.77,6.41) | |||||
| MPH-SR-STD | 0.8 | 0.24 | 0.74 | 0.34 | — | ||
| (0.30,1.73) | (0.08,0.60) | (0.22,2.59) | (0.11,0.98) | ||||
| MPH-SR-HD | 0.69 | 0.21 | 0.65 | 0.29 | 0.87 | — | |
| (0.23,1.73) | (0.06,0.59) | (0.23,1.73) | (0.09,0.94) | (0.22,3.33) | |||
Note: ATX = atomoxetine, BUP-SR = sustained release bupropion, HD = high dose, MPH = methylphenidate, OROS = osmotic-release oral system, STD = standard dose, SR = sustained release.
*Random-effects model. A relative risk greater than one (green) indicates that a statistically significantly higher proportion of participants in the row treatment achieved treatment response relative to the column treatment (e.g., significantly more participants who received ATX-STD achieved treatment response compared with placebo). A relative risk lower than one (red) indicates that a statistically significantly higher proportion of participants in the column treatment achieved treatment response relative to the row treatment (e.g., significantly more participants who received ATX-STD achieved treatment response compared with BUP-SR-STD). White indicates no statistically significant difference between treatments.
Among the other treatments, use of standard-dose sustained-release bupropion resulted in significantly fewer responders compared with standard-dose atomoxetine (RR 0.33, 95% CrI 0.11, 0.78). High- and standard-dose sustained-release methylphenidate also resulted in significantly fewer responders compared to standard-dose atomoxetine (RR 0.21 and 0.24, respectively). High-dose osmotic-release oral system methylphenidate was significantly better than both standard- and high-dose sustained-release methylphenidate at improving the number of clinical responders (Table 4B).
Clinician-reported clinical response. Among RCTs with a treatment duration of at least 12 weeks, clinical response was assessed by a clinician as a continuous measure in 15 RCTs (n = 3365) [25, 26, 32, 35, 36, 43, 44, 54, 58, 60, 62, 66, 69, 70, 80] and as a dichotomous measure in eight RCTs (n = 1902) [25, 26, 32, 44, 58, 59, 69, 70] (Table 2). Only half [25, 26, 44, 59] of the trials contributing data to the analysis of clinician- reported clinical response as a dichotomous measure were judged to be at low ROB for blinding compared to 40% of studies contributing data to the analysis of continuous data for this outcome. Further, only one study [26] was at low risk of bias across all ROB domains, reporting a significant improvement in clinician-reported clinical response among men (n = 25) who received 16 weeks of methylphenidate treatment but not among those who received placebo (n = 24).
The results of pairwise MA showed that, compared with placebo, the number of patients who showed a clinical response was significantly higher among those using any ADHD pharmacotherapy (RR 1.32, 95% CI 1.05 to 1.66, I2 = 58%). Further, the use of any ADHD pharmacotherapy revealed a significant moderate improvement in clinical response compared to placebo on the CAARS-O-SV scale (MD –3.89, 95%CI –4.49 to –2.76), although the I2 value associated with this analysis was 78%, which indicates a considerable amount of heterogeneity between studies (Fig 5).
Fig 5. Clinician-reported clinical response.
Clinical response among patients who received any ADHD pharmacotherapy (versus placebo) in trials with a treatment duration of at least 12 weeks. (A) Continuous measure of response. (B) Dichotomous (treatment response: yes versus no).
The evidence network for the effect of individual ADHD pharmacotherapies on clinician-reported clinical response (continuous) included 15 RCTs (15 two-arm trials) [25, 26, 32, 35, 36, 43, 44, 54, 58, 60, 62, 66, 69, 70, 80] involving 3365 participants who were randomized to seven pharmacotherapies, placebo, or no treatment (Fig 6A). The network for the number of responders (dichotomous) included 1902 participants randomized to seven pharmacotherapies, placebo, or no treatment in eight RCTs (seven 2-arm, one 3-arm) (Fig 6B) [25, 32, 44, 58, 59, 69, 70]. Consistency could not be formally evaluated for any NMA conducted because of a lack of closed loops informed by more than one RCT [19]. Additional information is contained within Appendix I in S2 File.
Fig 6. Network diagrams for patient-reported clinical response.
Treatment nodes are proportional to the number of patients who took the corresponding treatment, while the width of each edge is proportional to the number of trials included in the comparison. Intervention abbreviations: ATX = atomoxetine, GUAN = guanfacine, HD = high dose, MAS-XR = mixed amphetamine salts, MPH = methylphenidate, OROS = osmotic-release oral system, ER = extended release, SR = sustained release, STD = standard dose. (A) Continuous measure of response. (B) Dichotomous (treatment response: yes versus no).
Relative to placebo, we found that low-dose methylphenidate was significantly better at improving clinical response, as measured by clinicians, by both continuous and dichotomous measures (Table 5). Standard-dose atomoxetine, standard-dose sustained-release methylphenidate, and low-dose methylphenidate were significantly better than placebo at improving clinical response by continuous assessment, with no significant difference in the number of responders. Compared with patients on placebo and those on high-dose sustained release methylphenidate, the number of clinical responders was significantly higher in the low-dose methylphenidate group. Caution is urged when interpreting these results, however, given that all estimates for low-dose methylphenidate were informed by a single study [26] and the wide credible interval associated with the effect estimate for this treatment compared to its high-dose sustained-release counterpart (RR 8.43, 95% Crl 1.04 to 118.40) reflects a high level of uncertainty. No other significant differences relative to placebo or among the pharmacotherapies were detected.
Table 5. Clinician-reported clinical response in trials with a treatment duration of at least 12 weeks–indirect comparison of ADHD pharmacotherapies.
| A) CONTINUOUS measure of response | |||||||
| Mean difference (95% credible interval)* | |||||||
| Placebo | MAS-XR-HD | ATX-STD | GUAN-STD | MPH-OROS-STD | MPH-ER-STD | MPH-SR-STD | |
| Placebo | — | ||||||
| MAS-XR-HD | -4.2 | — | |||||
| (-12.1, 3.5) | |||||||
| ATX-STD | -3.7 | 0.6 | — | ||||
| (-6.7, -0.9) | (-7.8, 8.8) | ||||||
| GUAN-STD | -0.6 | 3.6 | 3.1 | — | |||
| (-9.4, 8.3) | (-8.2, 15.5) | (-6.1, 12.6) | |||||
| MPH-OROS-STD | -1.4 | 2.8 | 2.3 | -0.8 | — | ||
| (-7.0, 4.4) | (-6.7, 12.6) | (-3.9, 8.9) | (-11.4, 9.8) | ||||
| MPH-ER-STD | -3.9 | 0.3 | -0.2 | -3.3 | -2.5 | — | |
| (-11.5, 3.7) | (-10.6, 11.3) | (-8.3, 8.0) | (-15.1, 8.4) | (-12.2, 6.8) | |||
| MPH-SR-STD | -5.7 | -1.5 | -2 | -5.1 | -4.3 | -1.8 | — |
| (-11.2, -0.3) | (-11.1, 8.0) | (-8.1, 4.2) | (-15.5, 5.1) | (-12.4, 3.5) | (-11.1, 7.5) | ||
| MPH-LD | -10.4 | -6.2 | -6.8 | -9.9 | -9.1 | -6.5 | -4.7 |
| (-19.0, -2.1) | (-17.6, 5.2) | (-15.7, 2.2) | (-22.2, 2.4) | (-19.5, 0.9) | (-17.8, 4.8) | (-14.8, 5.3) | |
| Note: ATX = atomoxetine, GUAN = guanfacine, HD = high dose, LD = low dose, MAS-XR =mixed amphetamine salts, MPH = methylphenidate, OROS = osmotic-release oral system, ER = extended release, SR = sustained release, STD = standard dose. | |||||||
| *Random-effects model. Pooled mean differences expressed on the Conners’ ADULT ADHD Rating Scale-Self (CAARS-S), short form. A negative value indicates improvement in clinical response. Statistically significant changes are indicated by use of bold and colour (green indicates that the row treatment is significantly better than the column treatment). White indicates no significant difference between treatments. | |||||||
| B) DICHOTOMOUS (treatment response yes versus no) | |||||||
| Relative risk (95% credible interval)* | |||||||
| Placebo | ATX-STD | BUP-SR-STD | MPH-OROS-STD | MPH-ER-STD | MPH-SR-STD | MPH-SR- HD | |
| Placebo | — | ||||||
| ATX-STD | 1.99 | — | |||||
| (0.96, 4.14) | |||||||
| BUP-SR-STD | 0.72 | 0.37 | — | ||||
| (0.06, 3.27) | (0.02, 1.66) | ||||||
| MPH-OROS-STD | 1.51 | 0.78 | 2.01 | — | |||
| (0.18, 3.95) | (0.07, 2.32) | (0.16, 28.71) | |||||
| MPH-ER-STD | 1.58 | 0.87 | 2.21 | 1.1 | — | ||
| (0.42, 2.98) | (0.09, 2.42) | (0.20, 30.14) | (0.13, 10.74) | ||||
| MPH-SR-STD | 1.14 | 0.59 | 1.55 | 0.77 | 0.7 | — | |
| (0.11, 3.78) | (0.05, 2.12) | (0.11, 24.80) | (0.07, 8.49) | (0.06, 6.97) | |||
| MPH-SR-HD | 0.41 | 0.21 | 0.58 | 0.28 | 0.26 | 0.37 | — |
| (0.03, 2.70 | (0.01, 1.30) | (0.06, 5.20) | (0.02, 4.13) | (0.02, 3.69) | (0.02, 6.61) | ||
| MPH-LD | 3.57 | 1.73 | 4.68 | 2.26 | 2.04 | 2.98 | 8.43 |
| (1.13, 5.44) | (0.45, 3.85) | (0.84, 59.28) | (0.58, 20.17) | (0.54, 15.85) | (0.67, 30.78) | (1.04,118.40) | |
Note: ATX = atomoxetine, ER = extended release, HD = high dose, LD = low-dose, MPH = methylphenidate, OROS = osmotic-release oral system, SR = sustained release, STD = standard dose.
*Random-effects model. A relative risk greater than one (green) indicates that a statistically significantly higher proportion of participants in the row treatment achieved treatment response relative to the column treatment. A relative risk lower than one (red) indicates that a statistically significantly higher proportion of participants in the column treatment achieved treatment response relative to the row treatment. White indicates no statistically significant difference between treatments.
Executive function. No studies with a treatment duration of 12 weeks or longer assessed executive function. Data from 12 RCTs (n = 3024) that assessed executive function on a continuous scale after treatment for 2–10 weeks (Table 2) [27, 30, 33, 48–50, 52, 63, 67, 73, 74, 100] were analyzed by pair-wise MA. Just over half (58%) of these trials [30, 33, 48–50, 52, 100] were judged to be at low ROB for blinding. Compared with placebo, we found that the use of any ADHD pharmacotherapy was associated with a moderate statistically significant improvement in executive function (MD on BRIEF-A –5.72, 95% CI –7.15 to –4.29; I2 = 58%) (Appendix J in S2 File).
Quality of life. In total, quality of life was assessed in five RCTs with a treatment duration of 12 weeks or longer [25, 34–36, 70]. Of these, all involved the use of standard-dose atomoxetine compared with placebo or no treatment (n = 1862) (Table 2). Only one trial [25] was judged to be at low ROB for blinding; two others [34, 70] were judged to be at high ROB, and the remaining two [35, 36] were judged as unclear. Pair-wise MA showed that atomoxetine was associated with a small favourable improvement response when compared to placebo (MD on AAQoL scale 4.21, 95% CI 2.04 to 6.38) (Appendix K in S2 File).
Driving behaviour. Five studies evaluated driving behavior among participants with ADHD [23, 25, 34, 70, 84] (Appendix L in S2 File). Of these, three studies reported no significant difference in self-assessed driving behaviour following treatment with atomoxetine [25, 34, 70]. One study reported no self-assessed difference in driving anger following treatment with atomoxetine [84]. Two studies reported improved self-reported driving following treatment (lisdexamfetamine, atomoxetine) [23, 84].
Two studies reported no change in clinician-reported driving behaviour following treatment with atomoxetine [25, 84], while one study reported improved driving following treatment with atomoxetine [34]. Of note, the study that reported an improvement had a longer treatment duration (six months) compared to the studies that reported no difference (four or 12 weeks).
Harms
Network meta-analyses for harms were not robust owing to the large number of zero-event counts in the networks for each of these outcomes. As such, only pair-wise MAs were performed for serious adverse events, withdrawals due to adverse events, and treatment discontinuations. Hospitalizations and cardiovascular adverse events are summarized narratively. No studies reported on emergency room visits during the study period.
Serious adverse events. In total 33 studies reported serious adverse events; of these, five involved a treatment duration of at least 12 weeks. Among RCTs involving treatment for at least 12 weeks, three [32, 35, 44] reported the occurrence of serious adverse events during the treatment period, while two additional RCTs [26, 80] reported that no serious adverse events had occurred during the study (Table 2). Compared with placebo, there was no significant difference in the risk of a serious adverse event with use of an ADHD pharmacotherapy (RR 1.46, 95% CI 0.52 to 4.09; I2 = 0%) (Appendix M in S2 File). A total of 16 RCTs with any treatment duration reported the occurrence of serious adverse events [32, 34, 35, 44, 47, 49, 50, 52, 56, 63, 68, 72, 73, 75, 78, 82], while an additional 17 RCTs reported that no serious adverse events had occurred during the study [21, 26, 31, 33, 40, 41, 48, 53, 76–78, 80, 81, 88, 93, 101, 103]. Compared with placebo, there was no significant difference in the risk of a serious adverse event with use of an ADHD pharmacotherapy (RR 1.21, 95% CI 0.67 to 2.18; I2 = 0%).
Withdrawals due to adverse events. Seventeen RCTs with a treatment duration of at least 12 weeks reported withdrawals due to adverse events (n = 3650) [25, 26, 28, 32, 34–36, 44, 58–60, 62, 69, 70, 79, 80, 102]. Compared with placebo, there was a significant increase in withdrawals due to adverse events among participants who received an ADHD medication, with moderate heterogeneity between trials (RR 2.30, 95% CI 1.62 to 3.25; I2 = 37%) (Appendix N in S2 File). Among the 52 RCTs (n = 10,726) of any treatment duration [21–23, 25, 26, 28, 31–36, 39–41, 44, 47–50, 52, 53, 55, 56, 58, 59, 62, 63, 65, 67–73, 75, 76, 78–82, 89, 94, 95, 102] reporting on withdrawals, use of an ADHD pharmacotherapy was associated with a higher risk of withdrawal due to an adverse event (RR 2.54, 95% CI 2.14 to 3.03; I2 = 0%) compared with placebo.
Treatment discontinuation. Sixteen RCTs with a treatment duration of at least 12 weeks reported treatment discontinuations (n = 3568) [25, 26, 28, 32, 34–36, 44, 58–60, 62, 69, 70, 79, 80]. Compared with placebo, there was no significant difference in the number of discontinuations between participants who received an ADHD pharmacotherapy and those who received placebo with moderate heterogeneity between trials (RR 1.04, 95% CI 0.91 to 1.20; I2 = 63%) (Appendix O in S2 File). The effect estimate was similar among 52 RCTs (n = 9959) with any treatment duration (RR 1.10, 95% CI 1.00 to 1.21; I2 = 46%).
Hospitalization. Three studies reported hospitalizations during the study period [60, 72, 73]. In their 2007 study, Spencer et al. [72] reported that two patients randomized to treatment with extended-release methylphenidate each experienced a serious adverse event that required hospitalization (ulcerative colitis/hypovolemic shock, fever/loss of consciousness). Neither patient was withdrawn from the study. In their 2008 study [73], Spencer et al. also reported that one patient assigned to treatment with mixed amphetamine salt group was admitted to hospital with a possible transient ischemic attack. The patient was discharged the following day, with a diagnosis of Tourette syndrome with vocal tic. The investigator disagreed with this diagnosis and felt that transient ischemic attack could not be ruled out.
Levin and colleagues [60] reported that two participants, both in the placebo group, experienced serious adverse events requiring admission to hospital (sexual assault, pneumothorax). Neither of these events was considered by the investigators to be study-related.
Cardiovascular events. No studies reported myocardial infarction during the study period. One study [73] reported that one patient experienced a possible transient ischemic attack during the study period, as previously described. No studies reported on cardiovascular death during the treatment period.
Sensitivity analyses
After removing studies judged to be at unclear or high ROB for blinding for subjective outcomes, some important differences were noted. When only trials at low ROB for blinding were included in the pair-wise meta-analyses, there was no longer a significant difference between ADHD pharmacotherapy and placebo for clinical response (clinician- or patient-reported) (Appendix P in S2 File). When the effects of individual ADHD pharmacotherapies were analyzed by network meta-analysis, the beneficial effect of atomoxetine on clinical response (clinician-reported, continuous scale) was no longer evident (Appendix P in S2 File). Similarly, when analyzed as a dichotomous variable (responder yes v. no), the beneficial of effect of atomoxetine was no longer evident. However, in this analysis, a low-dose MPH showed a significant benefit relative to placebo, atomoxetine, bupropion, osmotic-release oral system methylphenidate, and sustained release methylphenidate. This finding was based on a limited number of trials (k = 4) and requires additional investigation.
We further examined the influence of heterogeneity on the effect estimates, for two pair-wise MAs that had considerable heterogeneity: patient-reported clinical response (dichotomous) and clinician-reported clinical response (continuous). We identified a single study [70] that was methodologically different than the others in that it randomized participants to 12 weeks of standard-dose atomoxetine or no treatment; all other trials included participants randomized to an ADHD pharmacotherapy or placebo. After removing this study from the MA of patient-reported clinical response (dichotomous scale), while the I2 value was reduced (76% to 59%), there was no substantial difference in the overall effect estimate (Appendix P in S2 File). Similarly, while the I2 value for clinician-reported clinical response (continuous measure) was reduced from 78% to 68% after removing this study, there was no substantial difference in the overall effect estimate.
Discussion
In this study, we performed a comprehensive systematic review of RCTs that assessed the benefits and harms of pharmacotherapies for ADHD in adults. We found that, as a class, ADHD pharmacotherapies were more effective than placebo at improving patient- and clinician-reported clinical response, quality of life, and executive function; however, the clinical importance of these changes is unclear, and when the analyses were restricted to studies at low risk of bias due to blinding, there was no significant difference between ADHD pharmacotherapy and placebo in the meta-analysis, and few differences were evident in the network meta-analyses. Furthermore, when assessed by the GRADE approach, the certainty of the findings for all outcomes was very low to low, indicating that we are uncertain whether there are true differences between ADHD pharmacotherapies owing to the low quality of the available evidence and the high level of uncertainty.
When all studies were considered, atomoxetine was associated with better clinical response compared to most other pharmacotherapies; however, we note that the beneficial effect of atomoxetine was not conserved when only studies at low-risk of bias for blinding were considered. Additionally, participants who received atomoxetine were more likely to discontinue treatment in both shorter- (up to 12 weeks) and longer-term studies. This is consistent with the findings of a previous review which reported increased discontinuation with atomoxetine treatment in shorter-term studies [104]. Similarly, Bushe et al. [104] also noted increased participant discontinuation after short-term treatment with osmotic-release methylphenidate compared with those who received placebo. Our finding of no significant difference in the risk of treatment discontinuation among participants who received this medication for at least 12 weeks may suggest that patients discontinue osmotic-release oral-system methylphenidate at an earlier time point.
Most of the RCTs included in this review included participants who were not naïve to ADHD treatment (Appendix E in S2 File). Although this is not surprising, it may complicate the generalization of the study findings to a more broad patient population and may underestimate harms. Similarly, the use of a “washout” period for participants with prior treatment experience complicates the interpretation of individual study findings: effectively, a potentially beneficial treatment may be removed during the washout period and reinstated after randomization, leading to “breaking the blind” and/or overestimation of treatment effects. Despite this, few of the included RCTs assessed whether the blind had been broken during the study period or assessed the impact on subjective outcomes. This is an important consideration for treatments such as methylphenidate, which are associated with detectable effects that may render it difficult to fully blind such studies. Because most trials did not involve use of an active treatment, participants may have been aware of group assignment despite a “double blind” design, which could have introduced bias that was not detected as part of our risk of bias assessment. This inherent problem of maintaining the blind in ADHD trials has been highlighted for studies involving children and adults [105, 106], and contributed to the retraction of a 2014 Cochrane review of methylphenidate for ADHD in adults [107].
We identified a recently published systematic review and NMA by Cortese et al. [108] on a similar topic; however, several methodological differences between studies make direct comparisons with our study difficult. For example, in our NMA, we considered individual pharmacotherapies, formulations, and dose classifications separately, while Cortese et al. [108] grouped together all ADHD pharmacotherapies, regardless of formulation or dose (although some restrictions were applied). Furthermore, whenever possible, we restricted our analyses of benefits to data from studies with a treatment duration of 12 weeks or longer, whereas Cortese et al.’s [108] primary endpoint included outcome data available at times that were “closest to 12 weeks.” In addition, while we completed sensitivity analyses to examine the impact of studies that were judged to be of unclear or high ROB, we included all RCTs regardless as to whether they were open label, single-, or double-blind. In contrast, Cortese et al. [108] limited inclusion to RCTs that were double-blind, which is similar to the approach we adopted in sensitivity analyses. Despite these methodological differences, our finding that atomoxetine was associated with a moderate improvement in clinician-reported clinical response compared with placebo was consistent with the findings of Cortese et al. [108] Similarly, we also found that sustained-release methylphenidate was significantly better than placebo at improving clinical response, which is consistent with Cortese’s [108] findings. However, the effect sizes for each outcome were in the small to moderate range and the clinical importance of such differences is unclear [8, 109].
Strengths and limitations
The strengths of this review include the use of established systematic review methodology [8] and a priori registration of the review protocol. By using NMA methodology, we were able to compare the relative effects of individual ADHD pharmacotherapies, some of which have not been compared in head-to-head trials. This review was undertaken to inform policy recommendations for coverage of ADHD pharmacotherapies on Canadian provincial formularies; however, because we included a broad range of ADHD pharmacotherapies, the results of our review may be of interest to decision-makers in other jurisdictions.
Several limitations should be considered. First, the treatment duration employed in the included RCTs was variable. To minimize heterogeneity due to treatment duration, we restricted the NMAs to studies with a minimum duration of 12 weeks. This approach is consistent with Canadian clinical guidelines to assess outcomes after 12 weeks of treatment; [2] however, it precludes making conclusions about the benefits of short-term treatment. Further, since treatment for ADHD may be life-long, the short-term duration of most trials limits their generalizability to the real-world context. The generalizability of the findings to a larger clinical population is also hampered by the exclusion of participants with psychiatric comorbidities from most included RCTs. Second, the geometry of the evidence networks precluded formal evaluation of inconsistency, and the findings of most outcomes were downgraded in the GRADE assessment because of incoherence, imprecision, indirectness, and within-study bias (risk of bias). We also note the potential for publication bias, as 3 RCTs were included but did not report outcomes of interest for this review. Of these, the NCT records for two studies [110, 111] list quality of life, executive function, and clinical response among the outcome measures; however, these data have not been reported. Furthermore, some RCTs reported clinical response (observer and self-reported) as a dichotomous variable (i.e., number of patients who experienced a clinical response). The categorization of a continuous variable (as “response” or “no response”) introduces additional issues such as information loss, variation in the chosen-cut-point, failure to consider variation in response, and may increase the chance of a false positive result [112]. Third, as with all network meta-analyses, there is a risk of type I error. In particular, because of repeated testing, meta-analyses may particularly susceptible to making claims of efficacy when there is no true effect [113]. Our analyses did not explicitly consider the possibility of type 1 error and this should be considered in the interpretation of the findings, particularly for those where the confidence or credible intervals narrowly includes the null value. Fourth, some commonly prescribed interventions were under-represented in the evidence networks owing to a lack of published RCTs that met the eligibility criteria. Fifth, more than half of the included RCTs were at unclear or high risk of bias for blinding, which may have had an impact on the reliability of subjective data. Additionally, few trials assessed whether blinding was maintained over the treatment period, which may further confound the interpretation of the individual trial results. Sixth, we included only English-language publications, which may have excluded some relevant trials. However, we compared our included studies list to that of a recent systematic review with no language restrictions and did not identify any relevant non-English RCTs [108]. Finally, limited data from RCTs were available for some outcomes, especially for harms and quality of life over a long treatment period. This is highlighted by the small number of RCTs reporting serious adverse events, withdrawals due to adverse events, and treatment discontinuations beyond 12 weeks of treatment.
Because AHDH is a chronic condition, this lack of robust long-term clinical studies represents an important gap in the evidence base, and additional evidence from non-randomized and observational studies may be required to inform policy and clinical decision making. Further, policy decisions about which therapies are most appropriate may need to consider additional relevant factors, which may include burden of disease, therapeutic impact, safety profile, and socioeconomic impact [114]. For individual patients, the choice between treatments should reflect shared decision-making between the clinician and patient, with consideration of the patient’s values, preferences, and individual circumstances, as well as the potential risks and benefits of the treatment options [115].
Conclusions
Overall, we found that ADHD pharmacotherapies, as a class, improved clinical response relative to placebo; however, most studies were at risk of at least one important source of bias, and the beneficial effects were primarily observed in English-language studies in which participants and/or investigators were aware of treatment assignment. While there was limited evidence of serious harms, harm outcomes were inadequately reported over a long treatment duration. Overall, the certainty of the evidence was very low to low across outcomes, and additional long-term high-quality RCTs are needed to inform on patient-important outcomes such as quality of life and adverse events over a long treatment duration.
Supporting information
(DOCX)
Appendix A: Literature search strategy. Appendix B: ADHD pharmacotherapies included in the systematic review. Appendix C: Eligible scales for review. Appendix D: Included studies. Appendix E: Detailed inclusion and exclusion criteria. Appendix F: Risk of bias assessment. Appendix G: Publication bias. Appendix H: GRADE assessment. Appendix I: Clinical response. Appendix J: Executive function. Appendix K: Quality of life. Appendix L: Driving behavior. Appendix M: Serious adverse events. Appendix N: Withdrawals due to adverse events. Appendix O: Treatment discontinuation. Appendix P: Sensitivity analyses.
(DOCX)
Acknowledgments
The authors thank Nazmun Nahar for assistance with reference management and the creation of tables and figures for this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by the Ontario Drug Policy Research Network, which is funded by a grant from the Ontario Ministry of Health and Long-Term Care Health System Research Fund.
References
- 1.Hesson J, Fowler K. Prevalence and Correlates of Self-Reported ADD/ADHD in a Large National Sample of Canadian Adults. J Atten Disord. 2015;22(2):191–200. 10.1177/1087054715573992 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Attention Deficit Hyperactivity Disorder Resource Alliance. Canadian ADHD Practice Guidelines 2011. 1–148 p. [Google Scholar]
- 3.Quintero J, Morales I, Vera R, Zuluaga P, Fernández A. The Impact of Adult ADHD in the Quality of Life Profile. J Atten Disord. 2017:108705471773304-. 10.1177/1087054717733046 [DOI] [PubMed] [Google Scholar]
- 4.Morkem R, Patten S, Queenan J, Barber D. Recent Trends in the Prescribing of ADHD Medications in Canadian Primary Care. J Atten Disord. 2017. 10.1177/1087054717720719 [DOI] [PubMed] [Google Scholar]
- 5.Coyle D, Lee K, Loncar M, Tingley K. Treatment of attention deficit hyperactivity disorder in adults: final pharmacoeconomic report. 2015. [Google Scholar]
- 6.Bolea-Alamanac B, Nutt DJ, Adamou M, Asherson P, Bazire S, Coghill D, et al. Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the British Association for Psychopharmacology. Journal of psychopharmacology (Oxford, England). 2014;28(3):179–203. Epub 2014/02/15. 10.1177/0269881113519509 . [DOI] [PubMed] [Google Scholar]
- 7.Canadian ADHD Resource Alliance (CADDRA): Canadian ADHD Practice Guidelines. Toronto, Ontario: CADDRA, 2018. [Google Scholar]
- 8.Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed: The Cochrane Collaboration; 2011.
- 9.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162(11):777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 10.McDonagh MS, Peterson K, Thakurta S, Low A. Drug Class Review: Pharmacologic Treatments for Attention Deficit Hyperactivity Disorder: Final Update 4 Report. Portland (OR): Oregon Health & Science University, 2011. [PubMed] [Google Scholar]
- 11.CADTH. Grey Matters Light Ottawa, ON: CADTH; 2015 [cited 2017]. Available from: https://www.cadth.ca/media/is/cadth_Handout_greymatters_light_e.pdf.
- 12.Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44. Epub 2017/10/21. 10.1016/j.jclinepi.2017.10.005 . [DOI] [PubMed] [Google Scholar]
- 13.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. Epub 2011/01/05. 10.1016/j.jclinepi.2010.04.026 . [DOI] [PubMed] [Google Scholar]
- 14.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082 Epub 2020/04/04. 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castells X, Ramos-Quiroga JA, Rigau D, Bosch R, Nogueira M, Vidal X, et al. Efficacy of Methylphenidate for Adults with Attention-Deficit Hyperactivity Disorder. CNS Drugs. 2011;25(2):157–69. 10.2165/11539440-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 16.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International Journal of Epidemiology. 2008;37(5):1148–57. 10.1093/ije/dyn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–17. 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS user manual. 2003.
- 19.Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2011;14(4):417–28. Epub 2011/06/15. 10.1016/j.jval.2011.04.002 . [DOI] [PubMed] [Google Scholar]
- 20.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33(5):641–56. 10.1177/0272989X12455847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay GG, Michaels MA, Pakull B. Simulated driving changes in young adults with ADHD receiving mixed amphetamine salts extended release and atomoxetine. J Atten Disord. 2009;12(4):316–29. 10.1177/1087054708322986 . [DOI] [PubMed] [Google Scholar]
- 22.Michelson D, Adler L, Spencer T, Reimherr FW, West SA, Allen AJ, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53(2):112–20. 10.1016/s0006-3223(02)01671-2 . [DOI] [PubMed] [Google Scholar]
- 23.Biederman J, Fried R, Hammerness P, Surman C, Mehler B, Petty CR, et al. The effects of lisdexamfetamine dimesylate on the driving performance of young adults with ADHD: a randomized, double-blind, placebo-controlled study using a validated driving simulator paradigm. J Psychiatr Res. 2012;46(4):484–91. Epub 2012/01/27. 10.1016/j.jpsychires.2012.01.007 . [DOI] [PubMed] [Google Scholar]
- 24.Biederman J, Fried R, Hammerness P, Surman C, Mehler B, Petty CR, et al. The effects of lisdexamfetamine dimesylate on the driving performance of young adults with ADHD: a randomized, double-blind, placebo-controlled study using a validated driving simulator paradigm. Journal of Psychiatric Research. 2012;46(4):484–. 10.1016/j.jpsychires.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 25.Durell TM, Adler LA, Williams DW, Deldar A, McGough JJ, Glaser PE, et al. Atomoxetine treatment of attention-deficit/hyperactivity disorder in young adults with assessment of functional outcomes: a randomized, double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol. 2013;33(1):45–54. Epub 2013/01/02. 10.1097/JCP.0b013e31827d8a23 . [DOI] [PubMed] [Google Scholar]
- 26.Schrantee A, Tamminga HG, Bouziane C, Bottelier MA, Bron EE, Mutsaerts H-JM, et al. Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: A randomized clinical trial. JAMA Psychiatry. 2016;73(9):955–62. 10.1001/jamapsychiatry.2016.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigal T, Childress A, Frick G, Yan B, Wigal S, Madhoo M. Effects of SHP465 mixed amphetamine salts in adults with ADHD in a simulated adult workplace environment. Postgrad Med. 2018:111–21. CN-01430940. 10.1080/00325481.2018.1389227 [DOI] [PubMed] [Google Scholar]
- 28.Konstenius M, Jayaram-Lindstrom N, Guterstam J, Beck O, Philips B, Franck J. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial. Addiction. 2014;109(3):440–9. Epub 2013/10/15. 10.1111/add.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223–8. 10.1097/00004714-200104000-00015 . [DOI] [PubMed] [Google Scholar]
- 30.Tenenbaum S, Paull JC, Sparrow EP, Dodd DK, Green L. An experimental comparison of Pycnogenol and methylphenidate in adults with Attention-Deficit/Hyperactivity Disorder (ADHD). J Atten Disord. 2002;6(2):49–60. 10.1177/108705470200600201 . [DOI] [PubMed] [Google Scholar]
- 31.Wilens TE, Haight BR, Horrigan JP, Hudziak JJ, Rosenthal NE, Connor DF, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry. 2005;57(7):793–801. 10.1016/j.biopsych.2005.01.027 . [DOI] [PubMed] [Google Scholar]
- 32.Young JL, Sarkis E, Qiao M, Wietecha L. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51–60. 10.1097/WNF.0b013e31820c00eb . [DOI] [PubMed] [Google Scholar]
- 33.Adler LA, Dirks B, Deas PF, Raychaudhuri A, Dauphin MR, Lasser RA, et al. Lisdexamfetamine dimesylate in adults with attention-deficit/ hyperactivity disorder who report clinically significant impairment in executive function: results from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(7):694–702. Epub 2013/08/16. 10.4088/JCP.12m08144 . [DOI] [PubMed] [Google Scholar]
- 34.Adler LA, Goodman DW, Kollins SH, Weisler RH, Krishnan S, Zhang Y, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–73. 10.4088/jcp.v69n0903 . [DOI] [PubMed] [Google Scholar]
- 35.Adler LA, Liebowitz M, Kronenberger W, Qiao M, Rubin R, Hollandbeck M, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212–21. 10.1002/da.20549 . [DOI] [PubMed] [Google Scholar]
- 36.Adler LA, Spencer T, Brown TE, Holdnack J, Saylor K, Schuh K, et al. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29(1):44–50. 10.1097/JCP.0b013e318192e4a0 . [DOI] [PubMed] [Google Scholar]
- 37.Adler LA, Spencer TJ, Levine LR, Ramsey JL, Tamura R, Kelsey D, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord. 2008;11(6):720–7. 10.1177/1087054707308490 . [DOI] [PubMed] [Google Scholar]
- 38.Adler LA, Zimmerman B, Starr HL, Silber S, Palumbo J, Orman C, et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. J Clin Psychopharmacol. 2009;29(3):239–47. 10.1097/JCP.0b013e3181a390ce . [DOI] [PubMed] [Google Scholar]
- 39.Arnold VK, Feifel D, Earl CQ, Yang R, Adler LA. A 9-week, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study to evaluate the efficacy and safety of modafinil as treatment for adults with ADHD. J Atten Disord. 2014;18(2):133–44. Epub 2012/05/24. 10.1177/1087054712441969 . [DOI] [PubMed] [Google Scholar]
- 40.Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Harpold T, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59(9):829–35. 10.1016/j.biopsych.2005.09.011 . [DOI] [PubMed] [Google Scholar]
- 41.Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Kotarski M, et al. A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2010;30(5):549–53. 10.1097/JCP.0b013e3181ee84a7 . [DOI] [PubMed] [Google Scholar]
- 42.Biehl S, Merz C, Dresler T, Heupel J, Reichert S, Jacob C, et al. Increase or decrease of fMRI activity in adult attention deficit/hyperactivity disorder: Does it depend on task difficulty? Int J Neuropsychopharmacol. 2016;19(10):1–10. 2017-25135-005. 10.1093/ijnp/pyw049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butterfield ME, Saal J, Young B, Young JL. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: A double blind, placebo-controlled study. Psychiatry Res. 2016;236:136–41. 10.1016/j.psychres.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 44.Casas M, Rosler M, Sandra Kooij JJ, Ginsberg Y, Ramos-Quiroga JA, Heger S, et al. Efficacy and safety of prolonged-release OROS methylphenidate in adults with attention deficit/hyperactivity disorder: a 13-week, randomized, double-blind, placebo-controlled, fixed-dose study. World J Biol Psychiatry. 2013;14(4):268–81. Epub 2011/11/24. 10.3109/15622975.2011.600333 . [DOI] [PubMed] [Google Scholar]
- 45.Durell TM, Adler LA, Williams DW, Deldar A, McGough JJ, Glaser PE, et al. Atomoxetine treatment of attention-deficit/hyperactivity disorder in young adults with assessment of functional outcomes: a randomized, double-blind, placebo-controlled clinical trial. Journal of Clinical Psychopharmacology. 2013;33(1):45–. 10.1097/JCP.0b013e31827d8a23 [DOI] [PubMed] [Google Scholar]
- 46.Fan L-Y, Chou T-L, Gau SS-F. Neural correlates of atomoxetine improving inhibitory control and visual processing in Drug-naïve adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2017;38:4850–64. 10.1002/hbm.23683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frick G, Yan B, Adler LA. Triple-Bead Mixed Amphetamine Salts (SHP465) in Adults With ADHD: Results of a Phase 3, Double-Blind, Randomized, Forced-Dose Trial. J Atten Disord. 2017:1 10.1177/1087054717696771 [DOI] [PubMed] [Google Scholar]
- 48.Ginsberg Y, Lindefors N. Methylphenidate treatment of adult male prison inmates with attention-deficit hyperactivity disorder: randomised double-blind placebo-controlled trial with open-label extension. Br J Psychiatry. 2012;200(1):68–73. Epub 2011/11/15. 10.1192/bjp.bp.111.092940 . [DOI] [PubMed] [Google Scholar]
- 49.Goodman DW, Starr HL, Ma Y-W, Rostain AL, Ascher S, Armstrong RB. Randomized, 6-Week, Placebo-Controlled Study of Treatment for Adult Attention-Deficit/Hyperactivity Disorder: Individualized Dosing of Osmotic-Release Oral System (OROS) Methylphenidate With a Goal of Symptom Remission. J Clin Psychiatry. 2017;78(01):105–14. 10.4088/JCP.15m10348 [DOI] [PubMed] [Google Scholar]
- 50.Goto T, Hirata Y, Takita Y, Trzepacz PT, Allen AJ, Song D-H, et al. Efficacy and Safety of Atomoxetine Hydrochloride in Asian Adults With ADHD: A Multinational 10-Week Randomized Double-Blind Placebo-Controlled Asian Study. J Atten Disord. 2017;21(2):100–9. 10.1177/1087054713510352 [DOI] [PubMed] [Google Scholar]
- 51.Hamedi M, Mohammdi M, Ghaleiha A, Keshavarzi Z, Jafarnia M, Keramatfar R, et al. Bupropion in adults with Attention-Deficit/Hyperactivity Disorder: a randomized, double-blind study. Acta Med Iran. 2014;52(9):675–80. Epub 2014/10/18. PubMed . [PubMed] [Google Scholar]
- 52.Huss M, Ginsberg Y, Tvedten T, Arngrim T, Philipsen A, Carter K, et al. Methylphenidate hydrochloride modified-release in adults with attention deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial. Adv Ther. 2014;31(1):44–65. Epub 2013/12/29. 10.1007/s12325-013-0085-5 ; PubMed Central PMCID: PMC3905180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kollins SH, English JS, Itchon-Ramos N, Chrisman AK, Dew R, O'Brien B, et al. A pilot study of lis-dexamfetamine dimesylate (LDX/SPD489) to facilitate smoking cessation in nicotine-dependent adults with ADHD. J Atten Disord. 2014;18(2):158–68. Epub 2012/04/18. 10.1177/1087054712440320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konstenius M, Jayaram-Lindstrom N, Beck O, Franck J. Sustained release methylphenidate for the treatment of ADHD in amphetamine abusers: a pilot study. Drug Alcohol Depend. 2010;108(1–2):130–3. 10.1016/j.drugalcdep.2009.11.006 . [DOI] [PubMed] [Google Scholar]
- 55.Kuperman S, Perry PJ, Gaffney GR, Lund BC, Bever-Stille KA, Arndt S, et al. Bupropion SR vs. methylphenidate vs. placebo for attention deficit hyperactivity disorder in adults. Ann Clin Psychiatry. 2001;13(3):129–34. 10.1023/a:1012239823148 . [DOI] [PubMed] [Google Scholar]
- 56.Lee SI, Song DH, Shin DW, Kim JH, Lee YS, Hwang JW, et al. Efficacy and safety of atomoxetine hydrochloride in Korean adults with attention-deficit hyperactivity disorder. Asia Pac Psychiatry. 2014;6(4):386–96. Epub 2014/10/28. 10.1111/appy.12160 . [DOI] [PubMed] [Google Scholar]
- 57.Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2001;9(1):83–90. 10.1037/1064-1297.9.1.83 . [DOI] [PubMed] [Google Scholar]
- 58.Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87(1):20–9. 10.1016/j.drugalcdep.2006.07.004 . [DOI] [PubMed] [Google Scholar]
- 59.Levin FR, Evans SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV. Treatment of methadone-maintained patients with adult ADHD: double-blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend. 2006;81(2):137–48. 10.1016/j.drugalcdep.2005.06.012 . [DOI] [PubMed] [Google Scholar]
- 60.Levin FR, Mariani JJ, Specker S, Mooney M, Mahony A, Brooks DJ, et al. Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder a randomized clinical trial. JAMA Psychiatry. 2015;72:593–602. 10.1001/jamapsychiatry.2015.41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matochik JA, Liebenauer LL, King AC, Szymanski HV, Cohen RM, Zametkin AJ. Cerebral glucose metabolism in adults with attention deficit hyperactivity disorder after chronic stimulant treatment. Am J Psychiatry. 1994;151(5):658–64. 10.1176/ajp.151.5.658 . [DOI] [PubMed] [Google Scholar]
- 62.McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, Brady KT. A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. Am J Addict. 2010;19(6):481–9. 10.1111/j.1521-0391.2010.00076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medori R, Ramos-Quiroga JA, Casas M, Kooij JJ, Niemela A, Trott GE, et al. A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63(10):981–9. 10.1016/j.biopsych.2007.11.008 . [DOI] [PubMed] [Google Scholar]
- 64.Ni HC, Shang CY, Gau SS, Lin YJ, Huang HC, Yang LK. A head-to-head randomized clinical trial of methylphenidate and atomoxetine treatment for executive function in adults with attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2013;16(9):1959–73. Epub 2013/05/16. 10.1017/S1461145713000357 . [DOI] [PubMed] [Google Scholar]
- 65.Paterson R, Douglas C, Hallmayer J, Hagan M, Krupenia Z. A randomised, double-blind, placebo-controlled trial of dexamphetamine in adults with attention deficit hyperactivity disorder. Aust N Z J Psychiatry. 1999;33:494–502. 10.1080/j.1440-1614.1999.00590.x . [DOI] [PubMed] [Google Scholar]
- 66.Philipsen A, Graf E, Tebartz van Elst L, Jans T, Warnke A, Hesslinger B, et al. Evaluation of the efficacy and effectiveness of a structured disorder tailored psychotherapy in ADHD in adults: study protocol of a randomized controlled multicentre trial. Atten Defic Hyperact Disord. 2010;2(4):203–12. Epub 2011/03/25. 10.1007/s12402-010-0046-7 . [DOI] [PubMed] [Google Scholar]
- 67.Reimherr FW, Hedges DW, Strong RE, Marchant BK, Williams ED. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245–51. Epub 2008/06/24. PubMed [PMC free article] [PubMed] [Google Scholar]
- 68.Retz W, Rosler M, Ose C, Scherag A, Alm B, Philipsen A, et al. Multiscale assessment of treatment efficacy in adults with ADHD: a randomized placebo-controlled, multi-centre study with extended-release methylphenidate. World J Biol Psychiatry. 2012;13(1):48–59. Epub 2010/12/16. 10.3109/15622975.2010.540257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosler M, Fischer R, Ammer R, Ose C, Retz W. A randomised, placebo-controlled, 24-week, study of low-dose extended-release methylphenidate in adults with attention-deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci. 2009;259(2):120–9. 10.1007/s00406-008-0845-4 . [DOI] [PubMed] [Google Scholar]
- 70.Sobanski E, Sabljic D, Alm B, Baehr C, Dittmann RW, Skopp G, et al. A randomized, waiting list-controlled 12-week trial of atomoxetine in adults with ADHD. Pharmacopsychiatry. 2012;45(3):100–7. Epub 2011/12/17. 10.1055/s-0031-1291319 . [DOI] [PubMed] [Google Scholar]
- 71.Spencer T, Biederman J, Wilens T, Doyle R, Surman C, Prince J, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456–63. 10.1016/j.biopsych.2004.11.043 . [DOI] [PubMed] [Google Scholar]
- 72.Spencer TJ, Adler LA, McGough JJ, Muniz R, Jiang H, Pestreich L, et al. Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1380–7. 10.1016/j.biopsych.2006.07.032 . [DOI] [PubMed] [Google Scholar]
- 73.Spencer TJ, Adler LA, Weisler RH, Youcha SH. Triple-bead mixed amphetamine salts (SPD465), a novel, enhanced extended-release amphetamine formulation for the treatment of adults with ADHD: a randomized, double-blind, multicenter, placebo-controlled study. J Clin Psychiatry. 2008;69(9):1437–48. Epub 2008/11/18. 10.4088/jcp.v69n0911 . [DOI] [PubMed] [Google Scholar]
- 74.Sutherland SM, Adler LA, Chen C, Smith MD, Feltner DE. An 8-week, randomized controlled trial of atomoxetine, atomoxetine plus buspirone, or placebo in adults with ADHD. J Clin Psychiatry. 2012;73(4):445–50. Epub 2012/02/09. 10.4088/JCP.10m06788 . [DOI] [PubMed] [Google Scholar]
- 75.Takahashi N, Koh T, Tominaga Y, Saito Y, Kashimoto Y, Matsumura T. A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of osmotic-controlled release oral delivery system methylphenidate HCl in adults with attention-deficit/hyperactivity disorder in Japan. World J Biol Psychiatry. 2014;15(6):488–98. Epub 2014/01/25. 10.3109/15622975.2013.868925 . [DOI] [PubMed] [Google Scholar]
- 76.Weisler RH, Biederman J, Spencer TJ, Wilens TE, Faraone SV, Chrisman AK, et al. Mixed amphetamine salts extended-release in the treatment of adult ADHD: a randomized, controlled trial. CNS Spectr. 2006;11(8):625–39. 10.1017/s1092852900013687 . [DOI] [PubMed] [Google Scholar]
- 77.Weisler RH, Greenbaum M, Arnold V, Yu M, Yan B, Jaffee M, et al. Efficacy and Safety of SHP465 Mixed Amphetamine Salts in the Treatment of Attention-Deficit/Hyperactivity Disorder in Adults: results of a Randomized, Double-Blind, Placebo-Controlled, Forced-Dose Clinical Study. CNS drugs. 2017;31(8):685‐97. CN-01458170. 10.1007/s40263-017-0455-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weisler RH, Pandina GJ, Daly EJ, Cooper K, Gassmann-Mayer C. Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs. 2012;26(5):421–34. Epub 2012/04/24. 10.2165/11631990-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 79.Weiss M, Hechtman L, Adult ARG. A randomized double-blind trial of paroxetine and/or dextroamphetamine and problem-focused therapy for attention-deficit/hyperactivity disorder in adults. J Clin Psychiatry. 2006;67(4):611–9. PubMed . [PubMed] [Google Scholar]
- 80.Wilens TE, Adler LA, Weiss MD, Michelson D, Ramsey JL, Moore RJ, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend. 2008;96(1–2):145–54. 10.1016/j.drugalcdep.2008.02.009 . [DOI] [PubMed] [Google Scholar]
- 81.Wilens TE, Spencer TJ, Biederman J, Girard K, Doyle R, Prince J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry. 2001;158(2):282–8. Epub 2001/02/07. 10.1176/appi.ajp.158.2.282 . [DOI] [PubMed] [Google Scholar]
- 82.Winhusen TM, Somoza EC, Brigham GS, Liu DS, Green CA, Covey LS, et al. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(12):1680–8. 10.4088/JCP.09m05089gry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bain EE, Robieson W, Pritchett Y, Garimella T, Abi-Saab W, Apostol G, et al. A randomized, double-blind, placebo-controlled phase 2 study of alpha4beta2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology. 2013;38(3):405–13. Epub 2012/10/04. 10.1038/npp.2012.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barkley RA, Anderson DL, Kruesi M. A pilot study of the effects of atomoxetine on driving performance in adults with ADHD. J Atten Disord. 2007;10(3):306–16. 10.1177/1087054706292122 . [DOI] [PubMed] [Google Scholar]
- 85.Barkley RA, Murphy KR, O'Connell T, Connor DF. Effects of two doses of methylphenidate on simulator driving performance in adults with attention deficit hyperactivity disorder. J Safety Res. 2005;36(2):121–31. 10.1016/j.jsr.2005.01.001 . [DOI] [PubMed] [Google Scholar]
- 86.Bouffard R, Hechtman L, Minde K, Iaboni-Kassab F. The efficacy of 2 different dosages of methylphenidate in treating adults with attention-deficit hyperactivity disorder. Can J Psychiatry. 2003;48(8):546–54. 10.1177/070674370304800806 . [DOI] [PubMed] [Google Scholar]
- 87.Bron TI, Bijlenga D, Boonstra AM, Breuk M, Pardoen WF, Beekman AT, et al. OROS-methylphenidate efficacy on specific executive functioning deficits in adults with ADHD: a randomized, placebo-controlled cross-over study. Eur Neuropsychopharmacol. 2014;24(4):519–28. Epub 2014/02/11. 10.1016/j.euroneuro.2014.01.007 . [DOI] [PubMed] [Google Scholar]
- 88.Herring WJ, Wilens TE, Adler LA, Baranak C, Liu K, Snavely DB, et al. Randomized controlled study of the histamine H3 inverse agonist MK-0249 in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2012;73(7):e891–8. Epub 2012/08/21. 10.4088/JCP.11m07178 . [DOI] [PubMed] [Google Scholar]
- 89.Jain U, Hechtman L, Weiss M, Ahmed TS, Reiz JL, Donnelly GA, et al. Efficacy of a novel biphasic controlled-release methylphenidate formula in adults with attention-deficit/hyperactivity disorder: results of a double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2007;68(2):268–77. Epub 2007/03/06. 10.4088/jcp.v68n0213 . [DOI] [PubMed] [Google Scholar]
- 90.Kooij JJ, Burger H, Boonstra AM, Van der Linden PD, Kalma LE, Buitelaar JK. Efficacy and safety of methylphenidate in 45 adults with attention-deficit/hyperactivity disorder. A randomized placebo-controlled double-blind cross-over trial. Psychol Med. 2004;34(6):973–82. 10.1017/s0033291703001776 . [DOI] [PubMed] [Google Scholar]
- 91.Martin PT, Corcoran M, Zhang P, Katic A. Randomized, double-blind, placebo-controlled, crossover study of the effects of lisdexamfetamine dimesylate and mixed amphetamine salts on cognition throughout the day in adults with attention-deficit/hyperactivity disorder. Clin Drug Investig. 2014;34(2):147–57. Epub 2013/12/04. 10.1007/s40261-013-0156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Marchant BK. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry. 2007;68(1):93–101. Epub 2007/02/08. 10.4088/jcp.v68n0113 . [DOI] [PubMed] [Google Scholar]
- 93.Spencer T, Biederman J, Wilens T, Faraone S, Prince J, Gerard K, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58(8):775–82. 10.1001/archpsyc.58.8.775 . [DOI] [PubMed] [Google Scholar]
- 94.Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155(5):693–5. 10.1176/ajp.155.5.693 . [DOI] [PubMed] [Google Scholar]
- 95.Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1995;52(6):434–43. 10.1001/archpsyc.1995.03950180020004 . [DOI] [PubMed] [Google Scholar]
- 96.Taylor FB, Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol. 2000;10(4):311–20. 10.1089/cap.2000.10.311 . [DOI] [PubMed] [Google Scholar]
- 97.Verster JC, Bekker EM, de Roos M, Minova A, Eijken EJ, Kooij JJ, et al. Methylphenidate significantly improves driving performance of adults with attention-deficit hyperactivity disorder: a randomized crossover trial. Journal of psychopharmacology (Oxford, England). 2008;22(3):230–7. 10.1177/0269881107082946 . [DOI] [PubMed] [Google Scholar]
- 98.Wender PH, Reimherr FW, Marchant BK, Sanford ME, Czajkowski LA, Tomb DA. A one year trial of methylphenidate in the treatment of ADHD. J Atten Disord. 2011;15(1):36–45. Epub 2010/01/15. 10.1177/1087054709356188 . [DOI] [PubMed] [Google Scholar]
- 99.Wender PH, Reimherr FW, Wood D, Ward M. A controlled study of methylphenidate in the treatment of attention deficit disorder, residual type, in adults. Am J Psychiatry. 1985;142(5):547–52. 10.1176/ajp.142.5.547 . [DOI] [PubMed] [Google Scholar]
- 100.Wigal T, Brams M, Frick G, Yan B, Madhoo M. A randomized, double-blind study of SHP465 mixed amphetamine salts extended-release in adults with ADHD using a simulated adult workplace design. Postgrad Med. 2018;130(5):481–93. 10.1080/00325481.2018.1481712 . [DOI] [PubMed] [Google Scholar]
- 101.Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J, et al. Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behav Brain Funct. 2010;6:34 10.1186/1744-9081-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Philipsen A, Jans T, Graf E, Matthies S, Borel P, Colla M, et al. Effects of Group Psychotherapy, Individual Counseling, Methylphenidate, and Placebo in the Treatment of Adult Attention-Deficit/Hyperactivity Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2015;72(12):1199 10.1001/jamapsychiatry.2015.2146 [DOI] [PubMed] [Google Scholar]
- 103.Biederman J, Mick E, Spencer T, Surman C, Faraone SV. Is response to OROS-methylphenidate treatment moderated by treatment with antidepressants or psychiatric comorbidity? A secondary analysis from a large randomized double blind study of adults with ADHD. CNS Neurosci Ther. 2012;18(2):126–32. Epub 2011/11/11. 10.1111/j.1755-5949.2010.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bushe C, Day K, Reed V, Karlsdotter K, Berggren L, Pitcher A, et al. A network meta-analysis of atomoxetine and osmotic release oral system methylphenidate in the treatment of attention-deficit/hyperactivity disorder in adult patients. Journal of psychopharmacology (Oxford, England). 2016;30(5):444–58. 10.1177/0269881116636105 [DOI] [PubMed] [Google Scholar]
- 105.Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):Cd007813 Epub 2011/06/17. 10.1002/14651858.CD007813.pub2 . [DOI] [PubMed] [Google Scholar]
- 106.Storebo OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev. 2015;(11):Cd009885 Epub 2015/11/26. 10.1002/14651858.CD009885.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boesen K, Saiz LC, Erviti J, Storebo OJ, Gluud C, Gotzsche PC, et al. The Cochrane Collaboration withdraws a review on methylphenidate for adults with attention deficit hyperactivity disorder. Evid Based Med. 2017;22(4):143–7. Epub 2017/07/15. 10.1136/ebmed-2017-110716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. The Lancet Psychiatry. 2018;5(9):727–38. 10.1016/S2215-0366(18)30269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92. Epub 2003/04/30. 10.1097/01.MLR.0000062554.74615.4C . [DOI] [PubMed] [Google Scholar]
- 110.Shur-Fen Gau S. The Effectiveness of Atomoxetine on Brain Imaging, Neuropsychological, and Social Functions in Adults With Attention Deficit/Hyperactivity Disorder: ClinicalTrials.gov; 2010 [updated November 24, 2010; cited 2020 September 23].
- 111.Collins S. Lisdexamfetamine dimesylate in the treatment of adult ADHD with anxiety disorder comorbidity ClinicalTrials.gov; 2017 [updated August 2, 2017; cited 2020 September 23]. Available from: clinicaltrials.gov/show/NCT01863459.
- 112.Altman DG, Royston P. The cost of dichotomising continuous variables. Bmj. 2006;332(7549):1080 Epub 2006/05/06. 10.1136/bmj.332.7549.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Del Re AC, Spielmans GI, Fluckiger C, Wampold BE. Efficacy of new generation antidepressants: differences seem illusory. PLoS One. 2014;8(6):e63509 Epub 2013/06/12. 10.1371/journal.pone.0063509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Angelis A, Linch M, Montibeller G, Molina-Lopez T, Zawada A, Orzel K, et al. Multiple Criteria Decision Analysis for HTA across four EU Member States: Piloting the Advance Value Framework. Soc Sci Med. 2020;246:112595 Epub 2019/12/25. 10.1016/j.socscimed.2019.112595 . [DOI] [PubMed] [Google Scholar]
- 115.Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. Jama. 2014;312(13):1295–6. Epub 2014/10/01. 10.1001/jama.2014.10186 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Appendix A: Literature search strategy. Appendix B: ADHD pharmacotherapies included in the systematic review. Appendix C: Eligible scales for review. Appendix D: Included studies. Appendix E: Detailed inclusion and exclusion criteria. Appendix F: Risk of bias assessment. Appendix G: Publication bias. Appendix H: GRADE assessment. Appendix I: Clinical response. Appendix J: Executive function. Appendix K: Quality of life. Appendix L: Driving behavior. Appendix M: Serious adverse events. Appendix N: Withdrawals due to adverse events. Appendix O: Treatment discontinuation. Appendix P: Sensitivity analyses.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.