Abstract
Background:
In response to the public health emergency created by the COVID-19 pandemic, American Heart Association volunteers and staff aimed to rapidly develop and launch a resource for the medical and research community to expedite scientific advancement through shared learning, quality improvement, and research. In less than 4 weeks after it was first announced on April 3, 2020, AHA’s COVID-19 CVD Registry powered by Get With The Guidelines® (GWTG) received its first clinical records.
Methods and Results:
Participating hospitals are enrolling consecutive hospitalized patients with active COVID-19 disease, regardless of CVD status. This hospital quality improvement program will allow participating hospitals and health systems to evaluate patient-level data including mortality rates, intensive care unit (ICU) bed days, and ventilator days from individual review of electronic medical records of sequential adult patients with active COVID-19 infection. Participating sites can leverage these data for onsite, rapid quality improvement and benchmarking versus other institutions. After 9 weeks, more than 130 sites have enrolled in the program and more than 4,000 records have been abstracted in the national dataset. Additionally, the aggregate dataset will be a valuable data resource for the medical research community.
Conclusions:
The AHA COVID-19 CVD Registry will support greater understanding of the impact of COVID-19 on cardiovascular disease and will inform best practices for evaluation and management of patients with COVID-19.
Keywords: cardiovascular disease, cardiovascular complications, infectious disease, outcomes research, clinical
Patients with active COVID-19 infection often present with respiratory symptoms, yet outcomes are worse in patients with pre-existing cardiovascular (CV) disease or cardiac risk factors than in those without.1 Furthermore, COVID-19 itself is associated with adverse CV outcomes, including heart failure, cardiogenic shock, arrhythmias, stroke, and thromboembolic disease1–8.
Current understanding of the CV implications of COVID-19 come primarily from single center data, often limited by small sample size, incomplete follow up, and non-systematic data collection, limiting the ability to draw robust inferences and to generalize the findings. Thus, the natural history of COVID-19 and CV outcomes across diverse populations and clinical settings are poorly understood. There is a lack of high-quality data on proposed therapies which has led to use of unproven therapies and variation in practice9. Although clinical trials are forthcoming, observational studies of current practices in COVID-19 management and their association with patient outcomes may provide early evidence about promising strategies to guide research and clinical care of COVID-19 patients.
Many professional societies quickly recognized the early need for a structured mechanism to systematically collect data to support their members and the patients they treat. In the months since the first case of COVID-19 was confirmed in United States, there have been more than 80 patient registries listed with the U.S. National Library of Medicine. Many of the newly launched COVID-19 registries focus on specific patient populations, including those with different forms of cancer, dermatologic manifestations, maternity patients, and more.
AHA’s COVID-19 CVD Registry was intentionally designed to capture cardiovascular data from hospitalized adult patients with active COVID-19 infection, including those without prior history of cardiovascular or cerebrovascular disease. By including a broad and diverse patient population across many U.S. hospitals and capturing data throughout their hospital course of care, hospitals and researchers will be equipped to identify variations in care that are associated with patient outcomes.
AHA’s COVID-19 CVD Registry addresses the need for systematic capture of real-world practice patterns and outcomes in care. The Registry aims to collect complete case data on patient care, comorbidities, treatments, in hospital outcomes and discharge destinations for all hospitalized adult patients with COVID-19 infections at participating sites. Given emerging evidence of the important role of cardiovascular biomarkers in identifying patients with COVID-19 at high risk for complications,10 the Registry will collect serial cardiac, thrombotic, and inflammatory biomarkers over the course of the patient’s hospitalization. Participating sites retrospectively review and abstract data from patients who were treated and discharged prior to the inception of the registry, and will continue to abstract data from clinical encounters with current and future COVID-19 patients throughout the duration of the pandemic.
Methods
Design
AHA’s COVID-19 CVD Registry powered By Get With The Guidelines (GWTG) is a quality improvement program built on the existing GWTG platform. The Registry is designed to fill the unique gap in understanding CV risk and outcomes that the COVID-19 pandemic has created. To build the Registry, AHA volunteers and staff conducted an environmental scan of the published literature, anecdotal evidence, and data elements captured in clinical care (Figure); through an iterative, consensus process, the Steering Committee curated a list of core data elements that inform current knowledge gaps. Benchmark reports will allow participating sites to evaluate their patterns of care and patient outcomes, and to identify opportunities to optimize care.
Figure:
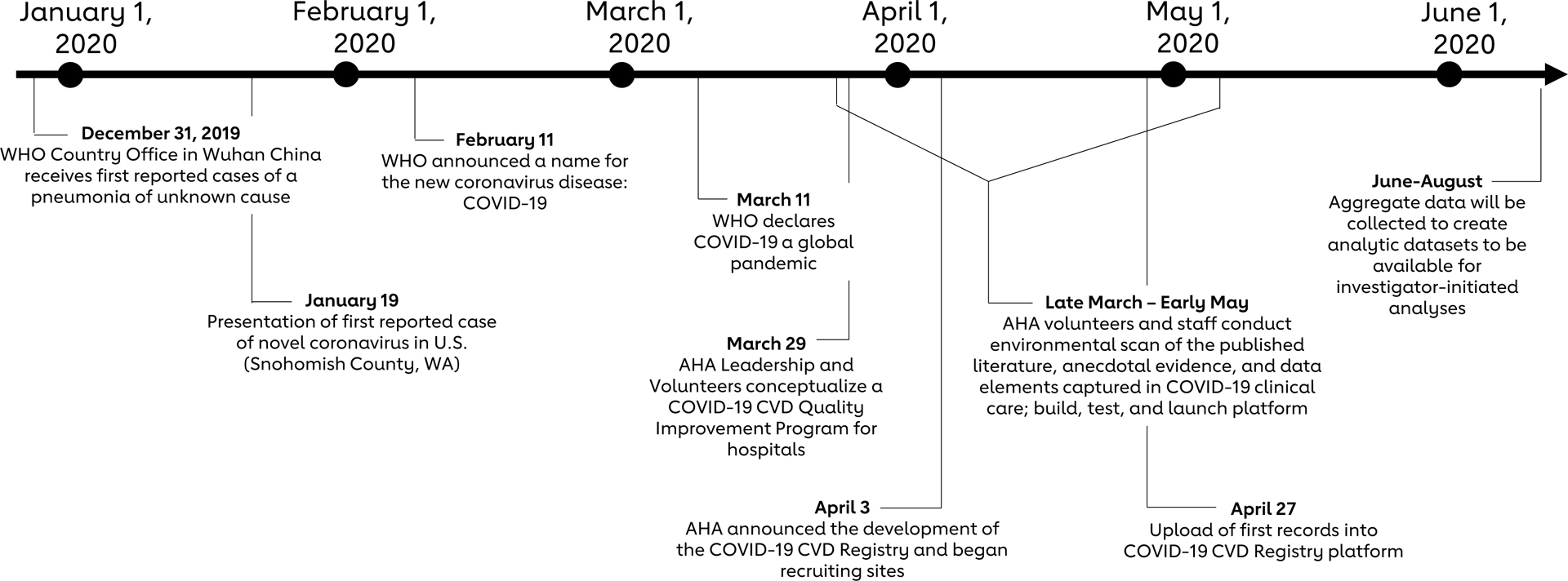
Abbreviated timeline of development for AHA’s COVID-19 CVD Registry Powered by Get With The Guidelines®. Following the first known reports of the novel coronavirus globally11, 12 and within the United States13, the American Heart Association COVID-19 CVD Registry Quality Improvement program and platform was conceptualized. Four weeks after the Registry was announced, the first records were uploaded into the platform. An aggregated, deidentified aggregate dataset will be available for investigator-initiated analyses.
Participating sites and eligible patient records
The COVID-19 CVD Registry is voluntary and open to all hospitals and health systems in the United States treating adult patients with active COVID-19 infections. Though 71% of sites currently enrolled are actively participating in one or more of AHA’s GWTG Quality Improvement programs, participation in an established GWTG program is not a prerequisite to enroll in the COVID-19 CVD Registry. On June 5, 2020, there were more than 130 hospitals, health systems, and medical centers enrolled from 30 states across the U.S. Additionally, more than 4,000 records and 18,000 patient days of serial labs were entered into the national dataset within the first five weeks that the data platform was available to participating sites. A current list of participating sites is available at www.heart.org/COVIDRegistry.
Case records should be uploaded from all consecutive patients 18 years of age or older hospitalized at each site with an acute COVID-19 diagnosis, regardless of hospital location or admitting department. Abstraction can begin any time after admission, but each record cannot be finalized in the registry until the patient has been discharged, since information through discharge is collected. Data from patients without active COVID-19, but with evidence of prior infection who were then subsequently hospitalized after the initial infection had resolved, will be excluded. To reduce the likelihood of introducing bias and to permit hospital performance comparisons, participating sites will submit data from all patients rather than a sample or subset of patients.
Study procedures
The Registry does not test clinical interventions, and individual patient care is entirely at the discretion of treating clinicians. Because data are abstracted into the Registry retrospectively and anonymously, individual patient informed consent is not required. The COVID-19 CVD Registry thus echoes existing AHA quality improvement programs, and the Institutional Review Boards (IRB) of participating sites will determine whether participation is exempt from IRB oversight or subject to review.
Data capture and monitoring
The COVID-19 CVD Registry currently has more than 200 data elements listed for sites to abstract and upload. See the Table for a representative list of the data elements. Additional information on the data elements, including the full case report form (CRF) and serial lab tracker form, is available at www.heart.org/COVIDRegistry and in the online data supplement. Participating sites will also be provided with coding instructions for uniform data definitions applied across all sites. Participating sites will have access to their own health system data to benchmark with the national dataset.
Table.
Data Elements in COVID-19 CVD Registry
| Category | Examples |
|---|---|
| Demographics | Sex, race/ethnicity, age |
| Medical history | Cardiac risk factors, COPD, autoimmune disorders |
| Medical therapy (prior to admission) | ACE inhibitors, steroids |
| Admission vital signs | Height, weight, blood pressure, heart rate, oxygen saturation |
| Laboratory values (admission) | Creatinine, AST, ALT, |
| Laboratory values (serial) | Troponin, BNP, D-dimer, C-reactive protein, Interleukin-6, lymphocyte count, creatinine |
| COVID Medical therapy (inpatient) | Hydroxychloroquine, lopinavir/ritonavir, remdesivir |
| Cardiovascular medical therapy | Anticoagulants, ACE inhibitor/ARB, statin, diabetes therapies |
| Inpatient interventions | Mechanical ventilation, mechanical circulatory support, |
| In-hospital outcomes | Mortality, stroke, myocardial infarction, arrhythmia, DVT/PE, length of stay |
AHA maintains an audit process enabling comprehensive review of participating sites’ submitted data for compliance with submission requirements. Error checks and warnings are present within the software platform. These real time checks alert users to any data quality issues and prevent saving records with erroneous data until the issues are resolved. Additionally, AHA selects a random sample of hospitals for chart re-abstraction to independently assess data quality. Audits may be conducted onsite or via remote monitoring. AHA will also notify program participants of any actions needed because of audit findings, which can include additional onsite training or conferences with AHA staff. Additionally, AHA receives data quality reports form Duke Clinical Research Institute (DCRI).
The registry aims to be comprehensive without being overly burdensome for data abstractors and sites will receive regular reports of their performance and benchmarking data to use for quality improvement purposes. To further facilitate participating sites’ quality improvement efforts, AHA has provided 50 open fields for sites or networks of sites to collect additional key data elements needed for local quality improvement.
Participating sites abstract data from electronic medical records into a secure, password-protected web-based, data entry platform housed at and maintained by IQVIA Inc. (Durham, NC). The Registry is collecting a Limited Data Set as defined by 45 CFR Section § 164.514(e). Date of birth allows for probabilistic linkages from the aggregated data set to other national data sets (e.g., Centers for Medicare & Medicaid Services claims data), as well as other AHA registries, to gain a better understanding of the overall impact of COVID-19.
To lower the barriers to entry and expedite quality improvement efforts needed to inform future clinical practice guidelines, the Registry is being offered at no cost for sites to enroll. Additionally, participating sites have access to educational and technical resources to help them fully leverage the platform. Sites began abstracting their data into AHA’s COVID-19 CVD Registry in late April 2020 (Figure).
As previously described, COVID-19-specific data elements have also been added to each of the existing GWTG modules for atrial fibrillation, coronary artery disease, heart failure, resuscitation, and stroke.14
National dataset
The structure of the COVID-19 CVD Registry will mirror the existing American Heart Association GWTG framework used to benchmark and improve care of various other CV conditions. Although COVID-19 treatment guidelines have yet to be developed and iterated to establish “best practice” standards of care for COVID-19 patients, a major goal of the registry is to gather a robust, nationally representative data source for aggregated analyses to inform clinical practice. To generate the national, aggregated limited dataset for investigator-initiated analyses, the data will be securely transferred from the IQVIA platform to the DCRI to create a standardized study database15, 16.
Innovation in research process to accelerate knowledge transfer
Given the need for the delivery of valid scientific knowledge in a very short time frame, the Steering Committee and AHA leadership recognized that traditional centralized methods for data analysis and publications would not meet the timeline needed to impact front line clinicians during the pandemic. A novel approach has been developed to facilitate rapid discovery and knowledge dissemination by leveraging the talents of site investigators. In addition to the traditional approach for GWTG registries in which statistical analyses are performed at a central analytical core, the national, aggregate deidentified dataset will be made available for approved analyses on AHA’s Precision Medicine Platform (PMP) at Precision.heart.org. PMP is a cloud-based platform that allows researchers to search and access data through an efficient governing process and analyze data in secure workspaces equipped with statistical analysis tools as well as machine learning and artificial intelligence software programs. Researchers and clinicians are also able to bring their own data to the workspace. The PMP provides clinicians and researchers ready access to the Registry data along with statistical tools and computational power in secure workspaces to enable research to move at a rapid pace.
The Registry will provide clinical scientists seeking to better understand the cardiovascular implications of the COVID-19 pandemic with an invaluable resource. By making the data available to many researchers concurrently on the PMP, AHA and the COVID-19 Steering Committee aim to accelerate the pace of research and scientific discovery through a burst science approach. This rapid development and dissemination of findings can inform and improve clinical practice during this pandemic.
Initially, the aggregated dataset will be available for investigator-initiated studies only for sites participating in the Registry. Site investigators wishing to study the data may submit their research proposal to AHA’s COVID Research and Publications Subcommittee for rapid review. The Committee will prioritize approval of proposals based on clinical importance, feasibility within the dataset, innovation, and other metrics. Applications from early career faculty and fellows in training, with appropriate supervision, are encouraged. Current, detailed information about analyzing the national COVID dataset and proposal form are available at www.heart.org/COVIDResearch.
Limitations
There are limitations that should be noted. First, AHA’s COVID-19 CVD Registry is a voluntary program and participation may be overrepresented by those hospitals and medical centers that have the capacity and resources to participate during the pandemic. To help reduce the barrier to participation, AHA has made this registry available at no cost and has created a data uploader and mapping tools to help lower the technical burden of manual data abstraction. Additionally, some centers are using medical students, research assistants, or other hospital personnel who are unable to perform regular onsite duties during the pandemic to abstract data, including by remote means. Second, the COVID-19 CVD Registry does not capture data on pediatric patients or those with prior COVID-19 infections. Lastly, the COVID-19 CVD Registry must balance data collection needs with resources required to abstract data, so some important variables are not collected or are only collected with limited detail. However, sites can leverage the additional 50 open fields to capture additional data elements that may be important to their patient populations.
Discussion
As health care providers grapple with the challenges of treating patients during the COVID-19 pandemic, there is a pressing need for the rapid development, verification, and dissemination of evidence-based clinical best practices to improve the quality of care and CV outcomes for patients infected with COVID-19.
Building on 20 years of successful delivery of quality improvement programs, the American Heart Association COVID-19 CVD Registry Powered by Get With The Guidelines® was rapidly developed to create a data repository for hospitals and health systems across the US. The Registry aims to capture all consecutive adult patients hospitalized with active COVID-19 infection with a focus on cardiovascular complications in this population. In particular, the serial biomarker data collected is a unique feature of the registry and should provide important insight into early signals of new or worsening cardiovascular or thrombotic conditions (Online Data Supplement).
The Registry facilitates individual site efforts to examine their own data for adult patients with active COVID-19 infections. Importantly, the Registry’s systematic data collection and data standards allow inter-hospital comparisons of patient case mix, disease severity, diagnostic and treatment patterns, and adverse outcomes. As such, the AHA’s national database will be a valuable data source to inform the development of evidence-based clinical practice. It leverages extensive infrastructure and a deep-rooted culture of quality improvement embedded within the GWTG program that spurs hospital performance improvement. This is evidenced by how quickly the registry progressed from inception on March 29, 2020 to the upload of its first records on April 27, 2020. Current enrollment information is available at www.heart.org/COVIDRegistry.
Additionally, by creating multiple points of entry for investigators to access and analyze the national dataset through the PMP, AHA strives to ensure that research and translation move rapidly. This will be accomplished by enabling by providing front line investigators with the data, computational power, analytic tools and project support to perform analyses of the dataset. Future releases will focus on improving efficiency of data collection through interoperability capacity building and leveraging opportunities for collaboration through linkages with other datasets. These aims are focused on the ultimate goals of informing factors associated with cardiovascular outcomes in COVID-19, supporting preparedness in the event of future pandemics, and developing potential therapeutic strategies to improve cardiovascular care and outcomes of patients admitted with COVID-19.
Supplementary Material
Acknowledgments:
The Get With The Guidelines® programs are provided by the American Heart Association.
Sources of Funding: AHA’s suite of Registries is funded by multiple industry sponsors. AHA’s COVID-19 CVD Registry is partially supported by The Gordon and Betty Moore Foundation.
Footnotes
Disclosures: Alger, Bolles, Hall, Rutan, Walchok, and Williams are employees of the American Heart Association. Drs. Bradley and Das have no relevant relationships to disclose (none). Dr. DeLemos receives Grant Support from Abbott Diagnostics and Roche Diagnostics, Consulting Income from Abbott Diagnostics, Ortho Clinical Diagnostics, Quidel Cardiovascular, Amgen, Regeneron, Eli Lilly and Novo Nordisk, and Janssen. Dr. Elkind discloses receiving study drug in-kind from the BMS-Pfizer Alliance for Eliquis and ancillary research funding from Roche for an NIH-funded trial of stroke prevention, and honoraria from UptoDate for chapters related to stroke; Dr. Elkind receives funding from National Institute for Neurological Disorders and Stroke, National Heart Lung and Blood Institute, Leducq Foundation, BMS-Pfizer Alliance for Eliquis, Roche. Dr. Rodriguez is funded by the National Heart, Lung, and Blood Institute (K01 HL 14460) and the American Heart Association/Robert Wood Johnson Harold Amos Medical Faculty Development Program; and serves as an advisor to HealthPals and has received consulting fees from NovoNordisk, the Medicines Company, and Janssen. Dr. Wang has received research grants to the Duke Clinical Research Institute from Abbott, AstraZeneca, Bristol Myers Squibb, Boston Scientific, Cryolife, Chiesi, Merck, Portola, and Regeneron, as well as consulting honoraria from AstraZeneca, Bristol Myers Squibb, and Cryolife.
References:
- 1.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A and Uriel N. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 2.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M and Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q, Yang K, Wang W, Jiang L and Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine. 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X and Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. DOI: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H and Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study Lancet(London, England). 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra MR, Desai SS, Kuy S, Henry TD and Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2007621 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S and Fifi JT. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382:e60 DOI: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendren NS, Drazner MH, Bozkurt B and Cooper LT, Jr. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation. 2020;141:1903–1914. DOI: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O’Donnell CJ and Warraich HJ. Prescription Fill Patterns for Commonly Used Drugs During the COVID-19 Pandemic in the United States. JAMA. 2020. DOI: 10.1001/jama.2020.9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B and Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020. DOI: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Rolling updates on coronavirus disease (COVID-19). Events as they happen. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed May 11, 2020. [Google Scholar]
- 12.World Health Organization. WHO Timeline - COVID-19. Available at: https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19. Accessed May 11, 2020.
- 13.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK and Washington State -nCo VCIT. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alger HM, Williams JH IV, Walchok JG, Bolles M, Fonarow GC and Rutan C. Role of Data Registries in the Time of COVID-19. Circ Cardiovasc Qual Outcomes. 2020;13:e006766 DOI: 10.1161/CIRCOUTCOMES.120.006766 [DOI] [PubMed] [Google Scholar]
- 15.Hong Y and LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–86. [DOI] [PubMed] [Google Scholar]
- 16.Lewis WR, Piccini JP, Turakhia MP, Curtis AB, Fang M, Suter RE, Page RL, 2nd and Fonarow GC. Get With The Guidelines AFIB: novel quality improvement registry for hospitalized patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2014;7:770–777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


